Application of New Al-Si Welding Filler with High Concentration of Copper and Magnesium: High-Temperature Strength and Anti-Corrosion Mechanism
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Microstructural and Element Distribution of SCM Weld Zone
3.2. Mechanical Properties of SCM Weld Zone in Different Environments
4. Conclusions
- (1)
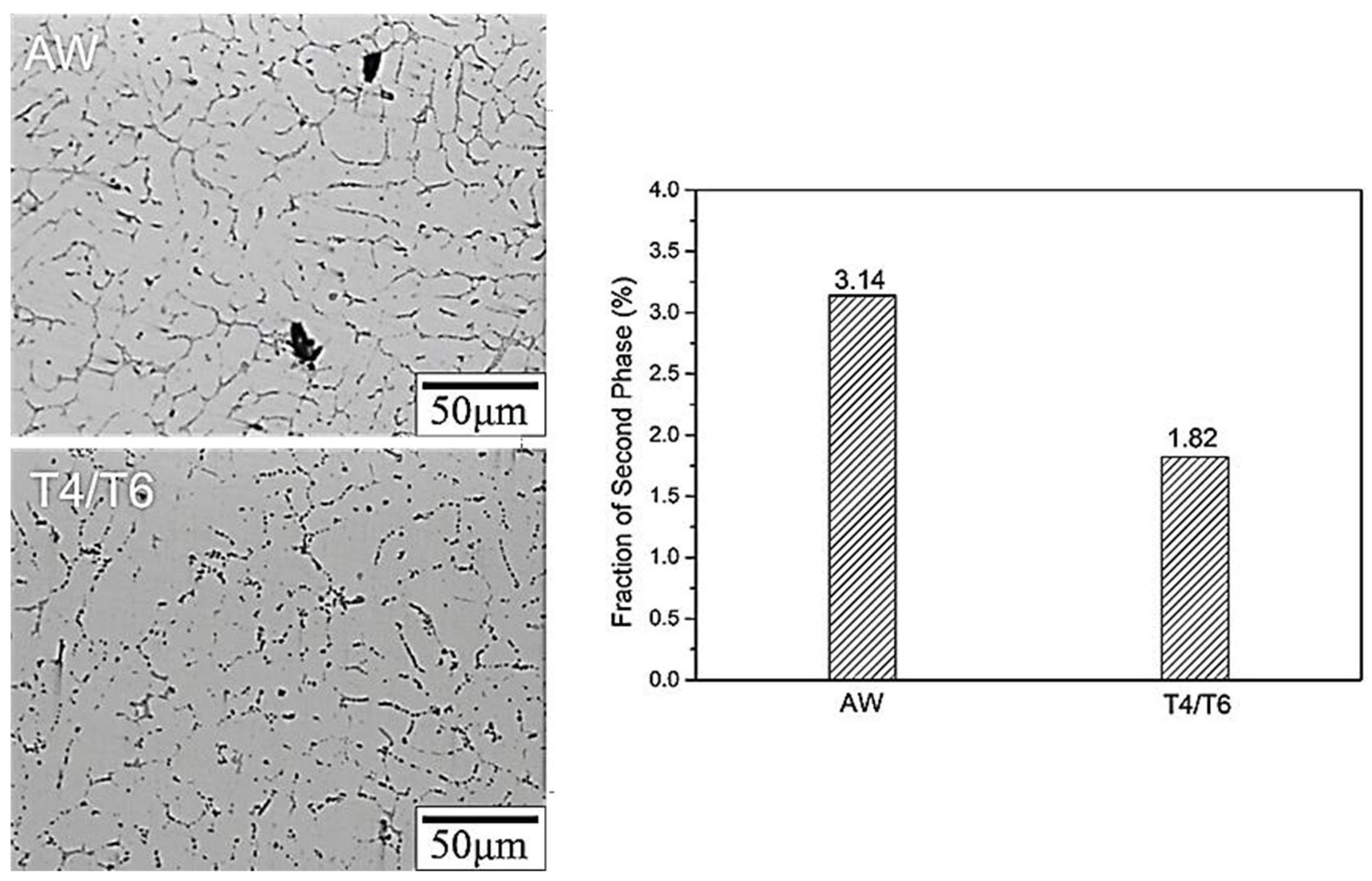
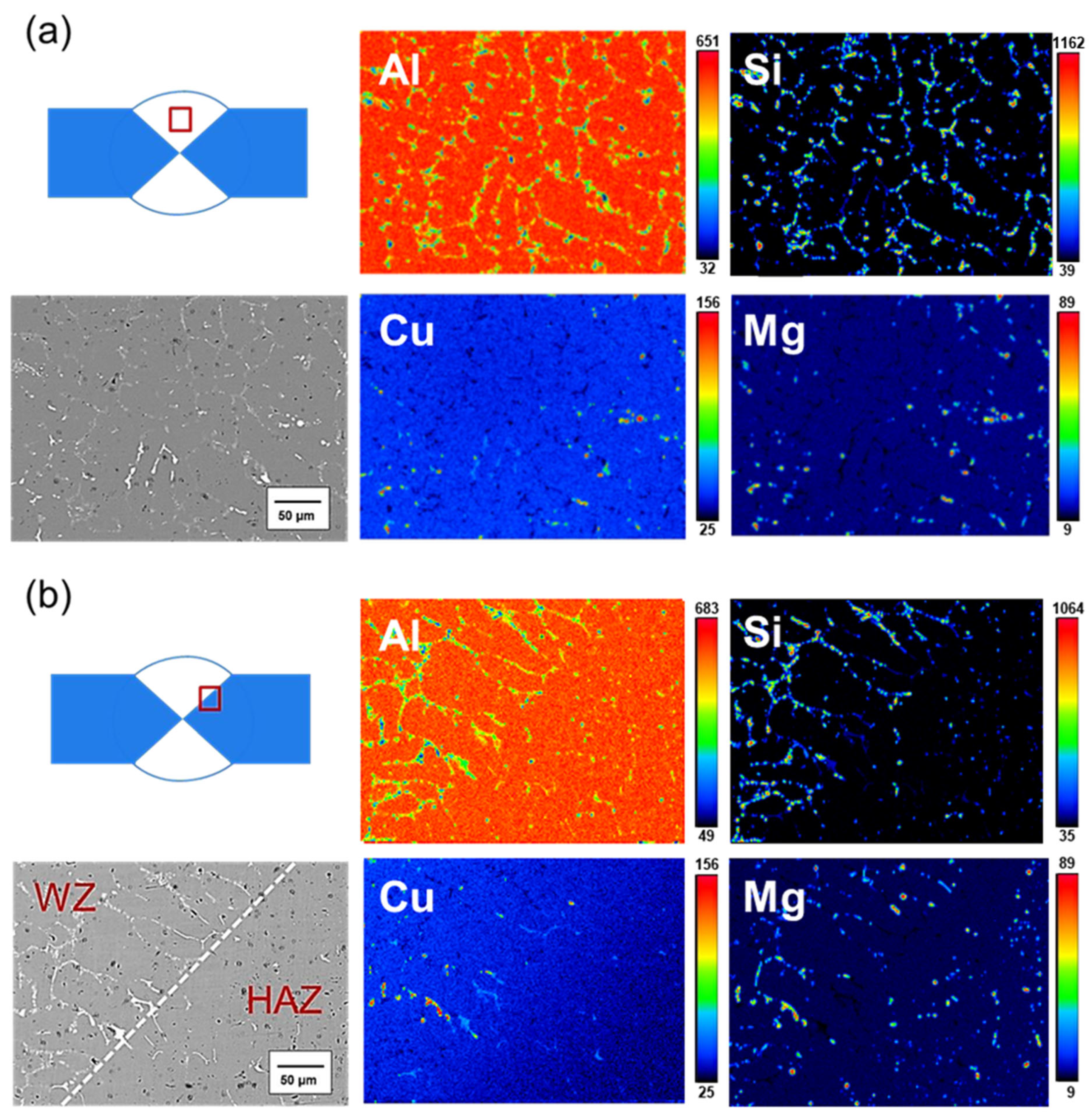
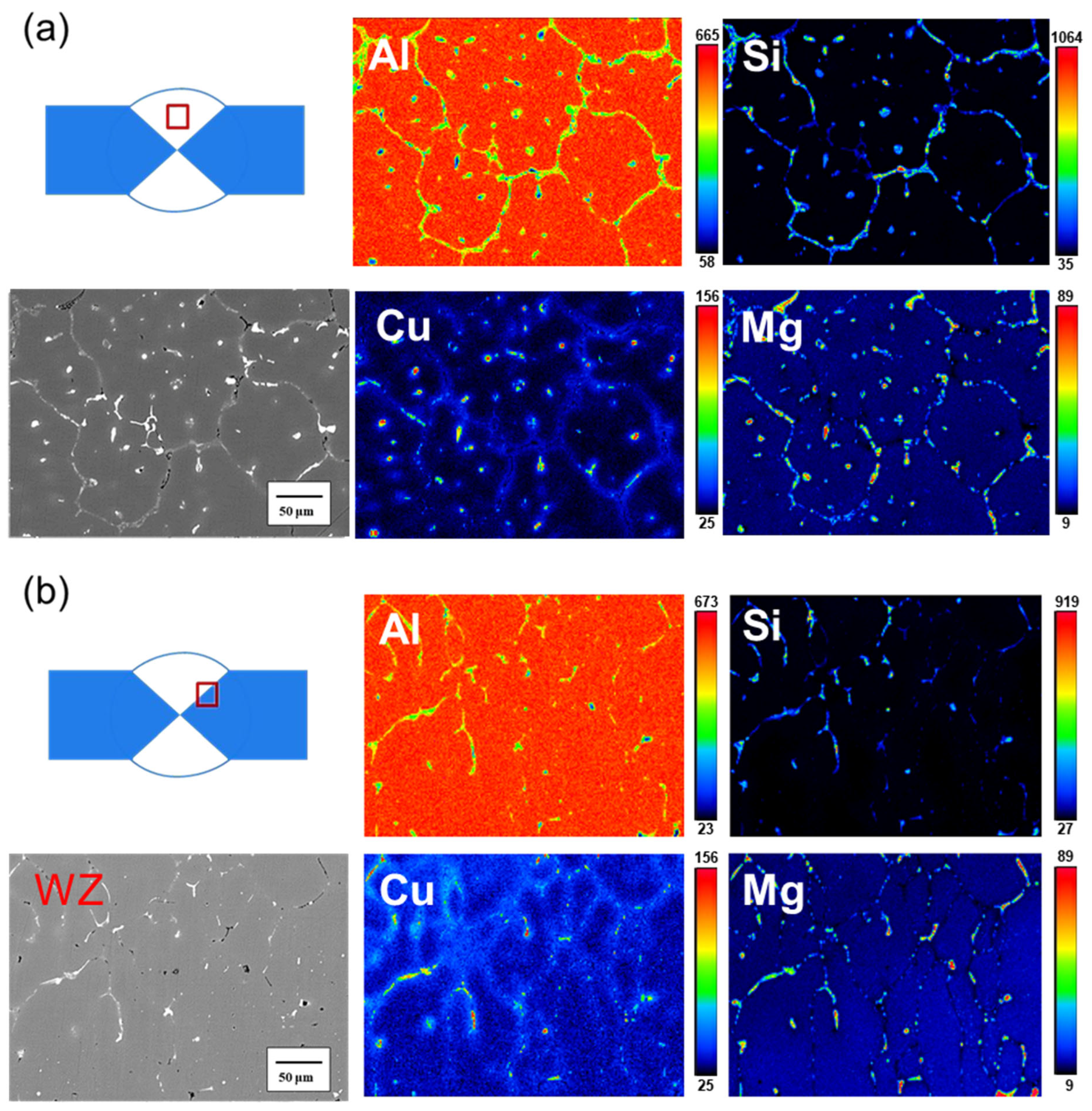
- The microstructure of the weld bead before heat treatment consisted of α-Al dendrites, similar to that of traditional cast A319. After heat treatment, the microstructure remained approximately the same, but the dendrites became larger. The junction of the weld bead and the substrate exhibited a more uniform arrangement.
- (2)
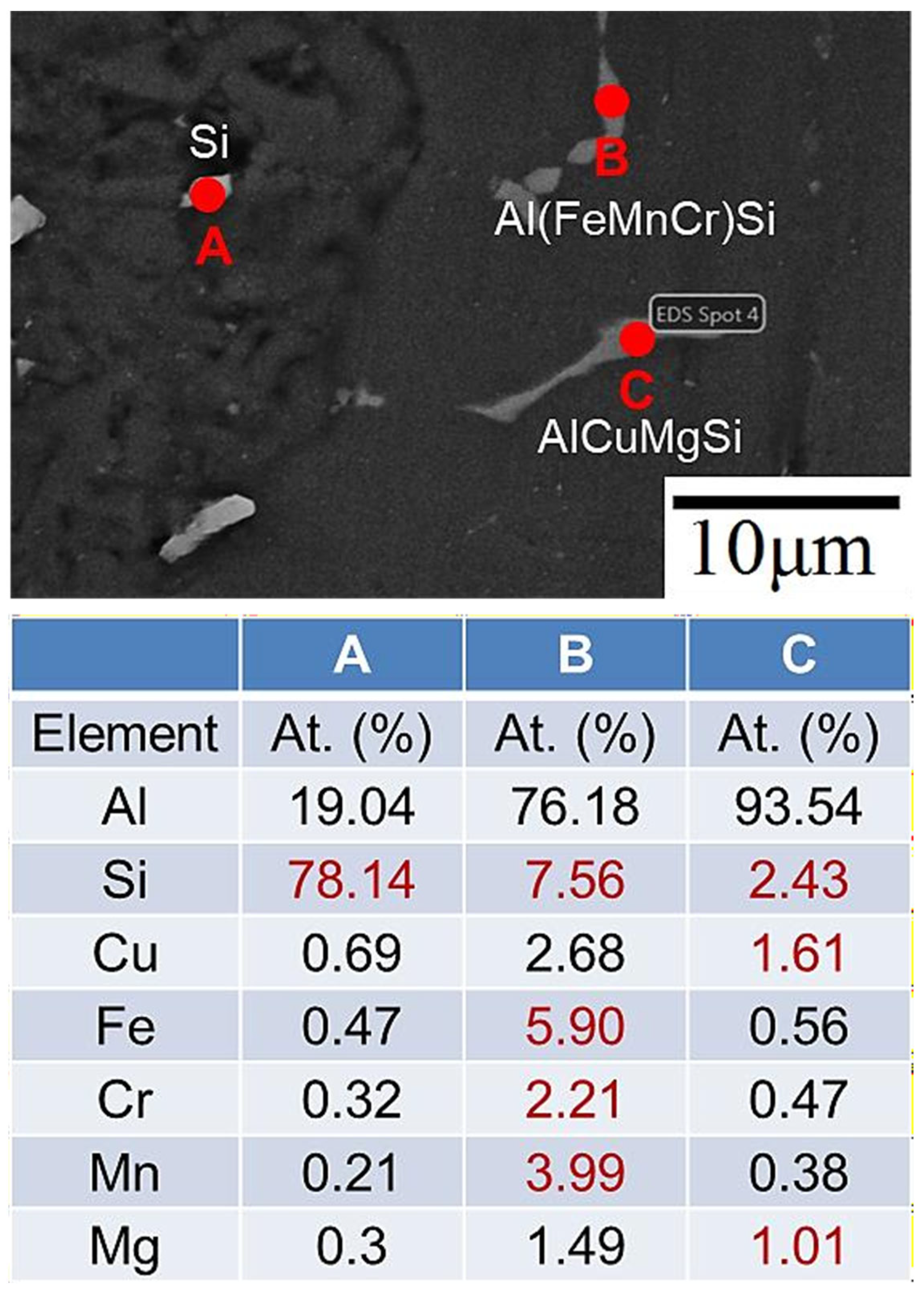
- Before heat treatment, Si particles and some Mg2Si phases segregated at the grain boundary. After heat treatment, Si, along with Mg and Cu, overlapped at the grain boundary, indicating that Si elements combined with other elements to form a phase.
- (3)
- After heat treatment, Si particles form a eutectic phase with Cu and Mg, leading to a substantial improvement in the specimen’s strength (from 200 MPa to 320 MPa). This results in no significant difference in strength between the weld bead and the 6061 substrate plate (310 MPa) after welding.
- (4)
- In terms of high-temperature mechanical properties, the UTS of the SCM-T4/T6 specimen was 250 MPa at 240 °C, surpassing the strength of the 6061 substrate plate after T4/T6 heat treatment at high temperatures (approximately 200 MPa).
- (5)
- Heat treatment enabled the SCM weld bead to reduce the percentage of grain boundaries and secondary phase particles (from 3.14% to 1.82%). This reduction effectively minimized galvanic corrosion, allowing the weld bead to maintain adequate strength after being immersed in a 3.5 wt.% NaCl solution for up to 3 days. However, when the remaining grain boundaries and secondary phase particles were preferentially corroded, embrittlement occurred (immersed for 7 days).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Sundarrajan, S. Optimization of pulsed TIG welding process parameters on mechanical properties of AA 5456 Aluminum alloy weldments. Mater. Des. 2009, 30, 1288–1297. [Google Scholar] [CrossRef]
- Wang, H.; Yang, F.; Zhang, Z.; Liu, L. Bonding mechanism of Al/steel interface formed by laser-TIG welding assisted riveting technology. Mater. Today Commun. 2020, 25, 101487. [Google Scholar] [CrossRef]
- Sokoluk, M.; Yao, G.; Pan, S.; Cao, C.; Li, X. High Strength Nanotreated Filler Material for TIG Welding of AA6061. In Light Metals 2020; Springer: Cham, Switzerland, 2020; pp. 380–385. [Google Scholar]
- Bansod, A.V.; Patil, A.P. Effect of welding processes on microstructure, mechanical properties, and corrosion behavior of low-Nickel austenitic stainless steels. Metallogr. Microstruct. Anal. 2017, 6, 304–314. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Wang, K.; Wang, H.; Hu, W. The Effect of Tungsten Inert Gas Welding on the Pitting Corrosion Behavior of 304 Stainless Steel. Int. J. Electrochem. Sci. 2020, 15, 3793–3806. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, J.; Ding, R. Ultrasonic vibration assisted tungsten inert gas welding of dissimilar magnesium alloys. J. Mater. Sci. Technol. 2018, 34, 2240–2245. [Google Scholar] [CrossRef]
- Bo, Q.I.N.; Yin, F.C.; Zeng, C.Z.; Xie, J.C.; Jun, S.H.E.N. Microstructure and mechanical properties of TIG/A-TIG welded AZ61/ZK60 magnesium alloy joints. Trans. Nonferrous Met. Soc. China 2019, 29, 1864–1872. [Google Scholar]
- Sinhmar, S.; Dwivedi, D.K. A study on corrosion behavior of friction stir welded and tungsten inert gas welded AA2014 aluminium alloy. Corros. Sci. 2018, 133, 25–35. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Lang, Q.; Song, G.; Liu, L. Microstructure evolution and deformation behavior of TIG welded 7075-T6 aluminum alloy followed by partial hot rolling. J. Manuf. Process. 2023, 94, 524–538. [Google Scholar] [CrossRef]
- Fauzi, E.I.; Jamil, M.C.; Samad, Z.; Muangjunburee, P. Microstructure analysis and mechanical characteristics of tungsten inert gas and metal inert gas welded AA6082-T6 tubular joint: A comparative study. Trans. Nonferrous Met. Soc. China 2017, 27, 17–24. [Google Scholar] [CrossRef]
- Baskutis, S.; Baskutiene, J.; Bendikiene, R.; Ciuplys, A. Effect of weld parameters on mechanical properties and tensile behavior of tungsten inert gas welded AW6082-T6 aluminium alloy. J. Mech. Sci. Technol. 2019, 33, 765–772. [Google Scholar] [CrossRef]
- Koprivica, A.; Bajić, D.; Šibalić, N.; Vukčević, M. Analysis of welding of aluminium alloy AA6082-T6 by TIG, MIG and FSW processes from technological and economic aspect. Machines. Technol. Mater. 2020, 14, 194–198. [Google Scholar]
- Kou, S. Solidification and liquation cracking issues in welding. JOM 2003, 55, 37–42. [Google Scholar] [CrossRef]
- Verma, R.P.; Pandey, K.N.; Sharma, Y. Effect of ER4043 and ER5356 filler wire on mechanical properties and microstructure of dissimilar aluminium alloys, 5083-O and 6061-T6 joint, welded by the metal inert gas welding. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2015, 229, 1021–1028. [Google Scholar] [CrossRef]
- ASM International Handbook Committee. Properties and selection: Nonferrous alloys and special-purpose materials. In ASM Metal Handbook; ASM International Handbook Committee: Almere, The Netherlands, 1992; Volume 2, p. 51. [Google Scholar]
- Ding, X.P.; Cui, H.; Zhang, J.X.; Li, H.X.; Guo, M.X.; Lin, Z.; Zhuang, L.Z.; Zhang, J.S. The effect of Zn on the age hardening response in an Al–Mg–Si alloy. Mater. Des. 2015, 65, 1229–1235. [Google Scholar] [CrossRef]
- Huang, B.C.; Hung, F.Y. Effect of Tensile Loading-Unloading Cyclic Plastic Deformation on Al-Si Alloy Manufactured through CCDR. Mater. Today Commun. 2023, 34, 104979. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. Phase relations and precipitation in Al–Mg–Si alloys with Cu additions. Prog. Mater. Sci. 2004, 49, 389–410. [Google Scholar] [CrossRef]
- Ozturk, F.; Sisman, A.; Toros, S.; Kilic, S.; Picu, R.C. Influence of aging treatment on mechanical properties of 6061 aluminum alloy. Mater. Des. 2010, 31, 972–975. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, B.; Bai, Z.; Wang, J.; Yin, Y. Study of the precipitation hardening behavior and intergranular corrosion of Al-Mg-Si alloys with differing Si contents. Metals 2017, 7, 387. [Google Scholar] [CrossRef]
- Haga, T.; Imamura, S.; Watari, H.; Nishida, S. Effect of Casting Conditions on Fluidity of Aluminum Alloy in Die Casting. In Proceedings of the International Conference on Leading Edge Manufacturing/Materials and Processing, Virtual, 3 September 2020; American Society of Mechanical Engineers: New York, NY, USA, 2020; Volume 83624, p. V001T05A013. [Google Scholar]
- Guzmán, I.; Granda, E.; Acevedo, J.; Martínez, A.; Dávila, Y.; Velázquez, R. Comparative in Mechanical Behavior of 6061 Aluminum Alloy Welded by Pulsed GMAW with Different Filler Metals and Heat Treatments. Materials 2019, 12, 4157. [Google Scholar] [CrossRef]
- Azhar, A.A.A.; Ibrahim, M.F.A.; Jalar, A.; Syarif, J.; Abdullah, S.; Rashdi, N.M.; Kornain, Z. Effects of different fillers on microstructure and tensile properties of welded AA6061-T6. Key Eng. Mater. 2011, 462, 1189–1193. [Google Scholar] [CrossRef]
- Othman, N.K.; Bakar, S.R.S.; Jalar, A.; Syarif, J.; Ahmad, M.Y. The effect of filler metals on mechanical properties of 6 mm AA 6061-T6 welded joints. Adv. Mat. Res. 2011, 154, 873–876. [Google Scholar] [CrossRef]
- Narsimhachary, D.; Bathe, R.N.; Padmanabham, G.; Basu, A. Influence of temperature profile during laser welding of aluminum alloy 6061 T6 on microstructure and mechanical properties. Mater. Manuf. Process. 2014, 29, 948–953. [Google Scholar] [CrossRef]
- Chu, Q.; Bai, R.; Jian, H.; Lei, Z.; Hu, N.; Yan, C. Microstructure, texture and mechanical properties of 6061 aluminum laser beam welded joints. Mater. Charact. 2018, 137, 269–276. [Google Scholar] [CrossRef]
- Fahimpour, V.; Sadrnezhaad, S.K.; Karimzadeh, F. Corrosion behavior of aluminum 6061 alloy joined by friction stir welding and gas tungsten arc welding methods. Mater. Des. 2012, 39, 329–333. [Google Scholar] [CrossRef]
- Öteyaka, M.Ö.; Ayrtüre, H. A study on the corrosion behavior in sea water of welds aluminum alloy by shielded metal arc welding, friction stir welding and gas tungsten arc welding. Int. J. Electrochem. Sci. 2015, 10, 8549–8557. [Google Scholar] [CrossRef]
- Mutombo, K.; Du Toit, M. Corrosion fatigue behavior of aluminium alloy 6061-T651 welded using fully automatic gas metal arc welding and ER5183 filler alloy. Int. J. Eng. Sci. 2011, 33, 1539–1547. [Google Scholar]
- Samuel, A.M.; Gauthier, J.; Samuel, F.H. Microstructural aspects of the dissolution and melting of Al2Cu phase in Al-Si alloys during solution heat treatment. Metall. Mater. Trans. A 1996, 27, 1785–1798. [Google Scholar] [CrossRef]
- Firouzdor, V.; Rajabi, M.; Nejati, E.; Khomamizadeh, F. Effect of microstructural constituents on the thermal fatigue life of A319 aluminum alloy. Mater. Sci. Eng. A 2007, 454, 528–535. [Google Scholar] [CrossRef]
- Salleh, M.S.; Omar, M.Z.; Syarif, J.; Alhawari, K.S.; Mohammed, M.N. Microstructure and mechanical properties of thixoformed A319 aluminium alloy. Mater. Des. 2014, 64, 142–152. [Google Scholar] [CrossRef]
- Ishak, M.; Noordin, N.F.M.; Razali, A.S.K.; Shah, L.H.A.; Romlay, F.R.M. Effect of filler on weld metal structure of AA6061 aluminum alloy by tungsten inert gas welding. IJAME 2015, 11, 2438–2446. [Google Scholar] [CrossRef]
- Camero, S.; Puchi, E.S.; Gonzalez, G. Effect of 0.1% vanadium addition on precipitation behavior and mechanical properties of Al-6063 commercial alloy. J. Mater. Sci. 2006, 41, 7361–7373. [Google Scholar] [CrossRef]
- Abbass, M.K.; Abd, H.H. A Comparison Study of Mechanical Properties between Friction StirWelding and TIG Welded Joints of Aluminum Alloy (Al 6061-T6). J. Eng. Technol. 2013, 31, 2701–2715. [Google Scholar] [CrossRef]
- Iacobescu, G. A Theoretical Model for Welding Process with Gaussian Heat Source-Part 1. Univ. Politeh. Buchar. Sci. Bull. Ser. D Mech. Eng. 2006, 68, 45–50. [Google Scholar]
- Braun, R. Investigation on microstructure and corrosion behavior of 6XXX series aluminium alloys. Mater. Sci. Forum 2006, 519, 735–740. [Google Scholar] [CrossRef]
- Han, Y.; Ma, K.; Li, L.; Chen, W.; Nagaumi, H. Study on microstructure and mechanical properties of Al–Mg–Si–Cu alloy with high manganese content. Mater. Des. 2012, 39, 418–424. [Google Scholar] [CrossRef]
- Zhen, L.; Fei, W.D.; Kang, S.B.; Kim, H.W. Precipitation behavior of Al-Mg-Si alloys with high silicon content. J. Mater. Sci. 1997, 32, 1895–1902. [Google Scholar] [CrossRef]
- Onoro, J.; Salvador, M.D.; Cambronero, L.E.G. High- temperature mechanical properties of aluminium alloys reinforced with boron carbide particles. Mater. Sci. Eng. A 2009, 499, 421–426. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, F.; Dai, Z.; Qiao, Y.; Chen, J.; Liu, W. Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints. Metals 2021, 11, 3. [Google Scholar] [CrossRef]
- Rahman, A.B.M.; Kumar, S.; Gerson, A. Galvanic corrosion of laser weldments of AA6061 aluminium alloy. Corros. Sci. 2007, 49, 4339–4351. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, X.; Gao, L.-X.; Joo, H.G.; Lee, K.Y. Effect of laser–arc hybrid welding on fracture and corrosion behavior of AA6061-T6 alloy. Mater. Sci. Eng. A 2011, 528, 2748–2754. [Google Scholar] [CrossRef]
- Gharavi, F.; Matori, K.A.; Yunus, R.; Othman, N.K.; Fadaeifard, F. Corrosion behavior of Al6061 alloy weldment produced by friction stir welding process. J. Mater. Res. Technol. 2015, 4, 314–322. [Google Scholar] [CrossRef]
- Shehadeh, L.M.; Jalham, I.S. The Effect of Adding Different Percentages of Manganese (Mn) and Copper (Cu) on the Mechanical Behavior of Aluminum. JJMIE 2016, 10, 19–26. [Google Scholar]
- Li, Y.J.; Brusethaug, S.; Olsen, A. Influence of Cu on the mechanical properties and precipitation behavior of AlSi7Mg0.5 alloy during aging treatment. Scr. Mater. 2006, 54, 99–103. [Google Scholar] [CrossRef]
- Feikus, F.J. Optimization of Al-Si cast-alloys for cylinder head applications. In Proceedings of the 102nd AFS Casting Congress, Atlanta, GA, USA, 10–13 May 1998; pp. 1–21. [Google Scholar]
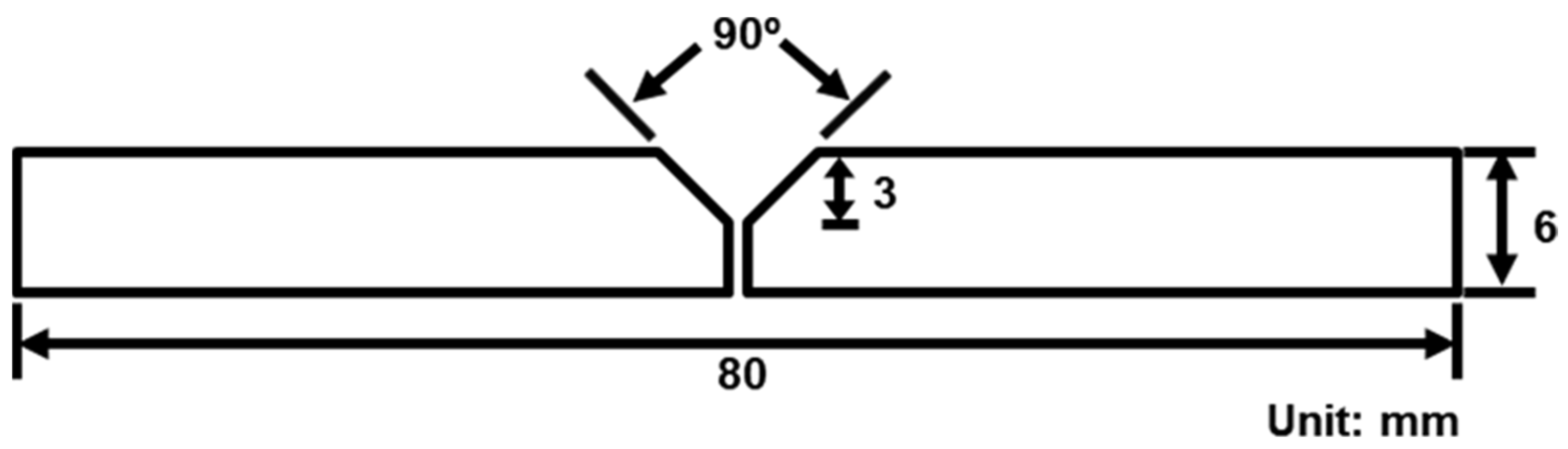
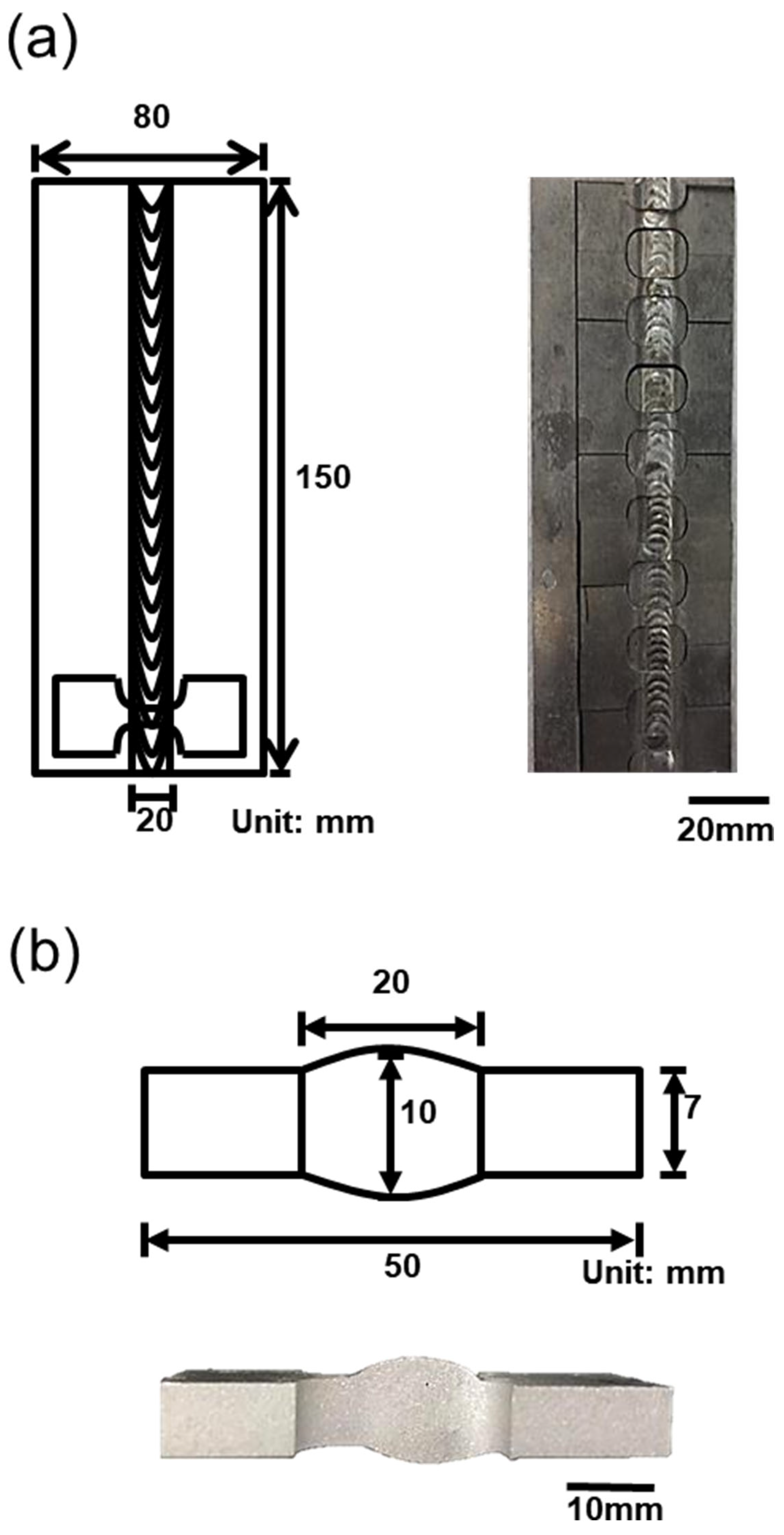

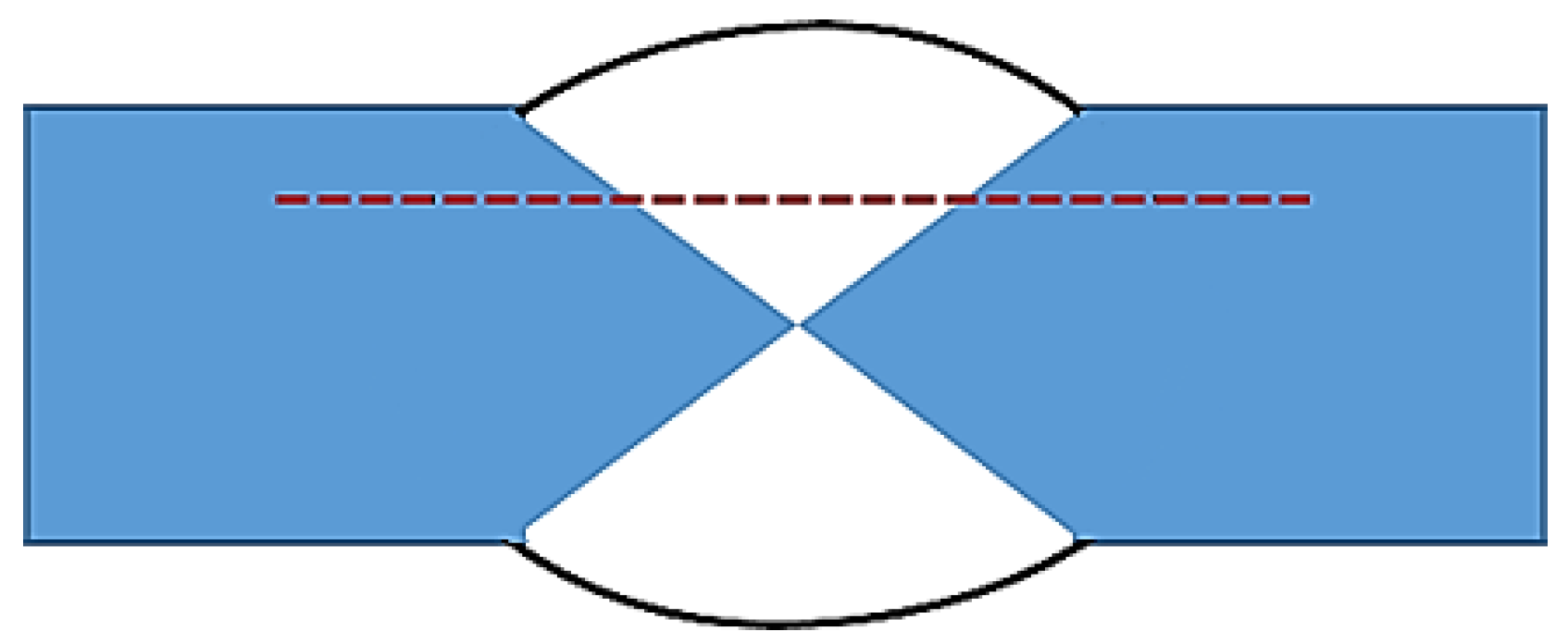

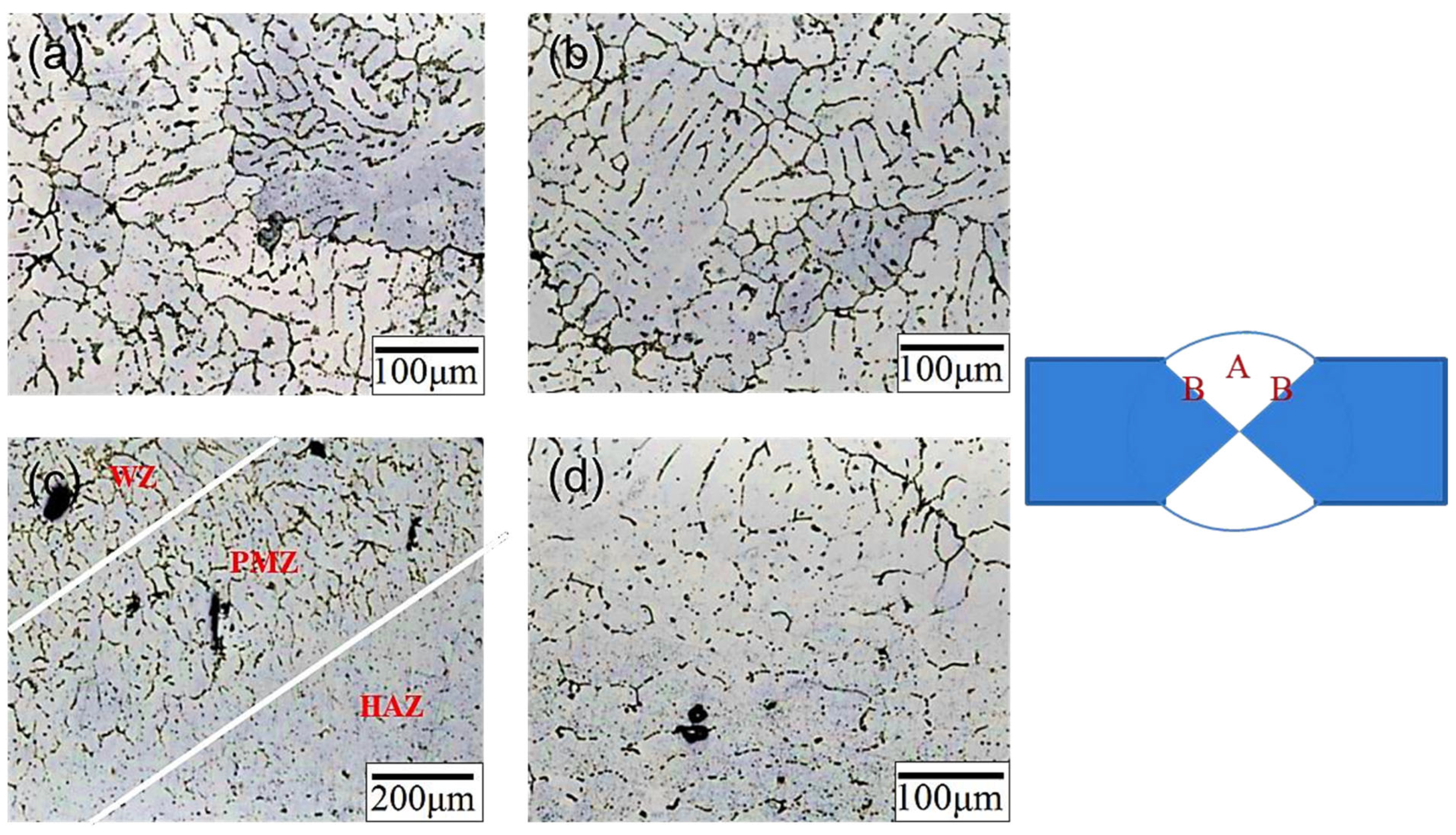





| Element (wt. %) | Si | Cu | Mg | Mn | Fe | Cr | Zr | Sr | Al |
|---|---|---|---|---|---|---|---|---|---|
| SCM | 8.80 | 3.90 | 0.35 | 0.26 | 0.14 | 0.13 | 0.08 | 0.05 | Bal. |
| A319 | 6.15 | 3.55 | 0.05 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | Bal. |
| 6061 | 0.65 | 0.30 | 1.10 | 0.10 | 0.40 | 0.20 | 0.00 | 0.00 | Bal. |
| YS (MPa) | UTS (MPa) | UE (%) | TE (%) | |
|---|---|---|---|---|
| Original filler | 210 ± 2.8 | 230 ± 3.0 | 4.6 ± 0.8 | 6.9 ± 0.8 |
| Heat-treated filler | 321 ± 1.6 | 376 ± 1.2 | 7.5 ± 0.5 | 8.4 ± 0.3 |
| SCM-AW | 165 ± 3.1 | 197 ± 4.2 | 4.9 ± 1.3 | 9.1 ± 0.6 |
| SCM-T4/T6 | 306 ± 2.4 | 321 ± 2.2 | 1.3 ± 0.1 | 3.6 ± 0.3 |
| 6061-T6 Standard [36] | 276 | 310 | 12.0 | 17.0 |
| YS (MPa) | UTS (MPa) | UE (%) | TE (%) | |
|---|---|---|---|---|
| RT | 306 ± 2.4 | 321 ± 2.2 | 1.3 ± 0.1 | 3.6 ± 0.3 |
| 180 °C | 286 ± 1.1 | 289 ± 1.7 | 0.5 ± 0.3 | 4.8 ± 0.4 |
| 240 °C | 249 ± 0.4 | 251 ± 0.4 | 0.4 ± 0.2 | 5.3 ± 0.5 |
| YS (MPa) | UTS (MPa) | UE (%) | TE (%) | |
|---|---|---|---|---|
| 0-day | 306 ± 2.4 | 321 ± 2.2 | 1.3 ± 0.1 | 3.6 ± 0.3 |
| 1-day | 301 ± 4.1 | 311 ± 3.2 | 0.9 ± 0.3 | 3.1 ± 0.5 |
| 3-day | 286 ± 0.5 | 289 ± 0.7 | 1.2 ± 0.2 | 4.1 ± 0.1 |
| 7-day | 269 ± 6.0 | 269 ± 6.0 | 0 | 0 |
| Strength of Filler | Strength of Welded | High Temperature Strength of Welded | Corrosion Resistance | Can Heat Treatment | |
|---|---|---|---|---|---|
| SCM-filler | ✓ | ✓ | ✓ | ✓ | ✓ |
| Traditional filler | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.-R.; Hung, F.-Y.; Pan, C.-Y. Application of New Al-Si Welding Filler with High Concentration of Copper and Magnesium: High-Temperature Strength and Anti-Corrosion Mechanism. Materials 2024, 17, 126. https://doi.org/10.3390/ma17010126
Zhao J-R, Hung F-Y, Pan C-Y. Application of New Al-Si Welding Filler with High Concentration of Copper and Magnesium: High-Temperature Strength and Anti-Corrosion Mechanism. Materials. 2024; 17(1):126. https://doi.org/10.3390/ma17010126
Chicago/Turabian StyleZhao, Jun-Ren, Fei-Yi Hung, and Chien-Yu Pan. 2024. "Application of New Al-Si Welding Filler with High Concentration of Copper and Magnesium: High-Temperature Strength and Anti-Corrosion Mechanism" Materials 17, no. 1: 126. https://doi.org/10.3390/ma17010126
APA StyleZhao, J.-R., Hung, F.-Y., & Pan, C.-Y. (2024). Application of New Al-Si Welding Filler with High Concentration of Copper and Magnesium: High-Temperature Strength and Anti-Corrosion Mechanism. Materials, 17(1), 126. https://doi.org/10.3390/ma17010126






