A Review on Cementitious and Geopolymer Composites with Lithium Slag Incorporation
Abstract
:1. Introduction
2. Physiochemical and Microscopic Analysis of LS
2.1. Physical Properties of Raw LS
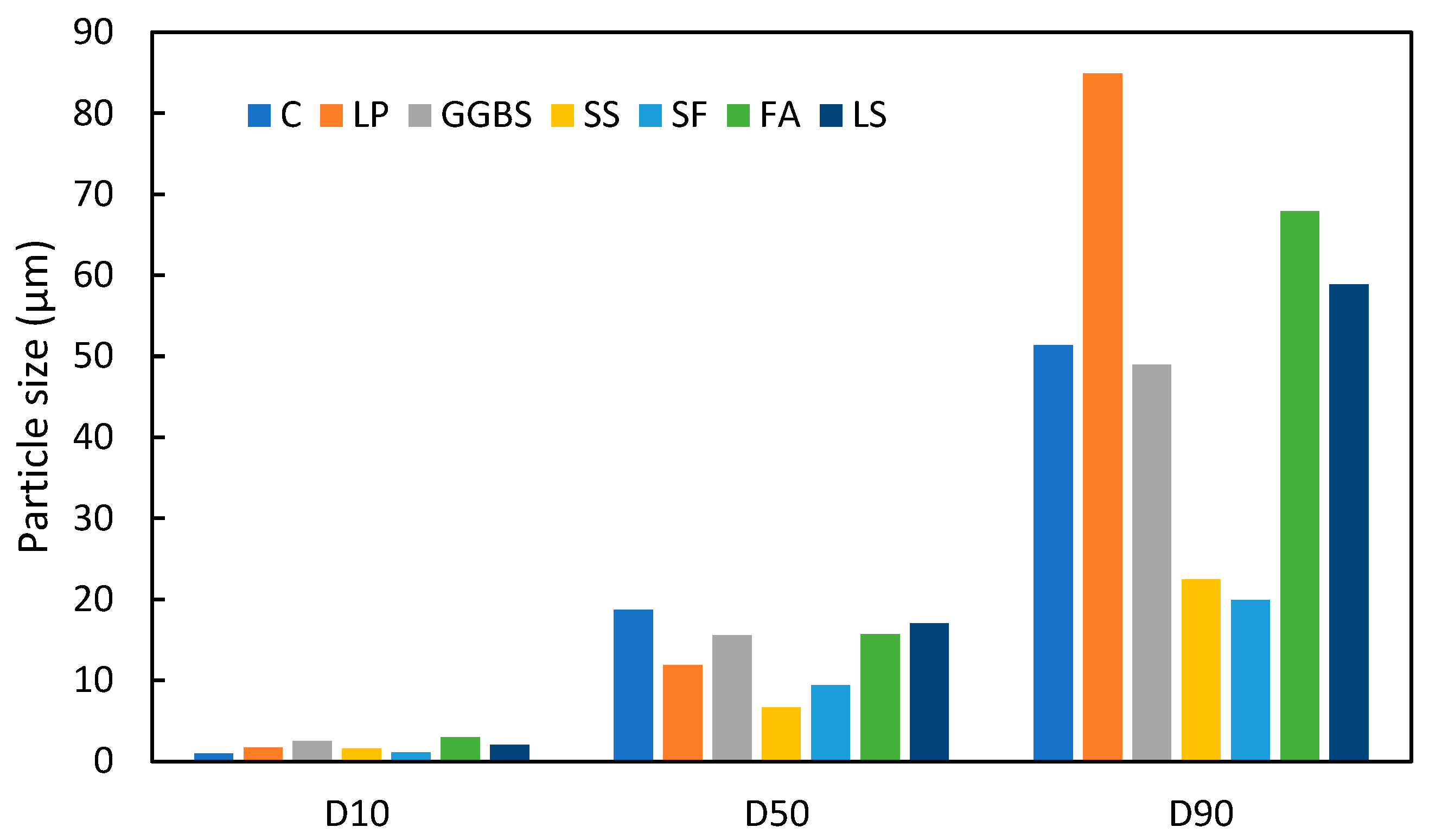
2.1.1. Particle Size Distribution
2.1.2. Density, Specific Surface Area, and Moisture Content
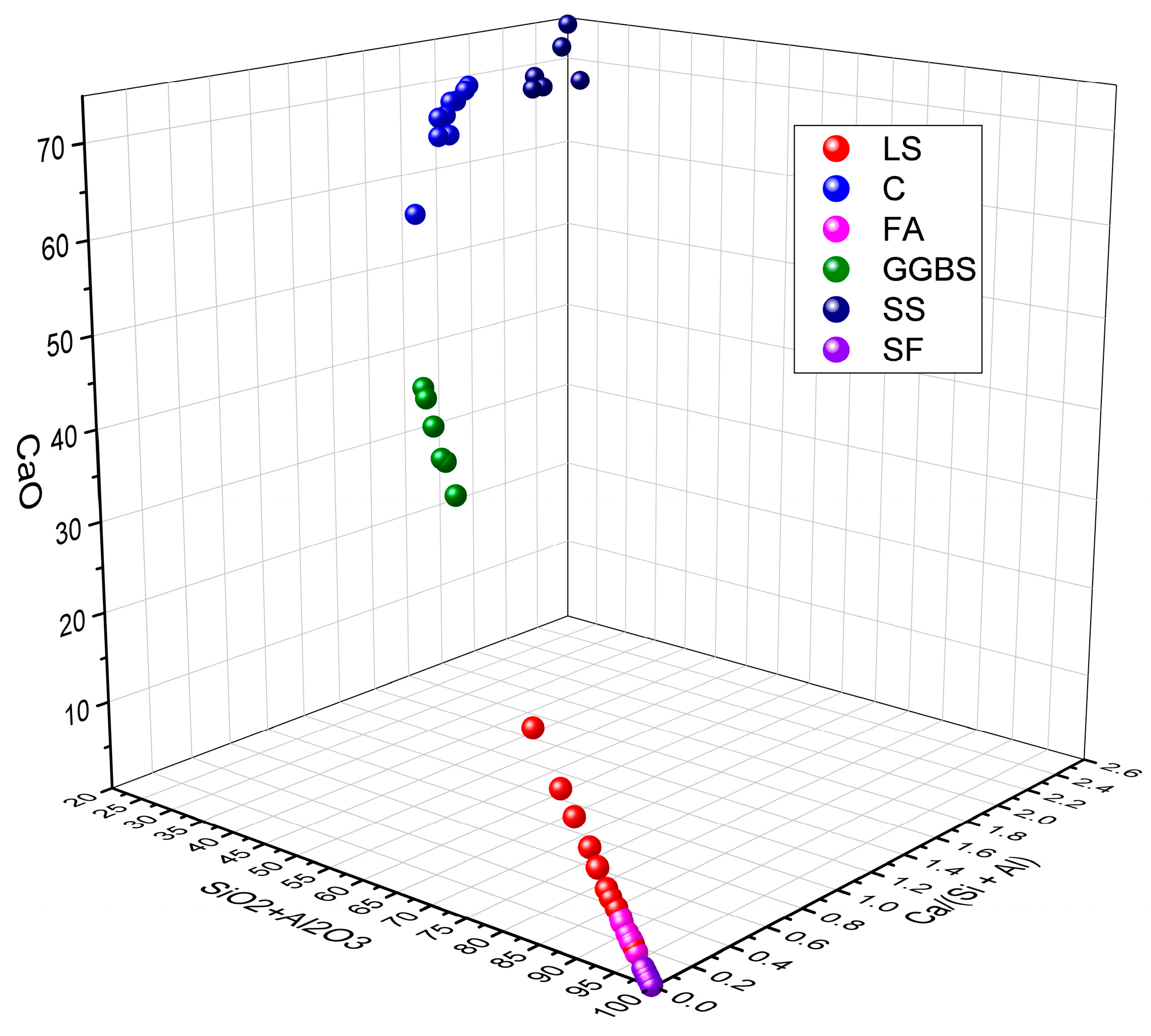
2.2. Chemical Properties of LS
2.2.1. Chemical Composition
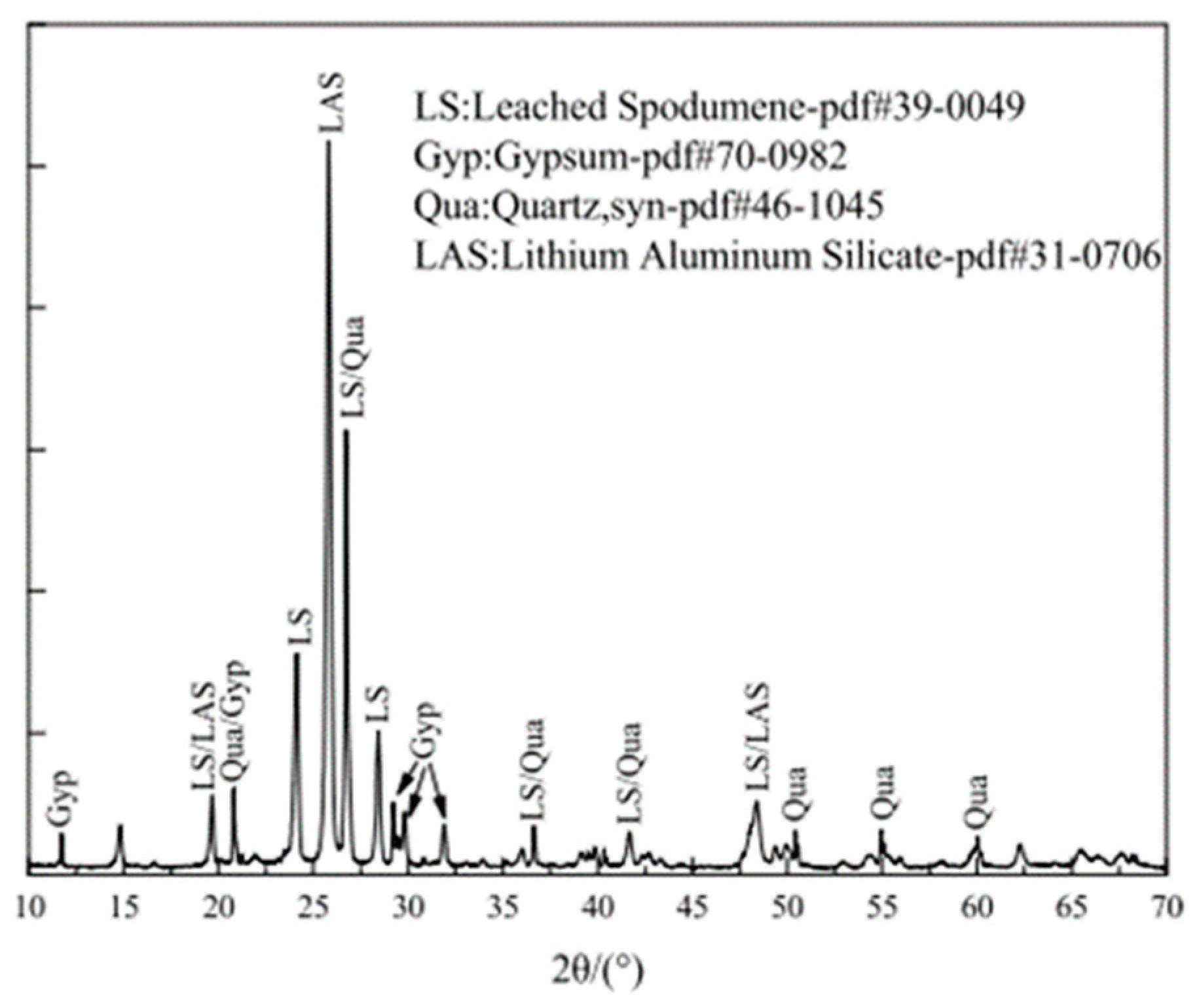
2.2.2. XRD Results
2.3. Microscopic Analysis of LS
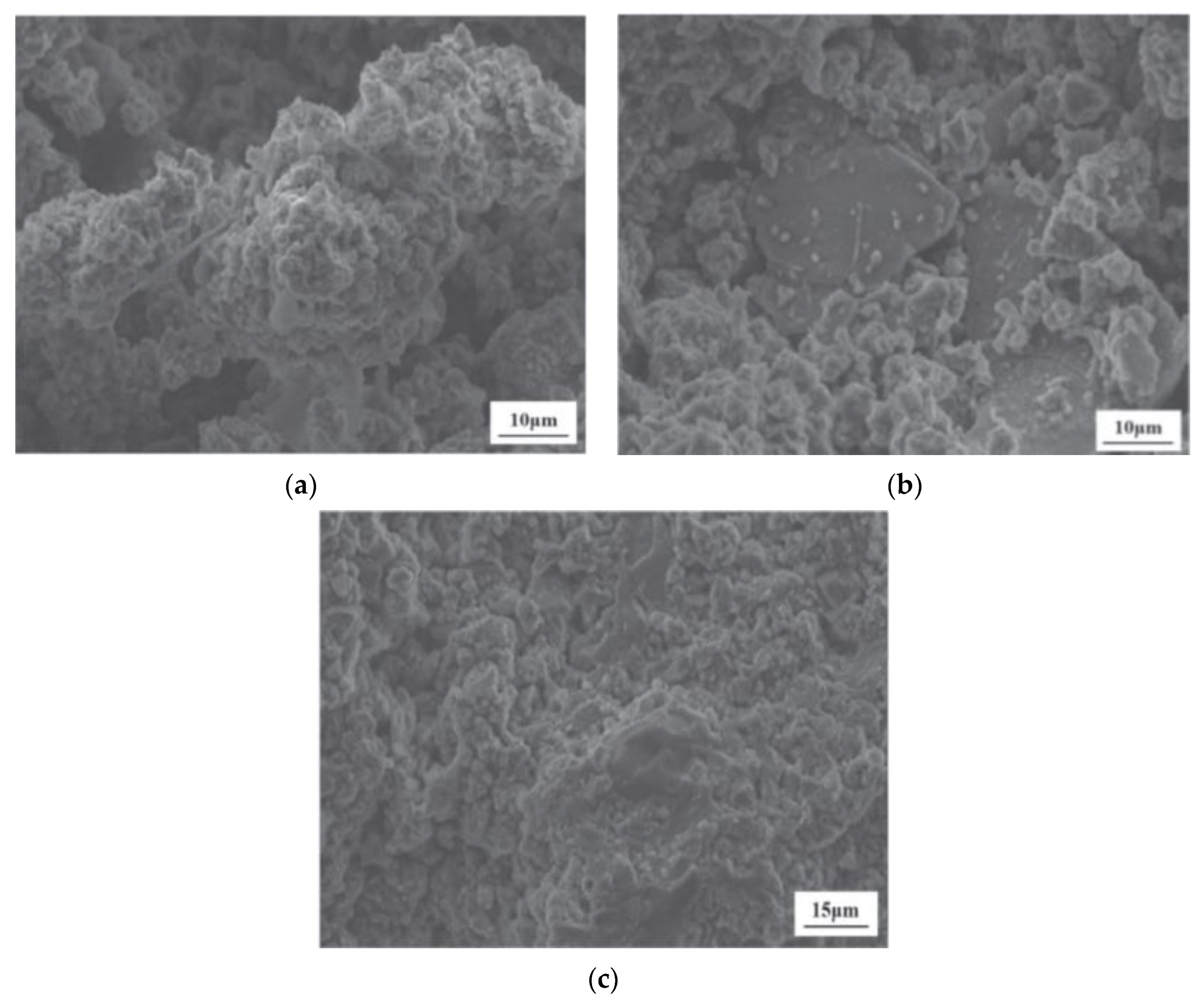
2.3.1. SEM-EDS Analysis
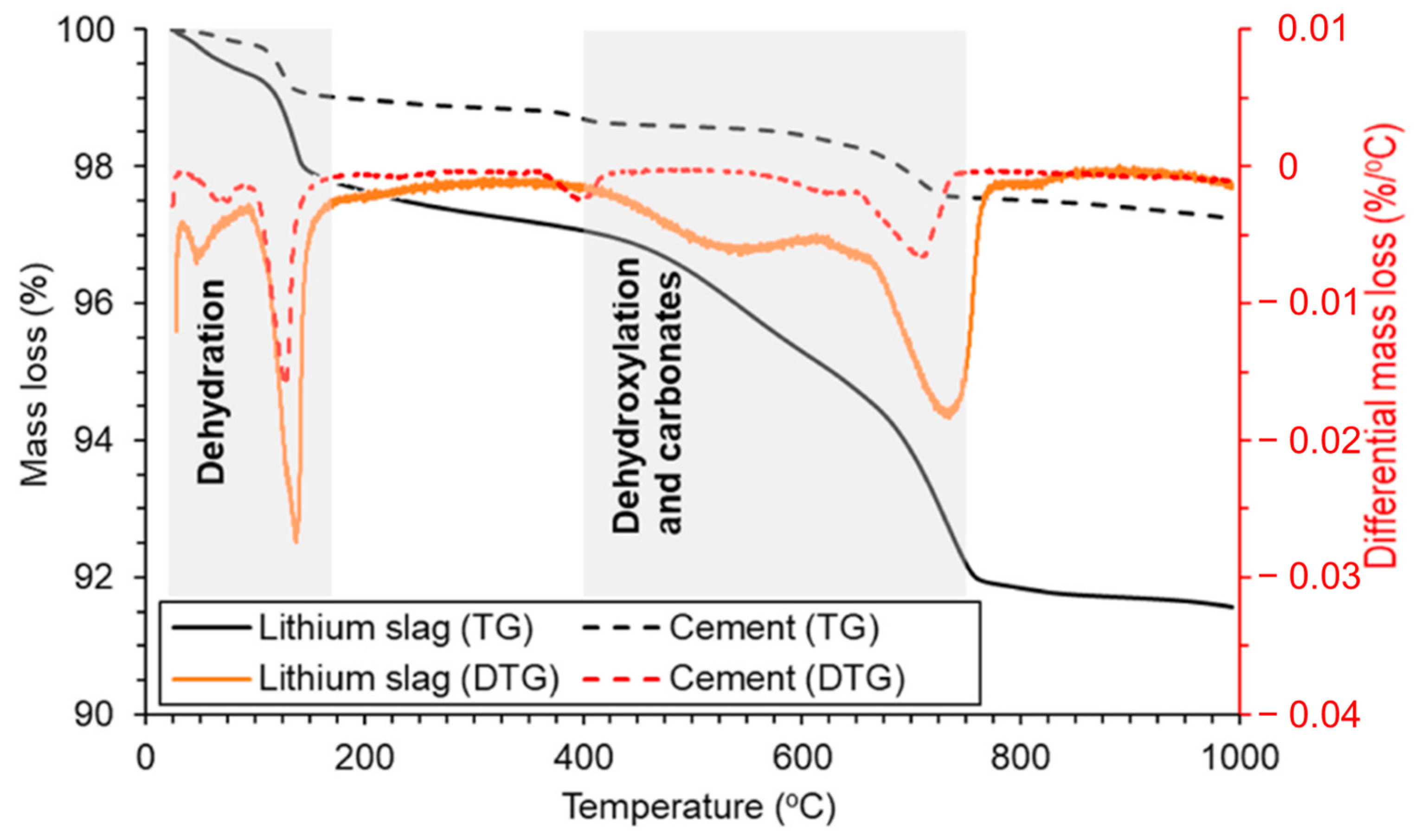
2.3.2. TG-DTG Analysis
2.3.3. NMR and XPS Analysis
3. Fresh State Properties of Cementitious Composites with LS Incorporation
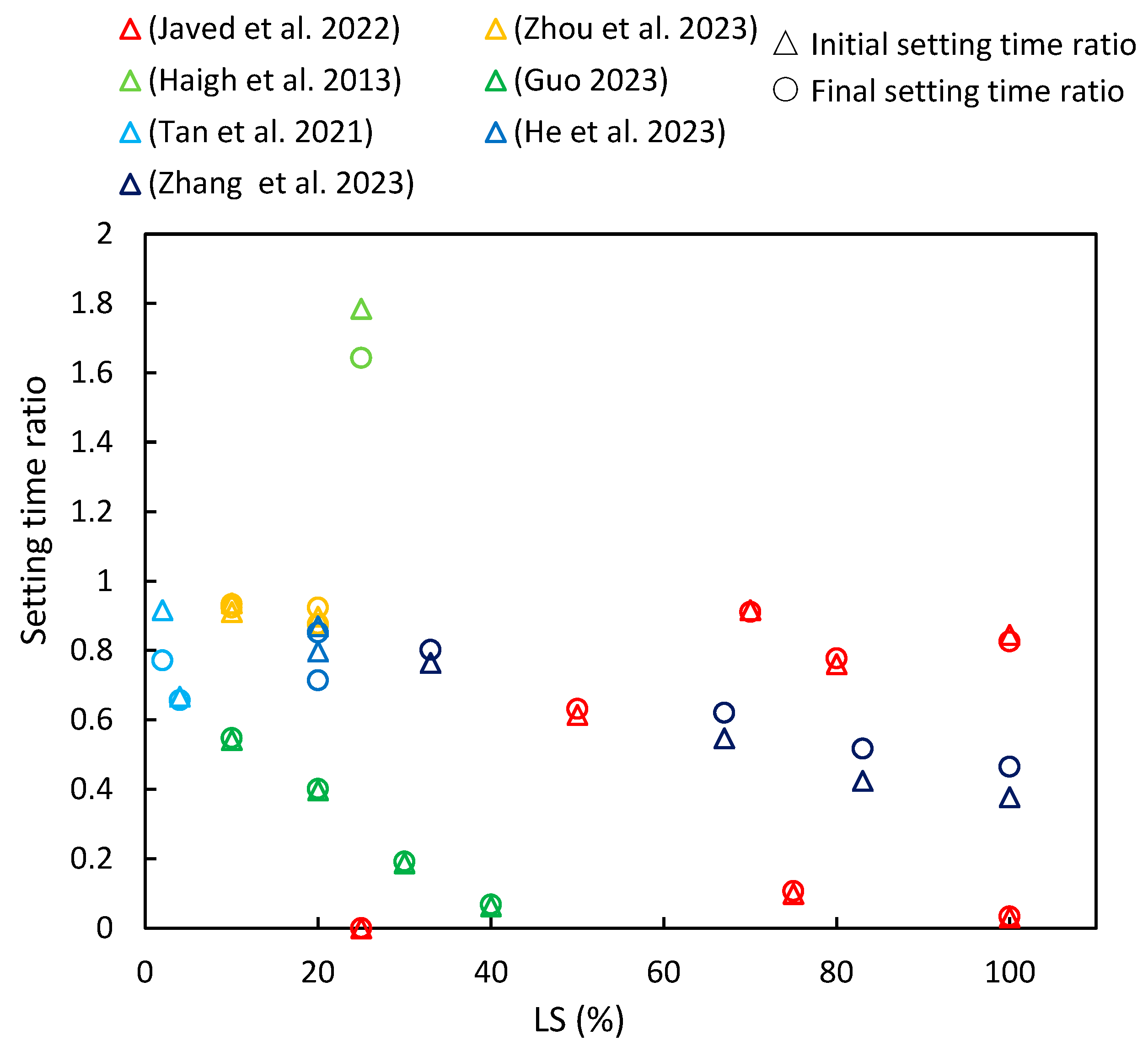
3.1. Setting Time
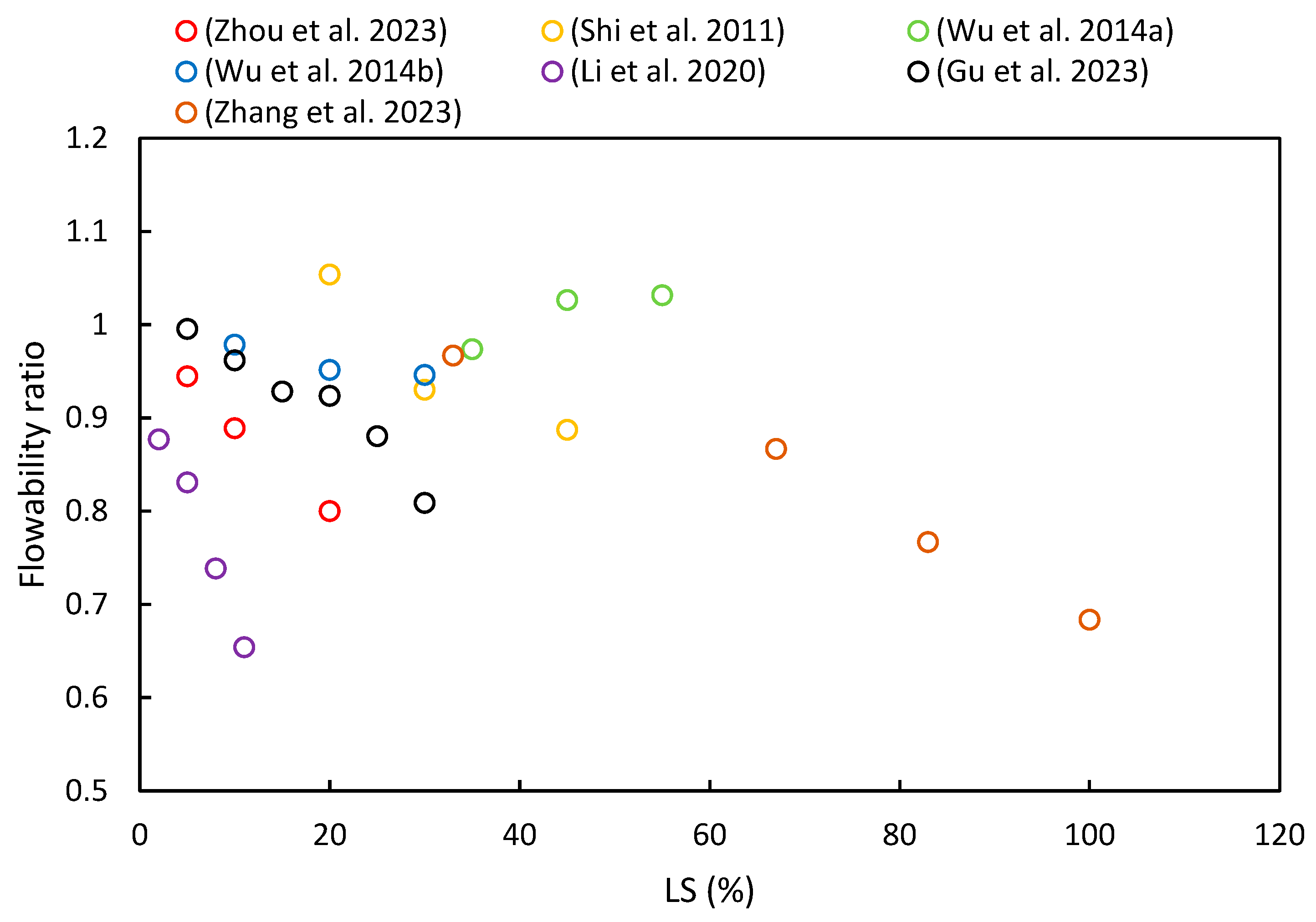
3.2. Flowability
3.3. Rheology
4. Mechanical Properties of Cementitious Composites with LS Incorporation
4.1. Compressive Strength
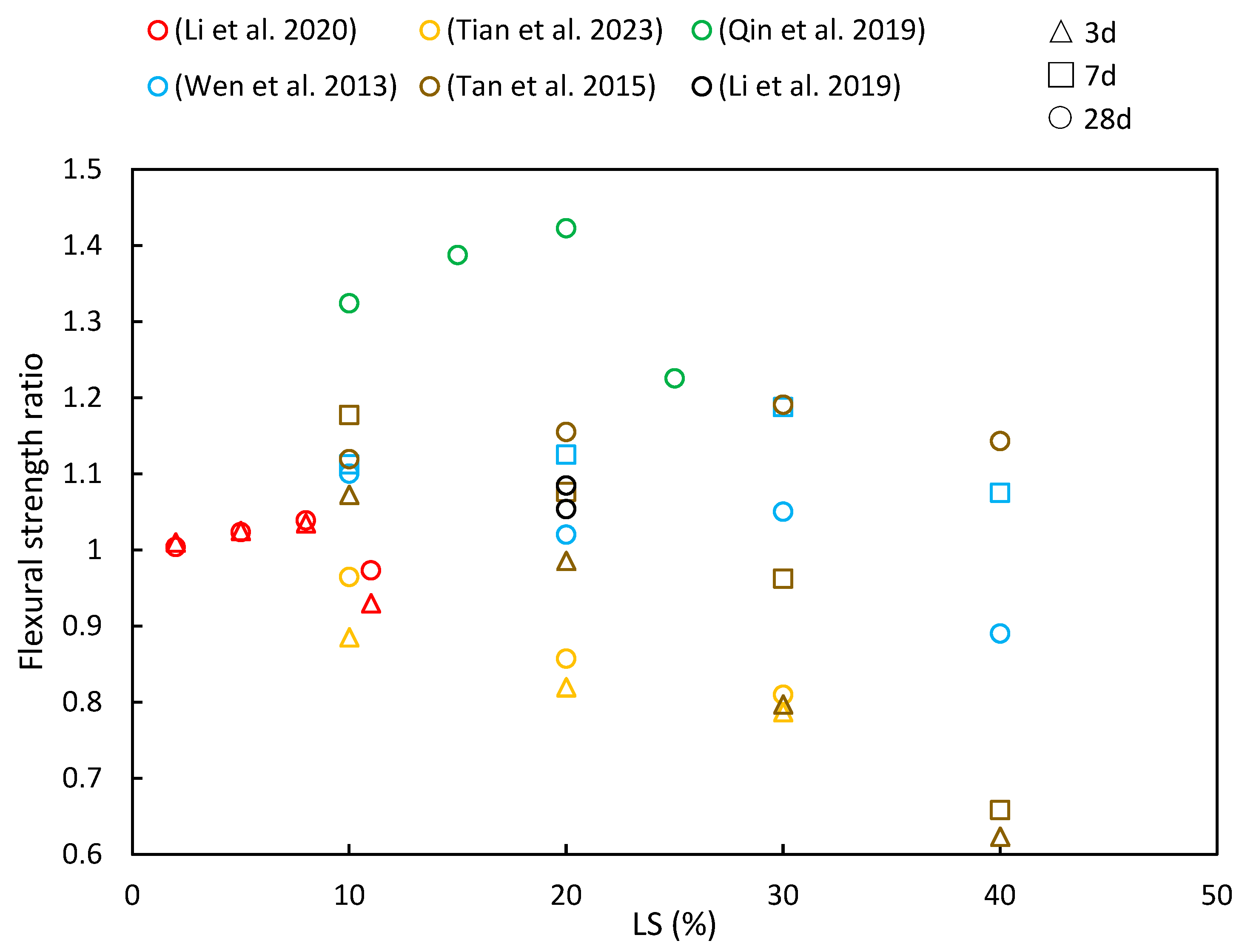
4.2. Flexural Strength
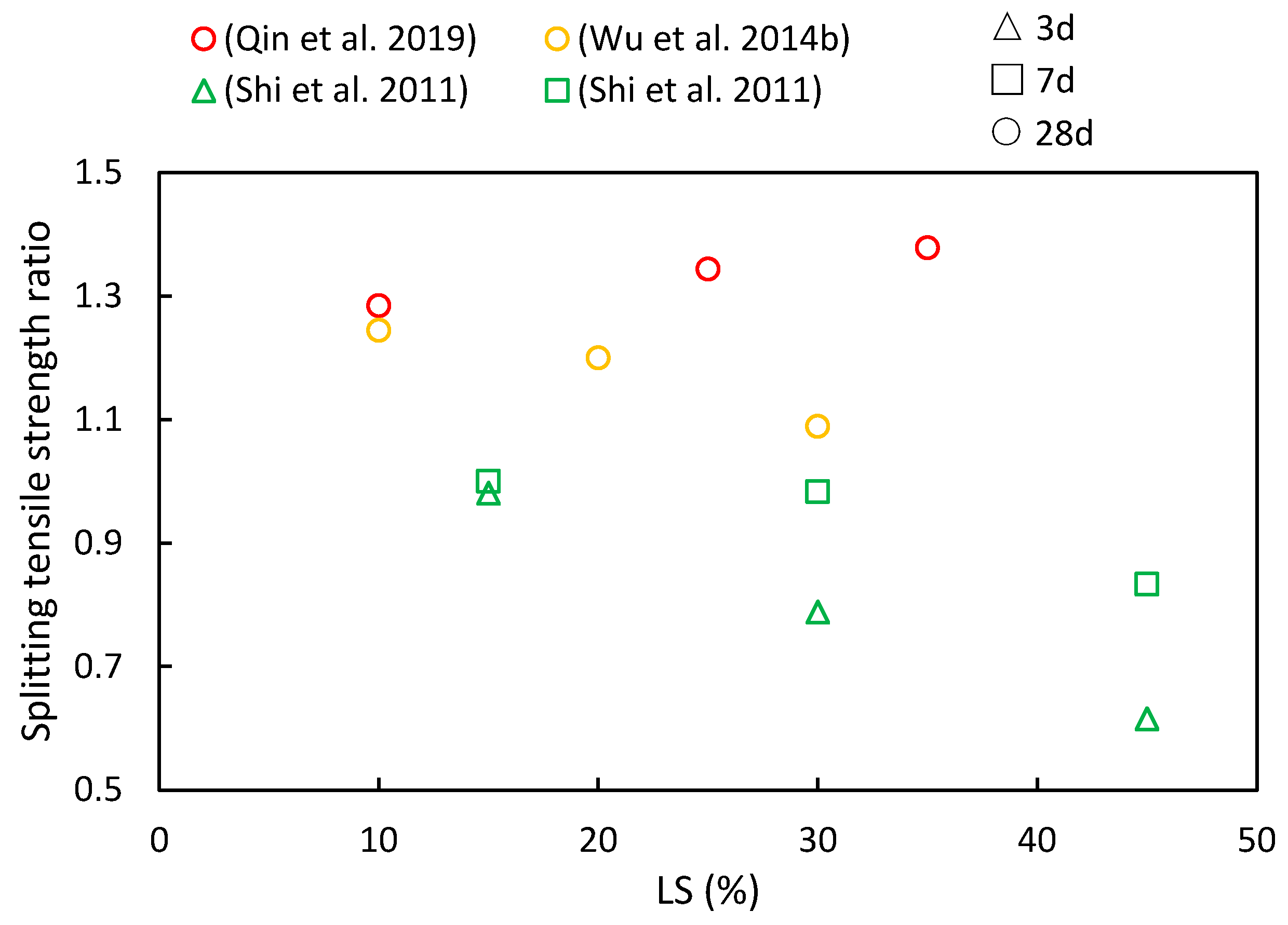
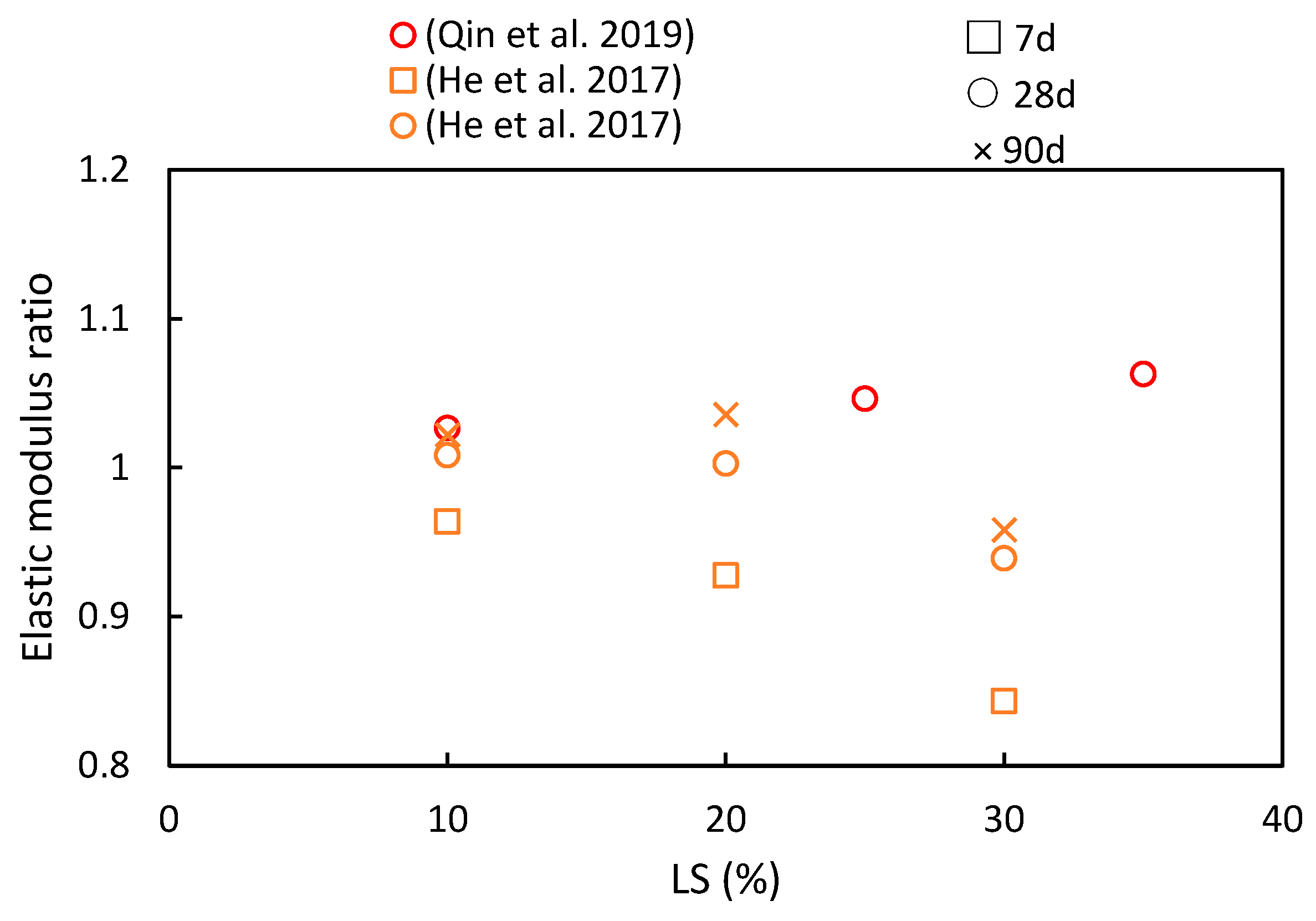
4.3. Splitting Tensile Strength and Elastic Modulus
5. Durability of Cementitious Composites with LS Incorporation
5.1. Chloride Resistance
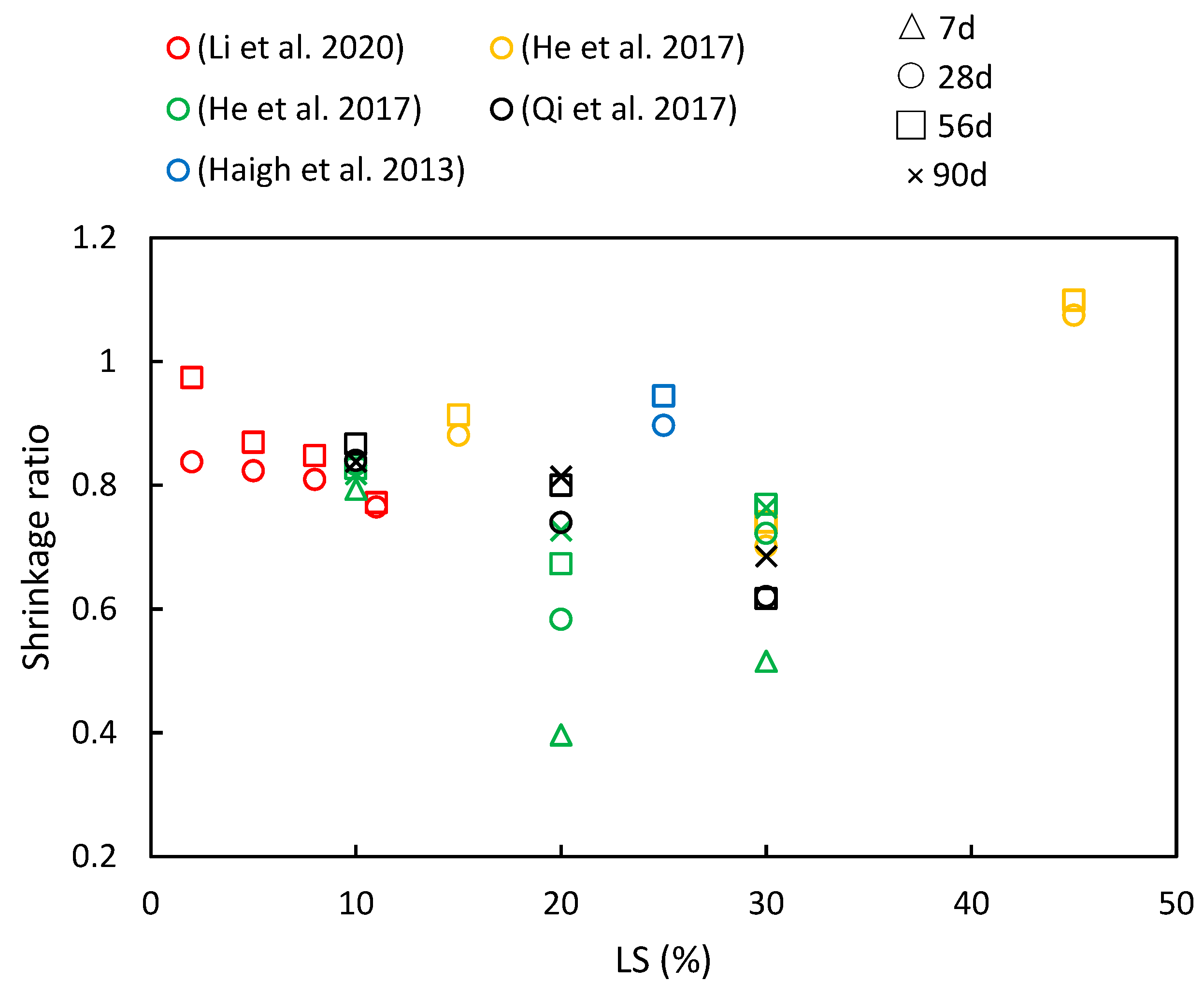
5.2. Shrinkage
5.3. Sulfate Attack and Carbonation
6. Chemical and Microstructural Investigations of Cementitious Composites with LS Incorporation
6.1. Hydration Heat
6.2. Pore Structure
6.3. XRD Analysis
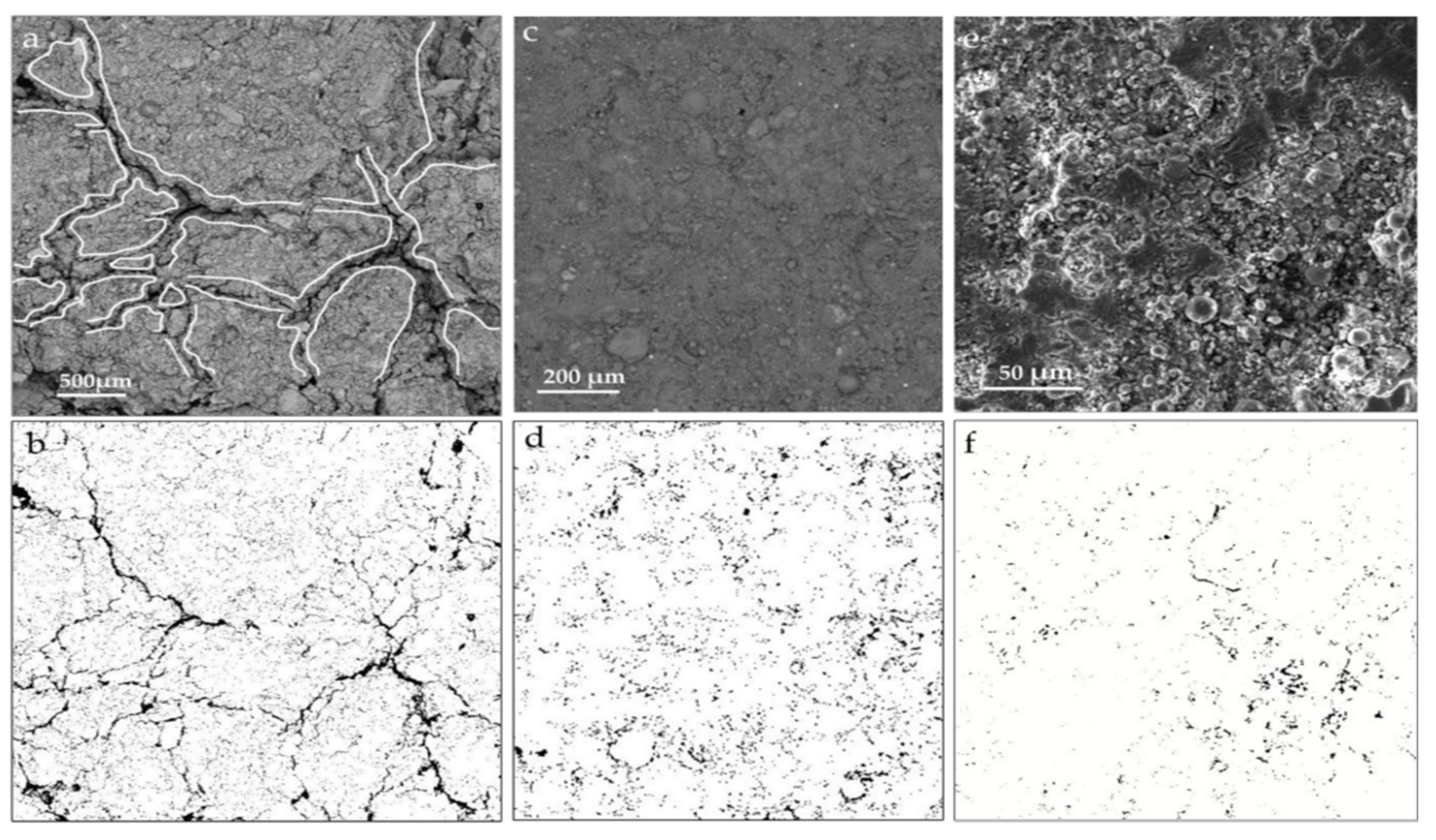
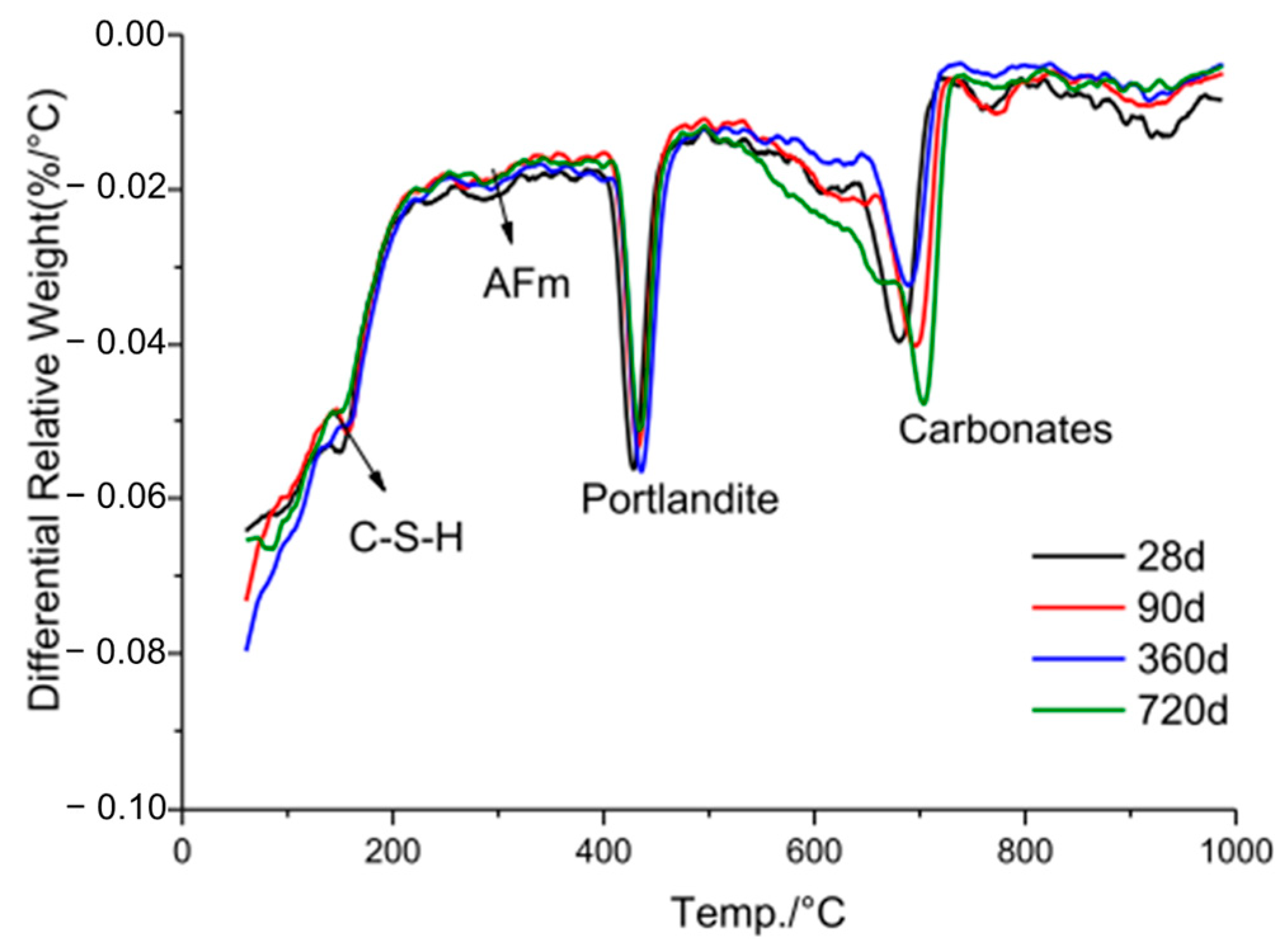
6.4. SEM Analysis
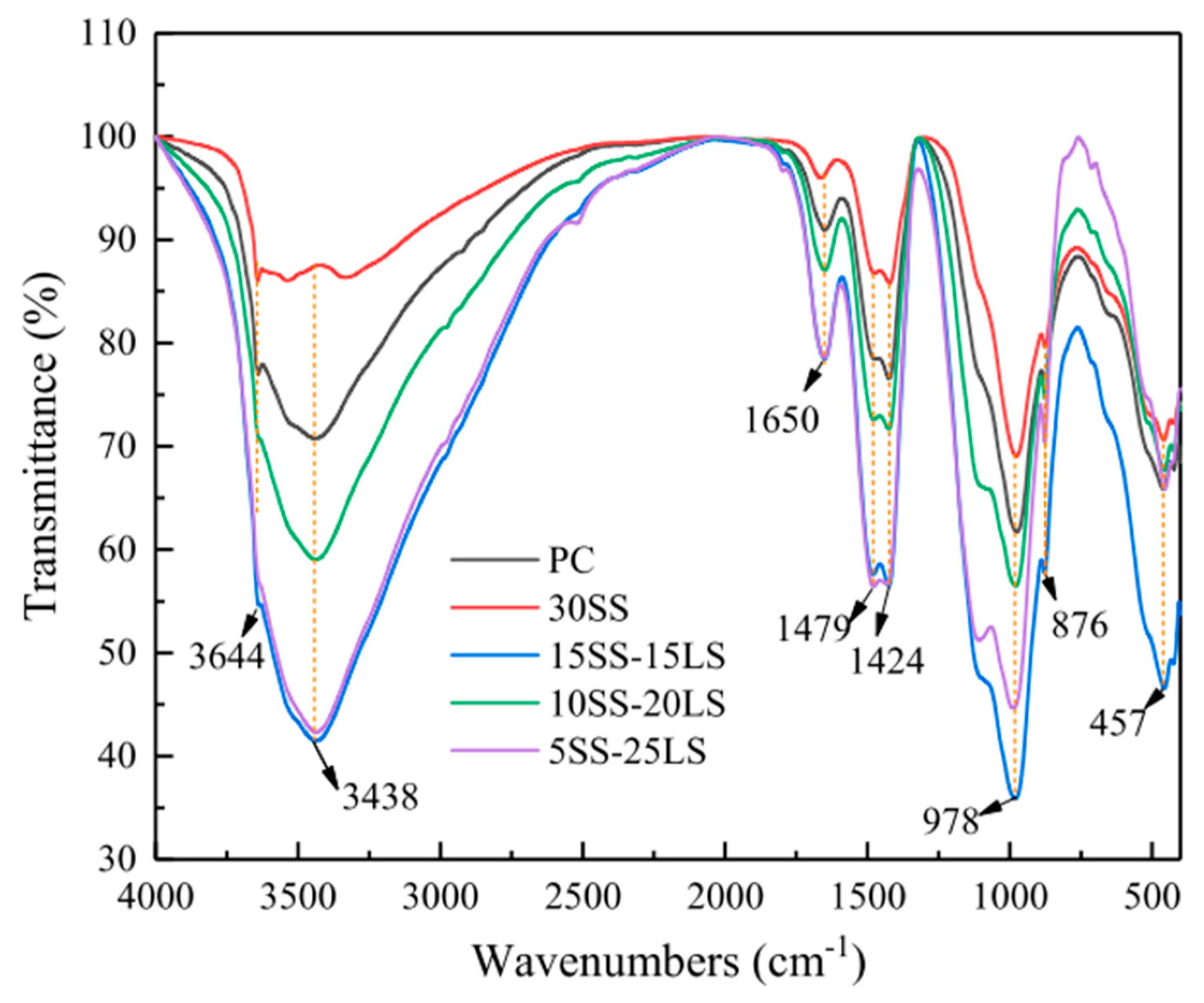
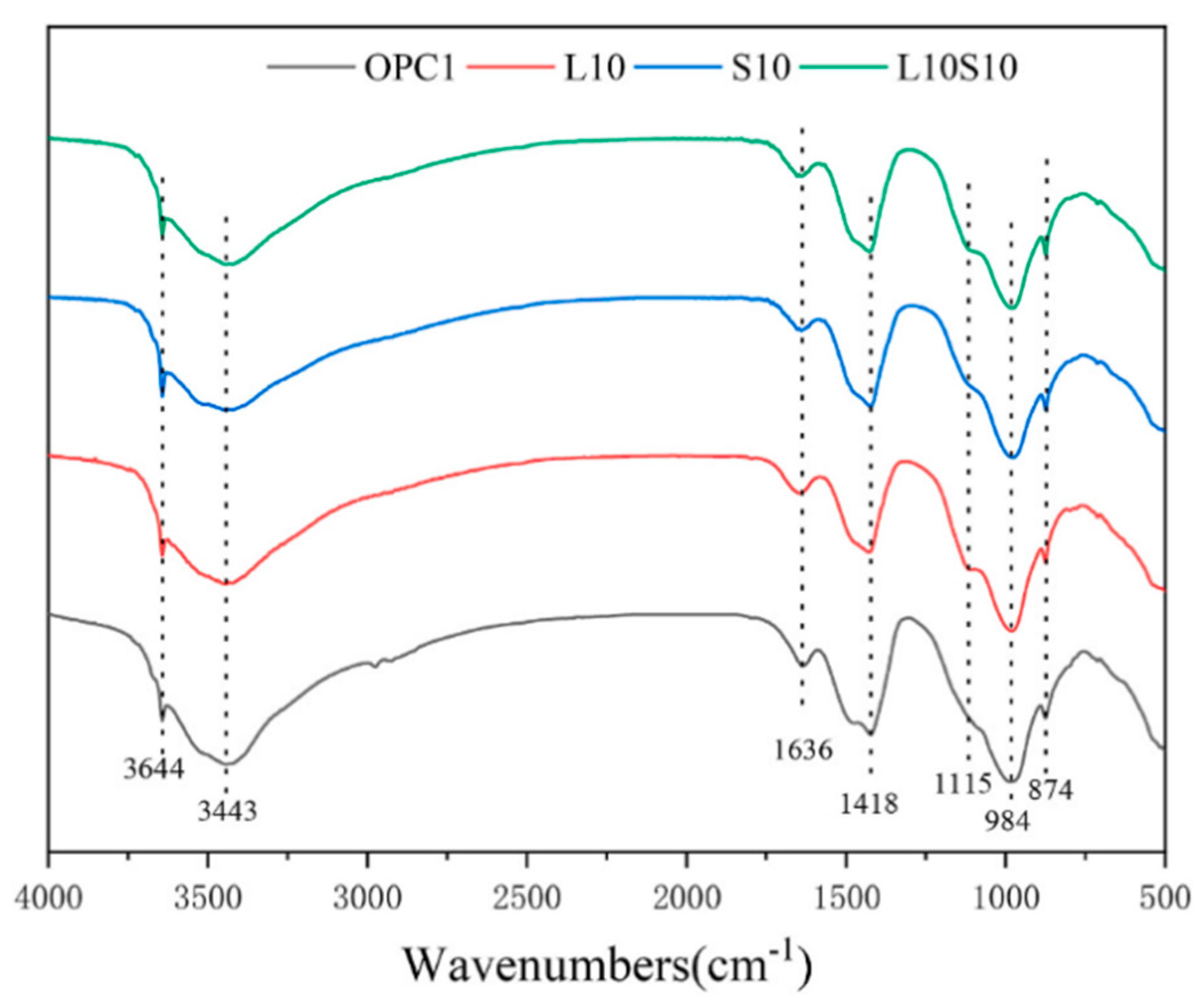
6.5. FTIR and TG Analysis
7. Cost, Energy, and Carbon Emission Comparisons
| Materials | Cost (USD/t) | Embodied CO2 (kg/t) | Embodied Energy (MJ/t) |
|---|---|---|---|
| LS | 10 [5] | 67 [118] | 2230 [22] |
| GGBS | 100 [119] | 67 [118] | 1590 [119] |
| MK | 220 [120] | 400 [121] | 2500 [120] |
| FA | 50 [122] | 8 [123] | 833 [121] |
| SF | 200 [124] | 14 [125] | 100 [126] |
| C | 467.5 [127] | 900 [128] | 5000 [129] |
| LP | 150 [130] | 75 [118] | 350 [121] |
8. Conclusions
- (1)
- The PSD of LS closely resembles that of FA and GGBS. This similarity suggests that LS can exhibit similar effects related to densification and nucleation when integrated into concrete, thus resembling the behavior of FA and other SCMs. Mechanical treatment of LS enhances the dissolution of aluminum, lithium, and silicon in LS, thereby expediting early hydration in LS–cement systems.
- (2)
- LS exhibits variations in SiO2 + Al2O3 and Ca/(Si + Al) within the ranges of 70.29–80.77% and 0.02–0.14%, respectively. This composition aligns LS with FA, which is characterized by high SiO2 and Al2O3 contents and a low CaO content. This similarity categorizes LS as a low-calcium precursor with chemical reactivity akin to that of FA.
- (3)
- In most of the literature examined, an increase in LS content was shown to lead to a reduction in the initial and final setting times of LS–cement and LS–geopolymer systems. Moreover, the studies determined that flowability decreased with an increase in LS content due to its irregular shape, strong water absorption characteristics, and elevated formation of AFt in the initial stages of hydration.
- (4)
- A recurring trend in most of the reviewed literature indicates that as LS content increases, the compressive strength, flexural strength, and splitting tensile strength ratios initially increase, with diminishing returns beyond a 30% threshold. This suggests an optimal LS content for achieving favorable mechanical properties. Additionally, with longer curing periods, there is a noticeable upward trend in the compressive strength, flexural strength, and splitting tensile strength ratios.
- (5)
- LS plays a crucial role in enhancing chloride ion migration resistance and reducing shrinkage in cementitious systems. Furthermore, as the composite ages, the resistance of the cementitious system to chloride ions becomes more robust. However, the behavior of drying shrinkage exhibits various trends.
- (6)
- The mechanisms through which LS operates within cementitious composites can be classified into three main categories. Firstly, there is the filling effect: the fine-grained nature of LS improves particle packing, and its fine particles act as pore blockers, thereby reducing interconnectivity between pores and effectively lowering porosity. Secondly, there is the pozzolanic effect: LS reacts with calcium hydroxide to generate additional hydration products, thereby refining large pores and bridging the gap between the paste and aggregates. Thirdly, there is the nucleation effect: LS provides nucleation sites, thereby promoting the preferential production and development of hydration products in these specific locations.
- (7)
- LS not only exhibits similar pozzolanic activity to FA, but it also comes at just one-fifth of the price of FA. This makes LS an economically attractive option for concrete or geopolymer production, thereby significantly reducing manufacturing costs. Moreover, the embodied CO2 and embodied energy of LS are comparable to GGBS, slightly higher than those of FA and SF, and significantly lower than those of MK, LP, and cement. Therefore, LS exhibits the potential for solid waste recycling and sustainable development.
9. Outlook
- (1)
- Current research on the grinding and chemical treatment of LS is limited. Further exploration is needed to enhance its utilization efficiency through physical and chemical modifications.
- (2)
- More research is required to understand the tensile properties of cementitious composites incorporating LS and their durability evolution in specific environments, such as freeze–thaw cycles and exposure to coupled acid–base and salt conditions.
- (3)
- Further exploration into the performance of LS in high-performance concrete, such as UHPC and engineered cementitious composites, is warranted.
- (4)
- The rheological properties of LS when incorporated into cement pastes and its subsequent performance in 3D printing applications deserve closer attention.
- (5)
- Investigation into the hydration mechanisms of LS when used in specialized cements, such as SAC and limestone calcined clay cement, requires further research.
- (6)
- The current quantitative research on the pozzolanic reactivity of LS is limited. A thorough assessment of the pozzolanic reactivity of LS is needed to confirm its suitability as an SCM in cement blends.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAM | Alkali-activated materials | PSD | Particle size distribution |
| AAS | Alkali-activated slag | SF | Silica fume |
| C | Cement | SSA | Specific surface area |
| DTG | Derivative thermogravimetric analysis | SS | Steel slag |
| EDS | Energy-dispersive spectroscopy | SCM | Supplementary cementitious material |
| FA | Fly ash | SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy | SAC | Sulfoaluminate cement |
| GGBS | Ground granulated blast slag | TIPA | Triisopropanolamine |
| LS | Lithium slag | TG | Thermogravimetry |
| LP | Limestone powder | TEA | Triethanolamine |
| MK | Metakaolin | UHPC | Ultra-high-performance concrete |
| NMR | Nuclear magnetic resonance | XRD | X-ray diffraction |
| OPC | Ordinary Portland cement | XRF | X-ray fluorescence |
| PCE | Polycarboxylate | XPS | X-ray photoelectron spectroscopy |
References
- Almeida, O.P.; Etherton-Beer, C.; Sanfilippo, F.; Page, A. Health morbidities associated with the dispensing of lithium to males and females: Cross-sectional analysis of the 10% Pharmaceutical Benefits Scheme sample for 2022. J. Affect. Disord. 2024, 344, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alfonso, J.E.; Moreno, G.; Valencia, C.; Sánchez, M.C.; Franco, J.M.; Gallegos, C. Influence of soap/polymer concentration ratio on the rheological properties of lithium lubricating greases modified with virgin LDPE. J. Ind. Eng. Chem. 2009, 15, 687–693. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Rabiea, E.A.; Abomostafa, H.M. Structural, optical, and magnetic studies on novel Pr- doped barium lithium fluoroborate glass system. J. Non-Cryst. Solids 2023, 619, 122533. [Google Scholar] [CrossRef]
- Juri, A.Z.; Song, X.-F.; Nakanishi, Y.; Dudley, J.; Jamieson, L.; Yin, L. Surface fractures in pre-crystallized and crystallized zirconia-containing lithium silicate glass-ceramics generated in ultrasonic vibration-assisted machining. J. Mech. Behav. Biomed. Mater. 2023, 147, 106132. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, S.F.; Chen, B.; Ahmad, M.R.; Haque, M.A. Development of Cleaner One-part geopolymer from lithium slag. J. Clean. Prod. 2021, 291, 125241. [Google Scholar] [CrossRef]
- Karrech, A.; Azadi, M.R.; Elchalakani, M.; Shahin, M.A.; Seibi, A.C. A review on methods for liberating lithium from pegmatities. Miner. Eng. 2020, 145, 106085. [Google Scholar] [CrossRef]
- Roy, T.; Plante, B.; Benzaazoua, M.; Demers, I. Geochemistry and mineralogy of a spodumene-pegmatite lithium ore at various mineral beneficiation stages. Miner. Eng. 2023, 202, 108312. [Google Scholar] [CrossRef]
- Kundu, T.; Rath, S.S.; Das, S.K.; Parhi, P.K.; Angadi, S.I. Recovery of lithium from spodumene-bearing pegmatites: A comprehensive review on geological reserves, beneficiation, and extraction. Powder Technol. 2023, 415, 118142. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Yliniemi, J.; Ismailov, A.; Levanen, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Recycling lithium mine tailings in the production of low temperature (700–900 °C) ceramics: Effect of ladle slag and sodium compounds on the processing and final properties. Constr. Build. Mater. 2019, 221, 332–344. [Google Scholar] [CrossRef]
- Rahman, S.A.; Shaikh, F.U.A.; Sarker, P.K. A comprehensive review of properties of concrete containing lithium refinery residue as partial replacement of cement. Constr. Build. Mater. 2022, 328, 127053. [Google Scholar] [CrossRef]
- Australian Lithium Production to Grow by 24.5% in 2022 as Capacity Expands. 2022. Available online: https://www.mining-technology.com/comment/australian-lithium-production/?cf-view (accessed on 27 July 2022).
- Liu, Z.; Wang, J.; Jiang, Q.; Cheng, G.; Li, L.; Kang, Y.; Wang, D. A green route to sustainable alkali-activated materials by heat and chemical activation of lithium slag. J. Clean. Prod. 2019, 225, 1184–1193. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Z.; Zhu, Y.; Huang, S.; Luo, Q.; Zhang, S. Effect of Lithium-Slag in the Performance of Slag Cement Mortar Based on Least-Squares Support Vector Machine Prediction. Materials 2019, 12, 1652. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, X.; He, C.; Ma, B.; Bai, Y.; Luo, Z. Utilization of lithium slag as an admixture in blended cements: Physico-mechanical and hydration characteristics. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 129–133. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Duan, P.; Ruan, S.; Zhang, Z.; Ge, W. The effects of lithium slag on microstructure and mechanical performance of metakaolin-based geopolymers designed by response surface method (RSM). Constr. Build. Mater. 2021, 299, 123950. [Google Scholar] [CrossRef]
- Guo, C.; Wang, R. Utilizing lithium slag to improve the physical-chemical properties of alkali-activated metakaolin-slag pastes: Cost and energy analysis. Constr. Build. Mater. 2023, 403, 133164. [Google Scholar] [CrossRef]
- Jayalath, A.; San Nicolas, R.; Sofi, M.; Shanks, R.; Ngo, T.; Aye, L.; Mendis, P. Properties of cementitious mortar and concrete containing micro-encapsulated phase change materials. Constr. Build. Mater. 2016, 120, 408–417. [Google Scholar] [CrossRef]
- Rupasinghe, M.; San Nicolas, R.; Mendis, P.; Sofi, M.; Ngo, T. Investigation of strength and hydration characteristics in nano-silica incorporated cement paste. Cem. Concr. Compos. 2017, 80, 17–30. [Google Scholar] [CrossRef]
- Rahman, S.; Dodd, A.; Khair, S.; Shaikh, F.; Sarker, P.; Hosan, M.A. Assessment of lithium slag as a supplementary cementitious material: Pozzolanic activity and microstructure development. Cem. Concr. Compos. 2023, 143, 105262. [Google Scholar] [CrossRef]
- Javed, U.; Shaikh, F.U.A.; Sarker, P.K. A comprehensive micro-nano investigative approach to study the development of aluminosilicate gel in binary blends of lithium slag geopolymer. Cem. Concr. Compos. 2024, 145, 105338. [Google Scholar] [CrossRef]
- Safari, A.; Lim, H. The benefit of delithiated beta spodumene to reduce the carbon footprint of cemented paste backfill. In Proceedings of the Paste 2023: 25th International Conference on Paste, Thickened and Filtered Tailings, University of Alberta, Edmonton, and Australian Centre for Geomechanics, Perth, Banff, AB, Canada, 29 April–3 May 2023; Wilson, G.W., Beier, N.A., Sego, D.C., Fourie, A.B., Reid, D., Eds.; University of Alberta, Edmonton, and Australian Centre for Geomechanics, Perth: Edmonton, NW, Canada, 2023; pp. 82–97. [Google Scholar]
- Javed, U.; Uddin Ahmed Shaikh, F.; Kumar Sarker, P. Microstructural investigation of thermo-mechanically processed lithium slag for geopolymer precursor using various characterization techniques. Constr. Build. Mater. 2022, 342, 127952. [Google Scholar] [CrossRef]
- Javed, U.; Shaikh, F.U.A.; Sarker, P.K. Microstructural investigation of lithium slag geopolymer pastes containing silica fume and fly ash as additive chemical modifiers. Cem. Concr. Compos. 2022, 134, 104736. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Y.; Dong, B.; Ren, J.; He, Y.; Wu, K.; Wang, Y. Lithium slag-based geopolymer synthesized with hybrid solid activators. Constr. Build. Mater. 2023, 365, 130070. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.; Qi, C.; Zhang, Q.; Xiao, C. Lithium slag and fly ash-based binder for cemented fine tailings backfill. J. Environ. Manag. 2019, 248, 109282. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Q.; Chen, Q.; Bian, J.; Qi, C.; Kang, Q.; Feng, Y. Mechanical and environmental characteristics of cemented paste backfill containing lithium slag-blended binder. Constr. Build. Mater. 2021, 271, 121567. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Liu, D.; Wang, M. Preparation of lightweight ceramsite from solid waste lithium slag and fly ash. Constr. Build. Mater. 2023, 398, 132419. [Google Scholar] [CrossRef]
- Borges, P.H.; Santos, F.A.; Milikic, N.; A Barsante, C. Lithium aluminosilicate residue as raw material in the production of sustainable concrete masonry units: A brazilian case. Open Constr. Build. Technol. J. 2016, 10, 418–430. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, X.; He, X.; Guo, Y.; Deng, X.; Su, Y.; Yang, J.; Wang, Y. Utilization of lithium slag by wet-grinding process to improve the early strength of sulphoaluminate cement paste. J. Clean. Prod. 2018, 205, 536–551. [Google Scholar] [CrossRef]
- Tan, H.; Li, M.; He, X.; Su, Y.; Zhang, J.; Pan, H.; Yang, J.; Wang, Y. Preparation for micro-lithium slag via wet grinding and its application as accelerator in Portland cement. J. Clean. Prod. 2020, 250, 119528. [Google Scholar] [CrossRef]
- Li, J.; Lian, P.; Huang, S.; Huang, L. Recycling of lithium slag extracted from lithium mica by preparing white Portland cement. J. Environ. Manag. 2020, 265, 110551. [Google Scholar] [CrossRef]
- Zhang, L.F.; Wang, R.Y. Experimental study on alkali-activated slag-lithium slag-fly ash environmental concrete. Adv. Mater. Res. 2011, 287, 1237–1240. [Google Scholar] [CrossRef]
- He, Z.-h.; Du, S.-g.; Chen, D. Microstructure of ultra high performance concrete containing lithium slag. J. Hazard. Mater. 2018, 353, 35–43. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, B.; Tan, H.; Liu, X.; Chen, P.; Luo, Z. Effect of TIPA on mechanical properties and hydration properties of cement-lithium slag system. J. Environ. Manag. 2020, 276, 111274. [Google Scholar] [CrossRef] [PubMed]
- Wen, H. Property research of green concrete mixed with lithium slag and limestone flour. Adv. Mater. Res. 2013, 765–767, 3120–3124. [Google Scholar] [CrossRef]
- He, Z.; Wu, R.; Yu, Y.; Gao, Y. Effect of Lithium Slag on Drying Shrinkage of Concrete with Manufactured-sand. J. Residuals Sci. Technol. 2017, 14, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, B.; Gu, X.; Han, D.; Wang, Q. Improving the performance of ultra-high performance concrete containing lithium slag by incorporating limestone powder. J. Build. Eng. 2023, 72, 106610. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, Q.; Han, D.; Li, Z. Iron ore tailings, phosphate slags, and lithium slags as ternary supplementary cementitious materials for concrete: Study on compression strength and microstructure. Mater. Today Commun. 2023, 36, 106644. [Google Scholar] [CrossRef]
- He, Y.; Zhang, G.; He, S.; Liu, S.; Jiang, M. Effect of C-S-H-PCE and TEA on performances of lithium slag-cement binder. J. Build. Eng. 2023, 78, 107659. [Google Scholar] [CrossRef]
- Argın, G.; Uzal, B. Enhancement of pozzolanic activity of calcined clays by limestone powder addition. Constr. Build. Mater. 2021, 284, 122789. [Google Scholar] [CrossRef]
- Haigh, M.; Dumitru, I.; Munn, B.; Papworth, F. Development of new high performance supplementary cementitious material—A lithium production by-product. In Proceedings of the CIA Binnual Conference, Queensland, Australia, 13 October 2013. [Google Scholar]
- He, Y.; Kang, Q.; Lan, M.; Peng, H. Mechanism and assessment of the pozzolanic activity of melting-quenching lithium slag modified with MgO. Constr. Build. Mater. 2023, 363, 129692. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.-X.; Li, L.; Wang, D.-M. Characteristics of alkali-activated lithium slag at early reaction age. J. Mater. Civ. Eng. 2019, 31, 4019312. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Liu, M.; Li, Y.; Tang, Y.; Zhuang, L.; Tian, B. Comprehensive utilization of waste residue from lithium extraction process of spodumene. Miner. Eng. 2021, 170, 106986. [Google Scholar] [CrossRef]
- Zhai, M.; Zhao, J.; Wang, D.; Wang, Y.; Wang, Q. Hydration properties and kinetic characteristics of blended cement containing lithium slag powder. J. Build. Eng. 2021, 39, 102287. [Google Scholar] [CrossRef]
- Karrech, A.; Dong, M.; Elchalakani, M.; Shahin, M. Sustainable geopolymer using lithium concentrate residues. Constr. Build. Mater. 2019, 228, 116740. [Google Scholar] [CrossRef]
- Yiren, W.; Dongmin, W.; Yong, C.; Dapeng, Z.; Ze, L. Micro-morphology and phase composition of lithium slag from lithium carbonate production by sulphuric acid process. Constr. Build. Mater. 2019, 203, 304–313. [Google Scholar] [CrossRef]
- Li, B.; Cao, R.; You, N.; Chen, C.; Zhang, Y. Products and properties of steam cured cement mortar containing lithium slag under partial immersion in sulfate solution. Constr. Build. Mater. 2019, 220, 596–606. [Google Scholar] [CrossRef]
- Li, J.; Huang, S. Recycling of lithium slag as a green admixture for white reactive powder concrete. J. Mater. Cycles Waste Manag. 2020, 22, 1818–1827. [Google Scholar] [CrossRef]
- He, Z.-h.; Li, L.-y.; Du, S.-g. Mechanical properties, drying shrinkage, and creep of concrete containing lithium slag. Constr. Build. Mater. 2017, 147, 296–304. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, J.; Li, Z.; Zhang, Y. The Mechanical Properties of Recycled Coarse Aggregate Concrete with Lithium Slag. Adv. Mater. Sci. Eng. 2019, 2019, 8974625. [Google Scholar] [CrossRef]
- Wu, F.F.; Shi, K.B.; Dong, S.K. Influence of concrete with lithium-slag and steel slag by early curing conditions. Key Eng. Mater. 2014, 599, 52–55. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, S. Ring method test on the early-age anti-cracking capability of high-performance lithium slag concrete. Appl. Mech. Mater. 2011, 94–96, 782–785. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.W.; Lan, X.Z.; Su, Y.S.; Wang, X.Y.; Wu, Y.D. Structural behavior of the stiffened double-skin profiled composite walls under compression. Steel Compos. Struct. 2019, 31, 1–12. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M.; Zhu, Z. Effect of Aluminium Substitution on Physical Adsorption of Chloride and Sulphate Ions in Cement-Based Materials. Materials 2023, 16, 6029. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Huang, S.; Zhou, Y.; Li, J.; Peng, W.; Wen, Y. Influence of lithium slag from lepidolite on the durability of concrete. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 12151. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, J.; Liu, K.; Lu, Y. Durability properties of recycled concrete with lithium slag under freeze-thaw cycles. Mater. Technol. 2021, 55, 171–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Guo, Z.; Li, D. Research on Performance Deterioration of Multi-walled Carbon Nanotube-lithium Slag Concrete under the Coupling Effect of Sulfate Attack and Dry-wet Cycles. Materials 2023, 16, 5130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gong, J.; Chen, B.; Gong, X.; Guo, W.; Zhang, Y.; Li, F. Mechanical Properties and Shrinkage of Ultrahigh-Performance Concrete Containing Lithium Carbonate and Nano-Calcium Carbonate. Adv. Civ. Eng. 2021, 2021, 6646272. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.; Xu, K.; Bi, L.; Chen, M.; Han, B. Corrosion behavior of concrete fabricated with lithium slag as corrosion inhibitor under simulated acid rain corrosion action. J. Clean. Prod. 2022, 377, 134300. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Zou, F.; Luo, H.; Zhou, Z.; Zhu, J.; Guo, G.; Zhong, Y. High performance C-A-S-H seeds from fly ash-carbide slag for activating lithium slag towards a low carbon binder. J. Environ. Manag. 2023, 345, 118658. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.B. The effects of particle size distribution and surface area upon cement strength development. Powder Technol. 2009, 188, 272–276. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, J.; Fan, J.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Lu, Z. Insight to workability, compressive strength and microstructure of lithium slag-steel slag based cement under standard condition. J. Build. Eng. 2023, 75, 107076. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rhee, K.Y. Evaluation of pozzolanic activity, heterogeneous nucleation, and microstructure of cement composites with modified bentonite clays. Constr. Build. Mater. 2022, 323, 126617. [Google Scholar] [CrossRef]
- Küçük, M.; Kinnarinen, T.; Timonen, J.; Mulari, O.; Häkkinen, A. Characterisation of Industrial Side Streams and Their Application for the Production of Geopolymer Composites. Minerals 2021, 11, 593. [Google Scholar] [CrossRef]
- Martins, A.C.P.; De Carvalho, J.M.F.; Costa, L.C.B.; Andrade, H.D.; de Melo, T.V.; Ribeiro, J.C.L.; Pedroti, L.G.; Peixoto, R.A.F. Steel slags in cement-based composites: An ultimate review on characterization, applications and performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- Šiler, P.; Krátký, J.; Kolářová, I.; Havlica, J.; Brandštetr, J. Calorimetric determination of the effect of additives on cement hydration process. Chem. Pap. 2013, 67, 213–220. [Google Scholar] [CrossRef]
- Sarkar, A.; Rano, R.; Mishra, K.K.; Sinha, I.N. Particle size distribution profile of some Indian fly ash—A comparative study to assess their possible uses. Fuel Process. Technol. 2005, 86, 1221–1238. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Mahmood, A.H.; Shaikh, F.U.A.; Sarker, P.K. Fresh state and hydration properties of high-volume lithium slag cement composites. Mater. Struct. 2023, 56, 91. [Google Scholar] [CrossRef]
- Odler, I. The BET-specific surface area of hydrated Portland cement and related materials. Cem. Concr. Res. 2003, 33, 2049–2056. [Google Scholar] [CrossRef]
- Sarbak, Z.; Stańczyk, A.; Kramer-Wachowiak, M. Characterisation of surface properties of various fly ashes. Powder Technol. 2004, 145, 82–87. [Google Scholar] [CrossRef]
- Yeau, K.Y.; Kim, E.K. An experimental study on corrosion resistance of concrete with ground granulate blast-furnace slag. Cem. Concr. Res. 2005, 35, 1391–1399. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and characterization of zeolite X from lithium slag. Appl. Clay Sci. 2012, 59–60, 148–151. [Google Scholar] [CrossRef]
- Gao, W.; Jian, S.; Li, X.; Tan, H.; Li, B.; Lv, Y.; Huang, J. The use of contaminated soil and lithium slag for the production of sustainable lightweight aggregate. J. Clean. Prod. 2022, 348, 131361. [Google Scholar] [CrossRef]
- Pfingsten, J.; Rickert, J.; Lipus, K. Estimation of the content of ground granulated blast furnace slag and different pozzolanas in hardened concrete. Constr. Build. Mater. 2018, 165, 931–938. [Google Scholar] [CrossRef]
- Ayala Valderrama, D.M.; Gómez Cuaspud, J.A.; Roether, J.A.; Boccaccini, A.R. Development and characterization of glass-ceramics from combinations of slag, fly ash, and glass cullet without adding nucleating agents. Materials 2019, 12, 2032. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, N.; Terzić, A.; Pezo, L.; Miličić, L.; Živojinović, D. Validation of energy-dispersive X-ray fluorescence procedure for determination of major and trace elements present in the cement based composites. Spectrochim. Acta Part B At. Spectrosc. 2019, 162, 105729. [Google Scholar] [CrossRef]
- Hossein, H.A.; Hamzawy, E.M.; El-Bassyouni, G.T.; Nabawy, B.S. Mechanical and physical properties of synthetic sustainable geopolymer binders manufactured using rockwool, granulated slag, and silica fume. Constr. Build. Mater. 2023, 367, 130143. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Evaluation of the suitability of ground granulated silico-manganese slag in Portland slag cement. Constr. Build. Mater. 2016, 125, 127–134. [Google Scholar] [CrossRef]
- Snellings, R.; Kazemi-Kamyab, H.; Nielsen, P.; Van den Abeele, L. Classification and milling increase fly ash pozzolanic reactivity. Front. Built Environ. 2021, 7, 670996. [Google Scholar] [CrossRef]
- Karrech, A.; Dong, M.; Skut, J.; Elchalakani, M.; Shahin, M.A. Management and valorisation of delithiated β-spodumene and its processing stream. Case Stud. Constr. Mater. 2021, 15, e00671. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Acid roasting of spodumene: Microwave vs. conventional heating. Miner. Eng. 2019, 138, 161–167. [Google Scholar] [CrossRef]
- Peltosaari, O.; Tanskanen, P.; Heikkinen, E.-P.; Fabritius, T. α→γ→β-phase transformation of spodumene with hybrid microwave and conventional furnaces. Miner. Eng. 2015, 82, 54–60. [Google Scholar] [CrossRef]
- Maslyk, M.; Dallos, Z.; Koziol, M.; Seiffert, S.; Hieke, T.; Petrović, K.; Kolb, U.; Mondeshki, M.; Tremel, W. A Fast and Sustainable Route to Bassanite Nanocrystals from Gypsum. Adv. Funct. Mater. 2022, 32, 2111852. [Google Scholar] [CrossRef]
- Bernal, S.A.; Juenger, M.C.; Ke, X.; Matthes, W.; Lothenbach, B.; De Belie, N.; Provis, J.L. Characterization of supplementary cementitious materials by thermal analysis. Mater. Struct. 2017, 50, 1–13. [Google Scholar] [CrossRef]
- Burris, L.E.; Juenger, M.C. Effect of calcination on the reactivity of natural clinoptilolite zeolites used as supplementary cementitious materials. Constr. Build. Mater. 2020, 258, 119988. [Google Scholar] [CrossRef]
- Tan, H.; Li, M.; He, X.; Su, Y.; Yang, J.; Zhao, H. Effect of wet grinded lithium slag on compressive strength and hydration of sulphoaluminate cement system. Constr. Build. Mater. 2021, 267, 120465. [Google Scholar] [CrossRef]
- Noor, L.; Tuinukuafe, A.; Ideker, J.H. A critical review of the role of ettringite in binders composed of CAC–PC–C and CSA–PC–C. J. Am. Ceram. Soc. 2023, 106, 3303–3328. [Google Scholar] [CrossRef]
- Matalkah, F.; Salem, T.; Shaafaey, M.; Soroushian, P. Drying shrinkage of alkali activated binders cured at room temperature. Constr. Build. Mater. 2019, 201, 563–570. [Google Scholar] [CrossRef]
- Witzleben, S.T. Acceleration of Portland cement with lithium, sodium and potassium silicates and hydroxides. Mater. Chem. Phys. 2020, 243, 122608. [Google Scholar] [CrossRef]
- Wu, F.F.; Shi, K.B.; Dong, S.K. Properties and Microstructure of HPC with Lithium-Slag and Fly Ash. Key Eng. Mater. 2014, 599, 70–73. [Google Scholar] [CrossRef]
- Dong, P.; Ahmad, M.R.; Chen, B.; Munir, M.J.; Kazmi, S.M.S. Preparation and study of magnesium ammonium phosphate cement from waste lithium slag. J. Clean. Prod. 2021, 316, 128371. [Google Scholar] [CrossRef]
- He, Z.; Chang, J.; Du, S.; Liang, C.; Liu, B. Hydration and microstructure of concrete containing high volume lithium slag. Mater. Express 2020, 10, 430–436. [Google Scholar] [CrossRef]
- Gu, X.; Wang, H.; Zhu, Z.; Liu, J.; Xu, X.; Wang, Q. Synergistic effect and mechanism of lithium slag on mechanical properties and microstructure of steel slag-cement system. Constr. Build. Mater. 2023, 396, 131768. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Balakrishna, M.N.; Chamberlain, D.A. Performance enhancement of self-compacting concrete in saline environment by hydrophobic surface protection. Can. J. Civ. Eng. 2019, 46, 677–686. [Google Scholar] [CrossRef]
- Biricik, Ö.; Mardani, A. Parameters affecting thixotropic behavior of self compacting concrete and 3D printable concrete; A state-of-the-art review. Constr. Build. Mater. 2022, 339, 127688. [Google Scholar] [CrossRef]
- Souza, M.T.; Ferreira, I.M.; de Moraes, E.G.; Senff, L.; de Oliveira, A.P.N. 3D printed concrete for large-scale buildings: An overview of rheology, printing parameters, chemical admixtures, reinforcements, and economic and environmental prospects. J. Build. Eng. 2020, 32, 101833. [Google Scholar] [CrossRef]
- He, Y.; You, C.; Jiang, M.; Liu, S.; Shen, J.; Hooton, R.D. Rheological performance and hydration kinetics of lithium slag-cement binder in the function of sodium sulfate. J. Therm. Anal. Calorim. 2023, 148, 11653–11668. [Google Scholar] [CrossRef]
- Prem, P.R.; Ravichandran, D.; Kaliyavaradhan, S.K.; Ambily, P. Comparative evaluation of rheological models for 3d printable concrete. Mater. Today Proc. 2022, 65, 1594–1598. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, Z.; Yuan, Q.; Jamaa, G.M.; Yang, C.; Zhu, X. Improving the rheological behavior of alkali-activated slag pastes by using low surface free energy mineral admixtures. Constr. Build. Mater. 2023, 392, 131879. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Hong, S.; Xing, F.; Dong, B. Properties and microstructure of lithium-slag-based geopolymer by one-part mixing method. Constr. Build. Mater. 2021, 273, 121723. [Google Scholar] [CrossRef]
- Targan, Ş.; Olgun, A.; Erdogan, Y.; Sevinç, V. Influence of natural pozzolan, colemanite ore waste, bottom ash, and fly ash on the properties of Portland cement. Cem. Concr. Res. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rukzon, S.; Sirivivatnanon, V. Resistance to chloride penetration of blended Portland cement mortar containing palm oil fuel ash, rice husk ash and fly ash. Constr. Build. Mater. 2008, 22, 932–938. [Google Scholar] [CrossRef]
- Guang-tai, Z.; Yong, C.; Hai-bo, L.; Xue-fan, L. Fractal characteristics of fiber lithium slag concrete cracks under sulfate attack. Chin. J. Eng. 2022, 44, 208. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, Y.C.; Arulrajah, A.; Sofi, M.; Sabri, Y. Reaction mechanism of alkali-activated brick clay mill residues. Constr. Build. Mater. 2022, 341, 127817. [Google Scholar] [CrossRef]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Mohamed, A.K. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Dorn, T.; Blask, O.; Stephan, D. Acceleration of cement hydration—A review of the working mechanisms, effects on setting time, and compressive strength development of accelerating admixtures. Constr. Build. Mater. 2022, 323, 126554. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Hooton, R.D.; Zhang, X.; He, S. Effects of TEA on rheological property and hydration performance of lithium slag-cement composite binder. Constr. Build. Mater. 2022, 318, 125757. [Google Scholar] [CrossRef]
- Haustein, E.; Kuryłowicz-Cudowska, A. The effect of fly ash microspheres on the pore structure of concrete. Minerals 2020, 10, 58. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Miah, M.J.; Patoary, M.M.H.; Paul, S.C.; Babafemi, A.J.; Panda, B. Enhancement of mechanical properties and porosity of concrete using steel slag coarse aggregate. Materials 2020, 13, 2865. [Google Scholar] [CrossRef]
- Schovanz, D.; Tiecher, F.; Hasparyk, N.P.; Kuperman, S.; Lermen, R.T. Evaluation of delayed ettringite formation through physical, mechanical, and microstructural assays. ACI Mater. J. 2021, 118, 101–109. [Google Scholar] [CrossRef]
- Flores-Ledesma, A.; Tejeda-Cruz, A.; Bucio, L.; Wintergerst, A.M.; Rodríguez-Chávez, J.A.; Moreno-Vargas, Y.A.; Arenas-Alatorre, J.A. Hydration products and bioactivity of an experimental MTA-like cement modified with wollastonite and bioactive glass. Ceram. Int. 2020, 46, 15963–15971. [Google Scholar] [CrossRef]
- Karim, M.R.; Chowdhury, F.I.; Zabed, H.; Saidur, M.R. Effect of elevated temperatures on compressive strength and microstructure of cement paste containing palm oil clinker powder. Constr. Build. Mater. 2018, 183, 376–383. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- Das, S.; Saha, P.; Jena, S.P.; Panda, P. Geopolymer concrete: Sustainable green concrete for reduced greenhouse gas emission—A review. Mater. Today Proc. 2022, 60, 62–71. [Google Scholar] [CrossRef]
- Samad, S.; Shah, A. Role of Binary Cement in Production of Environmentally Sustainable Concrete: A Critical Review. Int. J. Sustain. Built Environ. 2017, 6, 663–674. [Google Scholar] [CrossRef]
- Onn, C.C.; Mo, K.H.; Radwan, M.K.; Liew, W.H.; Ng, C.G.; Yusoff, S. Strength, carbon footprint and cost considerations of mortar blends with high volume ground granulated blast furnace slag. Sustainability 2019, 11, 7194. [Google Scholar] [CrossRef]
- Perez-Cortes, P.; Escalante-Garcia, J.I. Alkali activated metakaolin with high limestone contents—Statistical modeling of strength and environmental and cost analyses. Cem. Concr. Compos. 2020, 106, 103450. [Google Scholar] [CrossRef]
- Kumar, R.; Shafiq, N.; Kumar, A.; Jhatial, A.A. Investigating embodied carbon, mechanical properties, and durability of high-performance concrete using ternary and quaternary blends of metakaolin, nano-silica, and fly ash. Environ. Sci. Pollut. Res. 2021, 28, 49074–49088. [Google Scholar] [CrossRef]
- Request a Quote. Available online: https://www.boral.com.au/contact (accessed on 28 September 2023).
- Kumar, A.; Bheel, N.; Ahmed, I.; Rizvi, S.H.; Kumar, R.; Jhatial, A.A. Effect of silica fume and fly ash as cementitious material on hardened properties and embodied carbon of roller compacted concrete. Environ. Sci. Pollut. Res. 2022, 29, 1210–1222. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Shoubi, M.V.; Barough, A.S.; Amirsoleimani, O. Assessment of the roles of various cement replacements in achieving the sustainable and high performance concrete. Int. J. Adv. Eng. Technol. 2013, 6, 68. [Google Scholar]
- Kuruşcu, A.O.; Girgin, Z.C. Efficiency of structural materials in sustainable design. J. Civ. Eng. Archit. Oct. 2014, 8, 1260–1265. [Google Scholar]
- Available online: https://www.soilworx.com.au/contact-us/ (accessed on 28 September 2023).
- Anderson, J.; Moncaster, A. Embodied carbon of concrete in buildings, Part 1: Analysis of published EPD. Build. Cities 2020, 1, 198–217. [Google Scholar] [CrossRef]
- Henry, C.S.; Lynam, J.G. Embodied energy of rice husk ash for sustainable cement production. Case Stud. Chem. Environ. Eng. 2020, 2, 100004. [Google Scholar] [CrossRef]
- Factory Supply High Quality Limestone Calcium Carbonate Powder CaCO3 Powder. Available online: https://huabangmineral.en.made-in-china.com/product/TwQfZOLSbrUg (accessed on 30 September 2023).






| System | Specific Combination | References |
|---|---|---|
| Binary | LS-C | [29,30,31] |
| Ternary | LS-FA-C | [25,32] |
| LS-SF-C | [33] | |
| LS-TIPA-C | [34] | |
| LS-LP-C | [35,36] | |
| Quaternary | LS-GGBS-FA-C | [32] |
| LS-LP-SF-C | [37] | |
| LS-PS-SS-C | [38] | |
| LS-PCE-TEA-C | [39] |
| Reference | D10 (µm) | D50 (µm) | D90 (µm) |
|---|---|---|---|
| [38] | 1.56 | 13.00 | 81.00 |
| [45] | 2.90 | 7.10 | 42.80 |
| [39] | - | 11.80 | - |
| [61] | - | 30.39 | - |
| [37] | 0.84 | 6.24 | 28.00 |
| [63] | 2.74 | 25.26 | 83.65 |
| [43] | - | 4.53 | - |
| [19] | - | 38.00 | - |
| Average | 2.01 | 17.04 | 58.86 |
| Reference | SiO2 | Al2O3 | Fe2O3 | SO3 | CaO | MgO | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| [34] | 52.21 | 20.60 | 0.84 | 9.18 | 4.63 | 0.16 | 0.26 | 0.33 | 11.39 |
| [42] | 40.33 | 34.51 | 2.25 | - | 18.47 | 0.05 | - | - | - |
| [22] | 54.53 | 21.08 | 1.45 | 5.62 | 7.54 | 0.58 | 0.89 | 0.72 | 6.76 |
| [27] | 48.97 | 21.32 | 1.07 | 16.2 | 8.26 | 0.19 | 3.37 | ||
| [38] | 54.55 | 25.38 | 1.41 | 10.14 | 6.44 | 0.60 | 0.70 | 0.10 | - |
| [45] | 55.94 | 24.83 | 0.82 | 10.02 | 5.89 | 0.30 | 0.22 | - | - |
| [24] | 51.7 | 25.2 | 0.6 | 0.05 | 2.5 | 0.3 | 3.7 | - | 0.2 |
| [19] | 54.6 | 21.1 | 1.5 | 5.6 | 7.5 | 1.3 | 0.4 | 0.3 | - |
| [37] | 54.86 | 22.39 | 1.27 | 6.05 | 13.72 | 0.32 | 0.60 | 9.60 | |
| [63] | 53.92 | 21.13 | 1.55 | 11.19 | 11.11 | 0.40 | 0.24 | 0.14 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, H.; Rupasinghe, M.; Sofi, M.; Sharma, R.; Ranzi, G.; Mendis, P.; Zhang, Z. A Review on Cementitious and Geopolymer Composites with Lithium Slag Incorporation. Materials 2024, 17, 142. https://doi.org/10.3390/ma17010142
Gou H, Rupasinghe M, Sofi M, Sharma R, Ranzi G, Mendis P, Zhang Z. A Review on Cementitious and Geopolymer Composites with Lithium Slag Incorporation. Materials. 2024; 17(1):142. https://doi.org/10.3390/ma17010142
Chicago/Turabian StyleGou, Hongxiang, Madhuwanthi Rupasinghe, Massoud Sofi, Rajesh Sharma, Gianluca Ranzi, Priyan Mendis, and Zipeng Zhang. 2024. "A Review on Cementitious and Geopolymer Composites with Lithium Slag Incorporation" Materials 17, no. 1: 142. https://doi.org/10.3390/ma17010142
APA StyleGou, H., Rupasinghe, M., Sofi, M., Sharma, R., Ranzi, G., Mendis, P., & Zhang, Z. (2024). A Review on Cementitious and Geopolymer Composites with Lithium Slag Incorporation. Materials, 17(1), 142. https://doi.org/10.3390/ma17010142







