Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
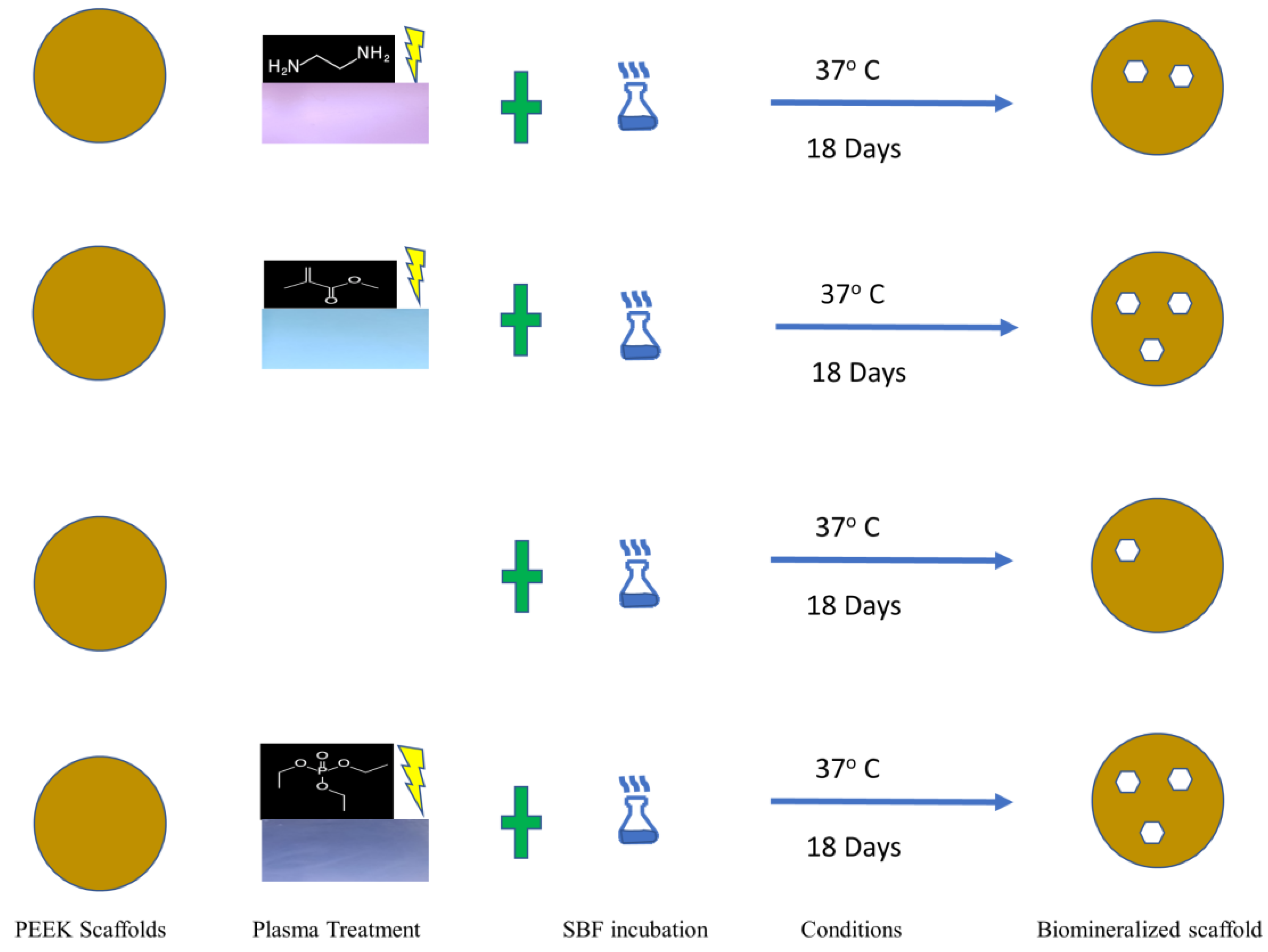
2.2. Scaffold Formation and Biomineralization Process
Accelerated Biomineralization of Scaffold
2.3. Characterization of Biomineralized PEEK
2.3.1. Chemical and Biomineralization Characterization
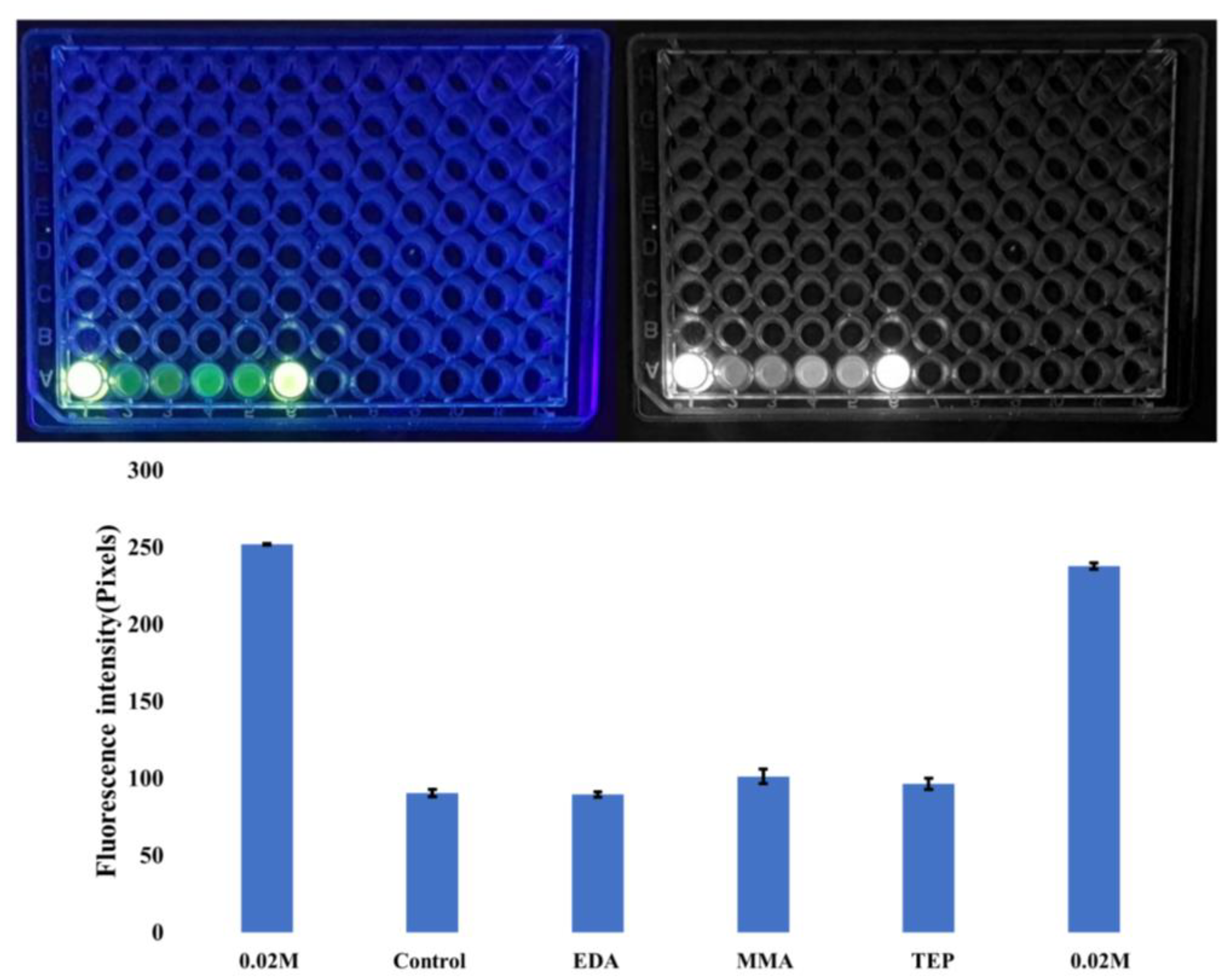
Calcein Staining
Fourier Infrared Spectroscopy (FTIR)
X-ray Photoelectron Spectroscopy
2.3.2. Morphological Analysis
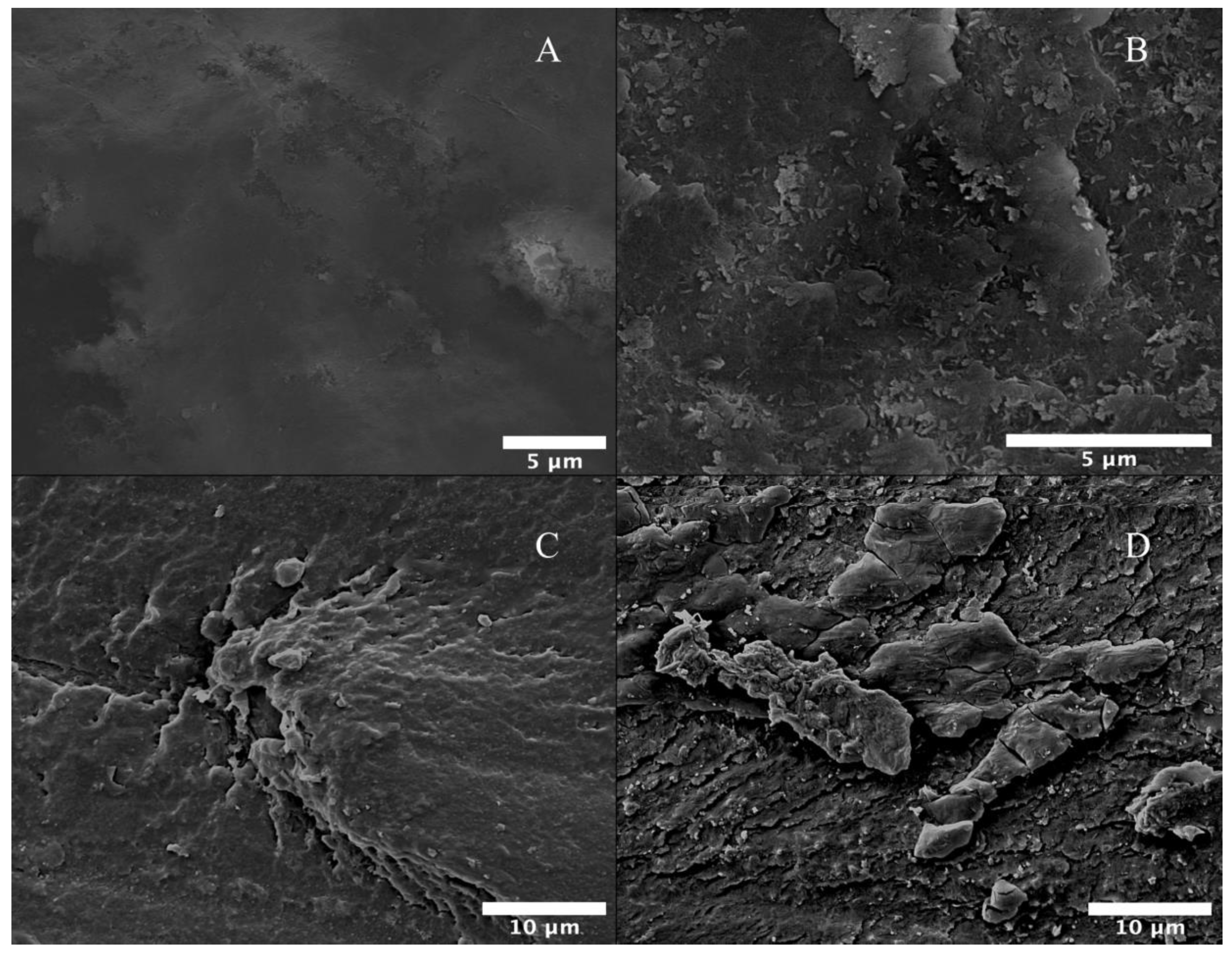
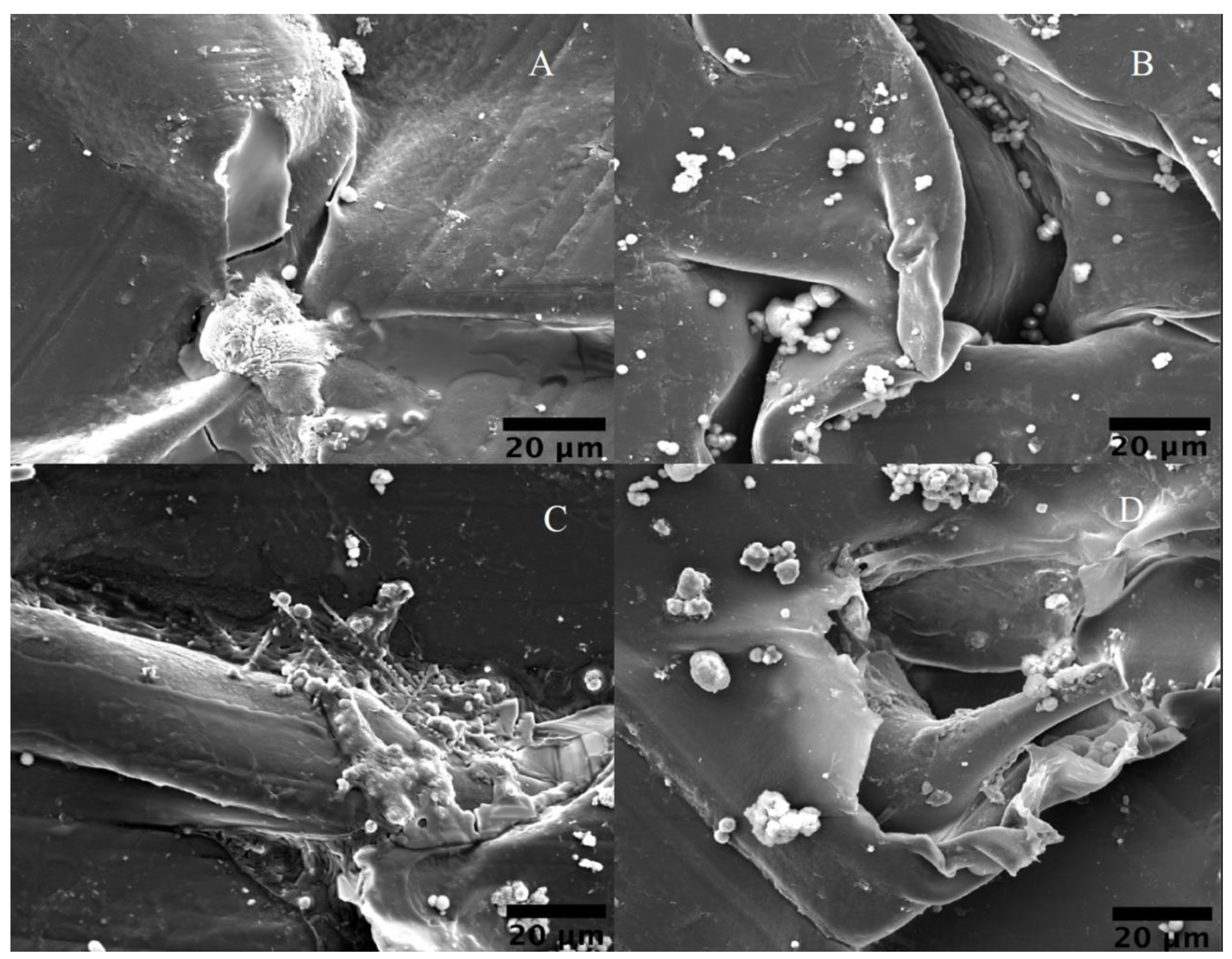
Scanning Electron Microscopy (SEM)
Water Contact Angle (WCA)
Surface Roughness Measurement
2.3.3. Biological Characterization
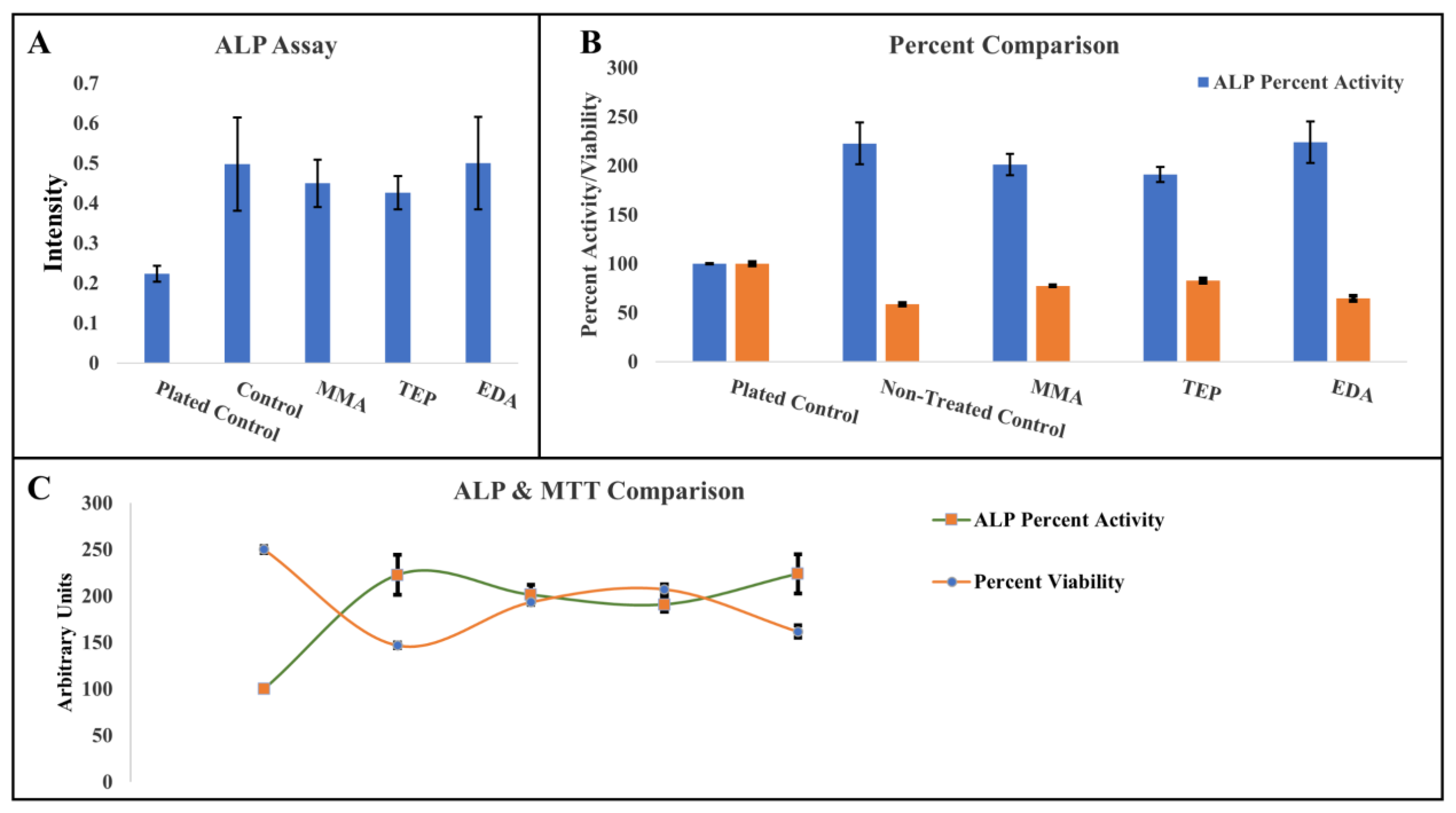
Alkaline Phosphatase (ALP) Absorbance
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Cell Assay
3. Results and Discussion
3.1. Wettability of Plasma-Treated PEEK
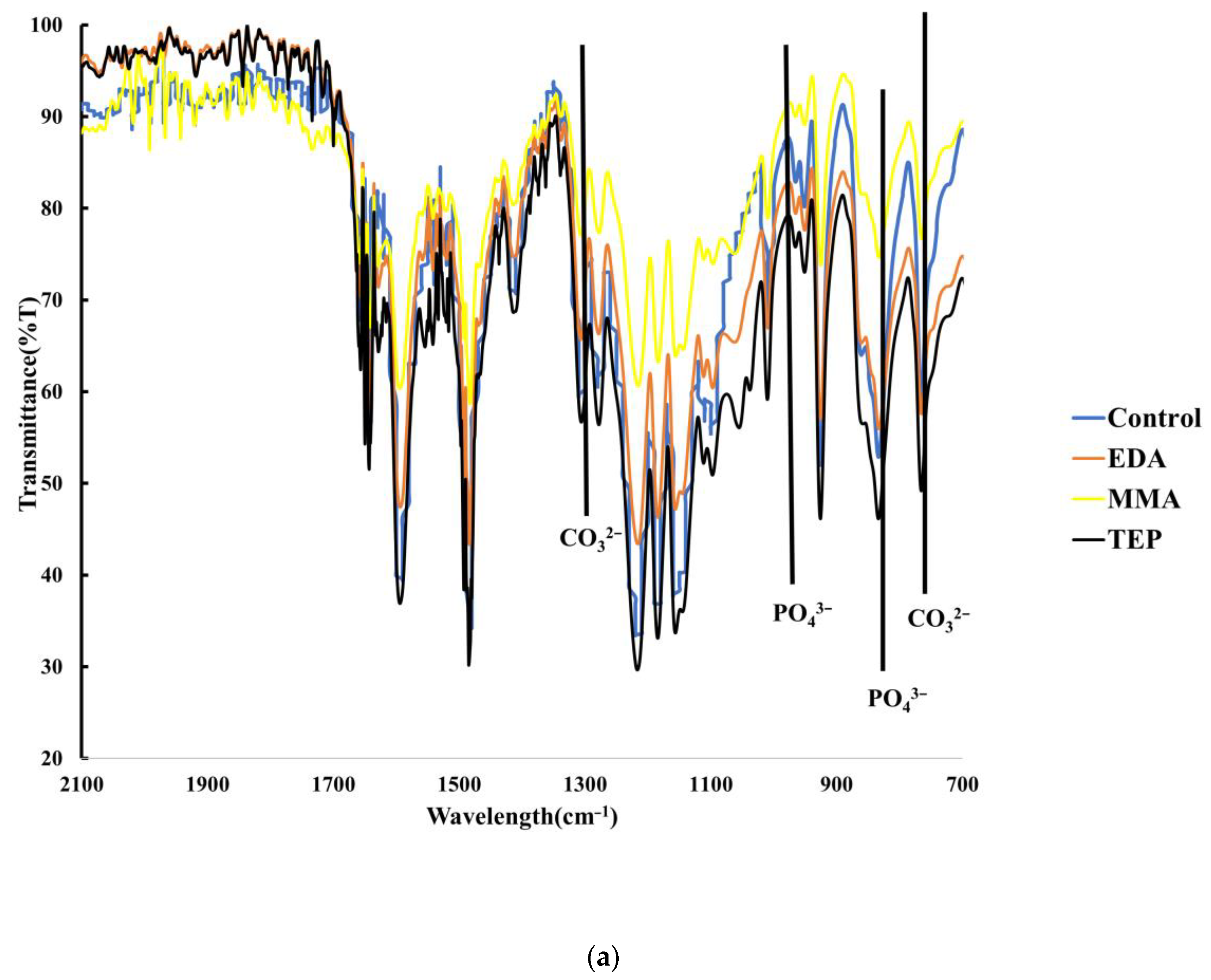
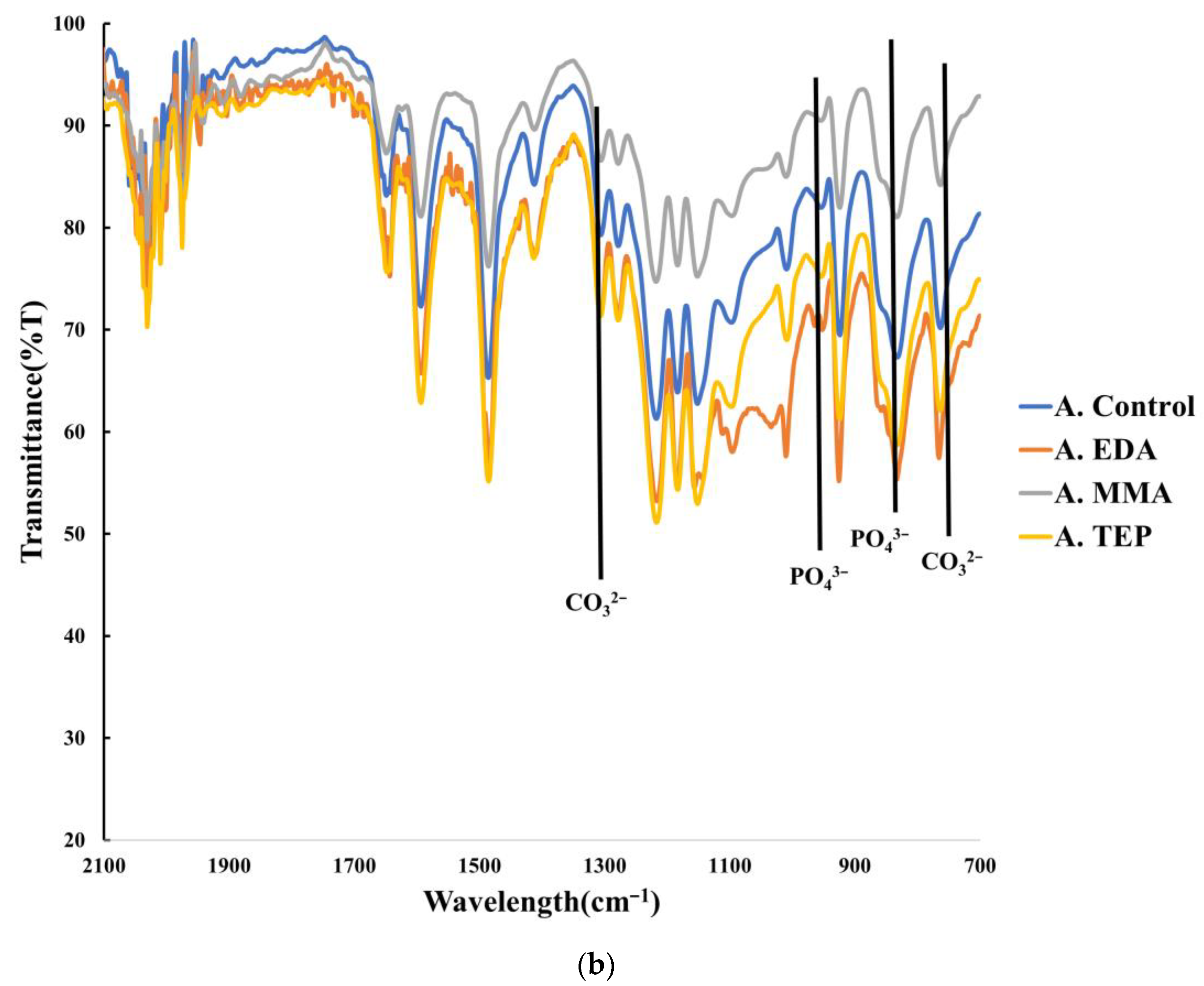
3.2. Chemical Characterization of Biomineralization
3.3. Biological and Morphological Characteristics of PEEK Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Xiong, C.; Zhang, L. Formation of bone-like apatite on plasma-carboxylated poly(etheretherketone) surface. Mater. Lett. 2014, 126, 147–150. [Google Scholar] [CrossRef]
- Wang, H.; Lu, T.; Meng, F.; Zhu, H.; Liu, X. Enhanced osteoblast responses to poly ether ether ketone surface modified by water plasma immersion ion implantation. Colloids Surf. B Biointerfaces 2014, 117, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wen, J.; Qian, S.; Cao, H.; Ning, C.; Pan, X.; Jiang, X.; Liu, X.; Chu, P.K. Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus. Biomaterials 2015, 51, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Poormoghadam, D.; Ghollasi, M.; Babavalian, H.; Tabasi, A.; Shams, M.; Goodarzi, V.; Salimi, A. Modification and characterization of an innovative polyvinyl alcohol-45S5 bioactive glass nanocomposite scaffold containing Donepezil hydrochloride for bone tissue engineering applications. Mater. Lett. 2021, 300, 130160. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Y.; Dai, K.; Chen, X.; Read, H.M.; Zeng, L.; Hang, F. Three dimensional printed bioglass/gelatin/alginate composite scaffolds with promoted mechanical strength, biomineralization, cell responses and osteogenesis. J. Mater. Sci. Mater. Med. 2020, 31, 77. [Google Scholar] [CrossRef]
- Fragal, E.H.; Cellet, T.S.P.; Fragal, V.H.; Witt, M.A.; Companhoni, M.V.P.; Ueda-Nakamura, T.; Silva, R.; Rubira, A.F. Biomimetic nanocomposite based on hydroxyapatite mineralization over chemically modified cellulose nanowhiskers: An active platform for osteoblast proliferation. Int. J. Biol. Macromol. 2019, 125, 133–142. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Abbas, M.; Alqahtani, M.S.; Alhifzi, R. Recent Developments in Polymer Nanocomposites for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 3312. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Winn, S.R.; Uludag, H.; Hollinger, J.O. Carrier Systems for Bone Morphogenetic Proteins. Clin. Orthop. Relat. Res. 1999, 367, S95–S106. [Google Scholar] [CrossRef]
- Liu, X.; Pettway, G.J.; McCauley, L.K.; Ma, P.X. Pulsatile release of parathyroid hormone from an implantable delivery system. Biomaterials 2007, 28, 4124–4131. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Sun, J.; Zhong, G.; Liu, C.; Wei, J. Hydroxyapatite nanoparticles as a controlled-release carrier of BMP-2: Absorption and release kinetics in vitro. J. Mater. Sci. Mater. Med. 2010, 21, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, J.; Xia, Y.; Yuan, Y.; Zhang, H.; Xu, S.; Lin, K. Loading BMP-2 on nanostructured hydroxyapatite microspheres for rapid bone regeneration. Int. J. Nanomed. Vol. 2018, 13, 4083–4092. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, P.W.; Schiegnitz, E.; Palarie, V.; Dau, M.; Frerich, B.; Al-Nawas, B. Influence of platelet-derived growth factor on osseous remodeling properties of a variable-thread tapered dental implant in vivo. Clin. Oral Implant. Res. 2017, 28, 201–206. [Google Scholar] [CrossRef]
- Oest, M.E.; Dupont, K.M.; Kong, H.-J.; Mooney, D.J.; Guldberg, R.E. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J. Orthop. Res. 2007, 25, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; López-Noriega, A.; Thompson, E.; Kelly, H.M.; Cryan, S.A.; O’Brien, F.J. Development of collagen–hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Release 2015, 198, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.R.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J.A. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R. 7—Cold plasma surface modification of biodegradable polymer biomaterials. In Biomaterials for Bone Regeneration; Dubruel, P., Van Vlierberghe, S., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 202–224. [Google Scholar]
- Mo, S.; Mehrjou, B.; Tang, K.; Wang, H.; Huo, K.; Qasim, A.M.; Wang, G.; Chu, P.K. Dimensional-dependent antibacterial behavior on bioactive micro/nano polyetheretherketone (PEEK) arrays. Chem. Eng. J. 2020, 392, 123736. [Google Scholar] [CrossRef]
- Mohammed, Z.; Jeelani, S.; Rangari, V. Low temperature plasma treatment of rice husk derived hybrid silica/carbon biochar using different gas sources. Mater. Lett. 2021, 292, 129678. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of Oxygen Plasma Nanotexturing of Organic Polymer Surfaces: From Stable Super Hydrophilic to Super Hydrophobic Surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef]
- Zhang, S.; Awaja, F.; James, N.; McKenzie, D.R.; Ruys, A.J. Autohesion of plasma treated semi-crystalline PEEK: Comparative study of argon, nitrogen and oxygen treatments. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 88–95. [Google Scholar] [CrossRef]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical development of simulated body fluids used in biomedical applications: A review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Kojic, N.; Rangger, C.; Özgün, C.; Lojpur, J.; Mueller, J.; Folman, Y.; Behrbalk, E.; Bakota, B. Carbon-Fibre-Reinforced PEEK radiolucent intramedullary nail for humeral shaft fracture fixation: Technical features and a pilot clinical study. Injury 2017, 48, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.A.; Lamorinière, S.; McGarry, P. Finite element investigation into the use of carbon fibre reinforced PEEK laminated composites for distal radius fracture fixation implants. Med. Eng. Phys. 2019, 67, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chloros, G.D.; Prodromidis, A.D.; Wilson, J.; Giannoudis, P.V. Fracture fixation in extremity trauma with carbon fiber-reinforced polyetheretherketone (CFR-PEEK) plates: Evidence today. Eur. J. Trauma Emerg. Surg. 2022, 48, 2387–2406. [Google Scholar] [CrossRef]
- Song, M.-J.; Amirian, J.; Linh, N.T.B.; Lee, B.-T. Bone morphogenetic protein-2 immobilization on porous PCL-BCP-Col composite scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45186. [Google Scholar] [CrossRef]
- Rezk, A.I.; Bhattarai, D.P.; Park, J.; Park, C.H.; Kim, C.S. Polyaniline-coated titanium oxide nanoparticles and simvastatin-loaded poly(ε-caprolactone) composite nanofibers scaffold for bone tissue regeneration application. Colloids Surf. B Biointerfaces 2020, 192, 111007. [Google Scholar] [CrossRef]
- Meskinfam, M.; Bertoldi, S.; Albanese, N.; Cerri, A.; Tanzi, M.C.; Imani, R.; Baheiraei, N.; Farokhi, M.; Farè, S. Polyurethane foam/nano hydroxyapatite composite as a suitable scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2018, 82, 130–140. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Liu, H.; Wen, W.; Li, H.; Zhou, C.; Luo, B. Biomineralization guided by polydopamine-modifed poly(L-lactide) fibrous membrane for promoted osteoconductive activity. Biomed. Mater. 2019, 14, 055005. [Google Scholar] [CrossRef]
- He, C.; Jin, X.; Ma, P.X. Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation. Acta Biomater. 2014, 10, 419–427. [Google Scholar] [CrossRef]
- Sabee, M.M.S.M.; Kamalaldin, N.; Yahaya, B.; Hamid, Z.A.A. Characterization and In Vitro Study of Surface Modified PLA Microspheres Treated with NaOH. J. Polym. Mater. 2016, 33, 191–200. [Google Scholar]
- Danilich, M.J.; Marchant, R.E. Radiofrequency Plasma Polymers Containing Ionic Phosphonate Groups: Effect of Monomer Structure and Carrier Gas on Properties and Deposition Rate. Plasmas Polym. 2002, 7, 127–149. [Google Scholar] [CrossRef]
- Lin, J.-C.; Tiong, S.-L.; Chen, C.-Y. Surface characterization and platelet adhesion studies on fluorocarbons prepared by plasma-induced graft polymerization. J. Biomater. Sci. Polym. Ed. 2000, 11, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Hilt, F.; Duday, D.; Gherardi, N.; Frache, G.; Bardon, J.; Choquet, P. Plasma Deposition of an Organophosphorus Coating at Atmospheric Pressure. Plasma Process. Polym. 2013, 10, 556–563. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Deposition and XPS and FTIR Analysis of Plasma Polymer Coatings Containing Phosphorus. Plasma Process. Polym. 2014, 11, 133–141. [Google Scholar] [CrossRef]
- Zain, N.M.; Hussain, R.; Kadir, M.R.A. Surface modification of yttria stabilized zirconia via polydopamine inspired coating for hydroxyapatite biomineralization. Appl. Surf. Sci. 2014, 322, 169–176. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Bradford, J.P.; Tucker, B.; Hernandez-Moreno, G.; Charles, P.; Thomas, V. Low-temperature inductively coupled plasma as a method to promote biomineralization on 3D printed poly(lactic acid) scaffolds. J. Mater. Sci. 2021, 56, 14717–14728. [Google Scholar] [CrossRef]
- Pillai, R.R.; Adhikari, K.R.; Gardner, S.; Sunilkumar, S.; Sanas, S.; Mohammad, H.; Thomas, V. Inkjet-printed plasma-functionalized polymer-based capacitive sensor for PAHs. Mater. Today Commun. 2023, 35, 105659. [Google Scholar] [CrossRef]
- ISO 23317; Implants for surgery in vitro evaluation for apatite-forming ability of implant materials. ISO: Geneva, Switzerland, 2014.
- Shi, X.; Jiang, J.; Sun, L.; Gan, Z. Hydrolysis and biomineralization of porous PLA microspheres and their influence on cell growth. Colloids Surf. B Biointerfaces 2011, 85, 73–80. [Google Scholar] [CrossRef]
- Ren, Y.; Sikder, P.; Lin, B.; Bhaduri, S.B. Microwave assisted coating of bioactive amorphous magnesium phosphate (AMP) on polyetheretherketone (PEEK). Mater. Sci. Eng. C 2018, 85, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Chenmiao, W.; Jian, W.; Song, W. The researches on cells adhesion on the biocompatible polymer surface with superwettability. Front. Bioeng. Biotechnol. 2016, 4, 1348. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Buck, E.; Manimunda, P.; Farivar, R.; Chromik, R.; Murshed, M.; Cerruti, M. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017, 47, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, H.; Xue, W.; Wang, Q. Effect of low temperature oxygen plasma treatment on microstructure and adhesion force of graphene. Appl. Surf. Sci. 2018, 428, 941–947. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Chodkowski, M.; Peréz-Huertas, S.; Wiechetek, Ł. Influence of Air Cold Plasma Modification on the Surface Properties of Paper Used for Packaging Production. Appl. Sci. 2022, 12, 3242. [Google Scholar] [CrossRef]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ahmad, A.U. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef]
- Bormashenko, E.; Legchenkova, I.; Navon-Venezia, S.; Frenkel, M.; Bormashenko, Y. Investigation of the Impact of Cold Plasma Treatment on the Chemical Composition and Wettability of Medical Grade Polyvinylchloride. Appl. Sci. 2021, 11, 300. [Google Scholar] [CrossRef]
- Abdulkareem, A.; Kasak, P.; Nassr, M.G.; Mahmoud, A.A.; Al-Ruweidi, M.; Mohamoud, K.J.; Hussein, M.K.; Popelka, A. Surface Modification of Poly(lactic acid) Film via Cold Plasma Assisted Grafting of Fumaric and Ascorbic Acid. Polymers 2021, 13, 3717. [Google Scholar] [CrossRef]
- Chang, W.; Mu, X.; Zhu, X.; Ma, G.; Li, C.; Xu, F.; Nie, J. Biomimetic composite scaffolds based mineralization of hydroxyapatite on electrospun calcium-containing poly(vinyl alcohol) nanofibers. Mater. Sci. Eng. C 2013, 33, 4369–4376. [Google Scholar] [CrossRef]
- Chen, S.; Guo, R.; Xie, C.; Liang, Q.; Xiao, X. Biomimetic mineralization of nanocrystalline hydroxyapatites on aminated modified polylactic acid microspheres to develop a novel drug delivery system for alendronate. Mater. Sci. Eng. C 2020, 110, 110655. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Shanas, N.A.; Bookbinder, S.; Nunes, M.; Krynska, B.; Pleshko, N. Fourier Transform Infrared Spectroscopy of Developing Bone Mineral: From Amorphous Precursor to Mature Crystal. Analyst 2019, 145, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, Y.; Ding, C.; Xie, J.; Li, J. Biomineralization and osteogenic differentiation modulated by substrate stiffness. Eur. Polym. J. 2020, 122, 109395. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Ionescu, I.C.; Ungureanu, E.; Berbecaru, A.; Zamfir, R.I.; Vladescu, A.; Vranceanu, D.M. Evaluation of surface modification techniques on the ability of apatite formation and corrosion behavior in synthetic body fluid: An in vitro study. Surf. Interfaces 2021, 22, 100866. [Google Scholar] [CrossRef]
- Murphy, W.L.; Kohn, D.H.; Mooney, D.J. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J. Biomed. Mater. Res. 2000, 50, 50–58. [Google Scholar] [CrossRef]
- Kim, S.H.; Thambi, T.; Phan, V.H.G.; Lee, D.S. Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Carbohydr. Polym. 2020, 233, 115832. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Wang, Z.; Wang, Y.; Dong, W.; Ma, W.; Zhao, S.; Sun, D. 3D-printed porous PEEK scaffold combined with CSMA/POSS bioactive surface: A strategy for enhancing osseointegration of PEEK implants. Compos. Part B Eng. 2022, 230, 109512. [Google Scholar] [CrossRef]
- Chen, J.; Chu, B.; Hsiao, B.S. Mineralization of hydroxyapatite in electrospun nanofibrous poly(L-lactic acid) scaffolds. J. Biomed. Mater. Res. Part A 2006, 79A, 307–317. [Google Scholar] [CrossRef]
- Vlădescu, A.; Pârâu, A.; Pană, I.; Cotru, C.M.; Constantin, L.R.; Braic, V.; Vrânceanu, D.M. In Vitro Activity Assays of Sputtered HAp Coatings with SiC Addition in Various Simulated Biological Fluids. Coatings 2019, 9, 389. [Google Scholar] [CrossRef]
- Nga, N.K.; Hoai, T.T.; Viet, P.H. Biomimetic scaffolds based on hydroxyapatite nanorod/poly(d,l) lactic acid with their corresponding apatite-forming capability and biocompatibility for bone-tissue engineering. Colloids Surf. B Biointerfaces 2015, 128, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Zamparini, F.; Valente, S.; Parchi, G.; Pasquinelli, G.; Taddei, P.; Prati, C. Green Hydrogels Composed of Sodium Mannuronate/Guluronate, Gelatin and Biointeractive Calcium Silicates/Dicalcium Phosphate Dihydrate Designed for Oral Bone Defects Regeneration. Nanomaterials 2021, 11, 3439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Nabiyouni, M.; Lin, B.; Bhaduri, S.B. Fabrication of novel poly(lactic acid)/amorphous magnesium phosphate bionanocomposite fibers for tissue engineering applications via electrospinning. Mater. Sci. Eng. C 2013, 33, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Ohuchi, F.S.; Hench, L.L. Compositional dependence of the formation of calcium phosphate films on bioglass. J. Biomed. Mater. Res. 1980, 14, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. Part A 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Takadama, H.; Hashimoto, M.; Mizuno, M.; Kokubo, T. Round-robin test of SBF for in vitro measurement of apatite-forming ability of synthetic materials. Phosphorus Res. Bull. 2004, 17, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-B.; Nakanishi, K.; Kokubo, T.; Soga, N.; Ohtsuki, C.; Nakamura, T.; Kitsugi, T.; Yamamuro, T. Dependence of Apatite Formation on Silica Gel on Its Structure: Effect of Heat Treatment. J. Am. Ceram. Soc. 2005, 78, 1769–1774. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, S.; Chen, J.; Zhao, Y.; He, F.; Li, Q.; Zou, L.; Shi, C. Biomimetic fabrication of icariin loaded nano hydroxyapatite reinforced bioactive porous scaffolds for bone regeneration. Chem. Eng. J. 2020, 394, 124895. [Google Scholar] [CrossRef]
- Yang, L.; Jin, S.; Shi, L.; Ullah, I.; Yu, K.; Zhang, W.; Bo, L.; Zhang, X.; Guo, X. Cryogenically 3D printed biomimetic scaffolds containing decellularized small intestinal submucosa and Sr2+/Fe3+ co-substituted hydroxyapatite for bone tissue engineering. Chem. Eng. J. 2022, 431, 133459. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, Y.; Guo, B.; Wang, Y.; Liu, W.; Sun, J.; Wang, X. Magnesium-organic framework-based stimuli-responsive systems that optimize the bone microenvironment for enhanced bone regeneration. Chem. Eng. J. 2020, 396, 125241. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in it, Their Composition and Applications. Front. Chem. 2019, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Mur, F.J.G. 8—Accelerating mineralization of biomimetic surfaces. In Biomineralization and Biomaterials; Aparicio, C., Ginebra, M.-P., Eds.; Woodhead Publishing: Boston, UK, 2016; pp. 267–289. [Google Scholar]


| C1s | O1s | P2p | N1s | |
|---|---|---|---|---|
| Neat | 77.5 | 18.9 | 0 | 2.6 |
| TEP | 47.9 | 41.3 | 10.2 | 0.6 |
| MMA | 63.0 | 30.9 | 0 | 0 |
| EDA | 73.6 | 10.2 | 0 | 14.0 |
| C1s | O1s | P2p | N1s | Ca2p | |
|---|---|---|---|---|---|
| Control | 87.1 | 8.4 | 0 | 0 | 0.9 |
| TEP | 57.0 | 28.5 | 1.8 | 4.2 | 1.5 |
| MMA | 86.8 | 10.7 | 0 | 1.2 | 0.8 |
| EDA | 75.0 | 15.8 | 0 | 1.9 | 1.2 |
| C1s | O1s | P2p | N1s | Ca2p | |
|---|---|---|---|---|---|
| A. Control | 73.2 | 20.6 | 0.7 | 2.1 | 1.0 |
| A. TEP | 76.7 | 20.6 | 2.1 | 0 | 0.6 |
| A. MMA | 60.8 | 29.7 | 3.4 | 4.8 | 1.3 |
| A. EDA | 78.8 | 16.2 | 0 | 2.6 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradford, J.P.; Hernandez-Moreno, G.; Pillai, R.R.; Hernandez-Nichols, A.L.; Thomas, V. Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization. Materials 2024, 17, 171. https://doi.org/10.3390/ma17010171
Bradford JP, Hernandez-Moreno G, Pillai RR, Hernandez-Nichols AL, Thomas V. Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization. Materials. 2024; 17(1):171. https://doi.org/10.3390/ma17010171
Chicago/Turabian StyleBradford, John P., Gerardo Hernandez-Moreno, Renjith R. Pillai, Alexandria L. Hernandez-Nichols, and Vinoy Thomas. 2024. "Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization" Materials 17, no. 1: 171. https://doi.org/10.3390/ma17010171
APA StyleBradford, J. P., Hernandez-Moreno, G., Pillai, R. R., Hernandez-Nichols, A. L., & Thomas, V. (2024). Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization. Materials, 17(1), 171. https://doi.org/10.3390/ma17010171







