Study on Quench Sensitivity during Isothermal Treatment of 7A65 Aluminum Alloy
Abstract
:1. Introduction
2. Material and Experiments
3. Results
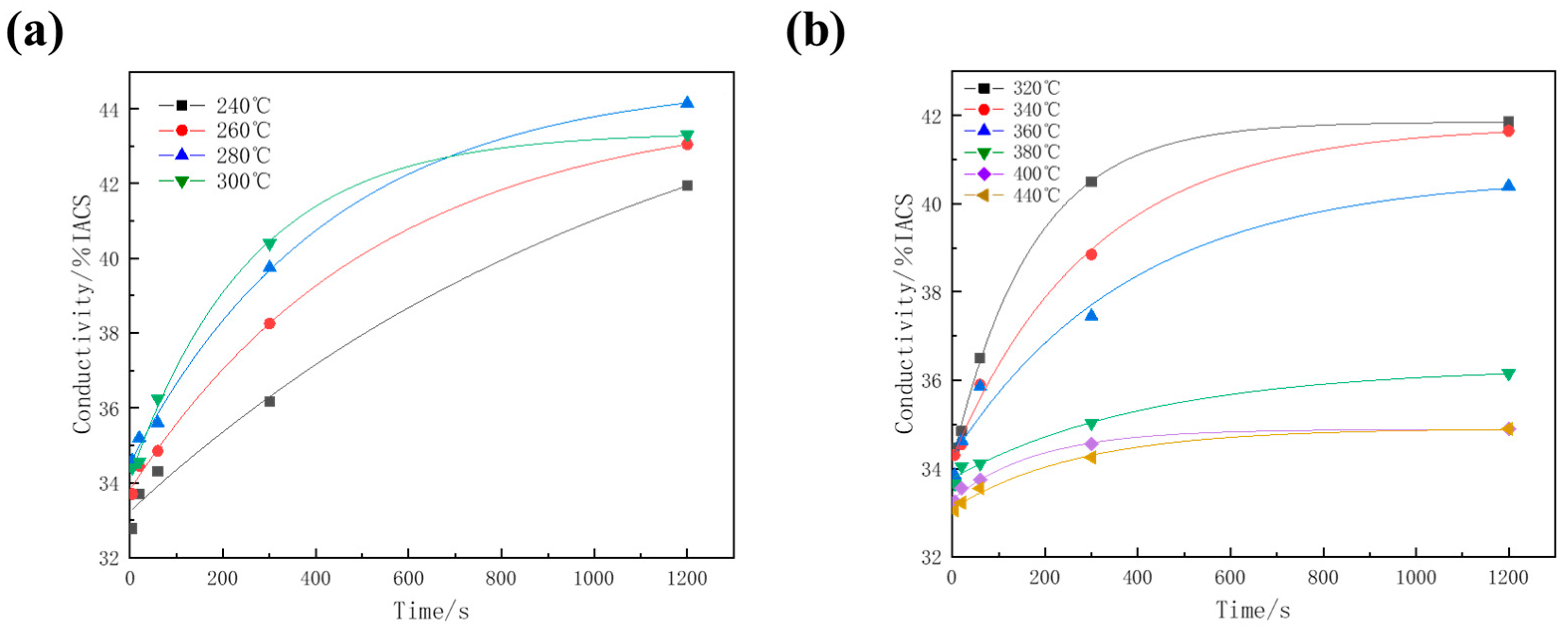
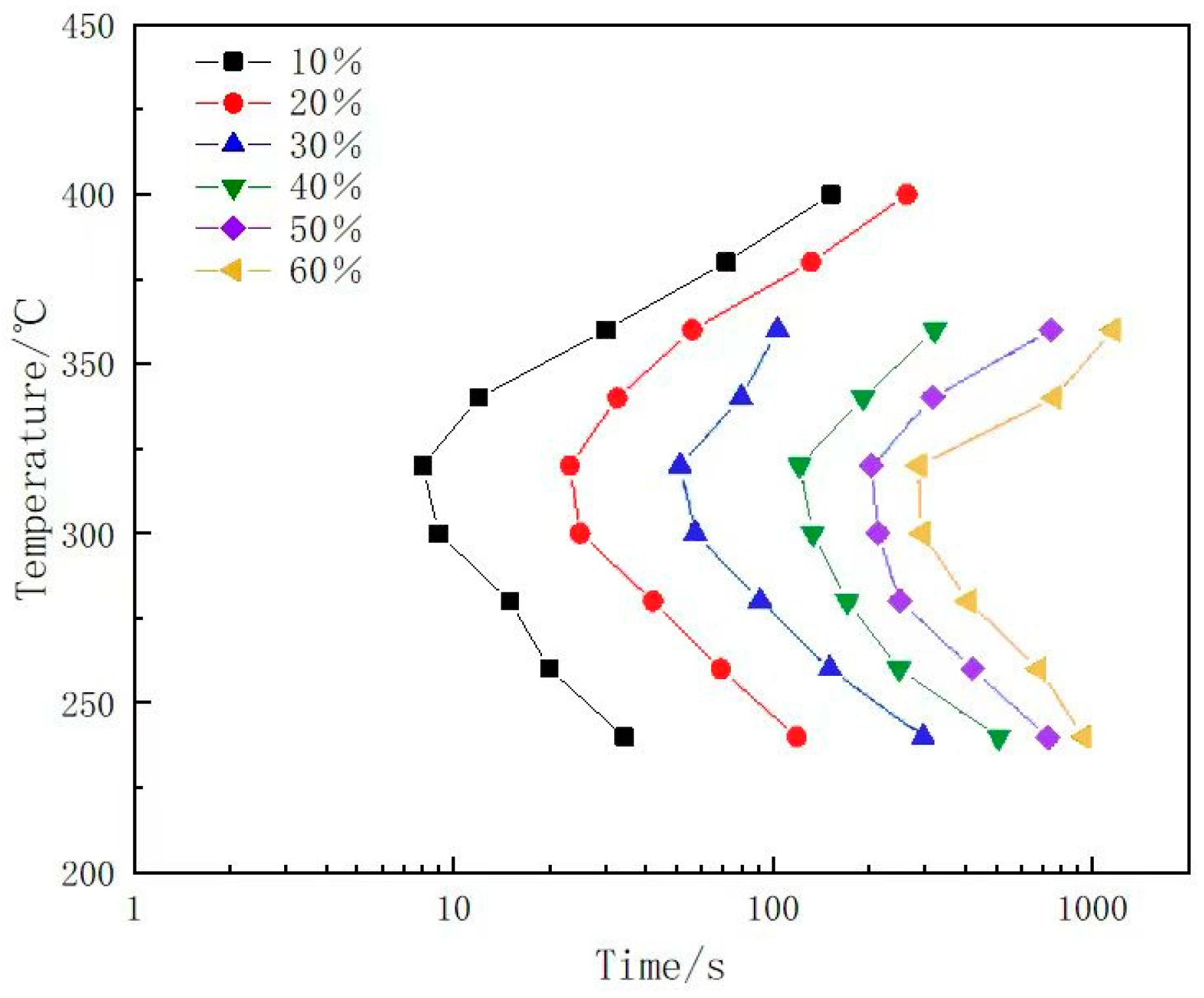
3.1. Conductivity Evolution and TTT Curve of 7A65 Aluminum Alloy
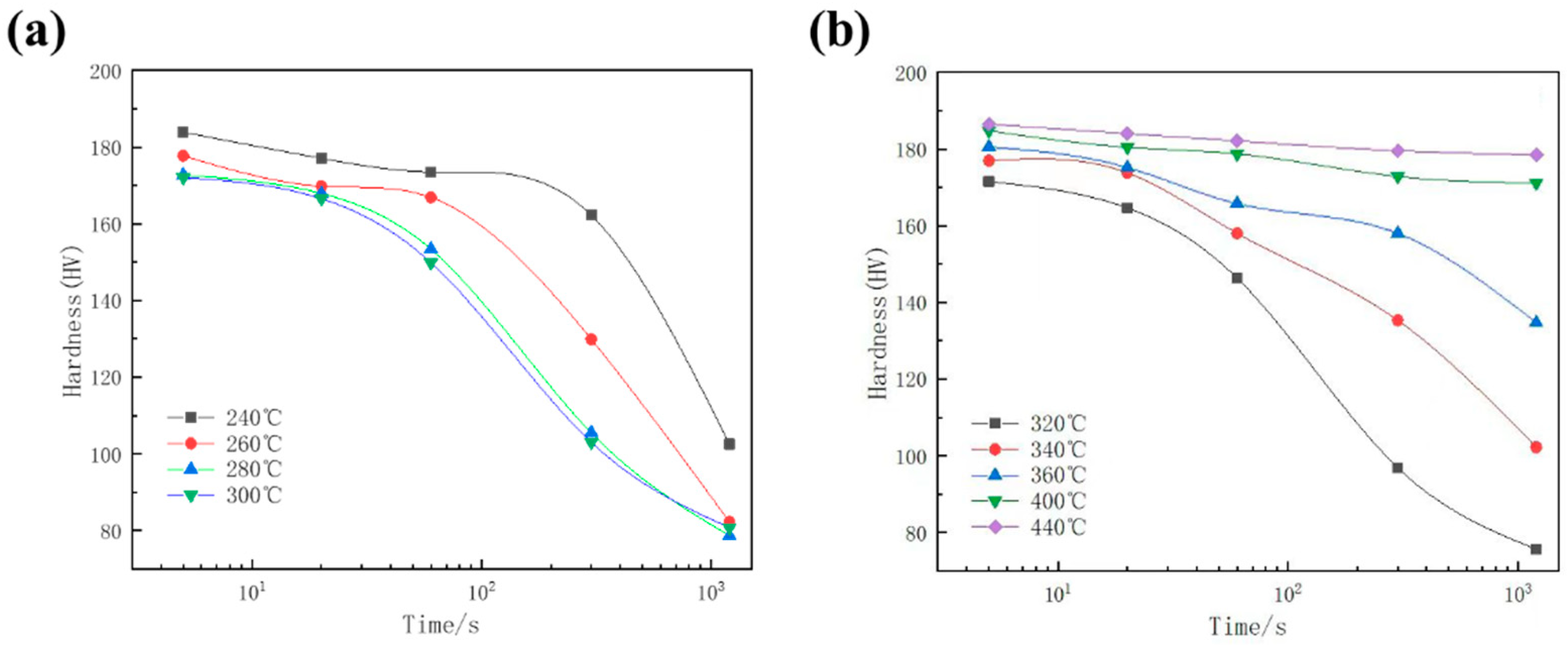
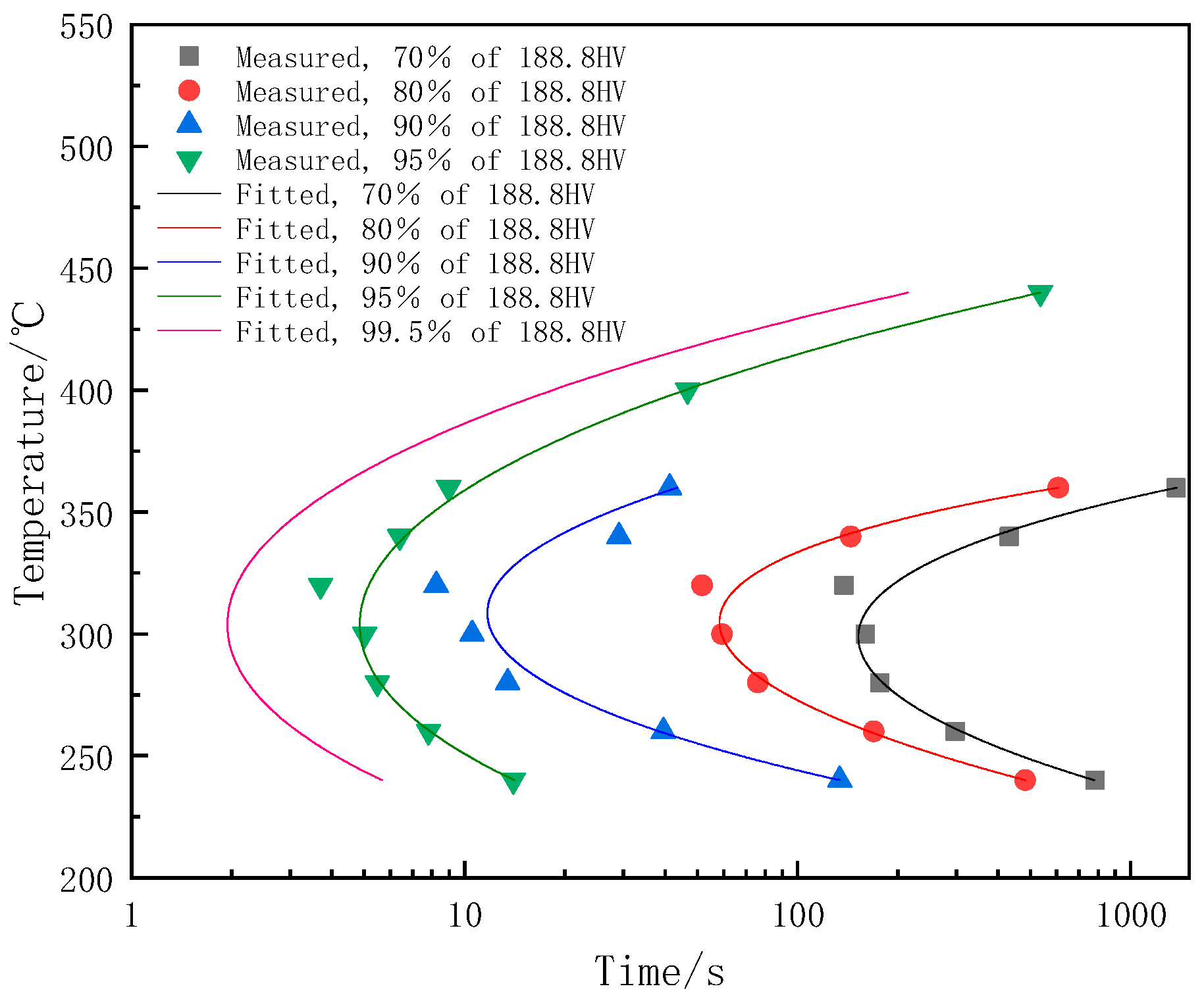
3.2. Hardness Evolution and the TTP Curve of 7A65 Aluminum Alloy
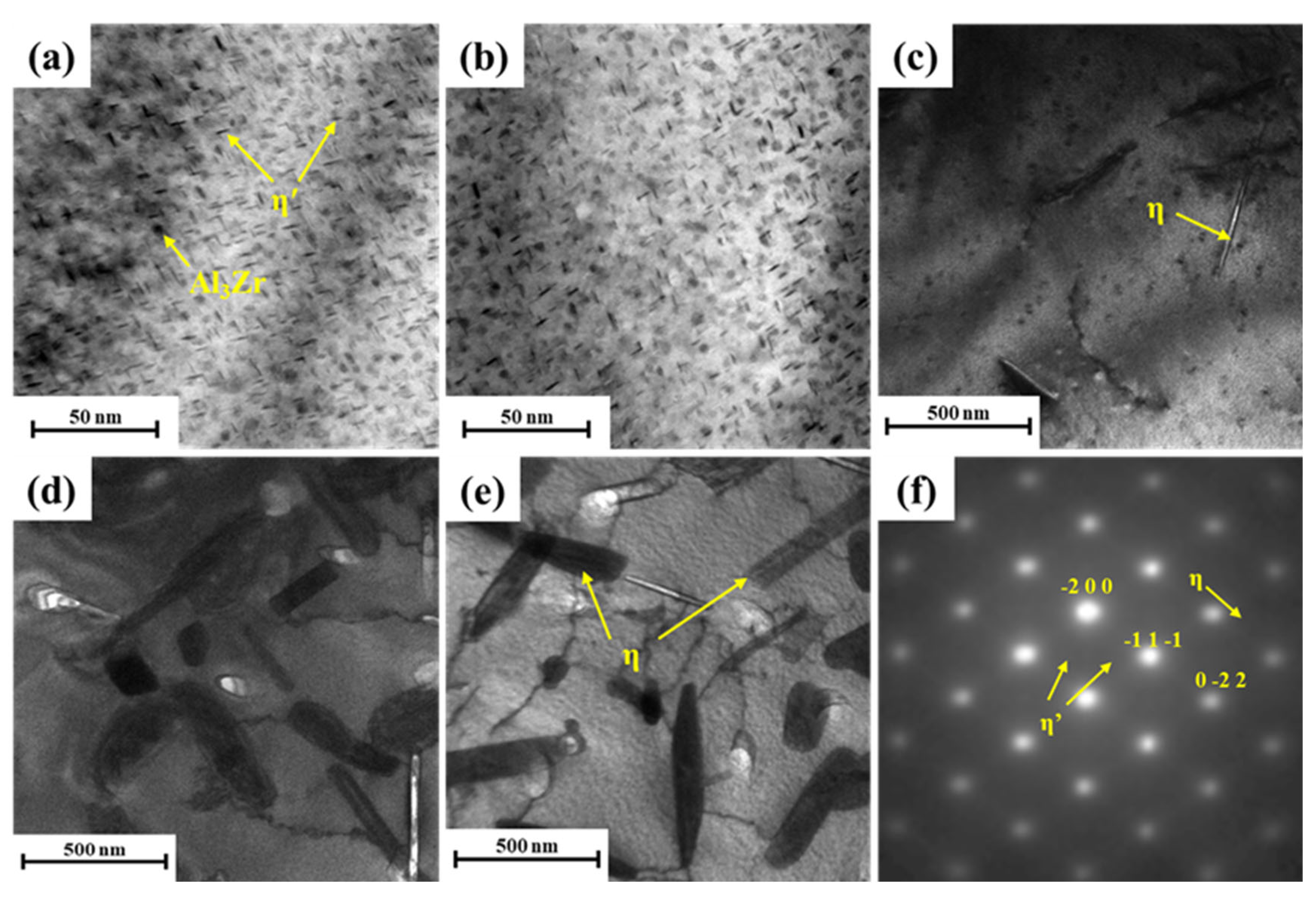
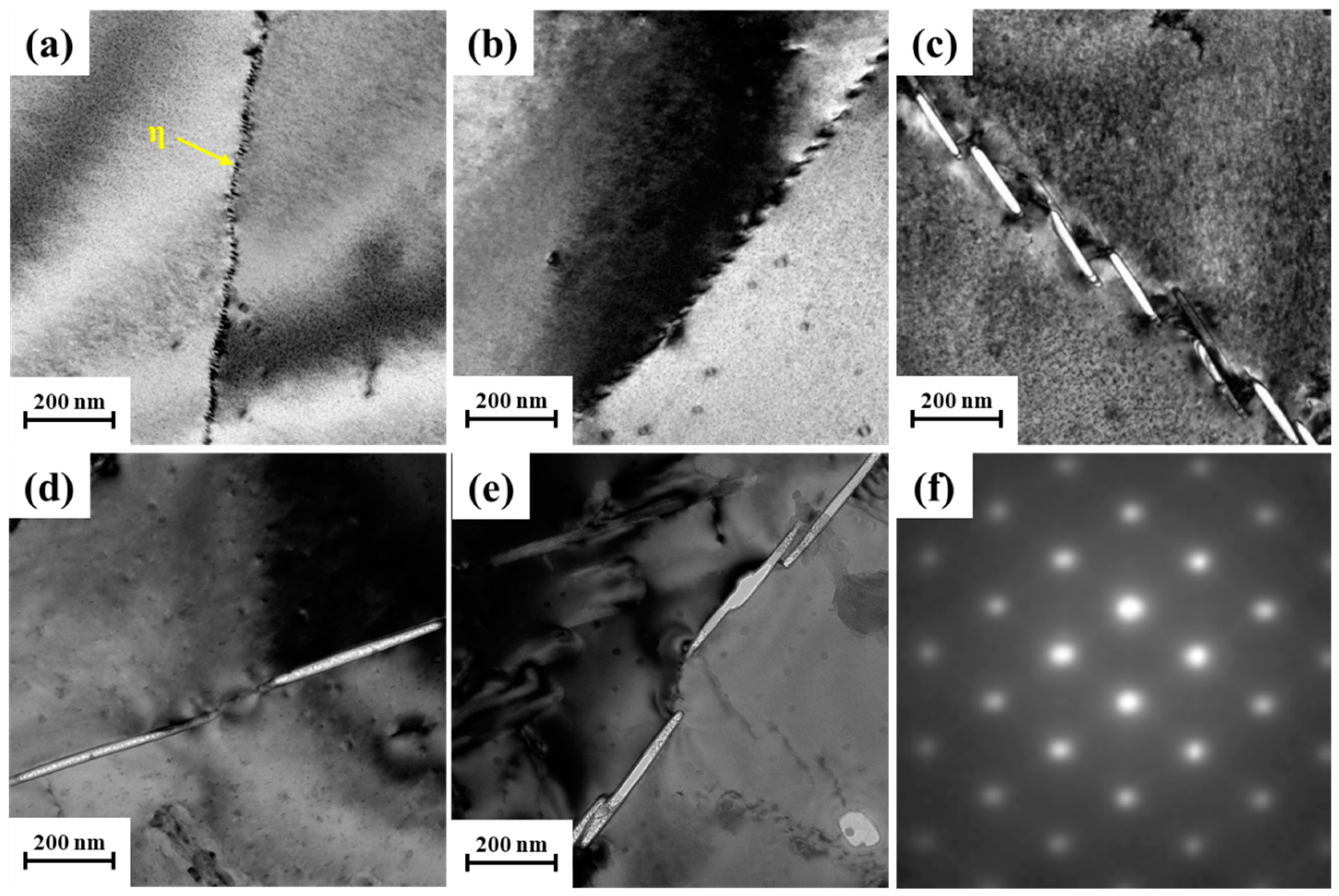
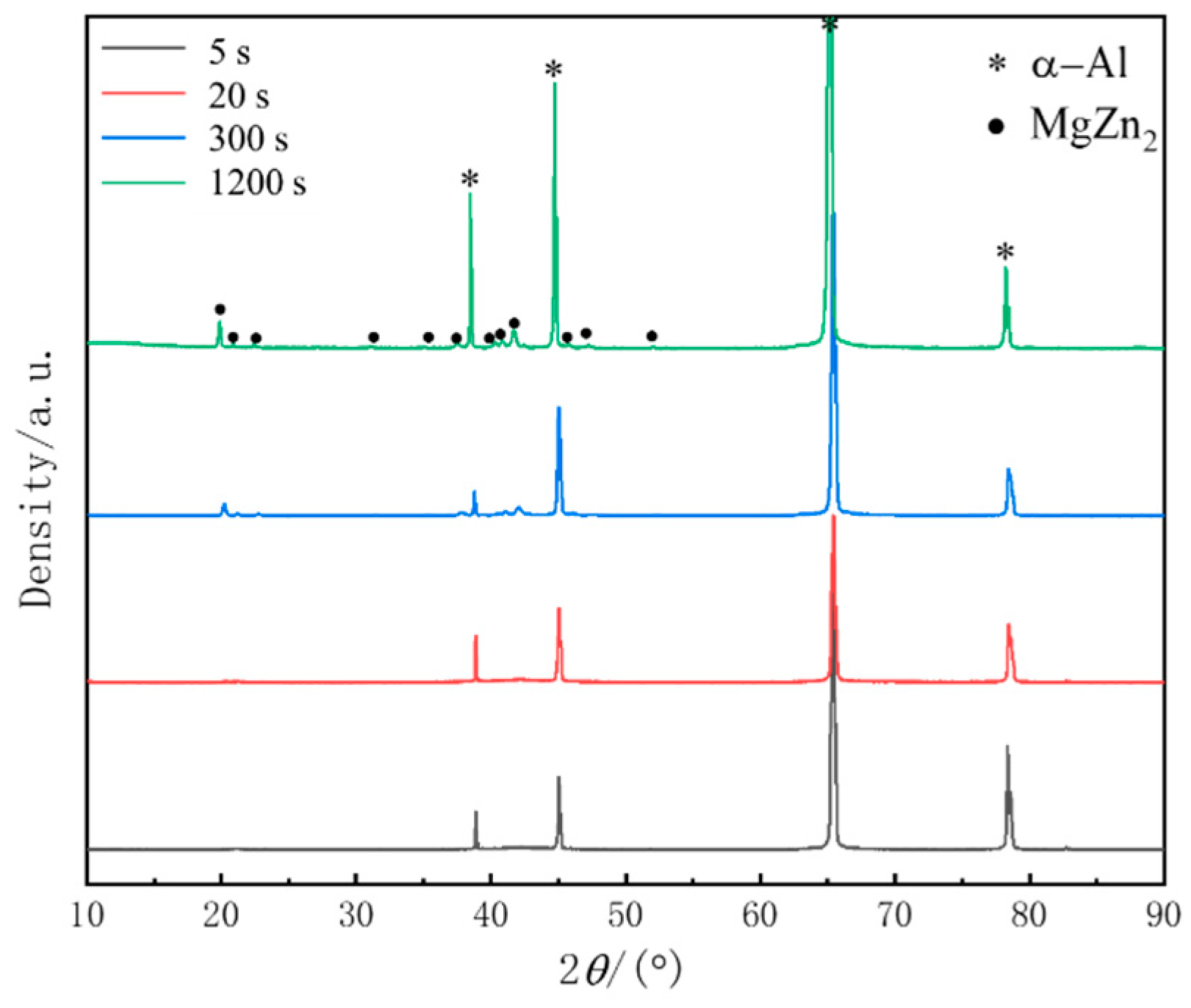
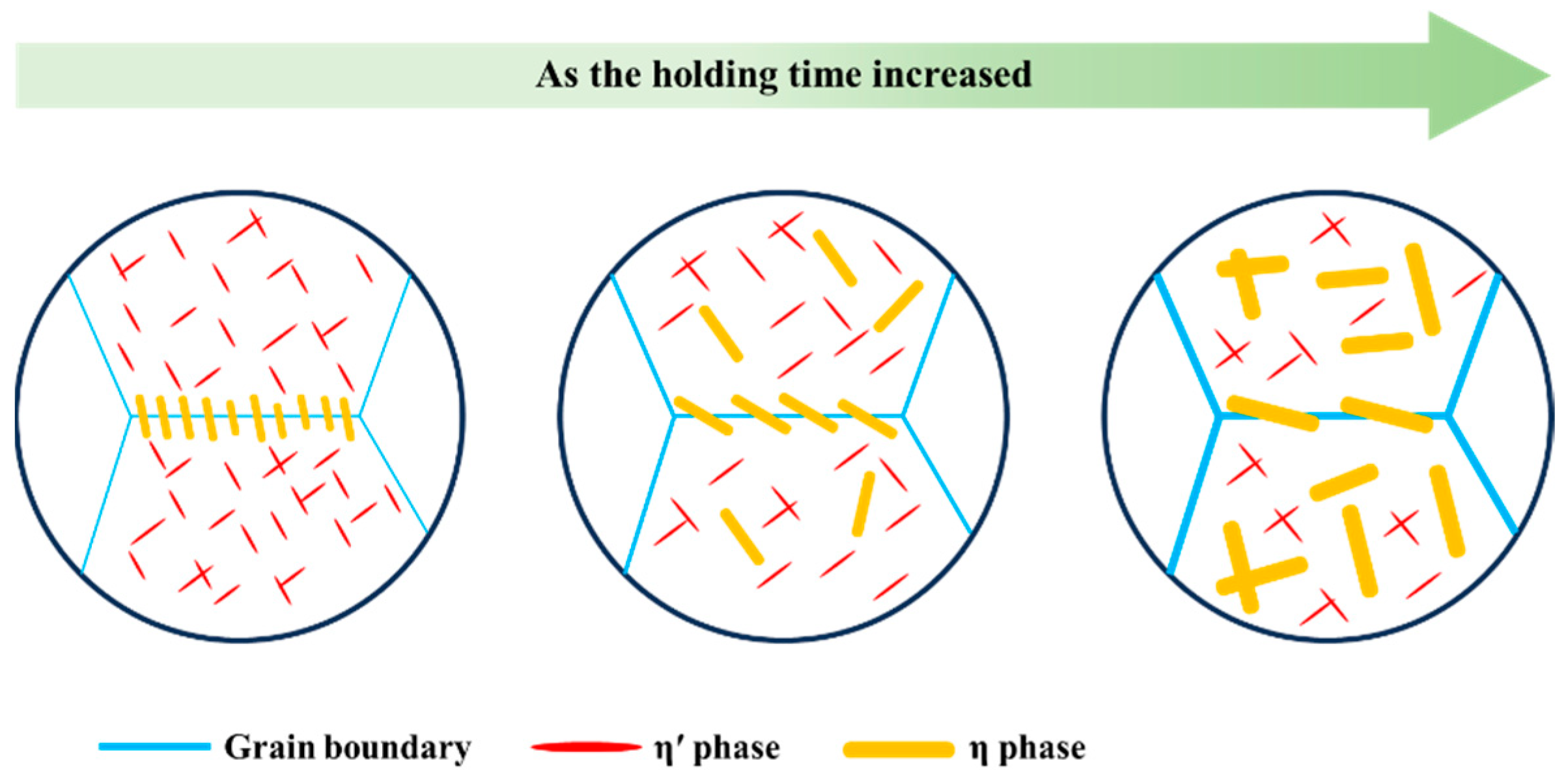
3.3. Microstructure Evolution during Isothermal Treatment
4. Discussion
5. Conclusions
- Severe premature precipitation occurred during isothermal treatment, and the nose temperature of the 7A65 aluminum alloy is 320 °C and the sensitive temperature range is from 240 °C to 360 °C. After isothermal treatment at 320 °C for only 60 s, the hardness of the sample decreased by nearly 15%.
- The η phases precipitated rapidly and grew within both the matrix and at the grain boundaries during isothermal treatment at the nose temperature. This severe premature precipitation depletes solute atoms, resulting in reduced quantities of nanoscale η′ phases and a broader PFZ.
- To achieve a balance between the mechanical properties and residual stresses of 7A65 aluminum alloy, it is necessary to increase the quenching cooling rate in the temperature range from 240 to 360 °C and appropriately reduce the cooling rate from the solid solution temperature to 360 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beura, V.K.; Sharma, A.; Karanth, Y.; Sharma, S.; Solanki, K. Corrosion behavior of 7050 and 7075 aluminum alloys processed by reactive additive manufacturing. Electrochim. Acta 2023, 470, 143357. [Google Scholar] [CrossRef]
- Peterson, L.A.; Horstemeyer, M.F.; Lacy, T.E.; Moser, R. Experimental characterization and constitutive modeling of an aluminum 7085-T711 alloy under large deformations at varying strain rates, stress states, and temperatures. Mech. Mater. 2020, 151, 103602. [Google Scholar] [CrossRef]
- Carvalho, A.L.M.; Renaudin, L.B.; Zara, A.J.; Martins, J. Microstructure analysis of 7050 aluminum alloy processed by multistage aging treatments. J. Alloys Compd. 2022, 907, 164400. [Google Scholar] [CrossRef]
- Korbel, A.; Bochniak, W.; Borowski, J.; Błaż, L.; Ostachowski, P.; Łagoda, M. Anomalies in precipitation hardening process of 7075 aluminum alloy extruded by KOBO method. J. Mater. Process. Technol. 2015, 216, 160–168. [Google Scholar] [CrossRef]
- Engler, O. Effect of precipitation state on plastic anisotropy in sheets of the age-hardenable aluminium alloys AA 6016 and AA 7021. Mater. Sci. Eng. A 2022, 830, 142324. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Yunxin, W.; Hai, G.; Liu, L. Determination of the Effect of Cold Working Compression on Residual Stress Reduction in Quenched Aluminium Alloy 2219 Block. Stroj. Vestn. J. Mech. Eng. 2019, 65, 311–318. [Google Scholar] [CrossRef]
- Lim, H.; Ko, D.; Ko, D.; Kim, B.M. Reduction of Residual Stress and Improvement of Dimensional Accuracy by Uphill Quenching for Al6061 Tube. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2014, 45, 472–481. [Google Scholar] [CrossRef]
- Olson, M.D.; Robinson, J.S.; Wimpory, R.C.; Hill, M.R. Characterisation of residual stresses in heat treated, high strength aluminium alloy extrusions. Mater. Sci. Technol. 2016, 32, 1427–1438. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Archambault, P.; Godard, D. High temperature precipitation kinetics and ttt curve of a 7xxx alloy by in-situ electrical resistivity measurements and differential calorimetry. Scr. Mater. 2000, 42, 675–680. [Google Scholar] [CrossRef]
- Evancho, J.W.; Staley, J.T. Kinetics of precipitation in aluminum alloys during continuous cooling. Metall. Trans. 1974, 5, 43–47. [Google Scholar] [CrossRef]
- Davydov, V.G.; Ber, L.B.; Kaputkin, E.Y.; Komov, V.I.; Ukolova, O.G.; Lukina, E.A. TTP and TTT diagrams for quench sensitivity and ageing of 1424 alloy. Mater. Sci. Eng. A 2000, 280, 76–82. [Google Scholar] [CrossRef]
- Xie, P.; Chen, K.; Chen, S.; Ye, S.; Jiao, H.; Huang, L. Study on quenching sensitivity of 7097 aluminum alloy. Mater. Res. Express 2020, 7, 16505. [Google Scholar] [CrossRef]
- Li, C.; Chen, D. Investigation on the Quench Sensitivity of 7085 Aluminum Alloy with Different Contents of Main Alloying Elements. Metals 2019, 9, 965. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, C.; Liu, S.; Deng, Y.L.; Zhang, X.M. Effect of homogenization time on quench sensitivity of 7085 aluminum alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2275–2281. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Pan, Q.; Li, M.; Ye, J.; Li, K.; Peng, Z.; Sun, Y. Investigation of microstructure evolution and quench sensitivity of Al–Mg–Si–Mn–Cr alloy during isothermal treatment. J. Alloys Compd. 2020, 826, 154144. [Google Scholar] [CrossRef]
- Tiryakioğlu, M.; Shuey, R.T. Quench sensitivity of 2219-T87 aluminum alloy plate. Mater. Sci. Eng. A 2010, 527, 5033–5037. [Google Scholar] [CrossRef]
- Milkereit, B.; Starink, M.J. Quench sensitivity of Al-Mg-Si alloys: A model for linear cooling and strengthening. Mater. Des. 2015, 76, 117–129. [Google Scholar] [CrossRef]
- Lervik, A.; Marioara, C.D.; Kadanik, M.; Walmsley, J.C.; Milkereit, B.; Holmestad, R. Precipitation in an extruded AA7003 aluminium alloy: Observations of 6xxx-type hardening phases. Mater. Des. 2020, 186, 108204. [Google Scholar] [CrossRef]
- Zhao, H.; Gault, B.; Ponge, D.; Raabe, D. Reversion and re-aging of a peak aged Al-Zn-Mg-Cu alloy. Scr. Mater. 2020, 188, 269–273. [Google Scholar] [CrossRef]
- Bendo, A.; Matsuda, K.; Nishimura, K.; Nunomura, N.; Tsuchiya, T.; Lee, S.; Marioara, C.; Tsuruc, T.; Yamaguchi, M.; Shimizud, K. The possible transition mechanism for the meta-stable phase in the 7xxx aluminium. Mater. Sci. Technol. 2020, 36, 1621–1627. [Google Scholar] [CrossRef]
- Knight, S.P.; Birbilis, N.; Muddle, B.C.; Trueman, A.R.; Lynch, S.P. Correlations between intergranular stress corrosion cracking, grain-boundary microchemistry, and grain-boundary electrochemistry for Al-Zn-Mg-Cu alloys. Corros. Sci. 2010, 52, 4073–4080. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Weyland, M.; Hutchinson, C.R. The effect of precipitate size on the yield strength-pitting corrosion correlation in Al-Cu-Mg alloys. Acta Mater. 2010, 58, 5941–5948. [Google Scholar] [CrossRef]
- Wang, H.; YI, Y.; Huang, S. Investigation of quench sensitivity of high strength 2219 aluminum alloy by TTP and TTT diagrams. J. Alloys Compd. 2017, 690, 446–452. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys, PARTI; Pergamon Press: Oxford, UK, 2002; p. 546. [Google Scholar]

| Coefficient | k2 (s) | k3 (J/mol) | k4 (K) | k5 (J/mol) |
|---|---|---|---|---|
| Value | 1.4 × 10−11 | 21,838 | 822 | 9381 |
| Temperature/°C | k | n |
|---|---|---|
| 240 | 0.01000 | 0.65146 |
| 260 | 0.03270 | 0.50379 |
| 280 | 0.03138 | 0.55567 |
| 300 | 0.03478 | 0.56484 |
| 320 | 0.04096 | 0.53904 |
| 340 | 0.03572 | 0.50111 |
| 360 | 0.02155 | 0.53257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Bao, L.; Huang, K.; Huang, S. Study on Quench Sensitivity during Isothermal Treatment of 7A65 Aluminum Alloy. Materials 2024, 17, 193. https://doi.org/10.3390/ma17010193
Li C, Bao L, Huang K, Huang S. Study on Quench Sensitivity during Isothermal Treatment of 7A65 Aluminum Alloy. Materials. 2024; 17(1):193. https://doi.org/10.3390/ma17010193
Chicago/Turabian StyleLi, Chen, Liangliang Bao, Ke Huang, and Shiquan Huang. 2024. "Study on Quench Sensitivity during Isothermal Treatment of 7A65 Aluminum Alloy" Materials 17, no. 1: 193. https://doi.org/10.3390/ma17010193




