Investigation of In Vitro Cytocompatibility of Zinc-Containing Coatings Developed on Medical Magnesium Alloys
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of MAO Samples
2.2. Surface Characterizations
2.3. Cell Culture
2.4. Cell Adhesion
2.5. CCK Assay
2.6. Alkaline Phosphatase (ALP) Activity
3. Results
3.1. Surface Characterizations of MAO Coatings
3.1.1. Morphology and Composition of MAO Coatings
3.1.2. XRD Analysis
3.1.3. Degradation Resistance
3.2. In Vitro Cytocompatibility
3.2.1. Cell Adhesion
3.2.2. Cytotoxicity
3.2.3. ALP Activity
4. Discussion
4.1. Formation of Zinc-Containing MAO Coatings
4.2. Property of Zinc-Containing Coatings
5. Conclusions
- Zn2+ ions entered into MAO coatings mainly by diffusion. XRD analysis shows that MAO coatings are mainly composed of Mg and MgO;
- MAO treatment can improve the degradation resistance of untreated magnesium alloys. However, the enhanced Na2[ZnEDTA] concentration decreases the coating thickness and degradation resistance;
- Zinc-containing MAO coatings have good cytocompatibility, and among the received WE43 substrate and zinc-containing MAO samples, the sample developed in the base solution added 6 g/L Na2[ZnEDTA] and achieved the best degradation resistance and osseogenic ability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narayanan, T.S.N.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, H.; Pan, H.; Zhao, Y. Insight into microbiologically induced corrosion performance of magnesium in tryptic soy broth with S. aureus and E. coli. J. Mater. Sci. Technol. 2022, 115, 221–231. [Google Scholar] [CrossRef]
- He, L.J.; Shao, Y.; Li, S.Q.; Cui, L.Y.; Ji, X.J.; Zhao, Y.B.; Zeng, R.C. Recent progress in layer-by-layer self-assembled coatings upon biodegradable magnesium alloys. Sci. China Mater. 2021, 64, 2093–2106. [Google Scholar] [CrossRef]
- Chen, S.S.; Song, P.D.; Yin, J.; Qi, K.; Li, H.D.; Hou, L.; Li, W.H. Enhancement of plasticity and corrosion resistance in a plasma electrolytic oxidation treated Mg-based amorphous alloy composite. J. Mater. Eng. Perform. 2023, 32, 2298–2306. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yan, C.W.; Wang, F.H.; Li, W.F. Electrochemical behaviors of anodized magnesium alloy AZ91D in chloride containing aqueous solution. Corr. Sci. 2005, 47, 2816–2831. [Google Scholar] [CrossRef]
- Qin, J.; Shi, X.T.; Li, H.Y.; Zhao, R.F.; Li, G.Q.; Zhang, S.F.; Ding, L.Y.; Cui, X.J.; Zhao, Y.; Zhang, R.F. Performance and failure process of green recycling solutions for preparing high degradation resistance coating on biomedical magnesium alloys. Green Chem. 2022, 24, 8113–8130. [Google Scholar] [CrossRef]
- Dai, W.B.; Li, C.Y.; He, D.; Jia, D.W.; Zhang, Y.M.; Tan, Z. Mechanism of residual stress and surface roughness of substrate on fatigue behavior of micro-arc oxidation coated AA7075-T6 alloy. Surf. Coat. Technol. 2019, 380, 125014. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, M.; Wen, S.; Mao, X.; Huo, W.G.; Guo, Y.Y.; Wang, Y.X. Microstructure and wear resistance of micro-arc oxidation ceramic coatings prepared on 2A50 aluminum alloys. Surf. Coat. Technol. 2020, 394, 125853. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Feng, T.; Cheng, Y.L. A Systematic Study of the Role of Cathodic Polarization and New Findings on the Soft Sparking Phenomenon from Plasma Electrolytic Oxidation of an Al-Cu-Li alloy. J. Electrochem. Soc. 2022, 169, 071505. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.J.; Qi, C.X.; Xue, Y.L.; Wan, Y.; Sun, H.L. Enhanced corrosion and tribocorrosion behavior of plasma electrolytic oxidized coatings on 5052 aluminum alloy with addition of pullulan to silicate electrolyte. J. Alloys Compd. 2023, 960, 170782. [Google Scholar] [CrossRef]
- He, X.R.; Feng, T.; Cheng, Y.L.; Hu, P.F.; Le, Z.Z.; Liu, Z.H.; Cheng, Y.L. Fast formation of a black inner α-Al2O3 layer doped with CuO on Al–Cu–Li alloy by soft sparking PEO process. Am. Ceram. Soc. 2023, 106, 7019–7042. [Google Scholar] [CrossRef]
- Dai, W.B.; Zhang, C.; Yue, H.T.; Li, Q.; Guo, C.G.; Zhang, J.Z.; Zhao, G.C.; Yang, X.L. A review on the fatigue performance of micro-arc oxidation coated Al alloys with micro-defects and residual stress. J. Mater. Res. Technol. 2023, 25, 4554–4581. [Google Scholar] [CrossRef]
- Bai, L.; Du, Z.; Du, J.; Yao, W.; Zhang, J.; Weng, Z.; Liu, S.; Zhao, Y.; Liu, Y.; Zhang, X.; et al. A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegration. Biomaterials 2018, 162, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, L.; Luan, J.; Wan, Y.; Li, R. Effect of carbon nanotubes additive on tribocorrosion performance of micro-arc oxidized coatings on Ti6Al4V alloy. Surf. Interfaces 2022, 28, 101626. [Google Scholar] [CrossRef]
- Qin, J.; Chen, Y.Q.; Chen, C.T.; Zhong, S.; Yan, Z.K.; Liu, W.J.; Wang, Y.B.; Lai, X.Y.; Zhao, Y.; Zhao, R.F.; et al. Preparation of HA-containing coating by one-step MAO on titanium alloys through synergistic effect of calcium gluconate and calcium glycerophosphate. Surf. Coat. Technol. 2023, 466, 129655. [Google Scholar] [CrossRef]
- Cao, G.Q.; Yang, L.; Yang, Y.Y.; Feng, L.Z.; Zhang, X.L.; Li, J.; Liu, B.D. Low-temperature selective catalytic reduction of NOx with NH3 over in-situ grown MnOx-Fe2O3/TiO2/Ti monolithic catalyst. J. Alloys Compd. 2023, 938, 168481. [Google Scholar] [CrossRef]
- Liu, Z.H.; Le, Z.Z.; He, X.R.; Cheng, Y.L.; Hu, P.F.; Cheng, Y.L. Plasma electrolytic oxidation of tantalum in an aluminate electrolyte: Effect of cathodic polarization and frequency. Ceram. Int. 2023, 49, 35042–35062. [Google Scholar] [CrossRef]
- Wang, Y.M.; Guo, J.W.; Shao, Z.K.; Zhuang, J.P.; Jin, M.S.; Wu, C.J.; Wei, D.Q.; Zhou, Y. A metasilicate-based ceramic coating formed on magnesium alloy by microarc oxidation and its corrosion in simulated body fluid. Surf. Coat. Technol. 2013, 219, 8–14. [Google Scholar] [CrossRef]
- Shi, X.T.; Wang, Y.; Li, H.Y.; Zhang, S.F.; Zhao, R.F.; Li, G.Q.; Zhang, R.F.; Sheng, Y.; Cao, S.Y.; Zhao, Y.J.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Li, B.; Huang, R.; Ye, J.; Liu, L.; Qin, L.; Zhou, J.; Zheng, Y.; Wu, S.; Han, Y. A self-healing coating containing curcumin for osteoimmunomodulation to ameliorate oseointegration. Chem. Eng. J. 2021, 403, 126323. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, Y.P.; Lv, Y.; Dong, Z.H.; Yang, L.; Zhang, E.L.; Hashimoto, T.; Zhou, X.R. PEO coating on Mg-Ag alloy: The incorporation and release of Ag species. J. Magnes. Alloys 2023, 11, 2182–2195. [Google Scholar] [CrossRef]
- Rizwan, M.; Alias, R.; Zaidi, U.Z.; Mahmoodian, R.; Hamdi, M. Surface modification of valve metals using plasma electrolytic oxidation for antibacterial applications: A review. J. Biomed. Mater. Res. Part A 2018, 106A, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Chen, C.T.; Liang, T.; Wang, Y.P.; Zhao, R.F.; Li, G.Q.; Bai, C.G.; Wu, Y.X.; Yu, F.L.; Sheng, L.Y.; et al. In vitro long-term antibacterial performance and mechanism of Zn-doped micro-arc oxidation coatings. Colloids Surf. B 2024, 233, 113634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Yang, L.; Lu, X.Q.; Lv, Y.; Jiang, D.; Yu, Y.; Peng, Z.; Dong, Z.H. Characterization and property of dual-functional Zn-incorporated TiO2 micro-arc oxidation coatings: The influence of current density. J. Alloys Compd. 2019, 810, 151893. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Yu, Y.; Lu, X.Q.; Lv, Y.; Jiang, D.; Peng, Z.; Zhou, J.; Zhang, X.; Sun, S.Q.; et al. Characterization and property of bifunctional Zn-incorporated TiO2 micro-arc oxidation coatings: The influence of different Zn sources. Ceram. Int. 2019, 45, 19747–19756. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhang, S.F.; Zhao, R.F.; Lou, J.; Zhang, R.F.; Huan, X.X.; Zhang, Y.J. Influences of Na2SiO3 and EDTA-ZnNa2 concentration on properties of zinc-containing coatings on WE43 magnesium alloys. Surf. Coat. Technol. 2018, 356, 108–122. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhu, Y.Y.; Zhao, R.F.; Zhang, S.F.; Lai, X.Y.; Wang, Y.B.; Yan, Z.K.; Liu, W.J.; Zhang, R.F. Investigation on influencing mechanism of processing parameters on corrosion resistance and zinc content of anodic coatings developed on magnesium alloys in near-neutral solutions. Coatings 2022, 12, 1286. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Radić, N.; Grbić, B.; Vasilić, R. MgO/ZnO coatings formed on magnesium alloy AZ31 by plasma electrolytic oxidation: Structural, photoluminescence and photocatalytic investigation. Surf. Coat. Technol. 2017, 310, 98–105. [Google Scholar] [CrossRef]
- Liu, D.Q.; Deng, J.T.; Liu, Z.D.; Jin, J.B.; Bi, Y.F.; Yang, J.J.; Zhou, S.F. Effects of ZnO nanoparticles addition to plasma electrolytic oxidation coatings on magnesium alloy: Microstructure, in vitro corrosion and antibacterial properties. J. Mater. Res. 2022, 37, 2897–2909. [Google Scholar] [CrossRef]
- Schmidt, J.; Pana, I.; Bystrova, A.; Dinu, M.; Dekhtyar, Y.; Vitelaru, C.; Gorohovs, M.; Marinescu, I.M.; Huri, P.Y.; Tamay, D.G.; et al. Zn doped CaP coatings used for controlling the degradation rate of MgCa1 alloy: In vitro anticorrosive properties, sterilization and bacteria/cell-material interactions. Colloids Surf. B 2023, 222, 113087. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V.V.; Zolotareva, N.V.; Novikova, O.V.; Petrov, B.I.; Lazarev, N.M.; Rumyantsev, R.V.; Lopatin, M.A.; Lopatina, T.I.; Kovylina, T.A.; Razov, E.N. Preparation of water-soluble zinc (Ⅱ) complexes with ethylenediaminetetraacetic acid: Molecular structure of zinc ethylenediaminetetraacetate trihydrate. Russ. J. Coord. Chem. 2023, 49, 206–217. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Chu, P.K.; Wang, L.; Pan, H.; Zheng, Y.; Wu, S.; Liu, X.; Cheng, K.M.C.; Wong, T.; et al. A functionalized TiO2/Mg2TiO4 nano-layer on biodegradable magnesium implant enables superior bone-implant integration and bacterial disinfection. Biomaterials 2019, 219, 119372. [Google Scholar] [CrossRef] [PubMed]
- Udoh, I.I.; Shi, H.W.; Daniel, E.F.; Li, J.Y.; Gu, S.H.; Liu, F.C.; Han, E.H. Active anticorrosion and self-healing coatings: A review with focus on multi-action smart coating strategies. J. Mater. Sci. Technol. 2022, 116, 224–237. [Google Scholar] [CrossRef]
- Xu, J.L.; Tao, S.C.; Ba, L.Z.; Luo, J.M.; Zheng, Y.F. Effects of Mo contents on the microstructure, properties and cytocompatibility of the microwave sintered porous Ti-Mo alloys. Mater. Sci. Eng. C 2019, 97, 156–165. [Google Scholar] [CrossRef]
- Guo, C.; Li, Y.; Qi, C.; Sun, H.; Xue, Y.; Wan, Y.; Zhang, D. Preparation and corrosion resistance characterization of MAO coating on AZ31B magnesium alloy formed in the mixed silicate and phosphate electrolytes with pectin as an additive. Surf. Coat. Technol. 2024, 476, 130209. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Daroonparvar, M.; Saebnoori, E.; Chami, A. In vitro degradation behavior, antibacterial activity and cytotoxicity of TiO2-MAO/ZnHA composite coating on Mg alloy for orthopedic implants. Surf. Coat. Technol. 2018, 334, 450–460. [Google Scholar] [CrossRef]
- Yang, C.; Cai, H.; Cui, S.H.; Huang, J.; Zhu, J.Y.; Wu, Z.C.; Ma, Z.Y.; Fu, R.K.Y.; Sheng, L.Y.; Tian, X.B.; et al. A zinc-doped coating prepared on the magnesium alloy by plasma electrolytic oxidation for corrosion protection. Surf. Coat. Technol. 2022, 433, 128148. [Google Scholar] [CrossRef]
- Feng, Y.S.; Ma, X.; Chang, L.; Zhu, S.J.; Guan, S.K. Characterization and cytocompatibility of polydopamine on MAO-HA coating supported on Mg-Zn-Ca alloy. Surf. Interface Anal. 2017, 49, 1115–1123. [Google Scholar] [CrossRef]
- Zhao, Q.M.; Li, G.Z.; Zhu, H.M. Preparation and Performance Characterization of Bioactive Coating on Magnesium Alloy. J. Biobased Mater. Bioenergy 2017, 11, 473–476. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Zhao, T.G. Effects of phosphates on microstructure and bioactivity of micro-arc oxidized calcium phosphate coatings on Mg-Zn-Zr magnesium alloy. Colloids Surf. B 2013, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nikody, M.; Li, J.P.; Balmayor, E.R.; Moroni, L.; Habibovic, P. The addition of zinc ions to polymer-ceramic composites accelerated osteogenic differentiation of human mesenchymal stromal cells. Biomater. Adv. 2023, 149, 213391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Gu, R.L.; Wang, F.L.; Zhao, X.; Yang, F.; Xu, Y.Q.; Yan, F.Y.; Zhu, Y.; Xia, D.D.; Liu, Y.S. 3D-Printed PCL/Zn scaffolds for bone regeneration with a dose-ependent effect on osteogenesis and osteoclastogenesis. Mater. Today Bio 2022, 13, 100202. [Google Scholar]


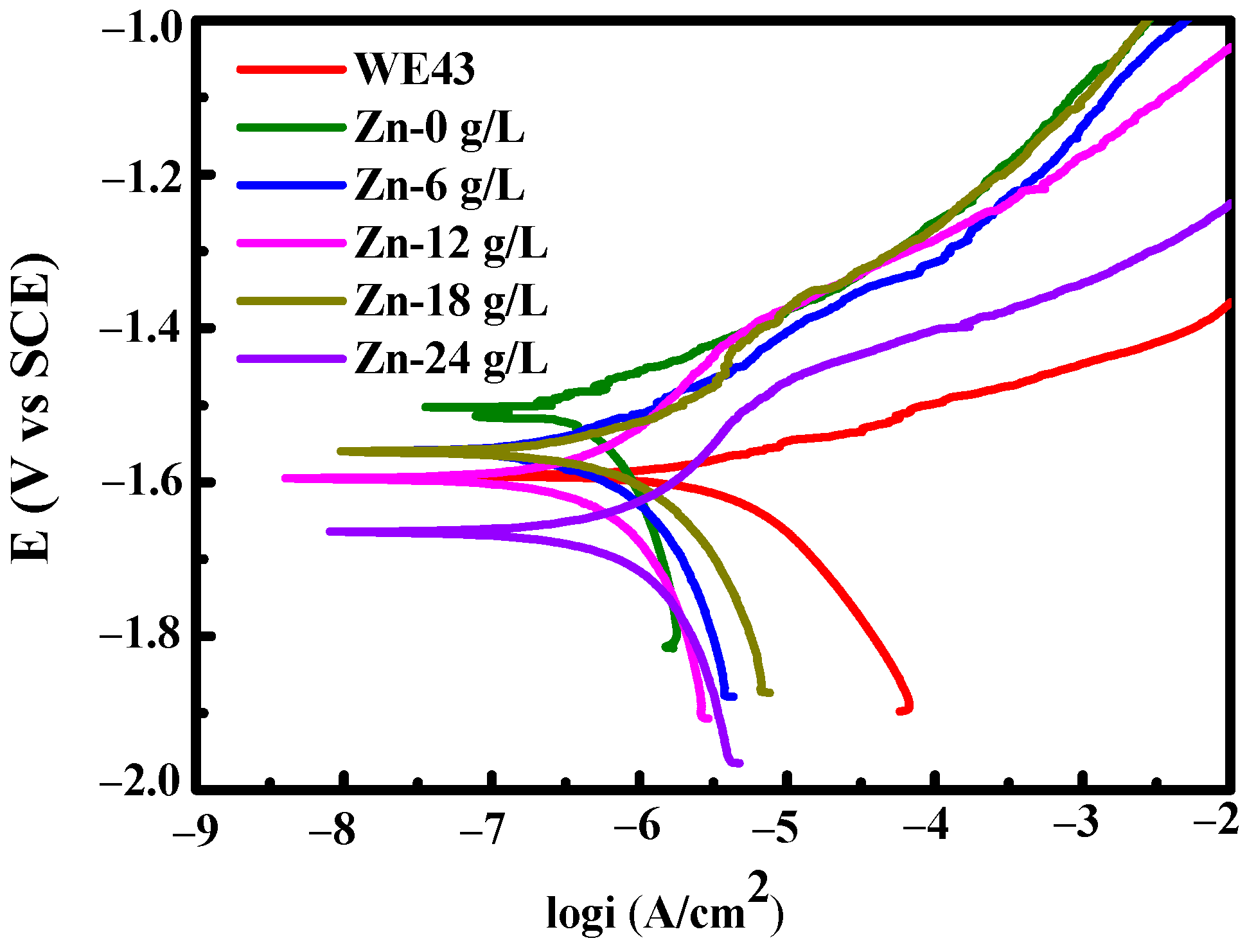
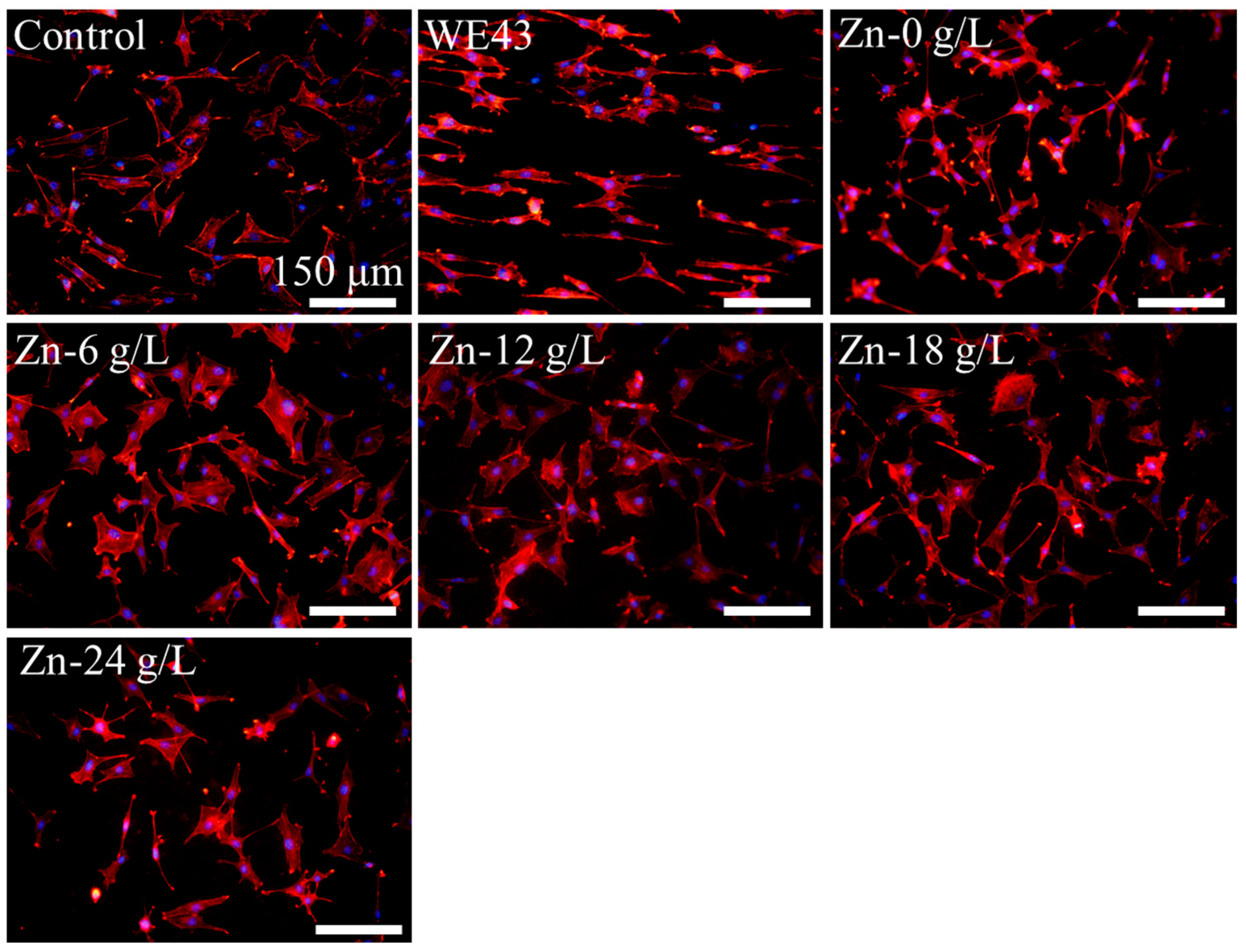
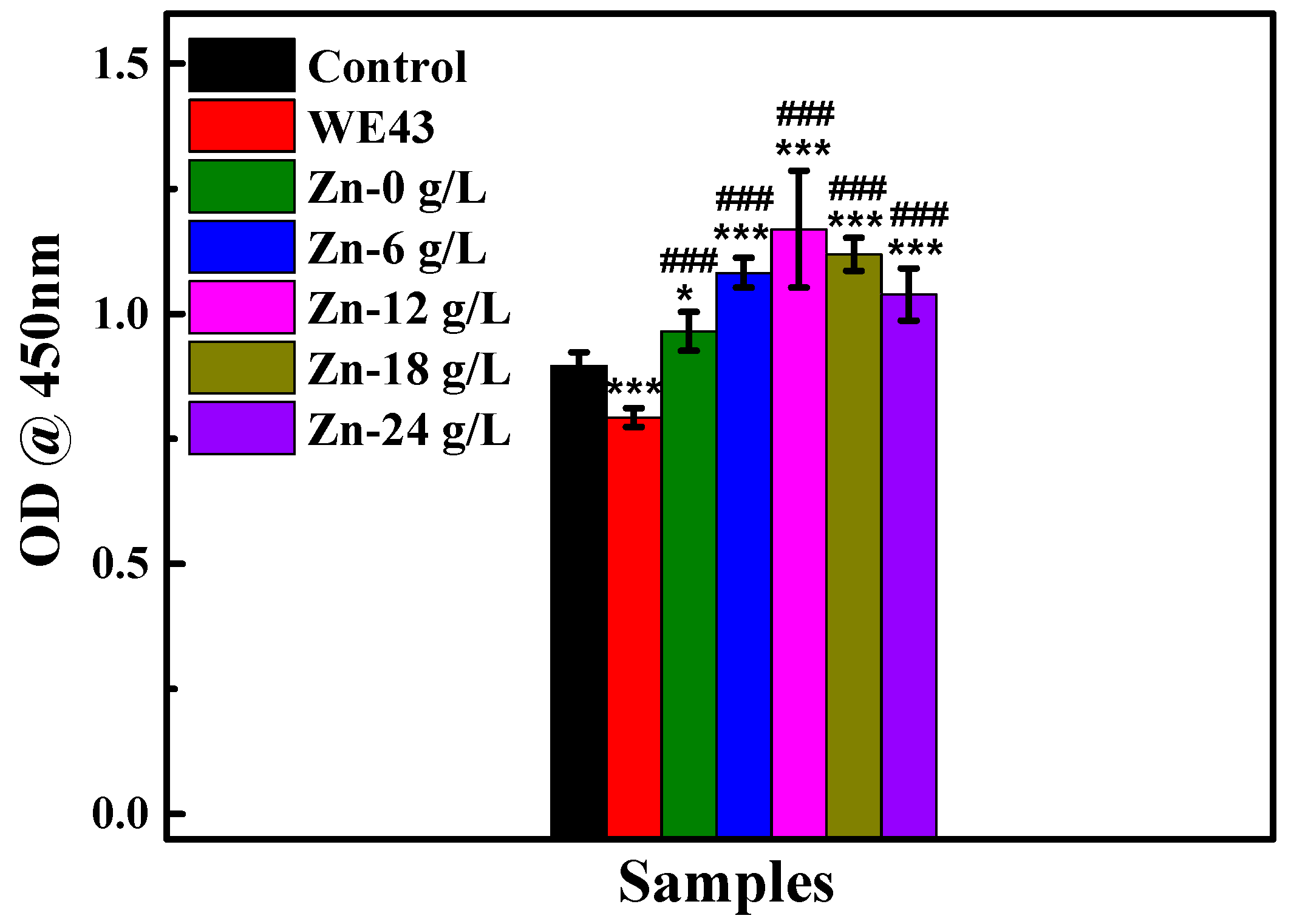
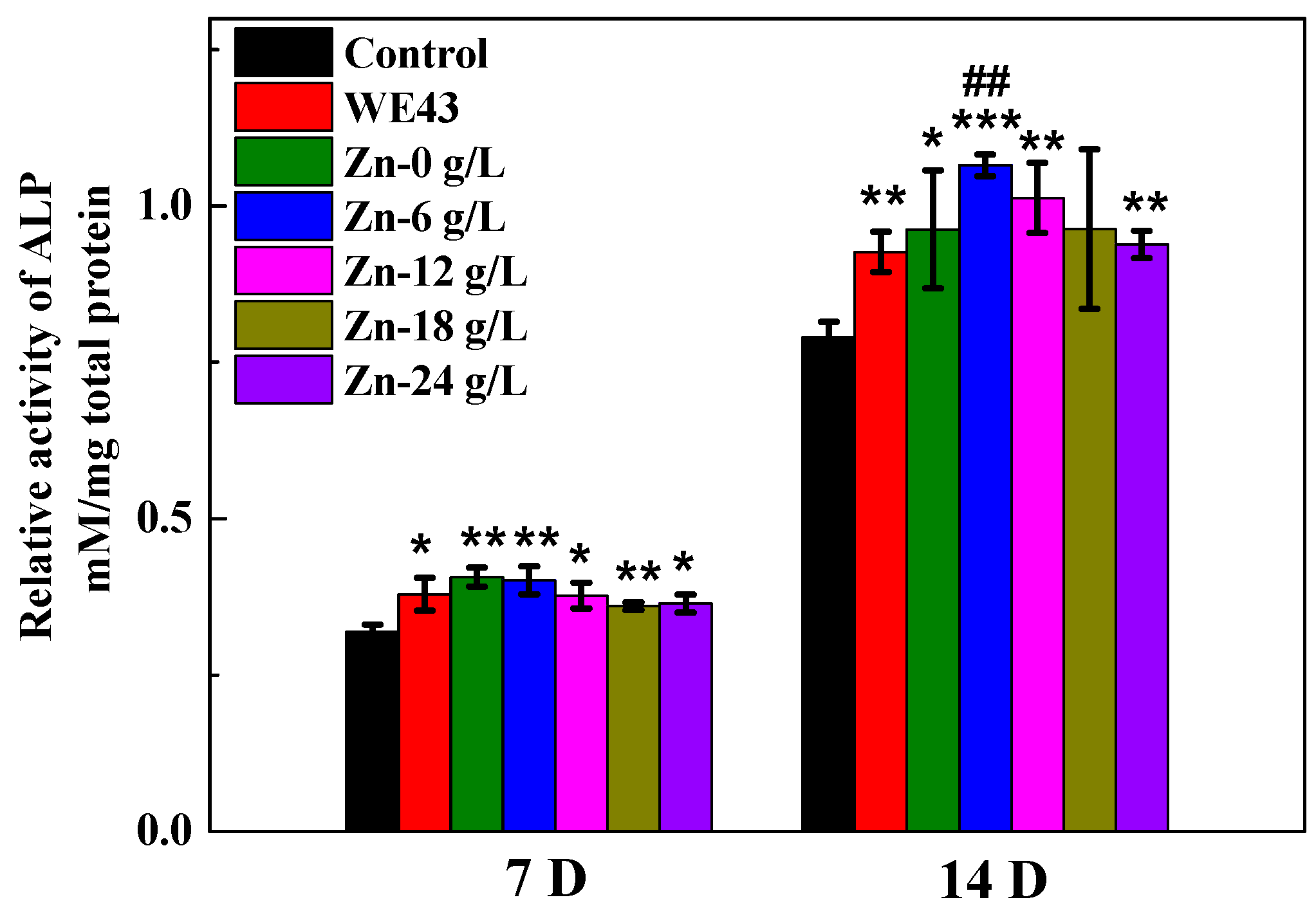
| Solutions | βa (mV/dec) | βc (mV/dec) | icorr (A·cm−2) | Ecorr (V) |
|---|---|---|---|---|
| Substrate | 313.21 | 316.26 | 1.13 × 10−5 | −1.6045 |
| Zn-0 g/L | 101 | 475.98 | 5.75 × 10−7 | −1.5028 |
| Zn-6 g/L | 135.89 | 285.77 | 6.84 × 10−7 | −1.5603 |
| Zn-12 g/L | 229.76 | 412.33 | 1.09 × 10−6 | −1.5952 |
| Zn-18 g/L | 221.46 | 311.66 | 1.18 × 10−6 | −1.5631 |
| Zn-24 g/L | 232.06 | 397.92 | 1.39 × 10−6 | −1.6655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Zhu, Y.; Yu, F.; Zhao, R.; Lai, X.; Jiang, H.; Xu, T.; Zhao, Y.; Zhang, R. Investigation of In Vitro Cytocompatibility of Zinc-Containing Coatings Developed on Medical Magnesium Alloys. Materials 2024, 17, 209. https://doi.org/10.3390/ma17010209
Wang Y, Liu Y, Zhu Y, Yu F, Zhao R, Lai X, Jiang H, Xu T, Zhao Y, Zhang R. Investigation of In Vitro Cytocompatibility of Zinc-Containing Coatings Developed on Medical Magnesium Alloys. Materials. 2024; 17(1):209. https://doi.org/10.3390/ma17010209
Chicago/Turabian StyleWang, Yun, Yuzhi Liu, Yuanyuan Zhu, Fanglei Yu, Rongfang Zhao, Xinying Lai, Haijun Jiang, Tianhong Xu, Ying Zhao, and Rongfa Zhang. 2024. "Investigation of In Vitro Cytocompatibility of Zinc-Containing Coatings Developed on Medical Magnesium Alloys" Materials 17, no. 1: 209. https://doi.org/10.3390/ma17010209





