Magnetron Sputtered Lead Titanates Thin Films for Pyroelectric Applications: Part 1: Epitaxial Growth, Material Characterization
Abstract
:1. Introduction
2. Materials and Methods
3. Pyroelectricity in Materials
4. Epitaxial Growth of Lead Titanates by Magnetron Sputtering
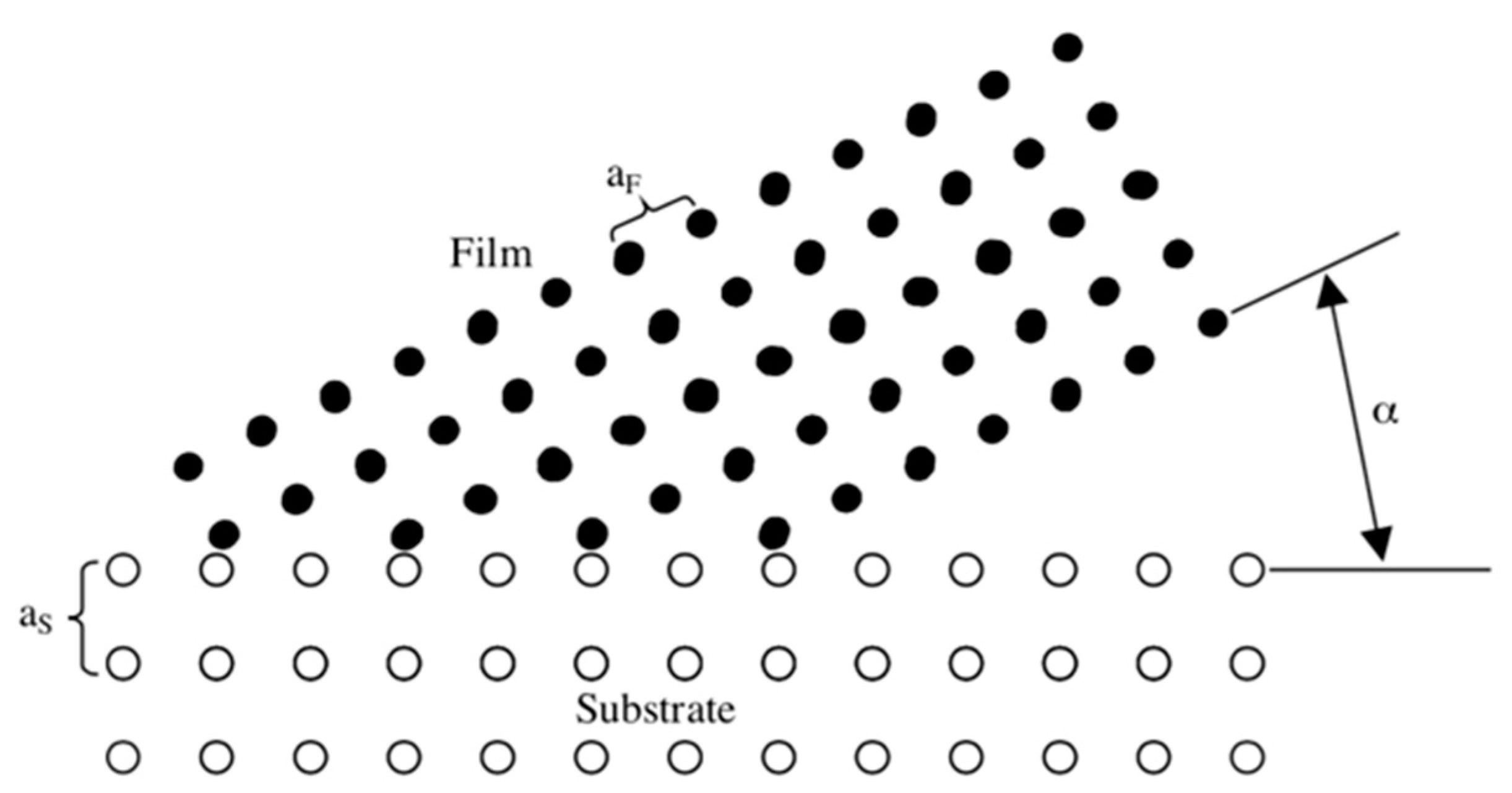
4.1. Epitaxy and the Parameters Influencing the Epitaxial Growth
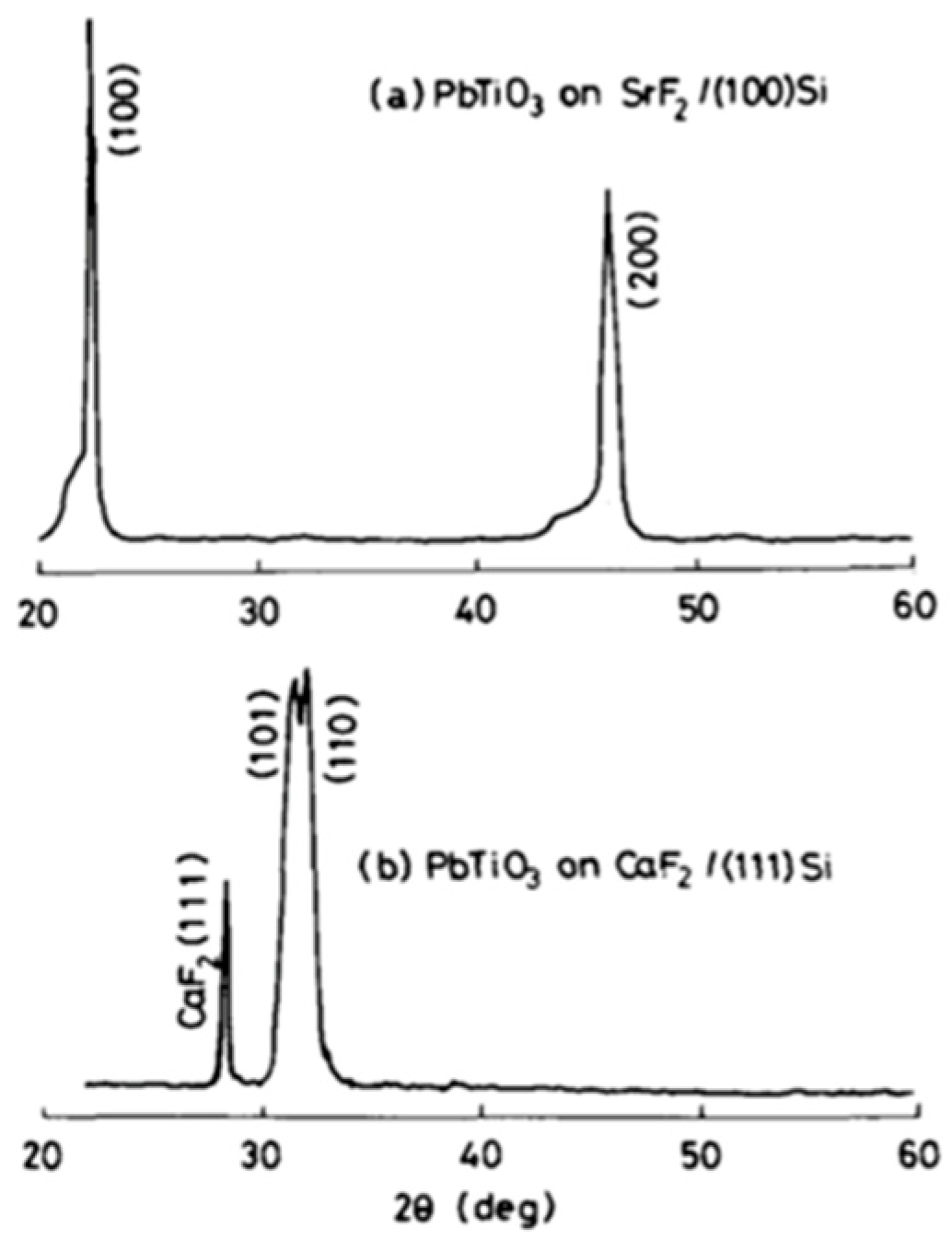
- (a)
- Thin films of the single crystalline phase are deposited epitaxially on appropriate single crystal substrates at Ts Tepi. The epitaxial temperature depends on both the film and the substrate material. In the case of magnetron sputtering of PT, Tepi575 °C.
- (b)
- Thin films of the polycrystal phase are deposited at Ts Tcrys.
- (c)
- The amorphous thin films deposited on the single crystal substrates also convert into the single-crystalline phase after the post-annealing at a temperature Tpan Tepi, by solid phase epitaxy as discussed in Section 4.2. In the case of ex situ magnetron sputtering of PT, Tpan750 °C.
4.2. Magnetron Sputtering and the Parameters Influencing the Sputtering Deposition Process
- Processes occurring at the source (target)
- 2.
- Processes between the target and the substrate
- 3.
- Processes occurring at the depositing film
4.3. Parameters Affecting Epitaxial Growth of Perovskite Lead Titanates by Magnetron Sputtering
- solid-state crystallization, i.e., low-temperature deposition of amorphous film followed by solid-state crystallization using post-annealing in [50]. These two routes are discussed below.
4.3.1. Growth Kinetics Using In Situ Substrate Heating
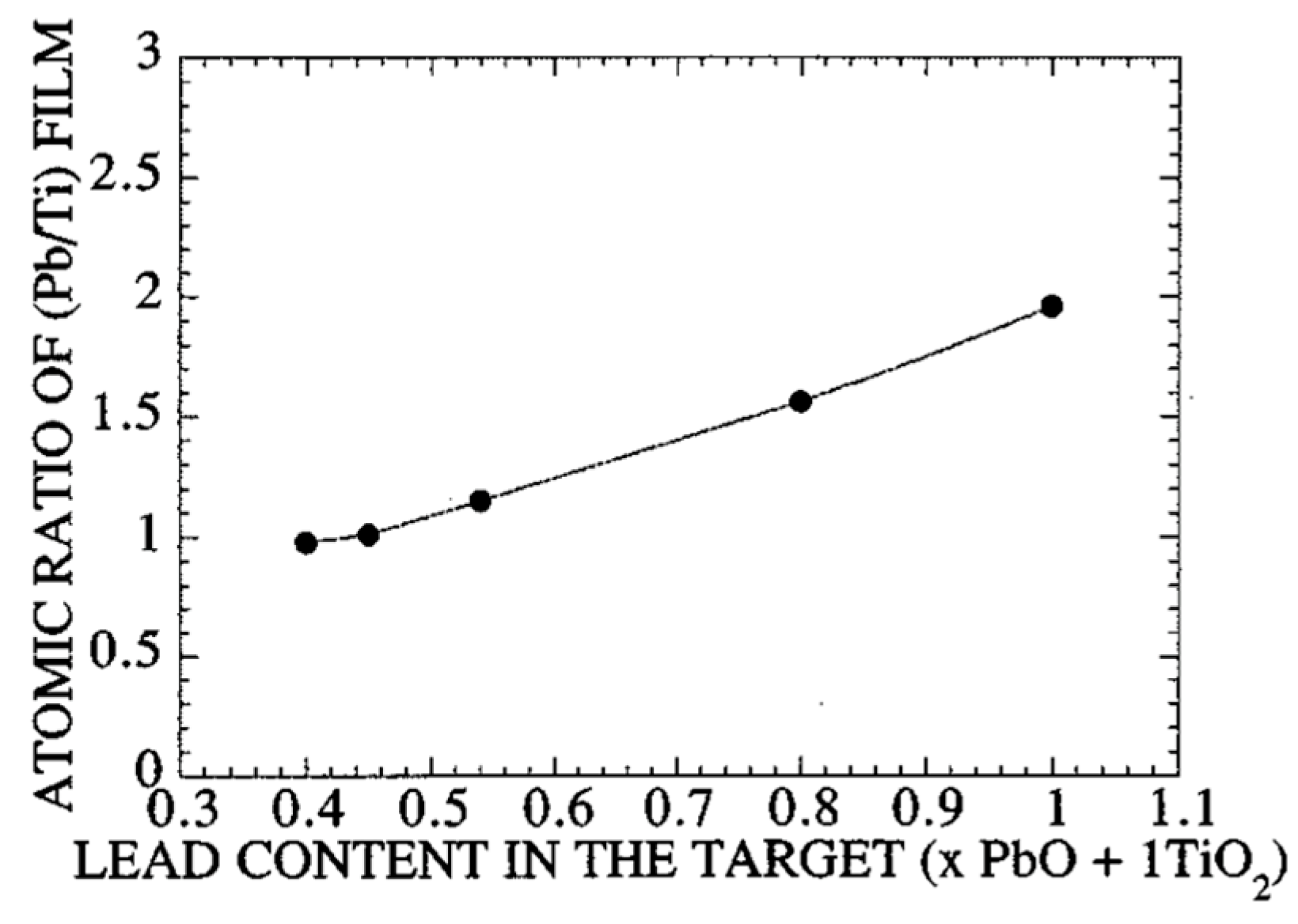
- Dependence of the PT film’s atomic ratio on the target’s lead content;
- 2.
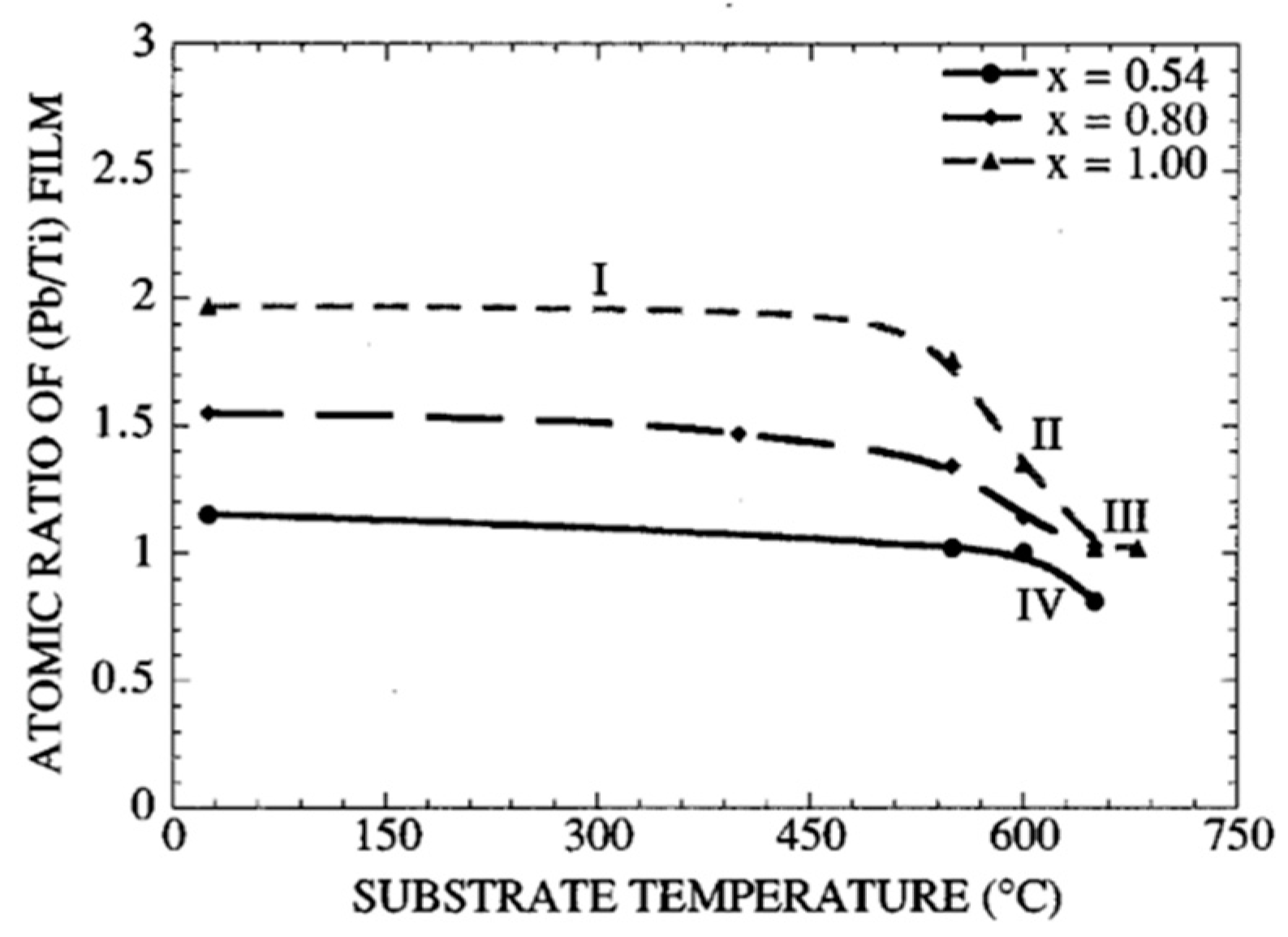
- Dependence of the film’s atomic ratio on the substrate’s growth temperature;
- 3.
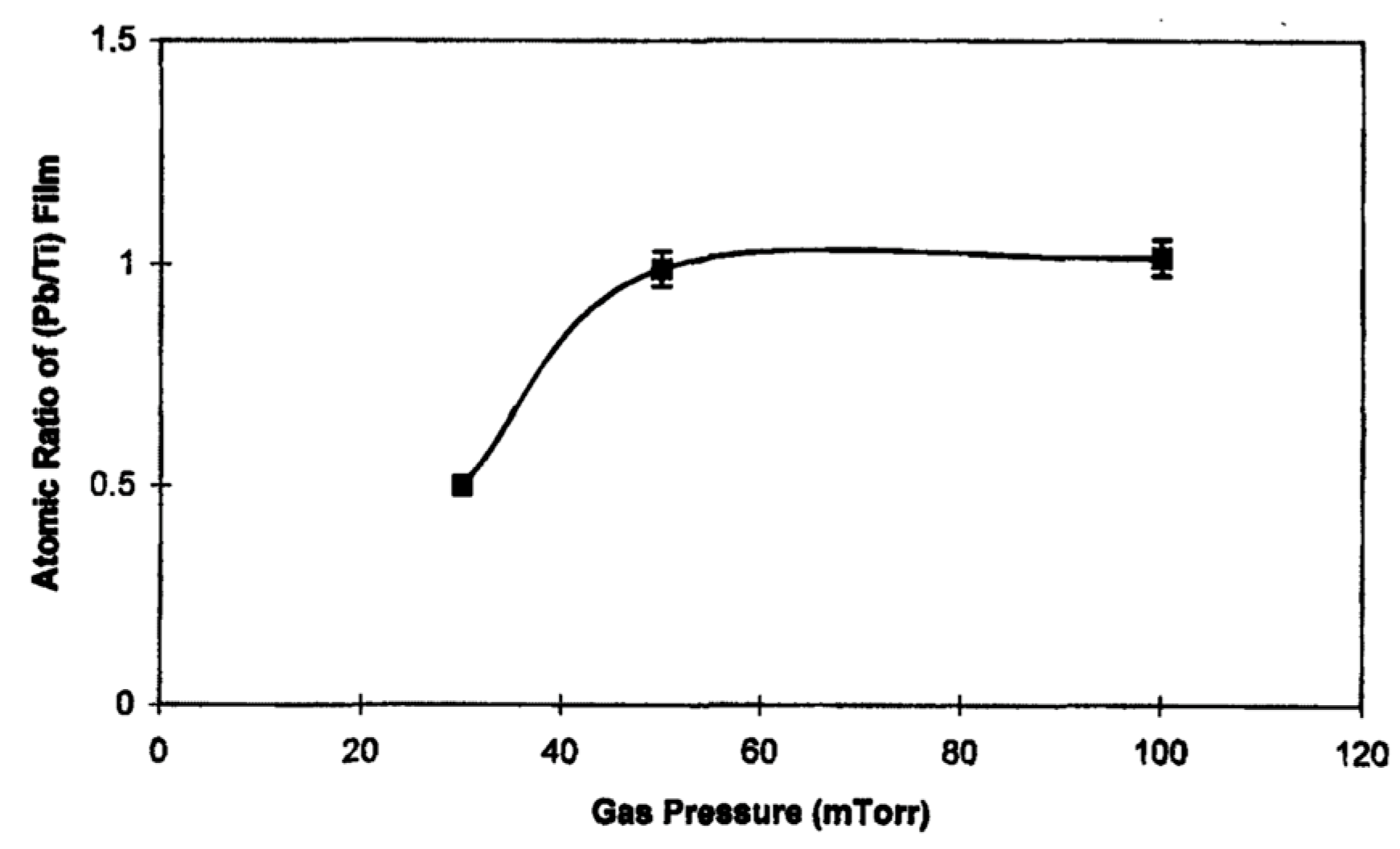
- The effect of gas pressure on the (Pb/Ti) ratio of the PT films;
- 4.
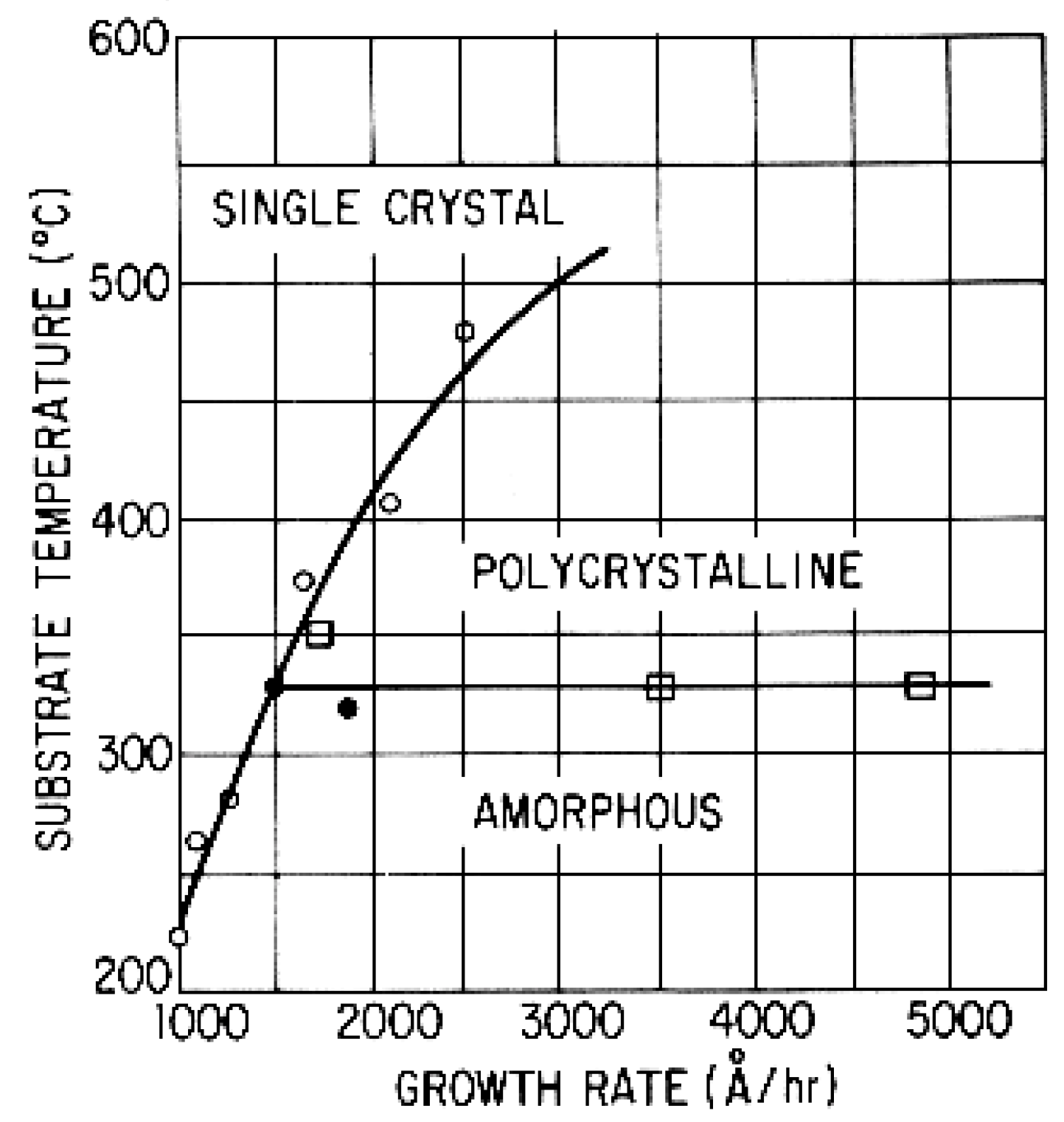
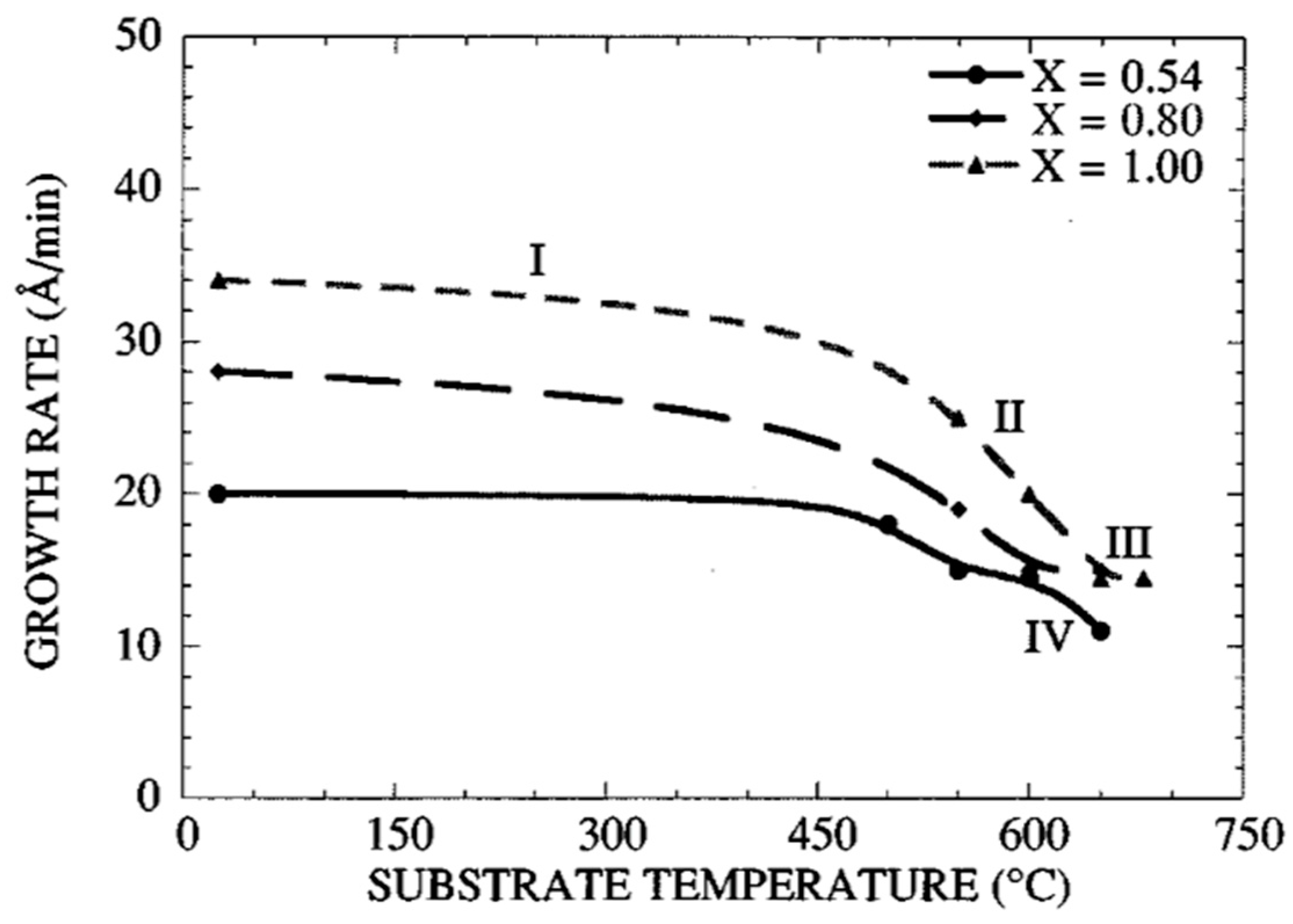
- Dependence of the growth rate on the substrate temperature;
- 5.
- Dependence of preferential orientation on ratio.
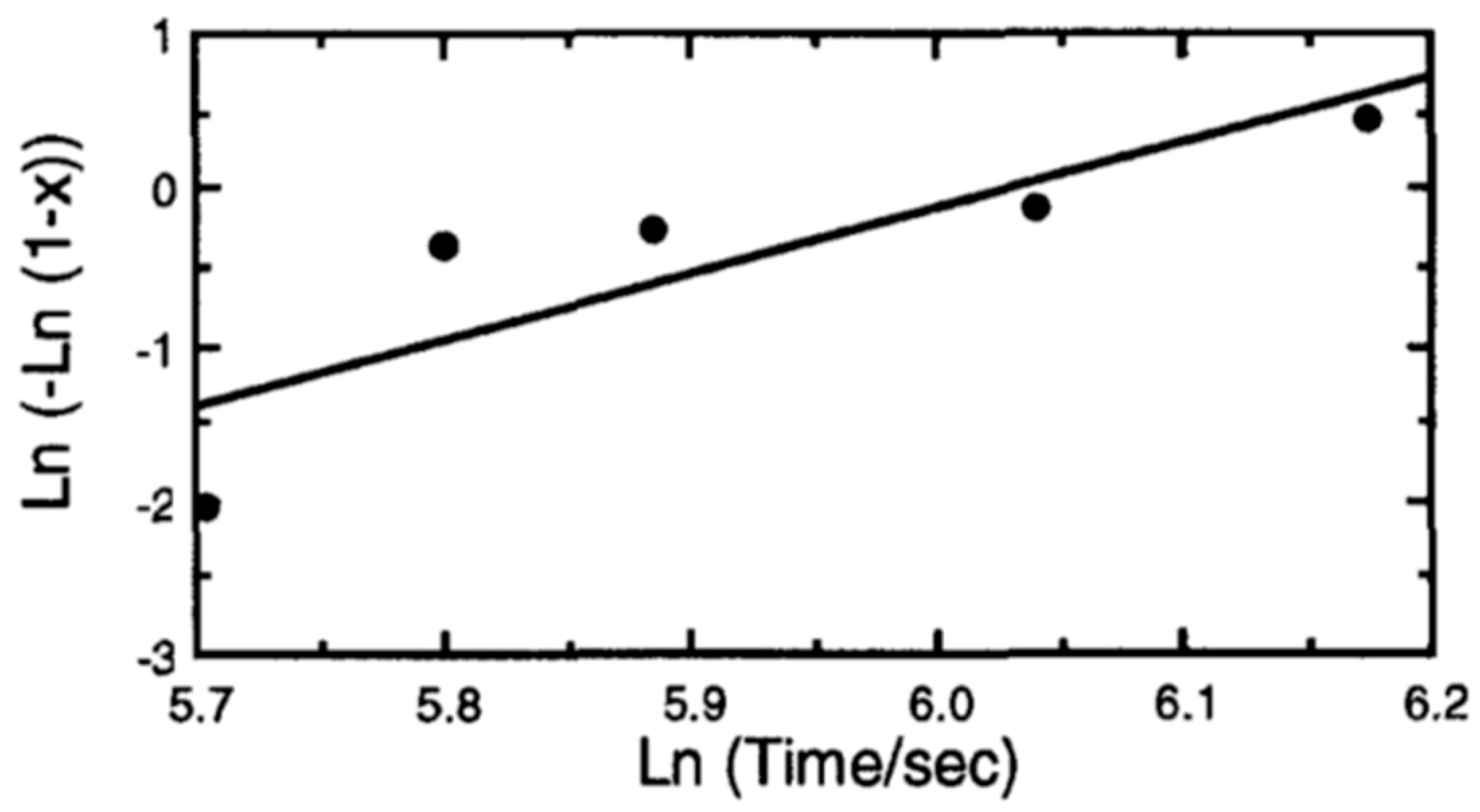
4.3.2. Solid State Crystallization
5. Discussion and Results
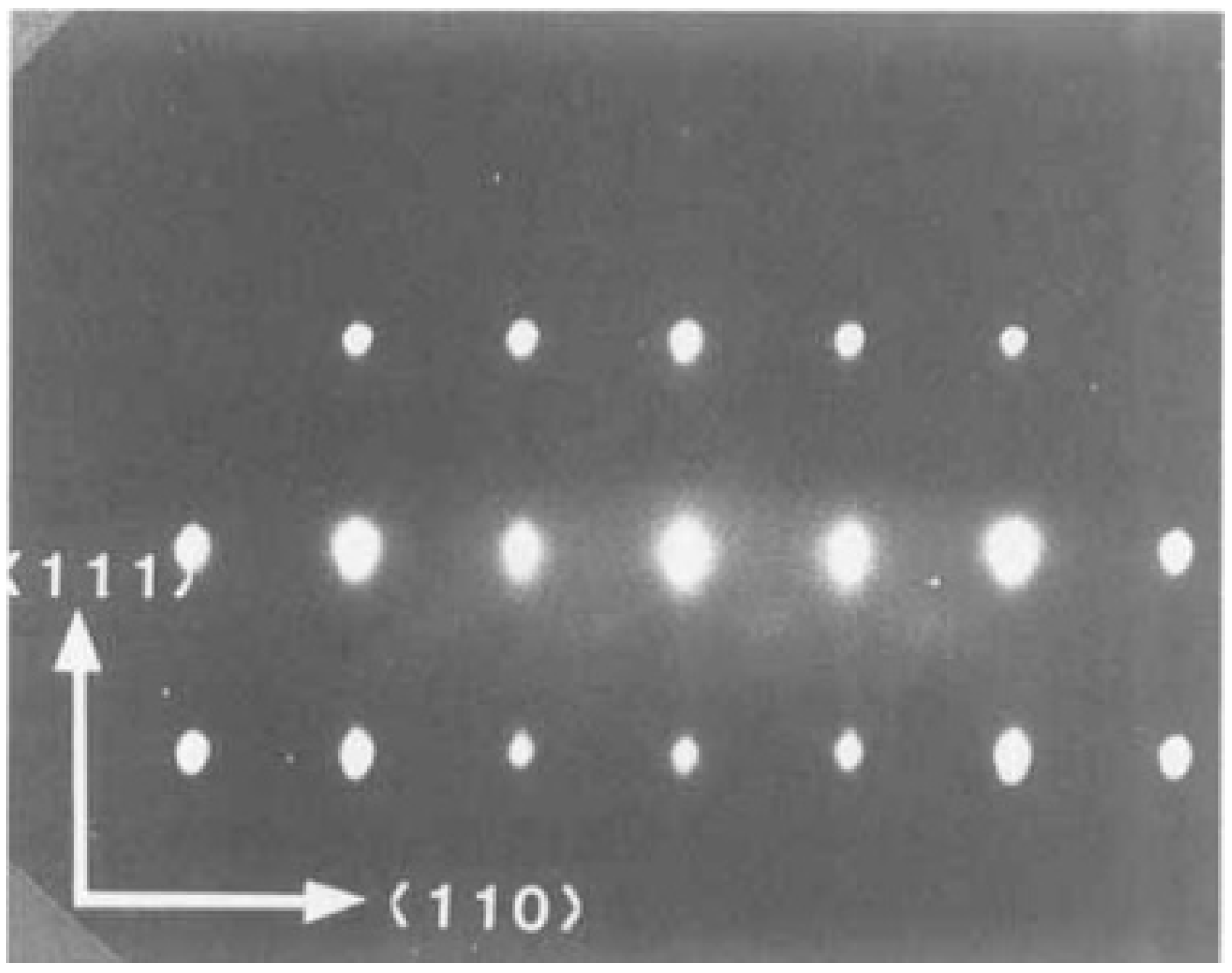
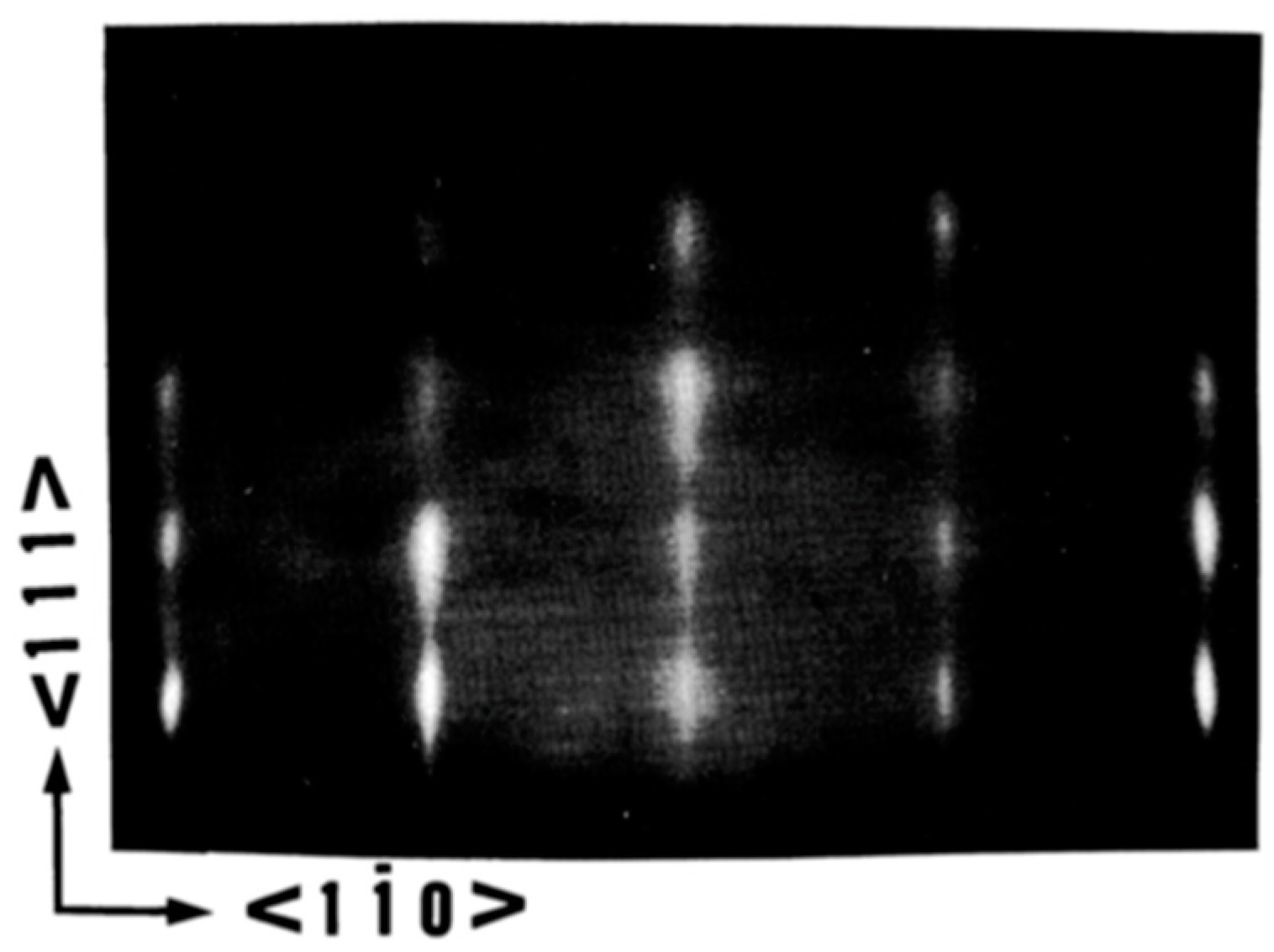
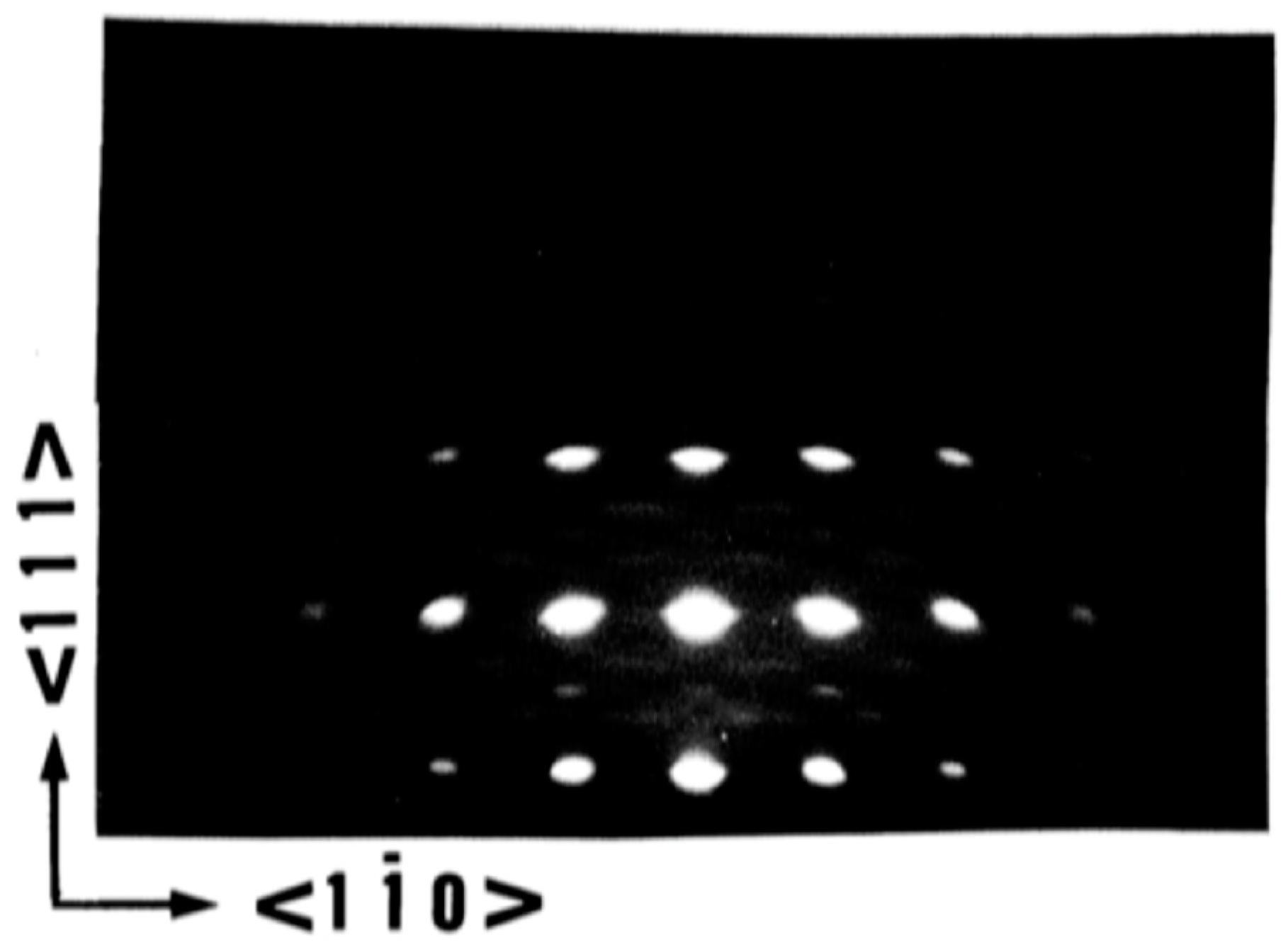
5.1. The Crystalline Quality of the Substrate/Buffer Layer
- PT grows in a layer-by-layer mode on TiO2-terminated SrTi (ST), but this converts to an island-growth mode; after approximately four monolayers of material, this is followed by a layer-by-layer mode if grown on SrRu on ST, which is SrO terminated.
- The island-like growth observed during PT growth on SrRu shows a typical “fingerprint” structure, where the islands have a certain height (1 nm) and grow only in lateral size until the islands coalesce. Transmission electron microscopy analysis does not show differences in the interface between PT grown on ST or grown on SrRu on ST. With a direct termination switch of SrO-terminated SrRu with a monolayer of Ti, PbTi grows layer by layer, as if it were grown on Ti-terminated ST. Only a difference in relaxation behavior is observed.
- By contrast, a termination switch of Ti-terminated ST with one monolayer of SrRu does not change the growth behavior as seen on a “thick” layer of SrRu. PT starts to grow in an island-like growth mode after only three monolayers of SrRu. This switch while the termination switch after one monolayer of SrRu is complete.
- Highly c-axis-oriented lanthanum-modified lead titanate (PLT) thin films were obtained by RF-magnetron sputtering under the conditions of low deposition rate and low gas pressure.
- Upon increasing La content, the degree of c-axis orientation of PLT films decreases, tetragonality c/a decreases, and Curie temperature decreases.
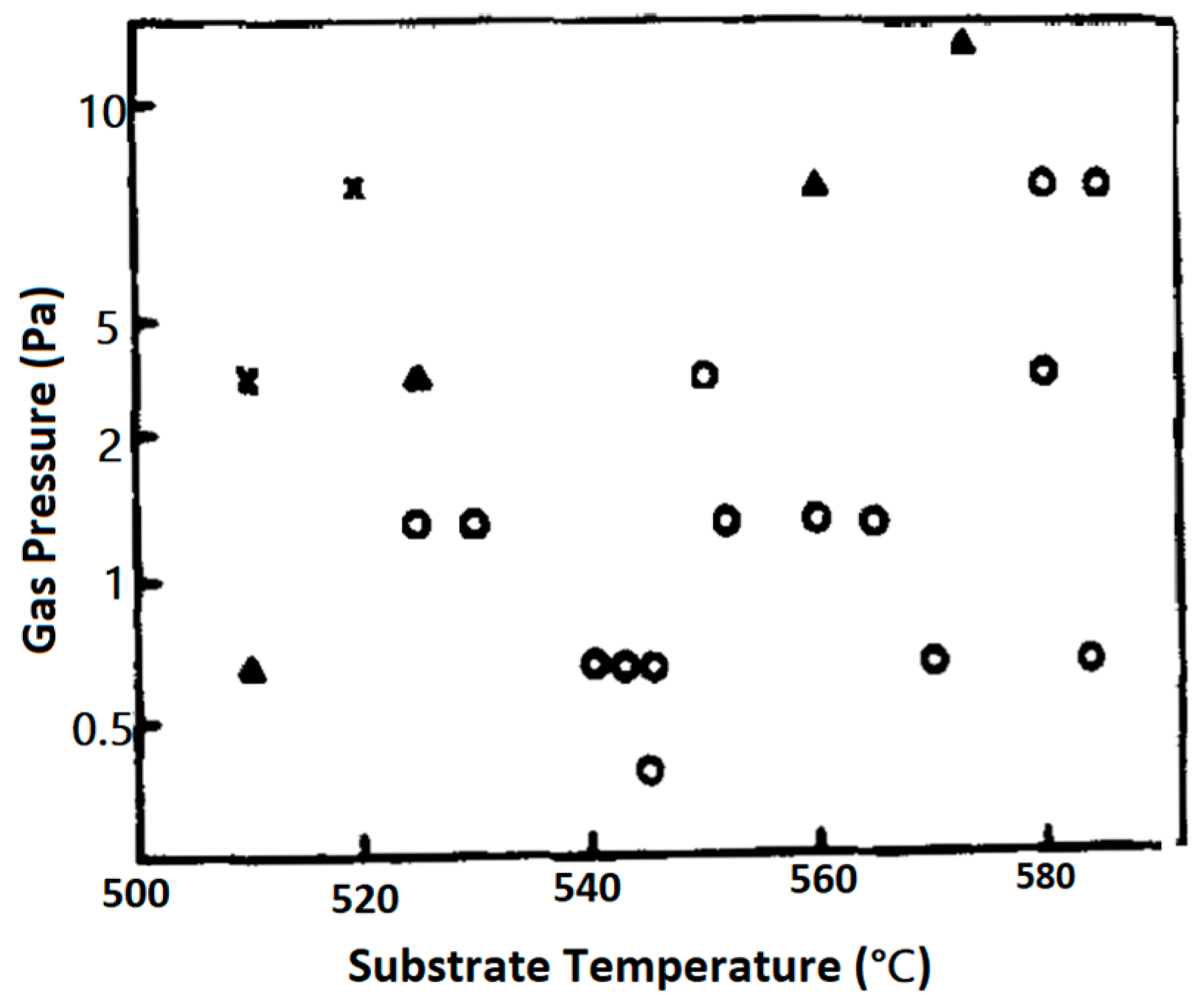
5.2. The Effect of (Growth) Substrate Temperature (Ts)
- The kinetic energy of the incident energetic plasma species irradiated on the substrate surface. The surface mobility of the ad-atoms is improved when the kinetic energy of the incident ad-atoms and/or the other energetic plasma species irradiated on the growing film surface is increased. This leads to an effective reduction of Ts [87].
- The incident angle of the irradiated species. This angle defines the amount of energy and/or momentum transferred to the species in the growing film.
- Tepi is reduced by the reduction of the film growth rate.
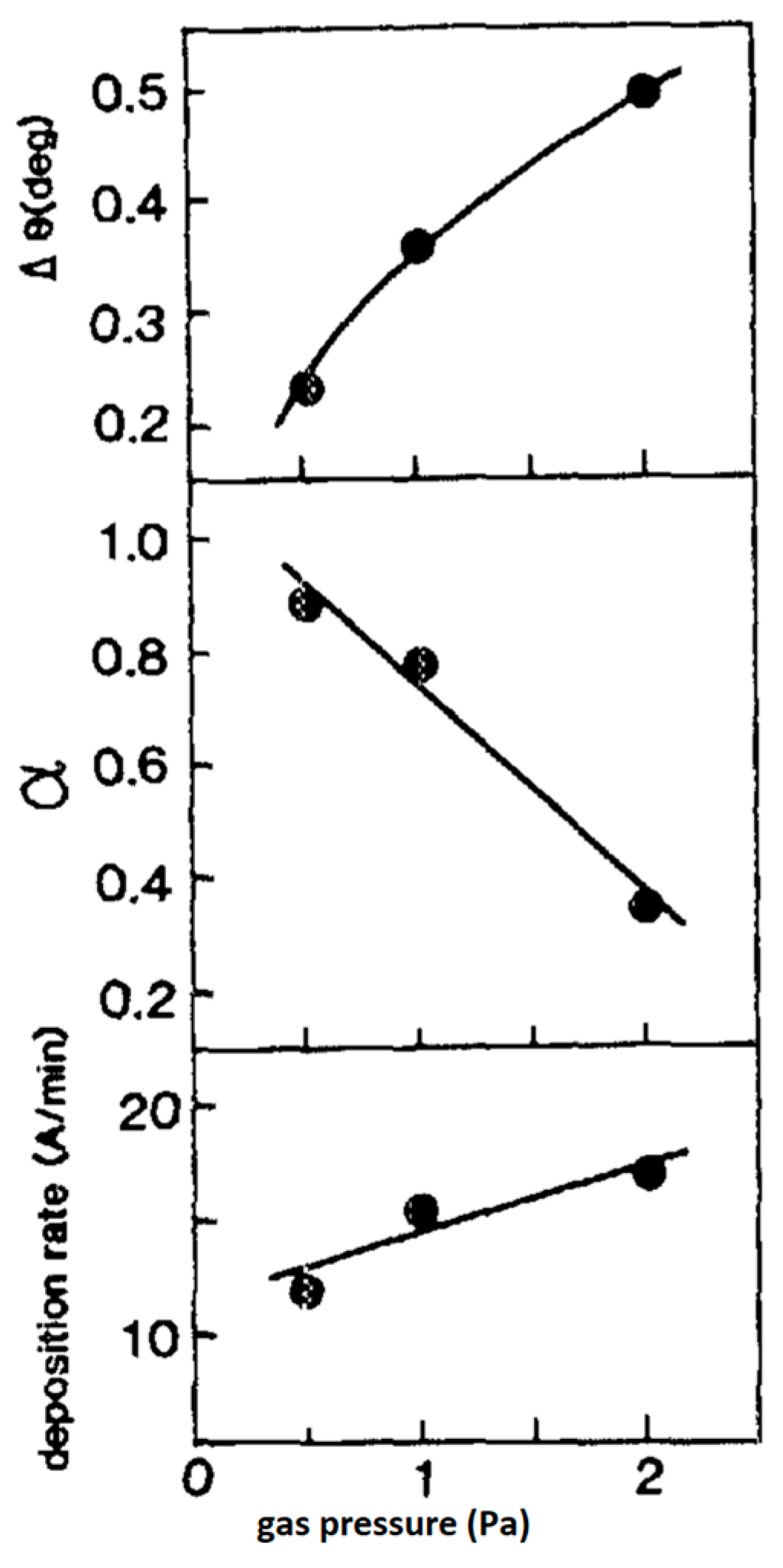
5.3. The Effect of Processing Gas
- The effect of processing gas composition
- 2.
- The oxygen partial pressure in the gas mixture
- 3.
- The effect of oxygen partial pressure in the gas mixture on the film’s morphology
- 4.
- The effect of oxygen total pressure on the film deposition rate;
- 5.
- The effect of the processing gas total pressure on film’s morphology.
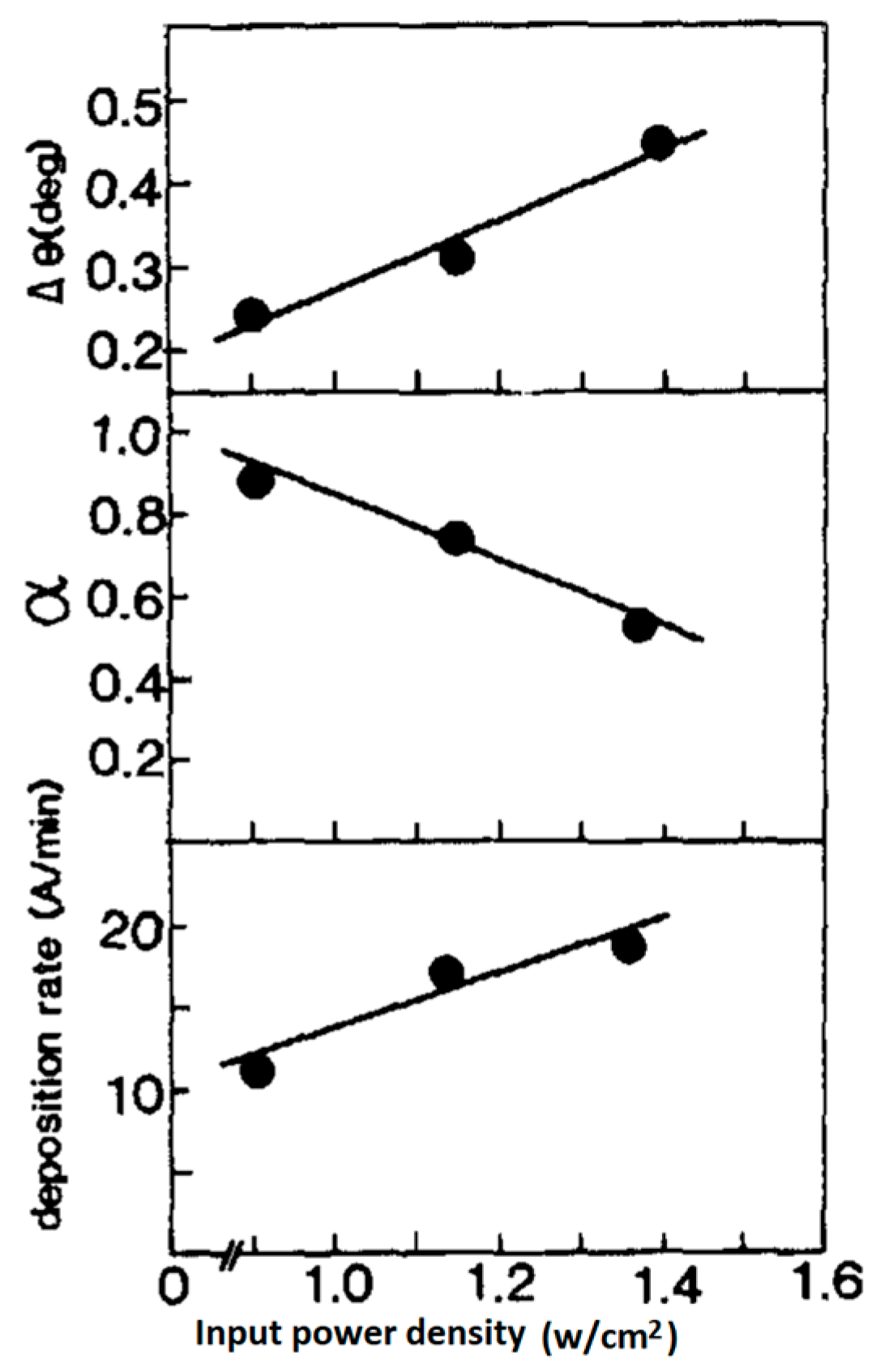
5.4. The Effect of RF Input Power on the Film’s Morphology
5.5. The Effect of Applied Negative DC Bias
- As the DC bias voltage to substrates increases in either negative or positive voltages, the Ti content and the thickness of PZT films both increase, compared with those prepared without applying DC bias voltage. However, using the negative bias voltage, the chemical composition of the PZT film comes closer to that of the composite target.
- Despite the redundant Pb content of the composite targets, the composition of Pb in PZT film is self-controlled.
- Epitaxial PZT films with excellent crystallinity are grown by applying 100 V bias voltage to substrates.
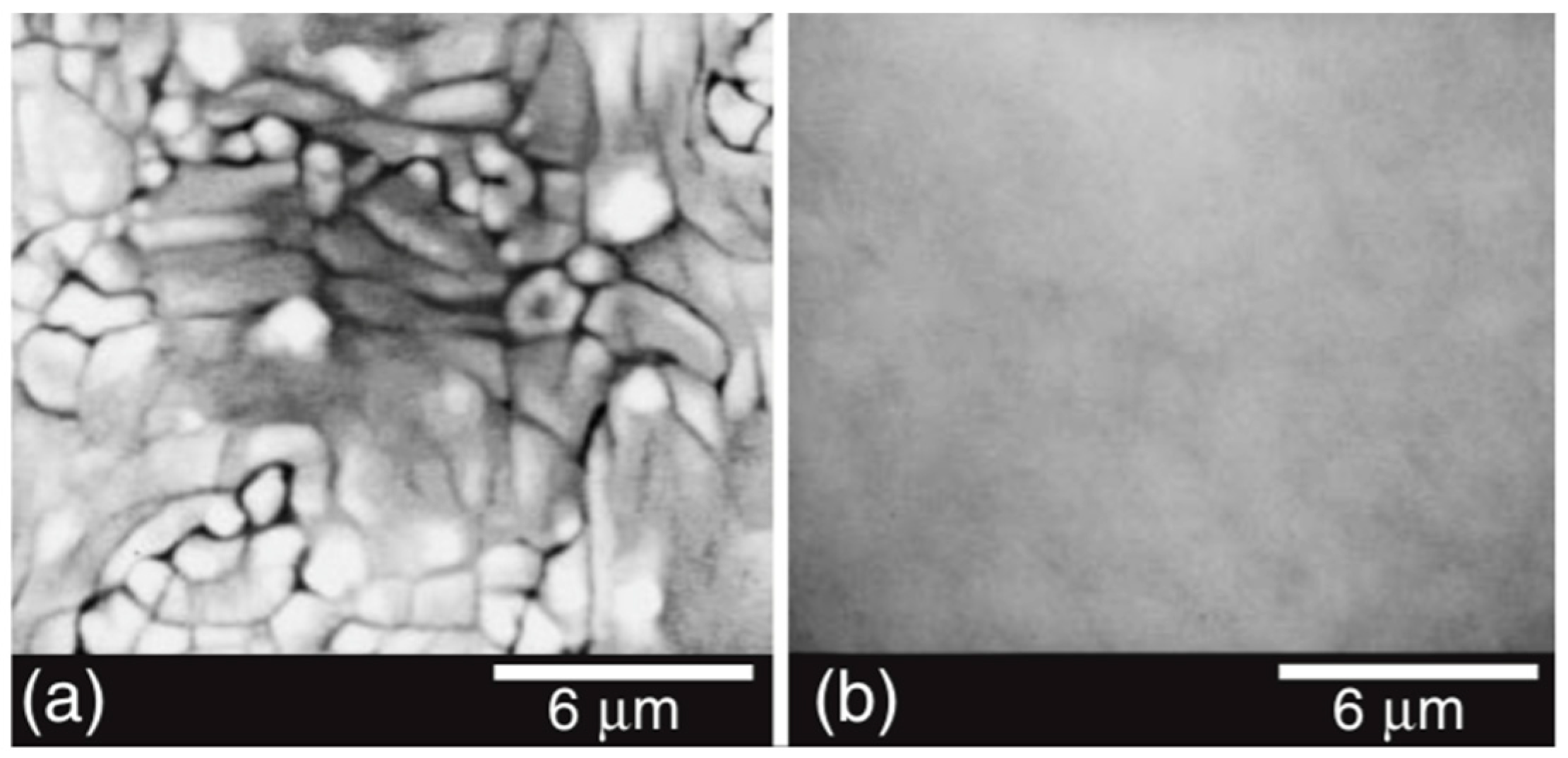
- As shown in Figure 27, clean, smooth PZT films without voids and pinholes are easily obtained.
5.6. The Effect of the Growing Film’s Thickness (t)
5.7. The Effect of the Cooling Rate
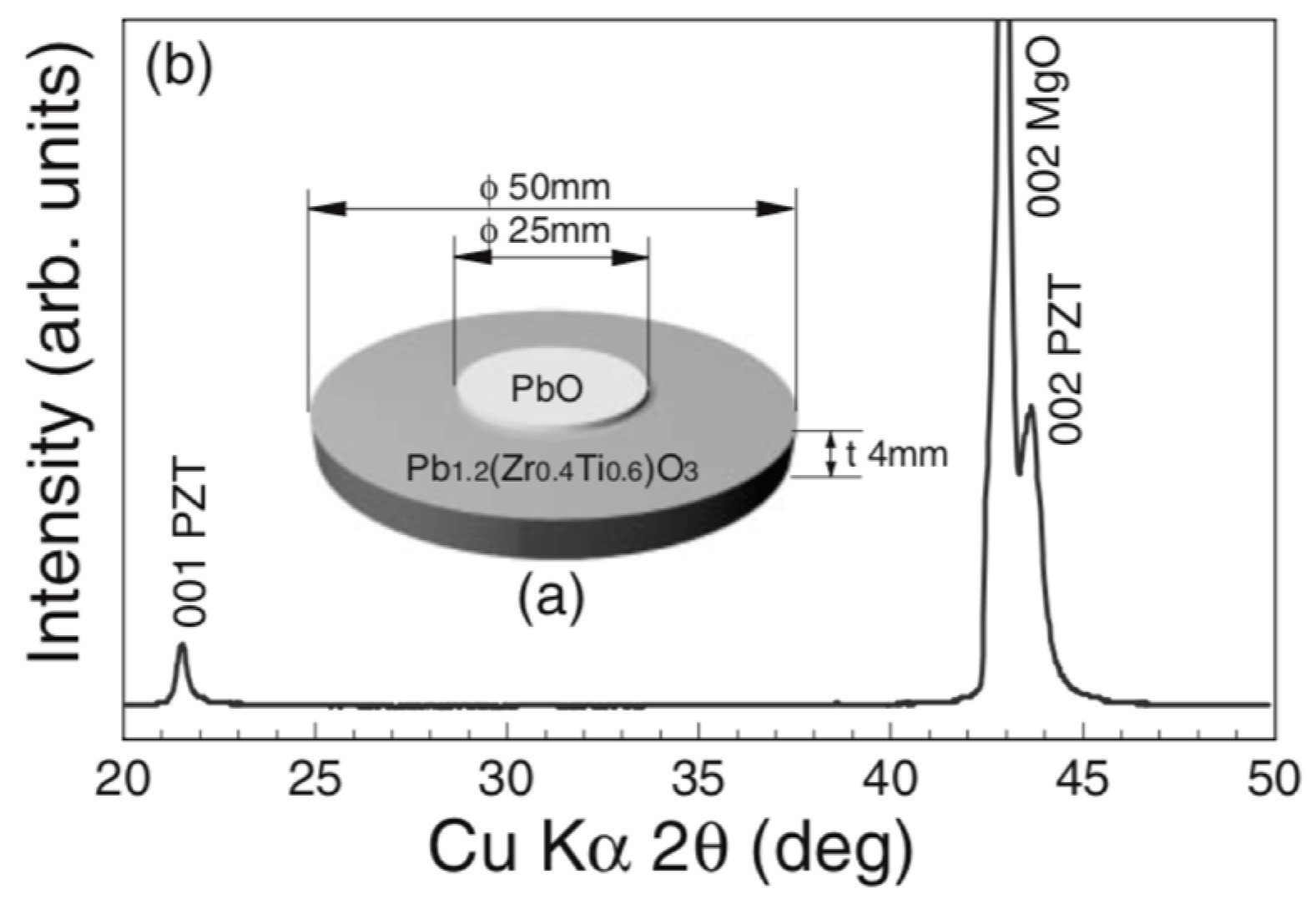
5.8. The Effect of the Excess PbO Content in the Source
6. Future Topics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, M.D.; Batra, A.K.; Guggilla, P.; Edwards, M.E.; Penn, B.G.; Currie, J.R. Pyroelectric Materials for Uncooled Infrared Detectors: Processing, Properties, and Applications (No. M-1273); NASA: Washington, DC, USA, 2010; pp. 1–57.
- Matsubara, S.; Shohata, N.; Mikami, M. Epitaxial growth of PbTiO3 on MgAl2O4/Si substrates. Jpn. J. Appl. Phys. 1985, 24, 10. [Google Scholar] [CrossRef]
- Nagao, N.; Takeuchi, T.T.; Iijima, K.I. Preparation and proper-ties of (Pb, La) TiO3 pyroelectric thin films by rf-magnetron sputtering. Jpn. J. Appl. Phys. 1993, 32, 4065. [Google Scholar] [CrossRef]
- Singh, K.; Singh, V.; Gupta, R.; Bamzai, K.K. Structural, dielectric, piezoelectric and ferroelectric behavior of rare earth double doped lead titanate ceramics synthesized by solid state method. J. Appl. Phys. 2014, 6, 8–14. [Google Scholar] [CrossRef]
- Castellano, R.N.; Feinstein, L.G. Ion-beam deposition of thin films of ferroelectric lead zirconate titanate (PZT). J. Appl. Phys. 1979, 50, 4406–4411. [Google Scholar] [CrossRef]
- Mochizuki, S.; Mihara, T.; Kimura, S.; Ishida, T. TfP205. Preparation of lead titanate thin films by reactive electron beam co-evaporation using ozone. Ferroelectrics 1992, 134, 319–323. [Google Scholar] [CrossRef]
- Roemer, A.; Essahlaoui, A.; Pons-Y-Moll, O.; Vincent, B.; Defourneau, R.M.; Seiler, W.; Boudrioua, A.; Millon, E. Growth of lead titanate thin film waveguides by pulsed-laser deposition. Thin Solid Films 2004, 453–454, 417–421. [Google Scholar] [CrossRef]
- Chen, C.; Ryder, D.F., Jr.; Spurgeon, W.A. Synthesis and micro-structure of highly oriented lead titanate thin films prepared by a Sol-Gel method. J. Am. Ceram. Soc. 1989, 72, 1495–1498. [Google Scholar] [CrossRef]
- Luo, W.G.; Ding, A.L.; Zhang, R.T.; Huang, Y.H.; Ge, M. Deposition and properties of PLT thin films by magnetron sputtering from powder target. In Proceedings of the ISAF ‘92: Proceedings of the Eighth IEEE International Symposium on Applications of Ferroelectrics, Greenville, SC, USA, 30 August–2 September 1992. [Google Scholar] [CrossRef]
- Okuyama, M.; Togami, Y.; Hamakawa, Y. Microwave effect in RF magnetron sputtering of PbTiO3. Appl. Surf. Sci. 1988, 33, 625–631. [Google Scholar] [CrossRef]
- Chopra, S.; Sharma, S.; Goel, T.C.; Mendiratta, R.G. Effect of annealing temperature on microstructure of chemically deposited calcium modified lead titanate thin films. Appl. Surf. Sci. 2004, 230, 207–214. [Google Scholar] [CrossRef]
- Pryds, N.; Esposito, V. Metal Oxide-based Thin Film Structures, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ichinose, N.; Hirao, Y.; Nakamoto, M.; Yamashita, Y. Pyroelectric Infrared Sensor Using Modified PbTiO3 and Its Applications. Jpn. J. Appl. Phys. 1985, 24, 178. [Google Scholar] [CrossRef]
- Pignolet, A.; Schmid, P.E.; Wang, L.; Lévy, F. Structure and Characterization of Sputtered Thin Films Based on Lead Titanate. MRS Proc. 1991, 230, 291. [Google Scholar] [CrossRef]
- Brewer, A.; Cho, K.H.; Saenrang, W.; Baek, S.H.; Frederick, J.C.; Eom, C.B. Uniform sputter deposition of high-quality epitaxial complex oxide thin films. J. Vac. Sci. Technol. Vac. Surf. Film. 2017, 35, 060607. [Google Scholar] [CrossRef]
- Mafi, E.; Calvano, N.; Patel, J.; Islam, M.S.; Hasan Khan, M.S.; Rana, M. Electro-Optical Properties of Sputtered Calcium Lead Titanate Thin Films for Pyroelectric Detection. Micromachines 2020, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Takayama, R.; Tomita, Y.; Iijima, K.; Ueda, I. Preparation and characteristics of pyroelectric infrared sensors made of c-axis oriented La-modified PbTi03 thin films. J. Appl. Phys. 1987, 61, 411–415. [Google Scholar] [CrossRef]
- Leng, W.J.; Yang, C.R.; Ji, H.; Zhang, J.H.; Tang, J.L.; Chen, H.W.; Gao, L.F. Electrical and optical properties of lanthanum-modified lead zirconate titanate thin films by radio-frequency magnetron sputtering. J. Appl. Phys. 2006, 100, 106102. [Google Scholar] [CrossRef]
- Matsubara, S.; Miura, S.; Miyasaka, Y.; Shohata, N. Preparation of epitaxial ABO3 perovskite-type oxide thin films on a (100) MgAl2O4/Si substrate. J. Appl. Phys. 1989, 66, 5826–5832. [Google Scholar] [CrossRef]
- Ye, C.; Tamagawa, T.; Schiller, P.; Polla, D.L. Pyroelectric PbTiO3 thin films for microsensor applications. Sens. Actuators A Phys. 1992, 35, 77–83. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, Z.; Fan, P. Characterization of PZT ferroelectric thin films by RF-magnetron sputtering. J. Phys. Conf. Ser. 2007, 61, 120–124. [Google Scholar] [CrossRef]
- Xie, Z.; Peng, B.; Meng, S.; Zhou, Y.; Yue, Z. High-Energy-Storage Density Capacitors of Bi(Ni1/2Ti1/2)O3-PbTiO3 Thin Films with Good Temperature Stability. J. Am. Ceram. Soc. 2013, 96, 2061–2064. [Google Scholar] [CrossRef]
- Kondo, M.; Sato, K.; Ishii, M.; Wakiya, N.; Shinozaki, K.; Kurihara, K. Electrooptic Properties of Lead Zirconate Titanate Films Prepared on Silicon Substrate. Jpn. J. Appl. Phys. 2006, 45, 7516–7519. [Google Scholar] [CrossRef]
- Kobayashi, M.; Jen, C.K. Piezoelectric thick bismuth titanate/lead zirconate titanate composite film transducers for smart NDE of metals. Smart Mater. Struct. 2004, 13, 951–956. [Google Scholar] [CrossRef]
- Kibe, T.; Inoue, T.; Namihira, T.; Kobayashi, M. High-temperature-immersion ultrasonic probe without delay line using PbTiO3/Pb (Zr, Ti) O3 ultrasonic transducer. Jpn. Soc. Appl. Phys. 2015, 54, 07HB09. [Google Scholar] [CrossRef]
- Gupta, K.M.; Gupta, N. Advanced Electrical and Electronics Materials: Processes and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; p. 362. [Google Scholar]
- Thakoor, S. High speed optoelectronic response from the edges of lead zirconate titanate thin film capacitors. Appl. Phys. Lett. 1993, 63, 3233–3235. [Google Scholar] [CrossRef]
- Kellati, M.; Sayouri, S.; Moudden, N.; Elaatmani, M.; Kaal, A.; Taibi, M. Structural and dielectric properties of La-doped lead titanate ceramics. Mater. Res. Bull. 2004, 39, 867–872. [Google Scholar] [CrossRef]
- Bastani, Y.; Bassiri-Gharb, N. Processing Optimization of Lead Magnesium Niobate-Lead Titanate Thin Films for Piezoelectric MEMS Application. J. Am. Ceram. Soc. 2012, 95, 1269–1275. [Google Scholar] [CrossRef]
- Anderson, L.K. Ferroelectrics in optical memories and displays: A critical appraisal. Ferroelectrics 1972, 3, 69–79. [Google Scholar] [CrossRef]
- Van Zant, P. Microchip Fabrication, 6th ed.; McGraw-Hill Education: New York, NY, USA, 2014; Available online: https://www.accessengineeringlibrary.com/content/book/9780071821018 (accessed on 1 April 2023).
- Speck, J.S.; Pompe, W. Domain configurations due to multiple misfit relaxation mechanisms in epitaxial ferroelectric thin films. I. Theory. J. Appl. Phys. 1994, 76, 466–476. [Google Scholar] [CrossRef]
- Speck, J.S.; Seifert, A.; Pompe, W.; Ramesh, R. Domain configurations due to multiple misfit relaxation mechanisms in epitaxial ferroelectric thin films. II. Experimental verification and implications. J. Appl. Phys. 1994, 76, 477–483. [Google Scholar] [CrossRef]
- Kwak, B.S.; Erbil, A.; Budai, D.J.; Chisholm, M.F.; Boatner, L.A.; Wilkens, B.J. Domain formation and strain relaxation in epitaxial ferroelectric heterostructures. Phys. Rev. B 1994, 49, 14865–14879. [Google Scholar] [CrossRef]
- Garg, A. Growth and Characterization of Epitaxial Oxide Thin Films; Cambridge University: Cambridge, UK, 2001. [Google Scholar]
- Smith, D.L. Thin-Film Deposition: Principles and Practice; McGraw-Hill: New York, NY, USA, 1995; p. 225. [Google Scholar]
- Greene, J.E. Epitaxial crystal growth by sputter deposition: Applications to semiconductors. Part 2. Crit. Rev. Solid State Mater. Sci. 1983, 11, 191. [Google Scholar] [CrossRef]
- Dey, S.K.; Alluri, P.V. PE-MOCVD of dielectric thin films: Challenges and opportunities. MRS Bull. 1996, 21, 44–48. [Google Scholar] [CrossRef]
- Tsuge, H.; Matsukara, N. In-situ low-temperature growth of YBa/sub2/Cu/sub3/O/sub7-x/films by reactive coevaporation. IEEE Trans. Magn. 1991, 27, 1009–1012. [Google Scholar] [CrossRef]
- Koster, G.; Huijben, M.; Rijnders, G. Epitaxial Growth of Complex Metal Oxides, 1st ed.; Elsevier: Boston, MA, USA, 2015. [Google Scholar] [CrossRef]
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings Science, Applications and Technology, 3rd ed.; William Andrew: Boston, MA, USA, 2009. [Google Scholar]
- Pai, Y.Y.; Tylan-Tyler, A.; Irvin, P.; Levy, J. Physics of SrTiO3-based heterostructures and nanostructures: A review. Rep. Prog. Phys. 2018, 81, 036503. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.L. Thin Film Phenomena, 1st ed.; McGraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Dawber, M. Sputtering techniques for epitaxial growth of complex oxides. In Epitaxial Growth of Complex Metal Oxides; Woodhead Publishing: Sawston, UK, 2015; pp. 31–45. [Google Scholar] [CrossRef]
- Swann, S. Magnetron sputtering. Phys. Technol. 1988, 19, 67–75. [Google Scholar] [CrossRef]
- Shiosaki, T.; Adachi, M.; Mochizuki, S.; Kawabata, A. Properties of sputter-deposited PbTiO3, Pb (Zr, Ti) O3, Pb2KNb5O15 films. Ferroelectrics 1985, 63, 227–234. [Google Scholar] [CrossRef]
- Okuyama, M.; Hamakawa, Y. PbTiO3 ferroelectric thin films and their pyroelectric application. Ferroelectrics 1991, 118, 261–278. [Google Scholar] [CrossRef]
- Ishida, M.; Matsunami, H.; Tanaka, T. Preparation and properties of ferroelectric PLZT thin films by rf sputtering. J. Appl. Phys. 1977, 48, 951–953. [Google Scholar] [CrossRef]
- Okuyama, M.; Matsui, Y.; Nakano, H.; Hamakawa, Y. PbTiO3 ferroelectric thin film gate fet for infrared detection. Ferroelectrics 1981, 33, 235–241. [Google Scholar] [CrossRef]
- Sreenivas, K.; Sayer, M.; Garrett, P. Properties of D.C. Magnetron-Sputtered Lead Zirconate Titanate Thin Film. Thin Solid Films 1989, 172, 251–267. [Google Scholar] [CrossRef]
- Ishibashi, K.; Hirata, K.; Hosokawa, N. Mass spectrometric ion analysis in the sputtering of oxide targets. J. Vac. Sci. Technol. Vac. Surf. Film. 1992, 10, 1718–1722. [Google Scholar] [CrossRef]
- Grace, J.M.; McDonald, D.B.; Reiten, M.T.; Olson, J.; Kampwirth, R.T.; Gray, K.E. Selective re-sputtering of bismuth in sputtered Bi-Sr-Ca-Cu-O films. J. Appl. Phys. 1991, 70, 3867–3875. [Google Scholar] [CrossRef]
- Faraz, T.; Knoops, H.C.M.; Verheijen, M.A.; Van Helvoirt, C.A.A.; Karwal, S.; Sharma, A.; Beladiya, V.; Szeghalmi, A.; Haus-mann, D.M.; Henri, J.; et al. Tuning Material Properties of Oxides and Nitrides by Substrate Biasing during Plasma-Enhanced Atomic Layer Deposition on Planar and 3D Substrate Topographies. ACS Appl. Mater. Interfaces 2018, 10, 13158–13180. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, M.; Ueda, T.; Hamakawa, Y. Preparation of Oriented PbTiO3 Ferroelectric Thin Films on Silicon. Jpn. J. Appl. Phys. 1985, 24, 619. [Google Scholar] [CrossRef]
- Xu, Y.; Mackenzie, J.D. Ferroelectric thin films prepared by sol-gel processing. Integr. Ferroelectr. 1992, 1, 17–42. [Google Scholar] [CrossRef]
- Jaber, B.; Remiens, D.; Thierry, B. Substrate temperature and target composition effects on PbTiO3 thin films produced in situ by sputtering. J. Appl. Phys. 1996, 79, 1182–1184. [Google Scholar] [CrossRef]
- Iijima, K.; Takayama, R.; Tomita, Y.; Ueda, I. Epitaxial growth and the crystallographic, dielectric, and pyroelectric properties of lanthanum-modified lead titanate thin films. J. Appl. Phys. 1986, 60, 2914–2919. [Google Scholar] [CrossRef]
- Jaber, B.; Dogheche, E.; Rèmiens, D.; Thierry, B. Influence of deposition parameters on physico-chemical and optical properties of sputtered PbTiO3 thin films. Integr. Ferroelectr. 1996, 13, 225–237. [Google Scholar] [CrossRef]
- Kim, S.; Kang, Y.; Baik, S. Growth of PbTiO3 thin films by r.f. sputtering on vicinal MgO(100) substrates. Thin Solid Films 1995, 256, 240–246. [Google Scholar] [CrossRef]
- Kim, S.; Kang, Y.; Baik, S. Sputter deposition of ferroelectric PbTiO3 thin films. Ferroelectrics 1994, 152, 1–6. [Google Scholar] [CrossRef]
- Takayama, R.; Tomita, Y.; Iijima, K.; Ueda, I. Pyroelectric linear array infrared sensors made of c-axis-oriented La-modified PbTiO3 thin films. J. Appl. Phys. 1988, 63, 5868–5872. [Google Scholar] [CrossRef]
- Wasa, K.; Adachi, H.; Kitabatake, M. Sputtering deposition process of perovskite Pb-Ti-O3 families. Ferroelectrics 1992, 137, 343–356. [Google Scholar] [CrossRef]
- Pignolet, A.; Schmid, P.E.; Wang, L.; Levy, F. Structure and electrical properties of sputtered lead titanate thin films. J. Phys. Appl. Phys. 1991, 24, 619–621. [Google Scholar] [CrossRef]
- Adachi, M.; Matsuzaki, T.; Yamada, T.; Shiosaki, T.; Kawabata, A. Sputter-Deposition of [111]-Axis Oriented Rhombohedral PZT Films and Their Dielectric, Ferroelectric and Pyroelectric Properties. Jpn. J. Appl. Phys. 1987, 26 Pt 1, 550–553. [Google Scholar] [CrossRef]
- Nam, S.M.; Tsurumi, T. In Situ Epitaxial Growth of Lead Zirconate Titanate Films by Bias Sputtering at High RF Power. Jpn. J. Appl. Phys. 2004, 43, 2672–2676. [Google Scholar] [CrossRef]
- Adachi, H.; Mitsuyu, T.; Yamazaki, O.; Wasa, K. Preparation of (Pb, La) TiO3-PbTiO3 Thin Films with Superlattice Structure. Jpn. J. Appl. Phys. 1987, 26, 15. [Google Scholar] [CrossRef]
- Schlom, D.G.; Marshall, A.F.; Sizemore, J.T.; Chen, Z.J.; Eckstein, J.N.; Bozovic, I.; Von Dessonneck, K.E.; Harris, J.S., Jr.; Bravman, J.C. Molecular beam epitaxial growth of layered Bi-Sr-Ca-Cu-O compounds. J. Cryst. Growth 1990, 102, 361–375. [Google Scholar] [CrossRef]
- Dogheche, E.; Jaber, B.; Rémiens, D. Optical waveguiding in epitaxial PbTiO3 thin films. Appl. Opt. 1998, 37, 4245–4248. [Google Scholar] [CrossRef]
- Velu, G.; Remiens, G. In situ deposition of sputtered PZT films: Control of the growth temperature by the sputtered lead flux. Vacuum 2000, 56, 199–204. [Google Scholar] [CrossRef]
- Rossnagel, S. Chapter 8: Sputtering and Sputter Deposition. In Handbook of Thin Film Deposition Processes and Technologies, 2nd ed.; Seshan, K., Ed.; Noyes Publications/William Andrew Publishing: Norwich, NY, USA, 2001; pp. 323–324. [Google Scholar]
- Mitsuyu, T.; Wasa, K.; Hayakawa, S. Rf-sputtered epitaxial films of Bi12TiO20 on Bi12GeO20 for optical waveguiding. J. Cryst. Growth 1977, 41, 151–156. [Google Scholar] [CrossRef]
- Matsui, Y.; Okuyama, M.; Hamakawa, Y. Preparation of PbTiO3 Ferroelectric Thin Film by Laser Annealing. Jpn. J. Appl. Phys. 1981, 20, 23. [Google Scholar] [CrossRef]
- Wang, L.; Pignolet, A.; Levy, F. Properties of Pb (ZrxTi1−x) O3 thin films prepared by r.f. magnetron sputtering and heat treatment. Mater. Res. Bull. 1990, 25, 1495–1501. [Google Scholar] [CrossRef]
- Thomas, R.; Mochizuki, S.; Mihara, T.; Ishida, T. Influence of Sputtering and Annealing Conditions on the Structure and Ferroelectric Properties of Pb (Zr, Ti) O3 Thin Films Prepared by RF Magnetron Sputtering. Jpn. J. Appl. Phys. 2001, 40 Pt 1, 5511–5517. [Google Scholar] [CrossRef]
- Shimizu, T.; Yokouchi, T.; Shiraishi, T.; Oikawa, T.; Krishnan, P.S.R.; Funakubo, H. Study on the effect of heat treatment conditions on metalorganic-chemical-vapor-deposited ferroelectric Hf0.5Zr0.5O2 thin film on Ir electrode. Jpn. J. Appl. Phys. 2014, 53, 09PA04. [Google Scholar] [CrossRef]
- Doremus, R.H. Rates of Phase Transformations, 1st ed.; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Jaffe, B.; Cook, W.R., Jr.; Jaffe, H. Piezoelectric Ceramics, 1st ed.; Academic Press: London, UK; New York, NY, USA, 1971. [Google Scholar]
- Coufal, H.J.; Grygier, R.K.; Horne, D.E.; Fromm, J.E. Pyro-electric calorimeter for photothermal studies of thin films and adsorbates. J. Vac. Sci. Technol. Vac. Surf. Film. 1987, 5, 2875–2889. [Google Scholar] [CrossRef]
- Shirane, G.; Hoshino, S. On the Phase Transition in Lead Titanate. J. Phys. Soc. Jpn. 1951, 6, 265–270. [Google Scholar] [CrossRef]
- Jangade, P.; Arjunwadkar, P.R.; Nagarbawadi, M.A. Structural characterization of lead titanate (PbTiO3) sample using FULLPROF. IOSR J. Appl. Phys. 2016, 8, 57–60. [Google Scholar] [CrossRef]
- “MgAl2O4”. Available online: https://www.msesupplies.com/products/magnesium-aluminate-mgal2o4-crystal?variant=22603198365754 (accessed on 21 September 2020).
- Erbil, A.; Kim, Y.; Gerhardt, R.A. Giant permittivity in epitaxial ferroelectric heterostructures. Phys. Rev. Lett. 1996, 77, 1628. [Google Scholar] [CrossRef]
- Sando, D. Strain and orientation engineering in ABO3 perovskite oxide thin films. J. Phys. Condens. Matter 2022, 34, 153001. [Google Scholar] [CrossRef]
- Ogawa, T.; Senda, A.S.; Kasanami, T.K. Controlling the crystal orientations of lead titanate thin films. Jpn. J. Appl. Phys. 1991, 30, 2145. [Google Scholar] [CrossRef]
- Kushida, K.; Takeuchi, H. Piezoelectricity of c-axis oriented PbTiO3 thin films. Appl. Phys. Lett. 1987, 50, 1800–1801. [Google Scholar] [CrossRef]
- Hayashi, S.; Iijima, K.; Hirao, T. Preparation of Pb-Based Ferroelectric Thin Films by Ion-and Photo-Assisted Deposition. MRS Online Proc. Libr. 1991, 243, 155–166. [Google Scholar] [CrossRef]
- Neifeld, R.A.; Hansen, A.; Pfeffer, R.; Potenziani, E.; Wilbur, W.; Basarab, D.; Lareau, R.; Shappirio, J.; Wrenn, C.; Calderon, L.; et al. Preparation of High Temperature Superconductor Thin Films on Si Based Substrates by Excimer Laser Ablation, Film-Substrate Interactions, and Plasma Processing Effects on Crystallinity. In Science and Technology of Thin Film Superconductors, 1st ed.; McConnell, R., Wolf, S., Eds.; Plenum Press: New York, NY, USA, 1989; pp. 45–51. [Google Scholar]
- Ramesh, R.; Aggarwal, S.; Auciello, O. Science and technology of ferroelectric films and heterostructures for non-volatile ferroelectric memories. Mater. Sci. Eng. R Rep. 2001, 32, 191–236. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Matsunaga, T.; Hassan, M.; Kanno, I.; Suzuki, T.; Wasa, K. Growth and structure of heteroepitaxial lead titanate thin films constrained by miscut strontium titanate substrates. J. Mater. Res. 2006, 21, 1261–1268. [Google Scholar] [CrossRef]
- Torii, K.; Kaga, T.; Kushida, K.; Takeuchi, H.; Takeda, E. Single-Target Sputtering Process for Lead Zirconate Titanate Thin Films with Precise Composition Control. Jpn. J. Appl. Phys. 1991, 30 Pt 1, 3562–3666. [Google Scholar] [CrossRef]
- Budiman, R.A.; Ruda, H.E. Relaxation model of coherent island formation in heteroepitaxial thin films. J. Appl. Phys. 2000, 88, 4586–4594. [Google Scholar] [CrossRef]


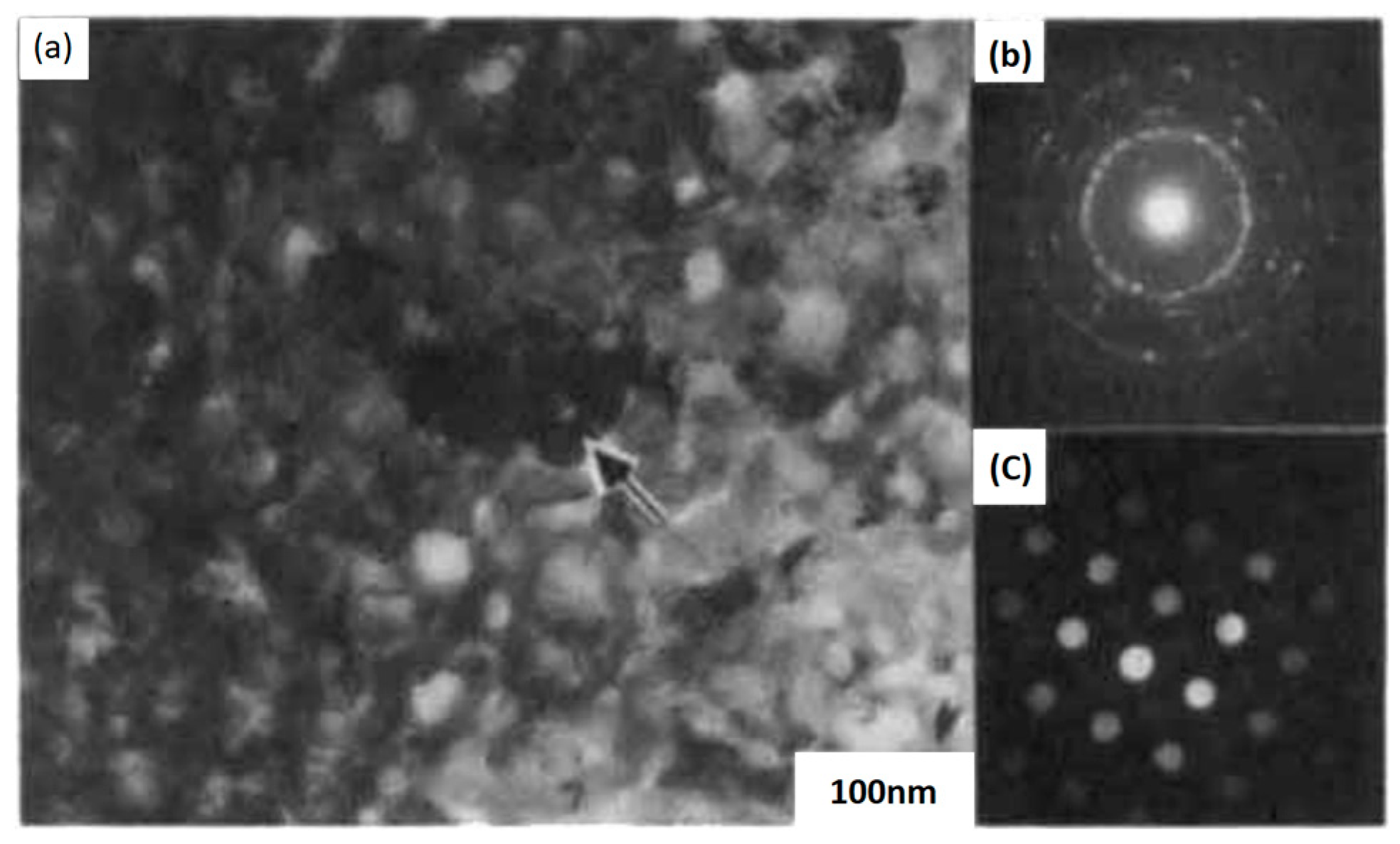
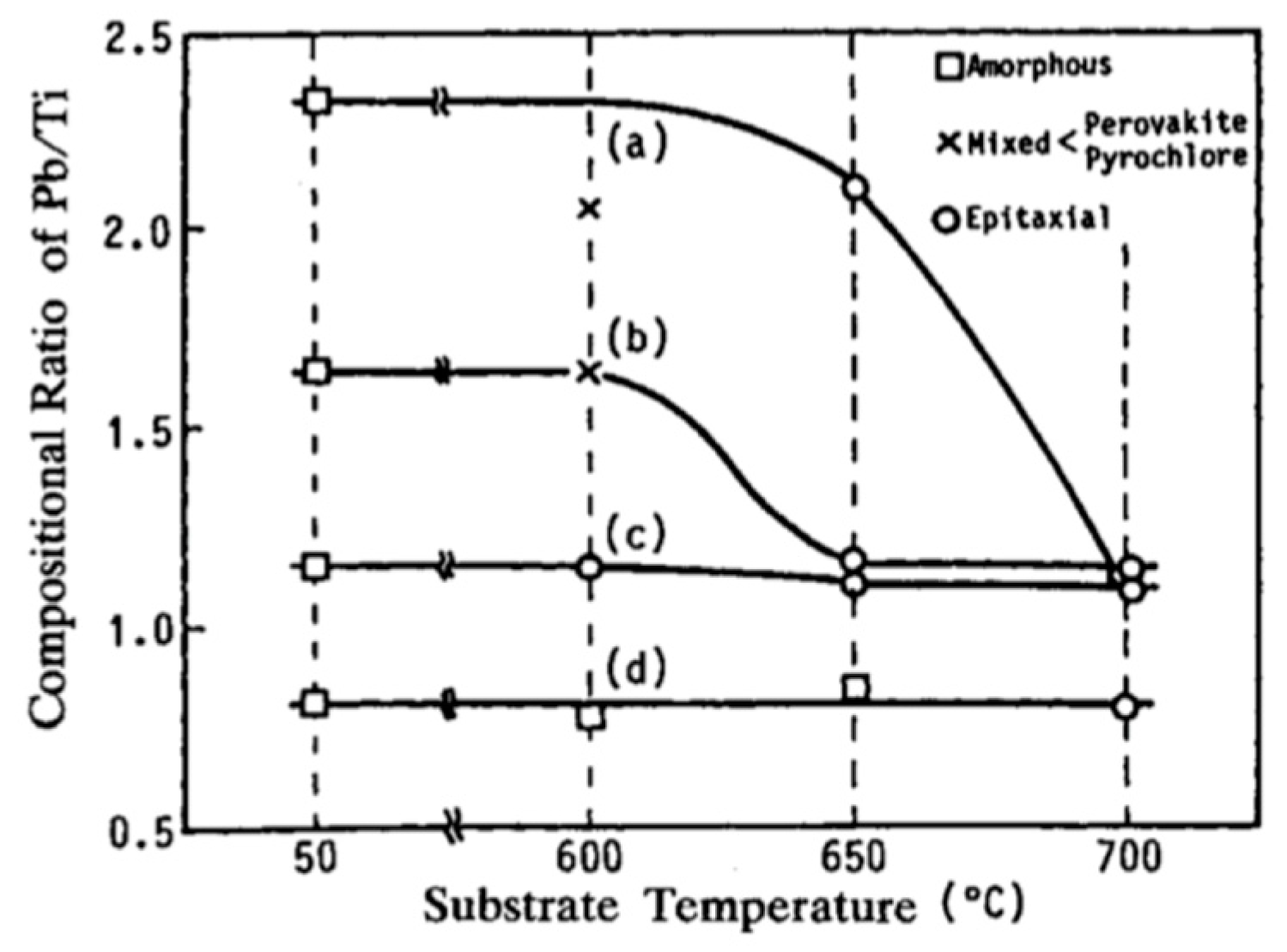
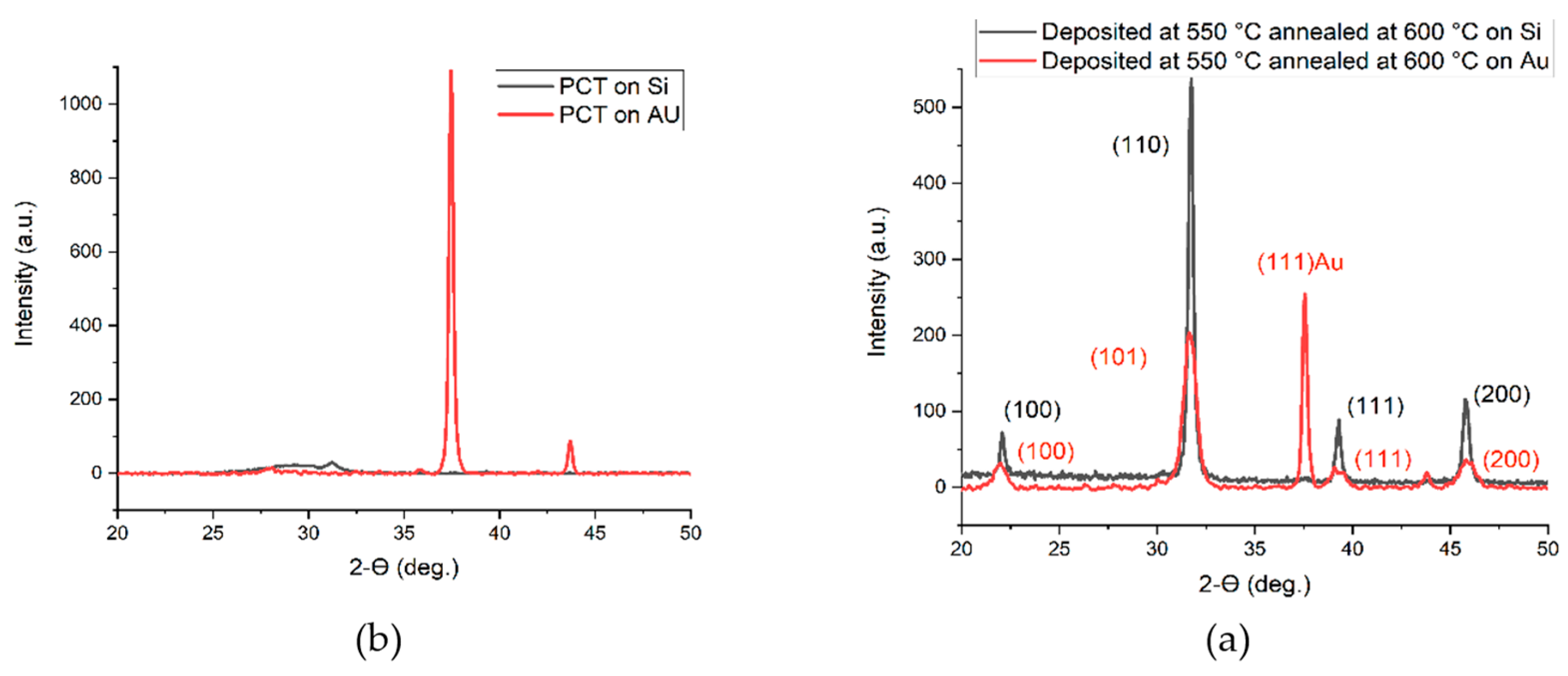

| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Target Diameter, in inch or mm [Target Composition (Form) + wt% or mol.%PbO] | Sputter-Deposition Processing Parameters | Substrate Material (Orientation) (Dst, mm) | Reference | ||||||
| Gas Mixture | P | Ts | RF (DC) Power Density or Power | ||||||
| (%) | (%) | °C | |||||||
| [Pb0.85 La0.15 Ti0.96O3 + 20 mol.% pbO](P) | 90 10 | 0–90 | 0.5 Pa | 425–475 | 4–9 | RF 1.3 W/cm2 | [3] | ||
| 75 mm [Pbl − xLa xTi 1−x/4O3 + 10 mol.% PbO] (p) . | 90 | 10 | 1 Pa | 600 | 2.5–3 | 2 W/cm2 | Pt/MgO or MgO (80 mm) | [17] | |
| [PT + 5 wt% PbO] (p) | 100 50 | 0 50 | 12–24 mTorr | 300–650 | N/A | RF 2–4 W/cm2 | (70 mm) | [59] | |
| [PT + 5 wt% PbO] (p) | 90 | 10 | 12–24 mTorr | 300 | N/A | RF 2–4 W/cm2 | MgO (70 mm) | [59] | |
| [PT + 5 wt% PbO] (p) | 90 | 10 | 12–24 mTorr | NA | N/A | RF 2–4 W/cm2 | Poly-Pt (70 mm) | [59] | |
| 80 mm [PT + 20 wt% PbO] (p) | 90 | 10 | 27 Pa | 350–500 | 3–4 | RF 3 W/cm2 | -Pt sheet-Si(wafer) Pt/Si(wafer)-Pt/mica (35–40 mm) | [49] | |
| 80 mm [(TiO2 + 10 W% excess Pb3O4] (p) | 90 | 10 | 200 mTorr | 350–550 | 3 | 150 W | -Pt/si(wafer)-Pt/mica (35–40 mm) | [49] | |
| 1′ [PbO + TiO2] (P) | 100 | 0 | 50–100 mTorr | 550–600 | N/A | RF 2.6 W/cm2 | Al2O3 (0001) (35 mm) | [56] | |
| 0.8PbO + 1TiO2 | N/A | N/A | N/A | 600–650 | N/A | N/A | SrTiO3 | [57] | |
| 0.54PbO + 1TiO2 | N/A | N/A | N/A | 550–600 | N/A | N/A | SrTiO3 | [57] | |
| 1PbO + 1TiO2 | N/A | N/A | N/A | 650–680 | N/A | N/A | SrTiO3 | [57] | |
| 75 mm [Pb1−x Lax Ti1−x/4 O3 + 10 mol.% PbO] (P) | 50 | 50 | 1 Pa | 600 | 1–2 | 2 W/cm2 | MgO coated with (100) Pt (80 mm) | [57] | |
| 75 mm [Pb1−x Lax Tix/4 O3 + 10 mol.% PbO] (p) | 90 | 10 | 1 Pa | 550–600 | 1–2 | RF 0.9–1.4 W/cm2 | MgO/(100) Pt (80 mm) | [57] | |
| 1′ [xPbO + TiO2] (p) | 100 | 0 | 50–100 mTorr | 550–600 | N/A | RF 2.6 W/cm2 | Al2O3 (0001) (35 mm) | [58] | |
| [PbTiO3 + 5 wt% PbO] (p) | 100 50 | 0 50 | 12–24 mTorr | 600 | 1.5 | 2–4 W/cm2 | (100) MgO (70 mm) | [59] | |
| [PT + 5 wt% PbO] (p) | 90 | 10 | 12–24 mTorr | 500–650 | N/A | 2–4 W/cm2 | MgO (70) | [60] | |
| [Pb (Zr0.9 Ti0.10 O3) + 20 wt% PbO] (p) | 80 | 20 | 20 mTorr | N/A | N/A | RF 150 W | [111] Pt | [60] | |
| Pb0.9 La0.1 Ti0.975 O3 | 90 | 10 | 1 Pa | 600 | 3–4 | 2 W/cm2 | (100) Pt/MgO (80 mm) | [61] | |
| Pb-Ti Composite | 50 | 50 | 0.6 mTorr | 150–300 | 3–60 | N/A | NA | [61] | |
| Perovskite compounds, metal composite, multi-target | 50 | 50 | 5 mTorr | Liq.N2 –700 | 5–7 | DC RF | Glass, Sapphire, MgO, SrTiO3 | [62] | |
| 90 mm [PT] (P) | 100 | 0 | 1 Pa | 500–650 | 4–6 | 3 W/cm2 | mm (100) Si or Pt on (100) Si or Si3Ti5 on (100) Si (60 mm) | [63] | |
| 80 mm [PT+ excess Pb3O4] | 90 | 10 | 27 Pa | 350–550 | 3–4 | 150 W | -Pt sheet-Si(wafer) -Pt/Si(wafer)-Pt/mica (35–40 mm) | [63] | |
| 100 mm [PT] (P, Sintered, 99.9%) | 90 | 10 | 4 Pa | 630 | N/A | 75 W | patterned Pt on SrTiO3 | [63] | |
| 3′ [PbLaZrTiO3] | 10 | 4 | 0.53 Pa | 476 | 60 | Pb: 0.69 W/cm2 Ti: 3.67 W/cm2 | Pt/Ti/SiO2/Si | [63] | |
| [Pb (Zr0.9Ti0.1) O3 + 20 wt% PbO] (P) | 80 | 20 | 20 mTorr | 610 | N/A | 150 W | (111) Pt | [64] | |
| Pbx (Zr0.4Ti0.6) O3 Ceramics: Composite ceramics: | 80 | 20 | 20 mTorr | 620 | 5 | 100 W, 200 W RF | (001) MgO single crystal (30) | [65] | |
| 60 mm [(Pb, Ti) wafers] | 67 | 33 | 3 Pa | 700 | N/A | DC Pb: 0.32 W/cm2 Ti: 6.37 W/cm2 | (100 mm) | [66] | |
| 100 mm [PbTiO3] (P) | N/A | N/A | N/A | 600 | 3–5 | N/A | (001) ST, miscut: 0.9°–10° (30 mm) | [67] | |
| (b) | |||||||||
| Notes | Reference | ||||||||
| Subs Pt/Ti/Glass, Target was Pb (Zr, Ti) O3 ceramic disk (dia mm), pre-sputtering duration min | [7] | ||||||||
| It used HVAC, base pressure = 10−6 Torr; substrate: cleaved MgO () annealed at 1200 for , in air. | [14] | ||||||||
| () Pt electrodes ( μm thickness) deposited on the () cleaved and polished. MgO single crystals, or on the () cleaved and polished MgO substrates. | [17] | ||||||||
| The target pressed on a quartz plate. | [49] | ||||||||
| Sputtering target consisted of cold-pressed mixed oxide powders of PbO and TiO2 (purity ) with composition: PbO TiO2, where and represent the mole concentration of PbO and TiO2, respectively. | [56] | ||||||||
| Subs Pt/Ti/SiO2/Si 100 mTorr, ambient, water-cooled, substrate Al2O3 [0001] at 700 °C. | [57] | ||||||||
| It used the bias sputtering. | [68] | ||||||||
| It used the UHV system with a base pressure of 10−6 Torr, () Cleaved MgO crystals were annealed for 3 h at 1200 C in air. Introducing O2 to processing gas reduced deposition rate. Optimum O2 content for stoichiometric PT is approximately . Amorphous films were obtained for the substrate temperature below 400 °C. Perovskite films were obtained above 500 °C. The epitaxial films that were highly axis oriented along the substrate surface normal were obtained above 600 °C. Their epitaxial relation was PbTiO3 ()/MgO (). | [60] | ||||||||
| Processing parameters for correct stoichiometry. | [63] | ||||||||
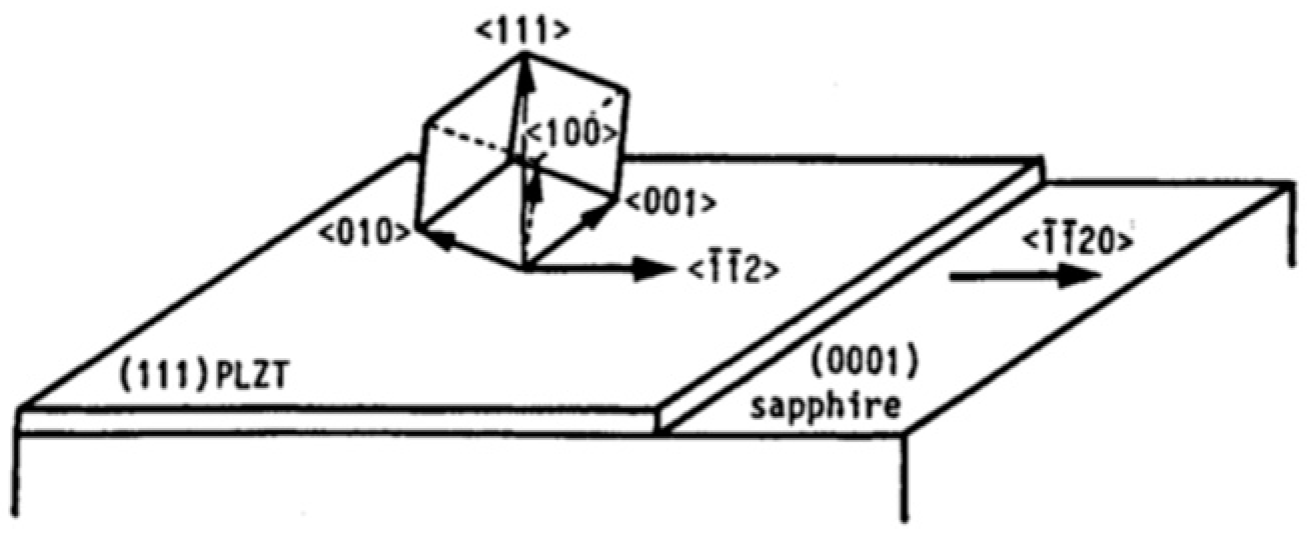
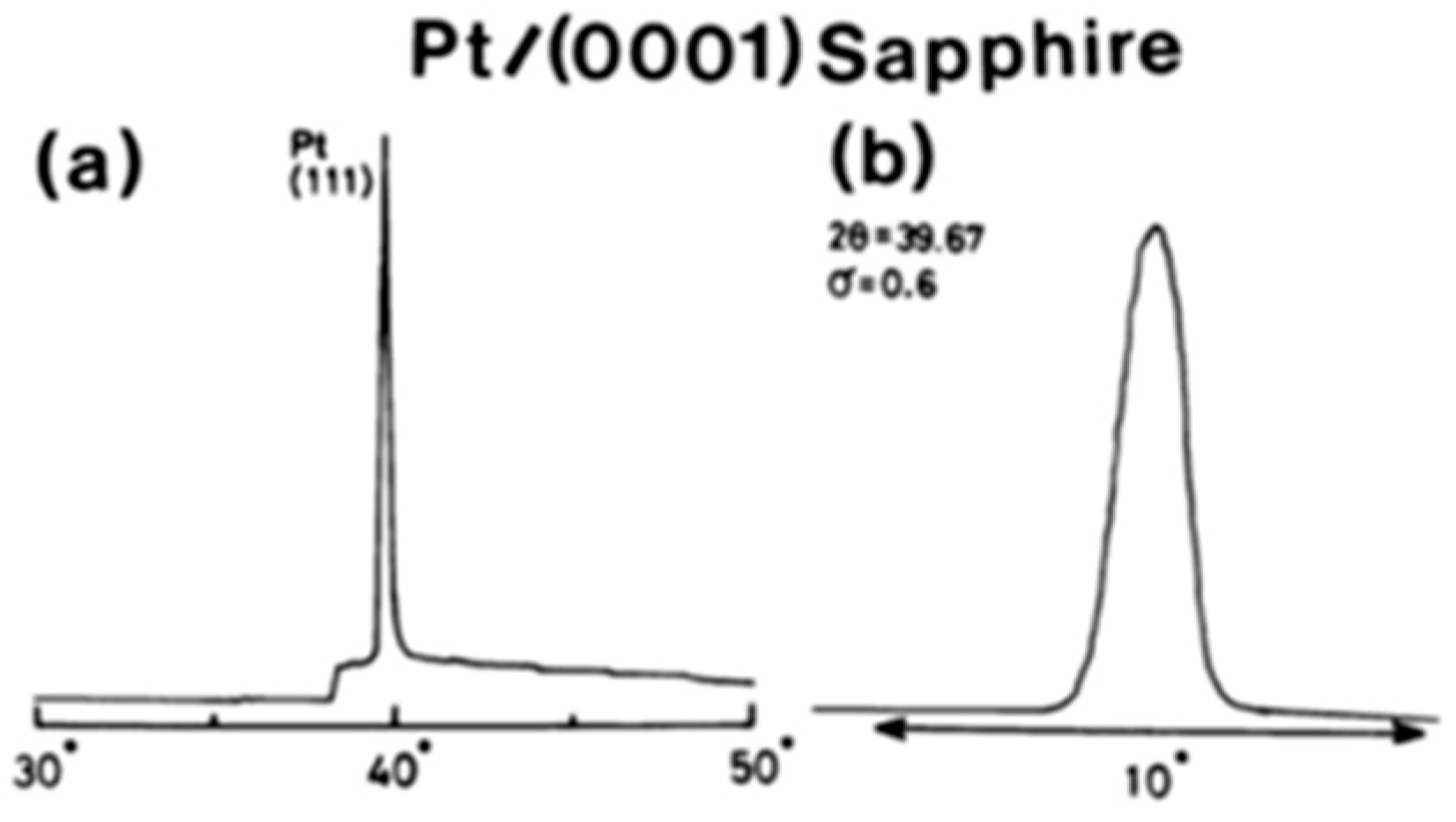
| To prepare [111]-oriented epitaxial PZT films, [111] oriented Pt films with a thickness of about μm were deposited epitaxially on () sapphire by RF magnetron sputtering at 450 °C. | [64] | ||||||||
| Deposition time was 30 min. | [65] | ||||||||
| It used the multi-target sputtering technique. | [66] | ||||||||
| The situ effects of platinized silicon substrates on the properties of PZT thin films by magnetron sputtering. | [69] | ||||||||
| Used cooling rate 100°/min, in air. | [67] | ||||||||
| Used bias voltage to substrate: , , , , , , . | [40] | ||||||||
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Target Diameter, in inch or mm [Target Composition (Form) + w% or mol.%PbO] | Sputter-Deposition Processing Parameters | Substrate Material & (SUBSTRATE to Target Distance) | Reference | |||||
| Gas Mixture (Ratio or %) | Pressure | Ts | RF (DC) Pw (W) or Pwd (W/cm2) | |||||
| (%) | (%) | or | (°C) | |||||
| 2.75′ [PbZr0.52Ti0.48 O3] 0.9(P) + [PbO] 0.1(P) | 9 | 1 | 2.7 Pa | 250 | 2.66 | 40 W | Pt/Ti/SiO2/Si (N/A) | [21] |
| 90 mm [PT + 10 w%? PbO] (P) | 100 | 0 | 0.8–1 Pa | N/A | 3–10 | 100–150 W | Pt/Si (60 mm) | [14] |
| 90 mm [PbZrO3 (P) + 10 w% PbO (P)] | 100 | 0 | N/A | N/A | N/A | N/A | N/A | [14] |
| [PT + 5 w% PbO] (P) | 100 50 | 50 50 | 12–24 mTorr | 300 | N/A | 2–4 W/cm2 | Pt (70 mm) | [60] |
| [Pb3O4 + TiO2] (P) | 90 | 10 | 200 mTorr | 350–450 | N/A | N/A | 3 3 mm Si, SiO2/Si, Pt/SiO2/Si | [72] |
| 90 mm [PbZrO3, PT mixture + 10 w% PbO] (P), near MPB PbZr0.53 Ti0.47O3 | 100 | N/A | 0.6–0.8 | N/A | 8–10 | 100–150 W | Synthetic Al2O3 or (100) Si covered with (111) Pt | [73] |
| (b) | ||||||||
| Target Diameter, inor [Target Composition (Form) + w% or mol.%PbO] | Post Annealing | Substrate Material & Substrate Target Distance (mm) | Reference | |||||
| Tpan () & Time (min) | Medium Gas (SCCM) | |||||||
| 2.75′ [PbZr0.52Ti0.48 O3] 0.9(P) + [PbO] 0.1(P) | 700 & 20 | Ar & O2 | Pt/Ti/SiO2/Si (50 mm) | [21] | ||||
| 90 mm [PT + 10 w%PbO] (P) | 800–820 | O2 | Pt/Si (60 mm) | [14] | ||||
| 90 mm [PbZrO3 (P) + 10 w% PbO (P)] | 600–650 | O2 | Pt/Si (60 mm) | [14] | ||||
| [PT + 5 w% PbO] (P) | 475 | N/A | MgO (70 mm) | [59] | ||||
| [PT + 5 w% PbO] (P) | 600–650 | N/A | Poly-Pt (70 mm) | [60] | ||||
| [Pb3O4 + TiO2] (P) | CW laser, 6 mm dia, 10–40 W, 1–10 s. | N/A | 3 3 mm Si, SiO2/Si, Pt/SiO2/Si | [72] | ||||
| 90 mm [PbZrO3, PT mixture + 10 w% PbO] (P), near MPB PbZr0.53 Ti0.47O3 | 600 | O2 (10) | Synthetic Al2O3 or (100) Si covered with (111) Pt | [73] | ||||
| 100 mm [Pb (Zr0.5 Ti0.5) O3] ceramic disc | 650 | O2 | Pt/Ti/corning 7059 (50 mm) | [74,75] | ||||
| Growth Dimension | Depletive Nucleation | Constant Nucleation | ||
|---|---|---|---|---|
| Interface | Diffusion | Interface | Diffusion | |
| 1-Dimensional | ||||
| 2-Dimensional | ||||
| 3-Dimensional | ||||
| Crystalline Material | Crystal System | Structure | Lattice Constant | Oxygen Distance | CTCE | Reference |
|---|---|---|---|---|---|---|
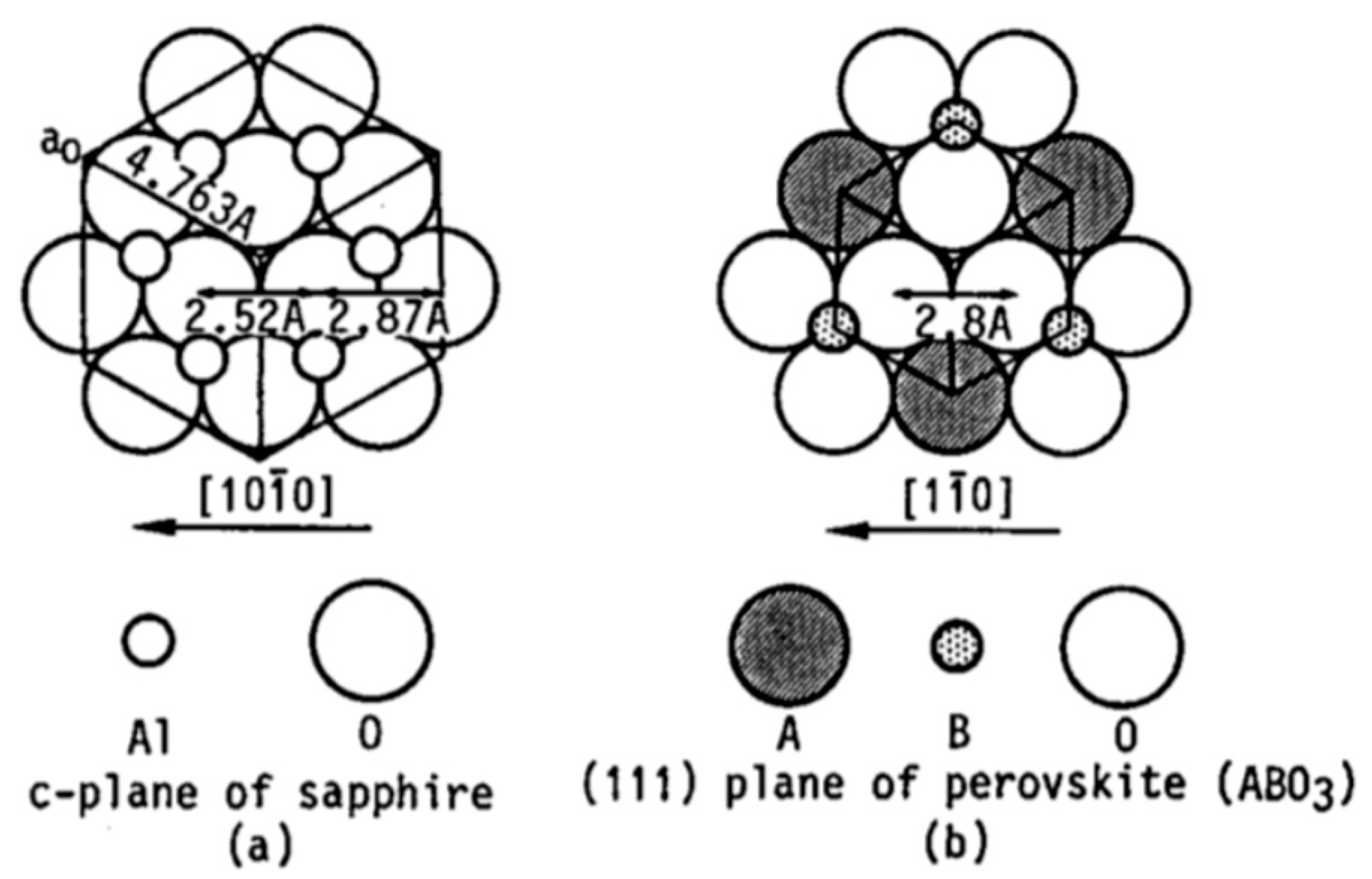
| Sapphire | Trigonal | Hexagonal | 2.75 | 4.5||to a-axis 5.3||to c-axis | [60,81] | |
| SrTi | Cubic | Perovskite | 2.76 | 9 | [81] | |
| PbTi | Tetragonal | Perovskite | 2.76 2.93 | 12 12 | [46,82] | |
| PbTi | Cubic | Perovskite | N/A | 12 | [46,82] | |
| PLZT | Cubic | Perovskite | N/A | N/A | [40,83] | |
| CaF2 | Cubic | NaCl | N/A | 18.85 | ||
| SrF2 | Cubic | NaCl | N/A | 18.4 | ||
| MgO | Cubic | NaCl | N/A | 13.8 | ||
| MgAl2 | Cubic | NA | 2.86 | 7.33 |
| Film Composition | ||
|---|---|---|
| PLZT (9/65/35) | 4.09 | 2.89 |
| PLZT (0/0/100):PT | 4.02 | 2.85 |
| PLZT (14/0/100) | 3.99 | 2.82 |
| PLZT (21/0/100) | 3.96 | 2.80 |
| PLZT (28/0/100) | 3.95 | 2.79 |
| PLZT (35/0/100) | 3.94 | 2.79 |
| PLZT (42/0/100) | 3.93 | 2.78 |
| Film/Buffer Layer | Substrate | Mismatch |
|---|---|---|
| PZT | Sapphire | 6.2 |
| Pt | Sapphire | 1.1 |
| PZT | Pt | 5 |
| Perovskite Thin Films | Temperature Relationship | Phase-Processing |
|---|---|---|
| Amorphous | Data | |
| Poly | ||
| Post-annealing | ||
| Single crystalline | (Vapor phase epitaxy) Post-annealing (Solid phase epitaxy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathipour, M.; Xu, Y.; Rana, M. Magnetron Sputtered Lead Titanates Thin Films for Pyroelectric Applications: Part 1: Epitaxial Growth, Material Characterization. Materials 2024, 17, 221. https://doi.org/10.3390/ma17010221
Fathipour M, Xu Y, Rana M. Magnetron Sputtered Lead Titanates Thin Films for Pyroelectric Applications: Part 1: Epitaxial Growth, Material Characterization. Materials. 2024; 17(1):221. https://doi.org/10.3390/ma17010221
Chicago/Turabian StyleFathipour, Morteza, Yanan Xu, and Mukti Rana. 2024. "Magnetron Sputtered Lead Titanates Thin Films for Pyroelectric Applications: Part 1: Epitaxial Growth, Material Characterization" Materials 17, no. 1: 221. https://doi.org/10.3390/ma17010221







