Investigation of the Chemical Composition, Microstructure, Density, Microhardness, and Elastic Modulus of the New β Ti-50Nb-xMo Alloys for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
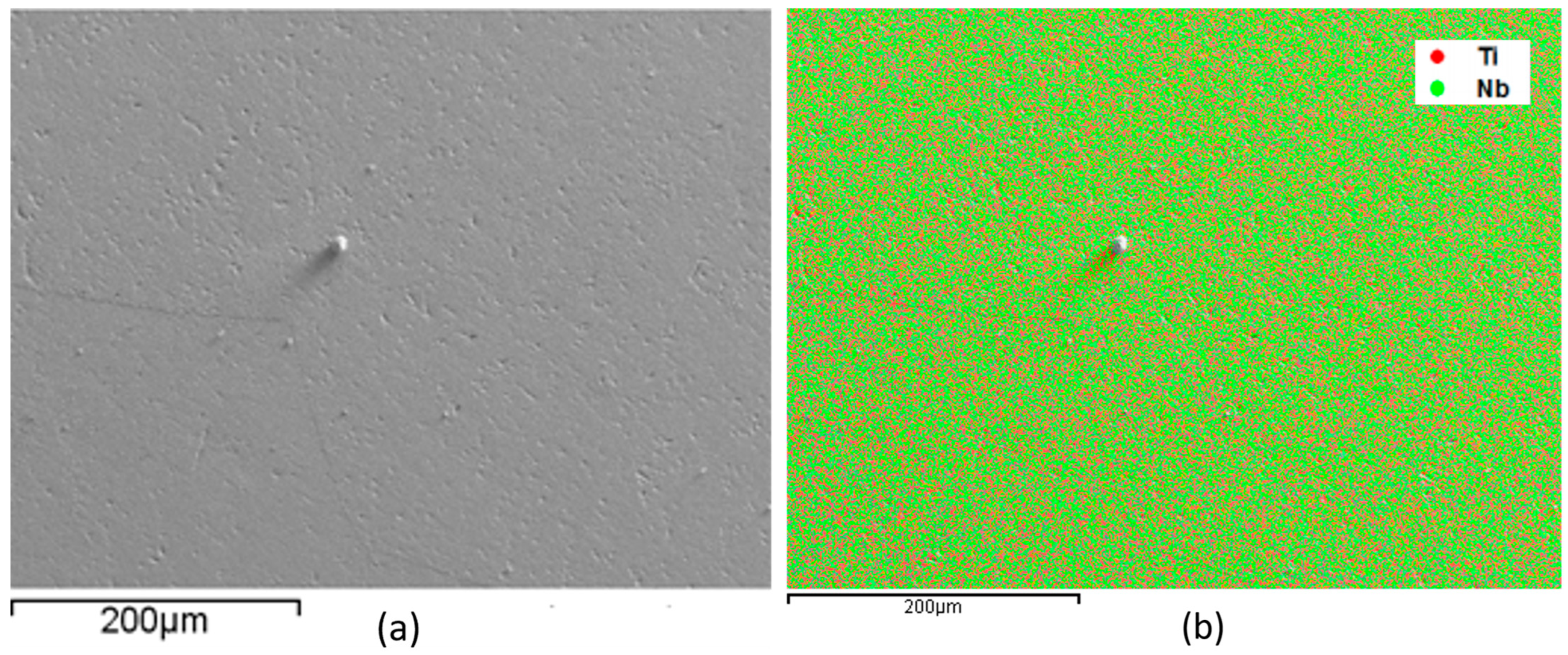
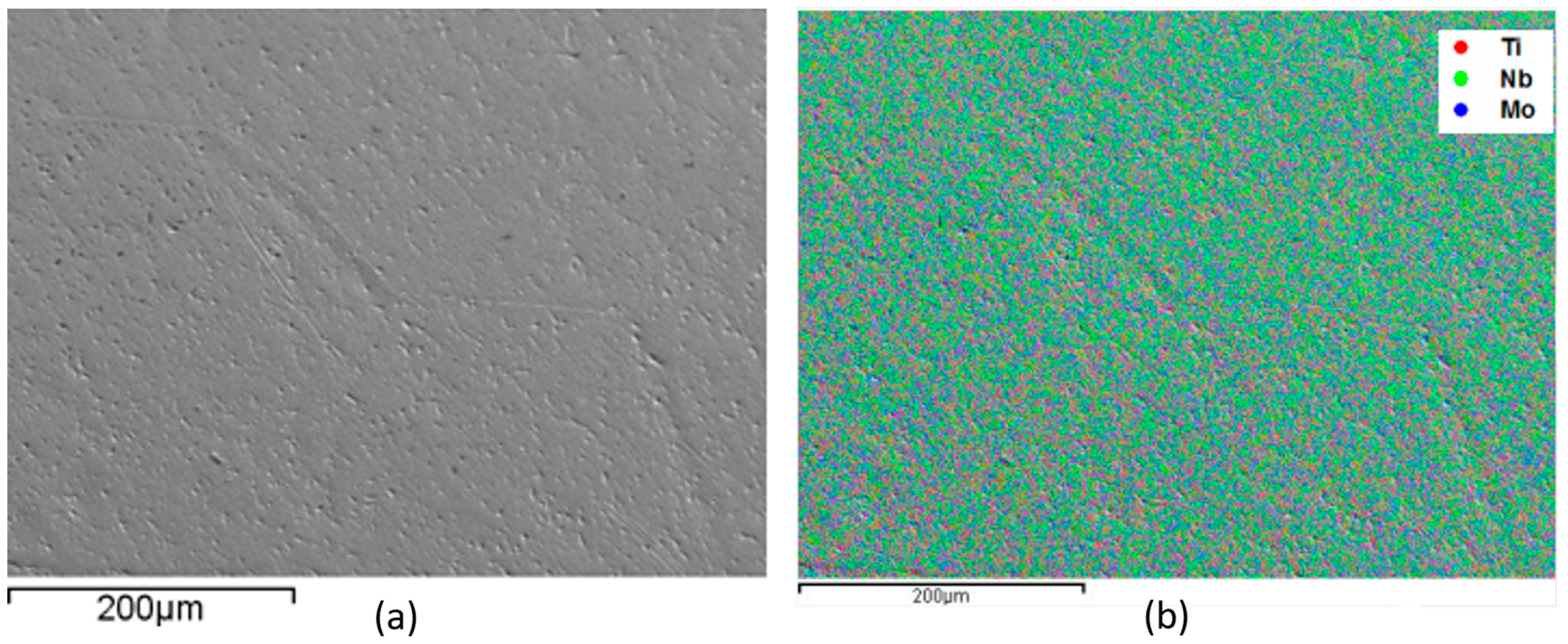
3. Results
4. Discussion
5. Conclusions
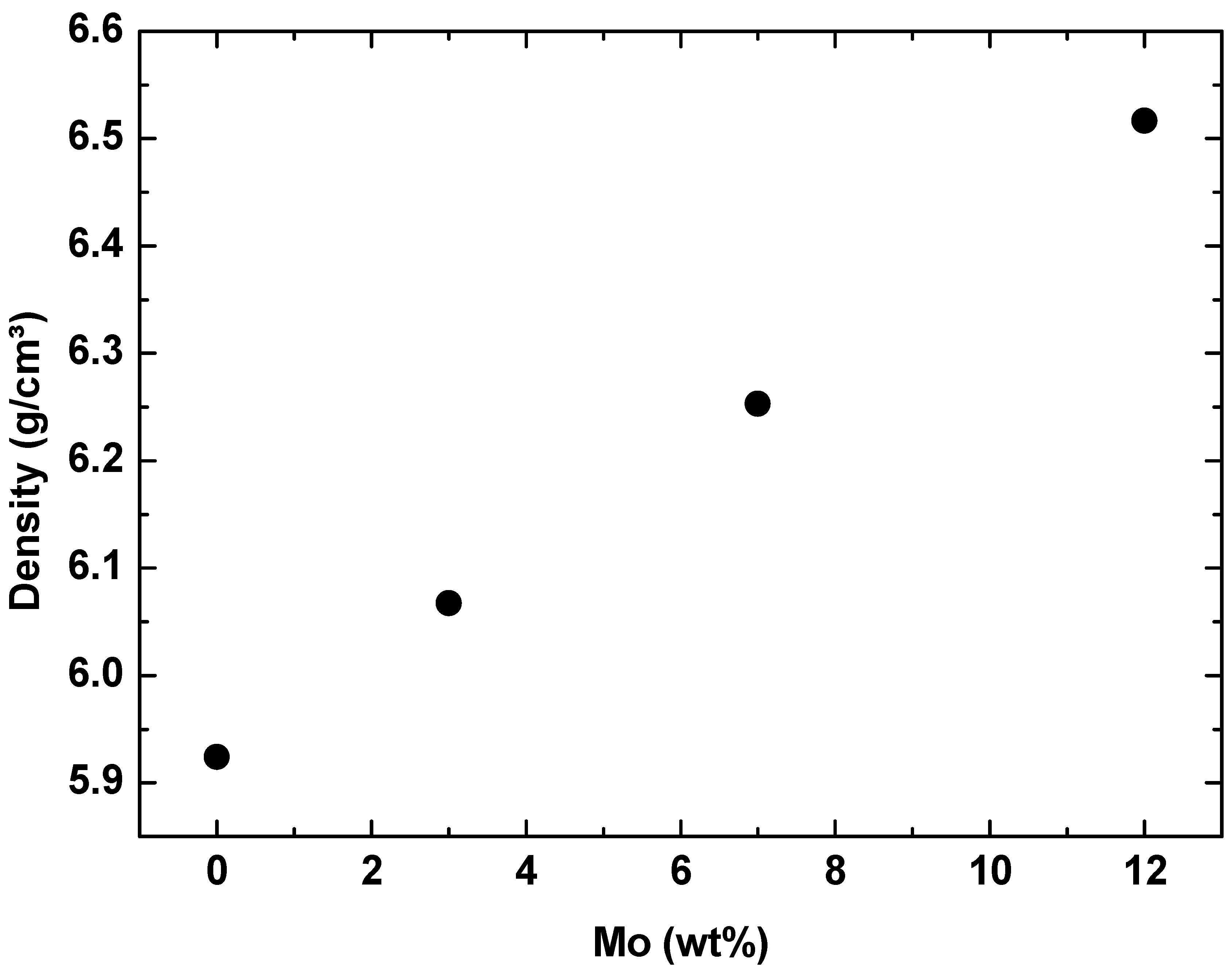
- The chemical composition results showed that the alloys produced in this study have good quality. In contrast, the experimental values obtained by the semi-quantitative EDS technique showed that the alloy elements (Ti, Nb, and Mo) have values close to the nominal ones. Furthermore, the density results showed that the alloys have suitable stoichiometry.
- The densities of Ti-50Nb-Mo alloys increase with the Mo content, with Mo having a higher density value than Ti.
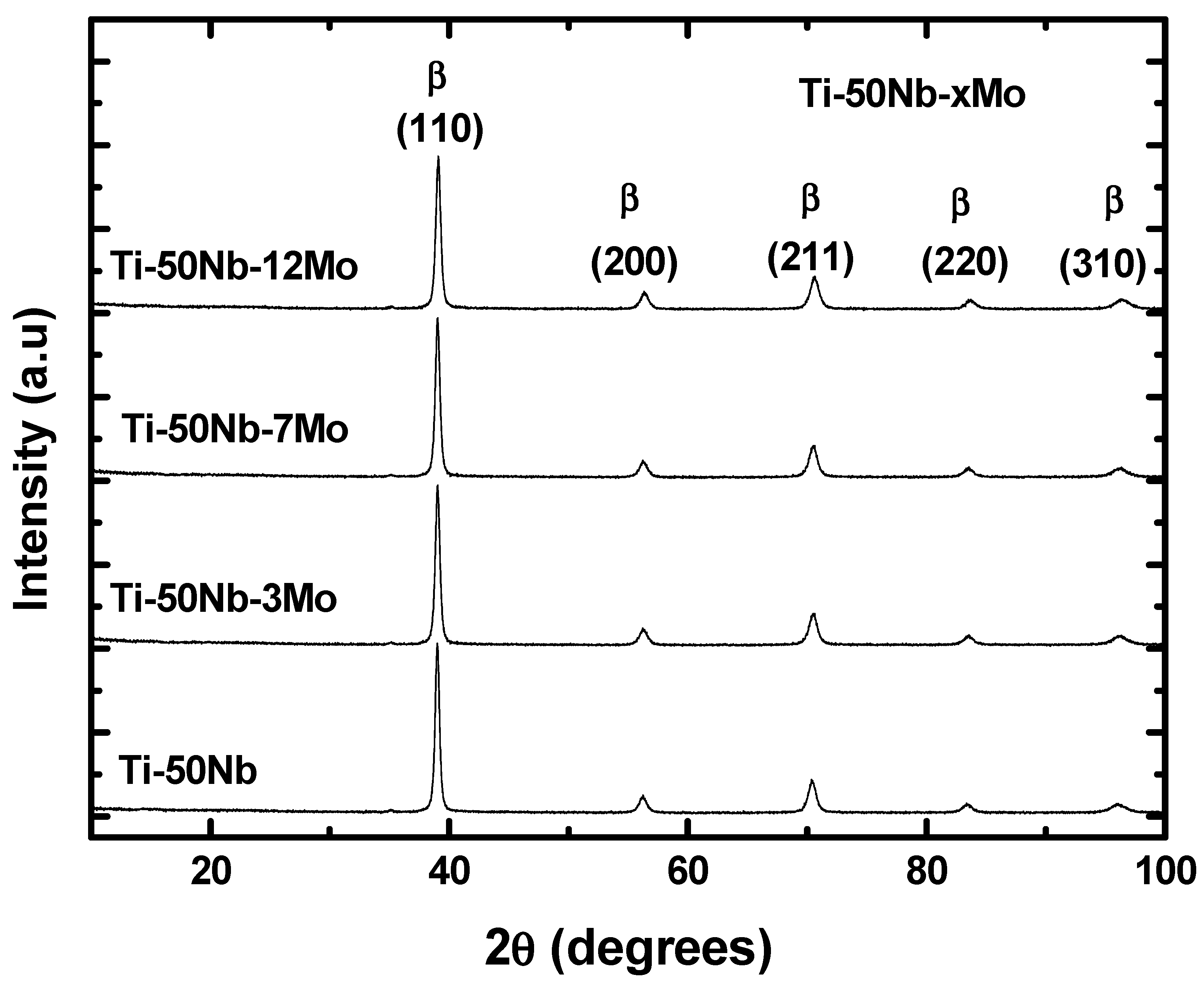
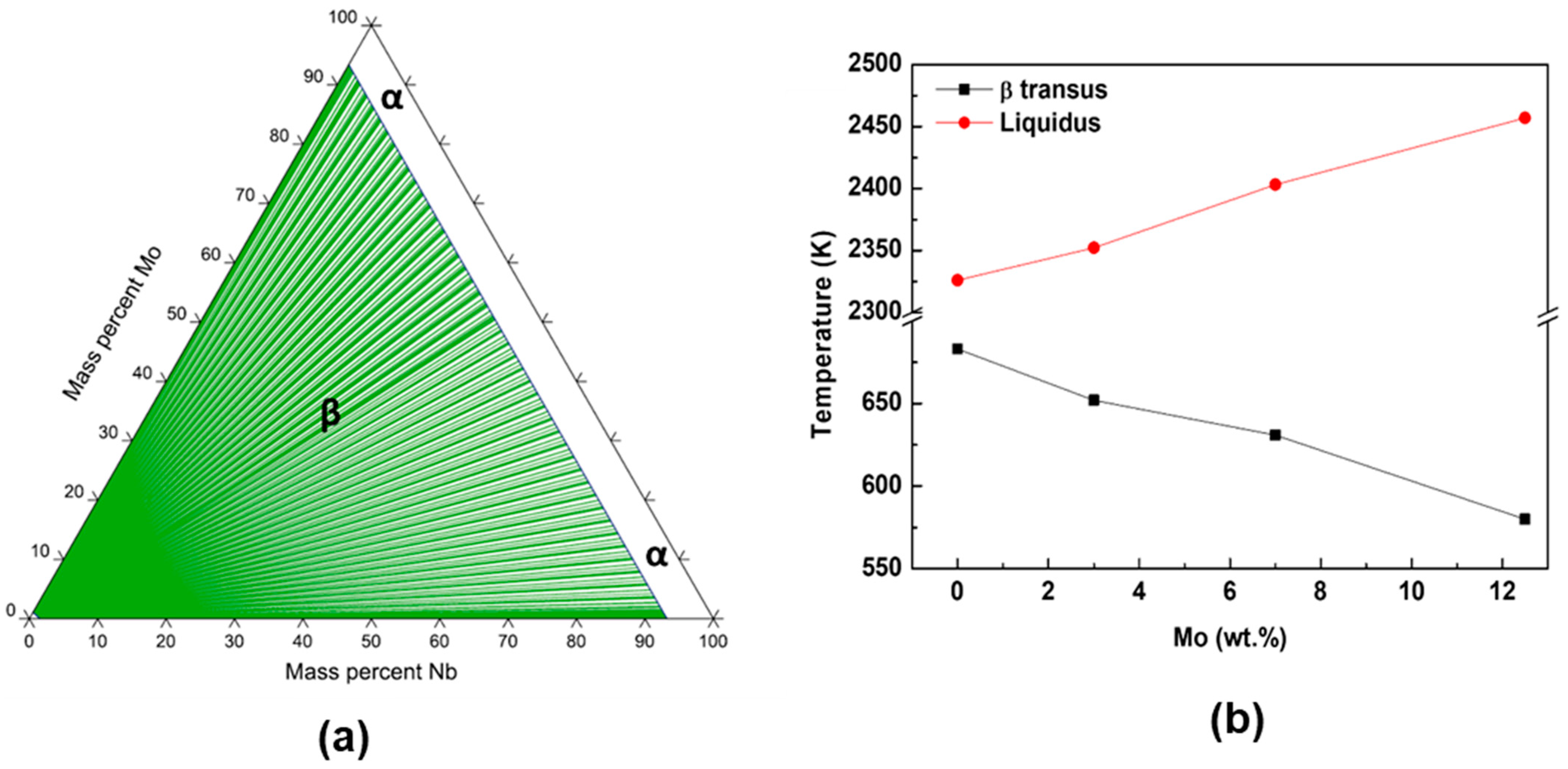
- All alloys have the body-centered cubic crystal structure, β, confirmed by X-ray diffraction, microscopy techniques, and thermo-calc simulation results.
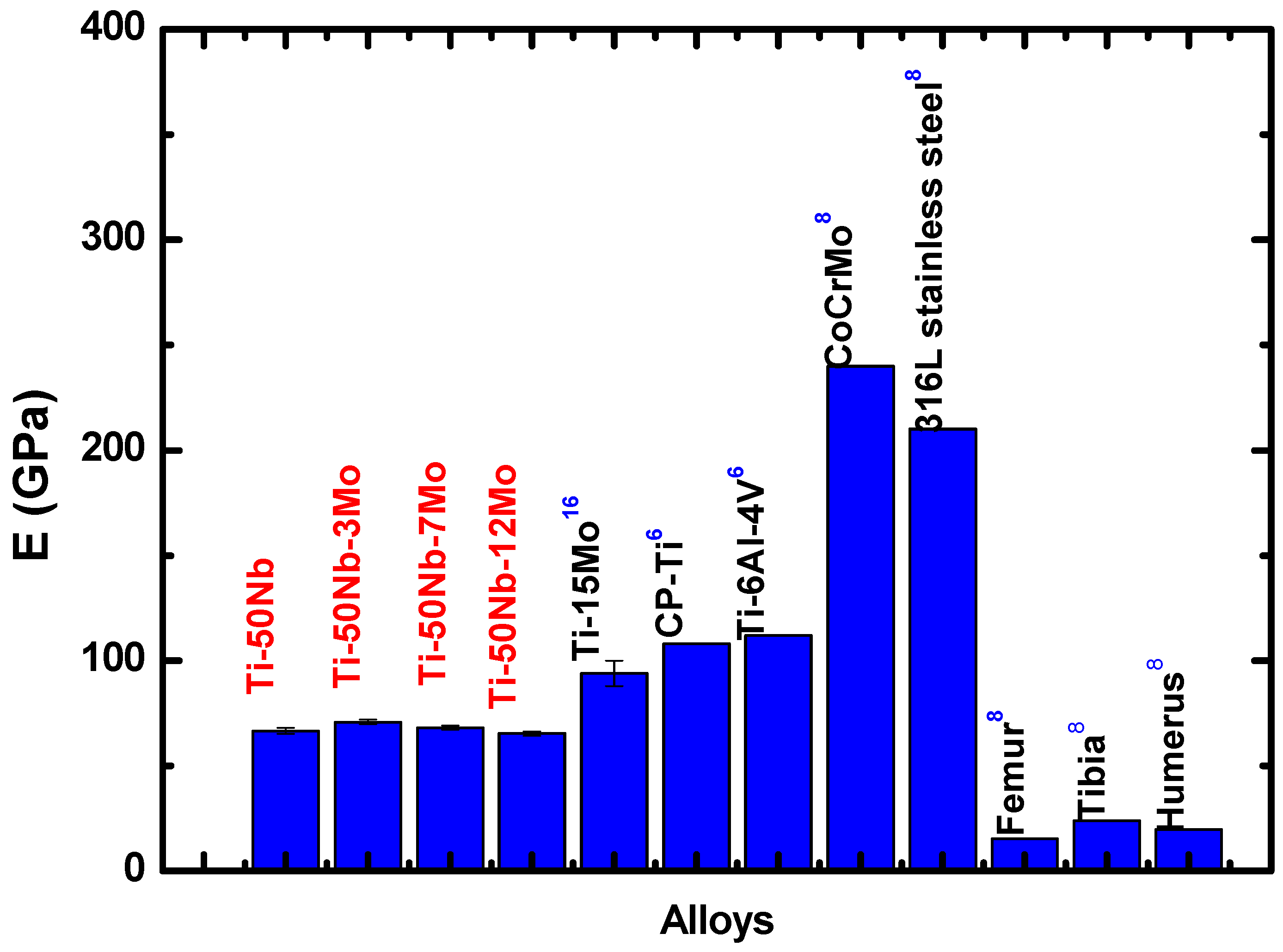
- The results show that the addition of molybdenum decreased elastic modulus.
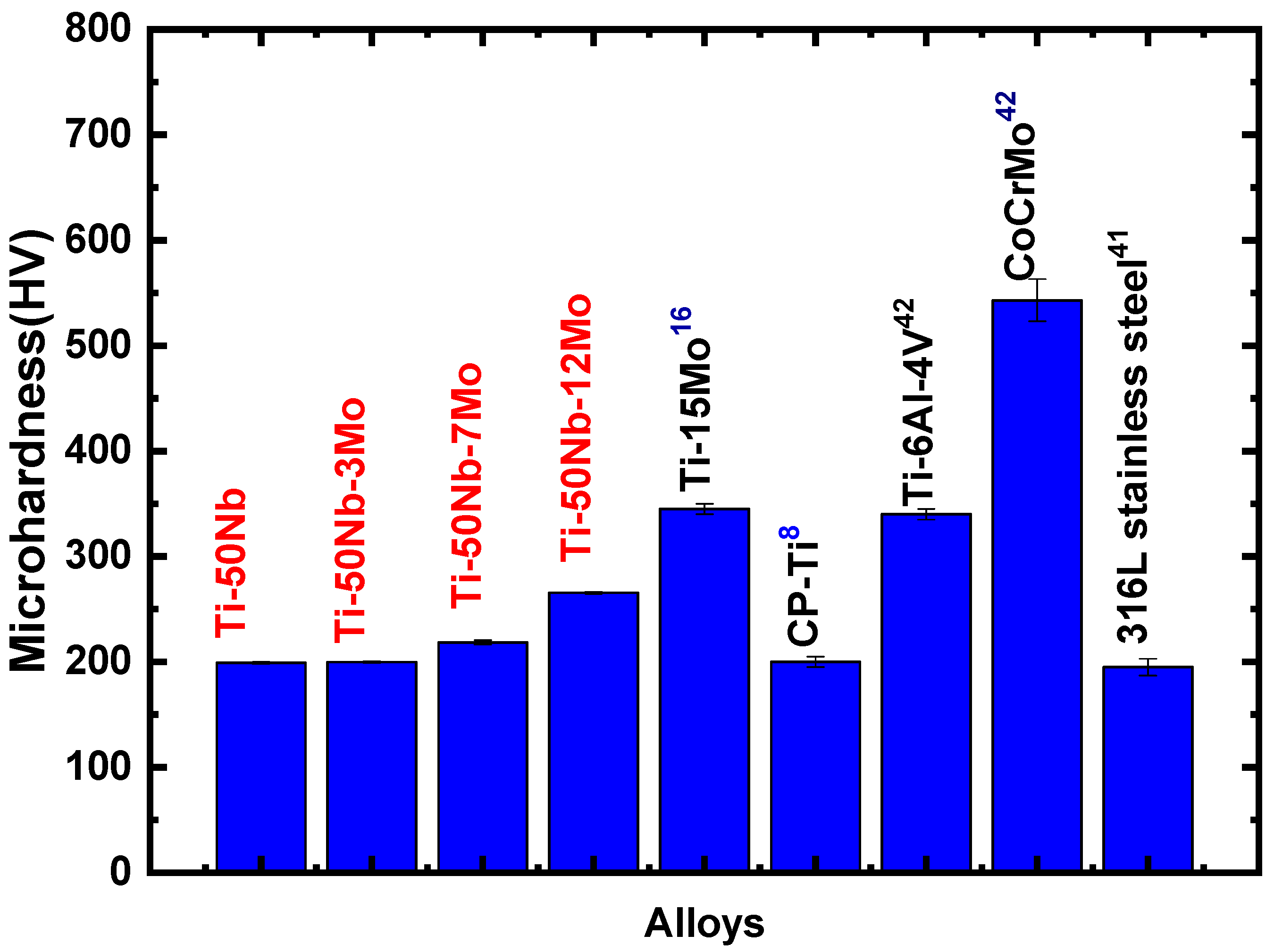
- The values found show such alloys as excellent candidates for biomaterial use.
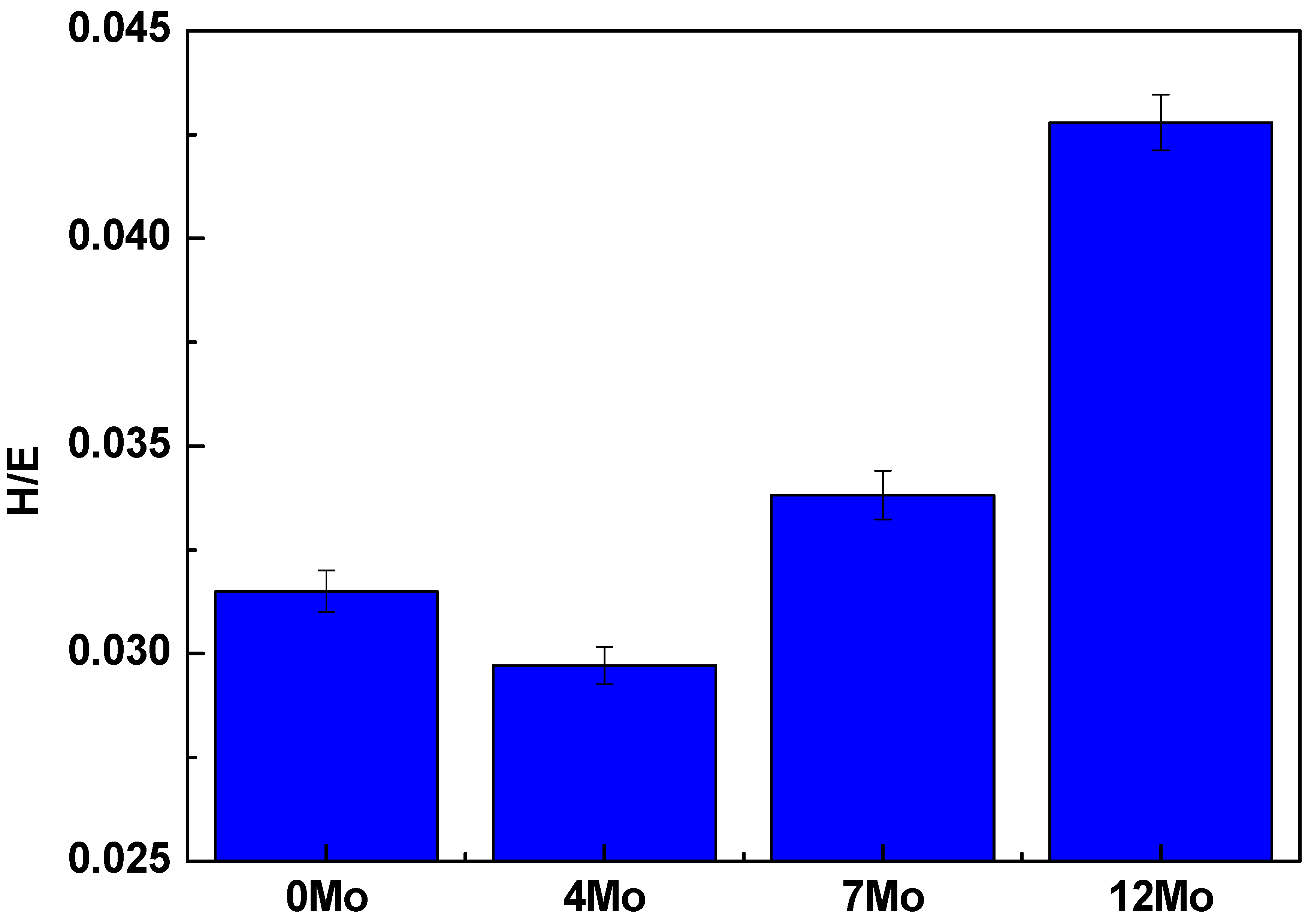
- However, among the alloys studied, the one with the best properties was the Ti-50Nb-12Mo alloy because it presented a lower elastic modulus and H/E ratio.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lakes, R.S. Biomaterials: An Introduction, 3rd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Lütjering, G.; Williams, J.C.; Gysler, A. Microstructure and Mechanical Properties of Titanium Alloys. In Microstructure and Properties of Materials; World Scientific: Singapore, 1998; pp. 1–77. [Google Scholar]
- Boyer, R.; Welsch, G.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1994. [Google Scholar]
- Collings, E.W. The Physical Metallurgy of Titanium Alloys; ASM International: Materials Park, OH, USA, 1989. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Al-Zain, Y.; Sato, Y.; Kim, H.Y.; Hosoda, H.; Nam, T.H.; Miyazaki, S. Room temperature aging behavior of Ti–Nb–Mo-based superelastic alloys. Acta Mater. 2012, 60, 2437–2447. [Google Scholar] [CrossRef]
- Al-Zain, Y.; Kim, H.Y.; Hosoda, H.; Nam, T.H.; Miyazaki, S. Shape memory properties of Ti–Nb–Mo biomedical alloys. Acta Mater. 2010, 58, 4212–4223. [Google Scholar] [CrossRef]
- Dahmani, M.; Fellah, M.; Hezil, N.; Benoudia, M.-C.; Abdul Samad, M.; Alburaikan, A.; Abd El-Wahed khalifa, H.; Obrosov, A. Structural and mechanical evaluation of a new Ti-Nb-Mo alloy produced by high-energy ball milling with variable milling time for biomedical applications. Int. J. Adv. Manuf. Technol. 2023, 129, 4971–4991. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, H.; Hao, C.; Zhao, J.; Wang, Q.; Liu, E.; Dong, C. First-principles calculations of elastic moduli of Ti–Mo–Nb alloys using a cluster-plus-glue-atom model for stable solid solutions. J. Mater. Sci. 2013, 48, 3138–3146. [Google Scholar] [CrossRef]
- Borborema, S.; de Holanda Ferrer, V.; Rocha, A.d.C.; Cossú, C.M.F.A.; Nunes, A.R.V.; Nunes, C.A.; Malet, L.; de Almeida, L.H. Influence of Nb Addition on α″ and ω Phase Stability and on Mechanical Properties in the Ti-12Mo-xNb Stoichiometric System. Metals 2022, 12, 1508. [Google Scholar] [CrossRef]
- Xu, L.-J.; Xiao, S.-L.; Tian, J.; Chen, Y.-Y. Microstructure, mechanical properties and dry wear resistance of β-type Ti−15Mo−xNb alloys for biomedical applications. Trans. Nonferrous Met. Soc. China 2013, 23, 692–698. [Google Scholar] [CrossRef]
- Jr, J.R.S.M.; Grandini, C.R. The influence of heat treatment on the structure and microstructure of Ti-15Mo-xNb system alloys for biomedical applications. Mater. Sci. Forum 2014, 783–786, 1255–1260. [Google Scholar]
- Martins, J.R.S., Jr.; Matos, A.A.; Oliveira, R.C.; Buzalaf, M.A.R.; Costa, I.; Rocha, L.A.; Grandini, C.R. Preparation and characterization of alloys of the Ti–15Mo–Nb system for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 639–648. [Google Scholar] [CrossRef]
- Ho, W.F.; Ju, C.P.; Chern Lin, J.H. Structure and properties of cast binary Ti–Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef]
- Morinaga, M. Electronic Approach to Alloy Design; Toyota Physical & Chemical Research Institute: Nagakute, Japan, 2011; pp. 1–6. [Google Scholar]
- Abdel-Hady, M.; Hinoshita, K.; Morinaga, M. General approach to phase stability and elastic properties of β-type Ti-alloys using electronic parameters. Scr. Mater. 2006, 55, 477–480. [Google Scholar] [CrossRef]
- Morinaga, M.; Yukawa, H. Alloy design with the aid of molecular orbital method. Bull. Mater. Sci. 1997, 20, 805–815. [Google Scholar] [CrossRef]
- Martins, G.V.; Silva, C.R.M.; Nunes, C.A.; Trava-Airoldi, V.J.; Borges, L.A.; Machado, J.P.B. Beta Ti-45Nb and Ti-50Nb Alloys Produced by Powder Metallurgy for Aerospace Application. Mater. Sci. Forum 2010, 660–661, 405–409. [Google Scholar] [CrossRef]
- Kuroda, P.A.B. Preparation, Microstructural Characterization, and Selected Mechanical Properties of Ti-20Zr-2.5Mo and Ti-20Zr-7.5Mo Used as Biomaterial. Mater. Sci. Forum 2016, 869, 946–951. [Google Scholar] [CrossRef]
- ASTM E384-16; Standard Test Method for Microindentation Hardness of Materials. ASTM: West Conshohocken, PA, USA, 2016.
- ASTM E1876-15; Standard Test Method for Dynamic Young’s Modulus, Shear Modulus, and Poisson’s Ratio by Impulse Excitation of Vibration. ASTM: West Conshohocken, PA, USA, 2015.
- Martins Júnior, J.R.S.; Nogueira, R.A.; Araújo, R.O.d.; Donato, T.A.G.; Arana-Chavez, V.E.; Claro, A.P.R.A.; Moraes, J.C.S.; Buzalaf, M.A.R.; Grandini, C.R. Preparation and characterization of Ti-15Mo alloy used as biomaterial. Mater. Res. 2011, 14, 107–112. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Pedroso, B.L.T.; Pontes, F.M.L.; Grandini, C.R. Effect of Titanium Addition on the Structure, Microstructure, and Selected Mechanical Properties of As-Cast Zr-25Ta-xTi Alloys. Metals 2021, 11, 1507. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. A review—Metastable β titanium alloy for biomedical applications. J. Eng. Appl. Sci. 2023, 70, 25. [Google Scholar] [CrossRef]
- Kanapaakala, G.; Subramani, V. A review on β-Ti alloys for biomedical applications: The influence of alloy composition and thermomechanical processing on mechanical properties, phase composition, and microstructure. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2022, 237, 1251–1294. [Google Scholar] [CrossRef]
- Hayyawi, A.R.; Al-Ethari, H.; Haleem, A.H. Development of β-Ti Alloys for Biomedical Applications—A Review. In Proceedings of the 2022 13th International Conference on Mechanical and Aerospace Engineering (ICMAE), Bratislava, Slovakia, 20–22 July 2022; pp. 1–6. [Google Scholar]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.-H. A review on alloy design, biological response, and strengthening of β-titanium alloys as biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef]
- Cardoso, G.C.; de Almeida, G.S.; Corrêa, D.O.G.; Zambuzzi, W.F.; Buzalaf, M.A.R.; Correa, D.R.N.; Grandini, C.R. Preparation and characterization of novel as-cast Ti-Mo-Nb alloys for biomedical applications. Sci. Rep. 2022, 12, 11874. [Google Scholar] [CrossRef]
- Morinaga, M.; Murata, Y.; Yukawa, H. Molecular orbital approach to alloy design. In Applied Computational Materials Modeling: Theory, Simulation and Experiment; Bozzolo, G., Noebe, R.D., Abel, P.B., Vij, D.R., Eds.; Springer: Boston, MA, USA, 2007; pp. 255–306. [Google Scholar]
- Cardoso, G.C.; Kuroda, P.A.B.; Grandini, C.R. Influence of Nb Addition on the Structure, Microstructure, Vickers Microhardness, and Young’s Modulus of new β Ti-xNb-5Mo alloys system. J. Mater. Res. Technol. 2023, 25, 3061–3070. [Google Scholar] [CrossRef]
- Ling, J.; Huang, D.; Bai, K.; Li, W.; Yu, Z.; Chen, W. High-throughput development and applications of the compositional mechanical property map of the β titanium alloys. J. Mater. Sci. Technol. 2021, 71, 201–210. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 87th ed.; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- He, G.; Liu, P.; Tan, Q. Porous titanium materials with entangled wire structure for load-bearing biomedical applications. J. Mech. Behav. Biomed. Mater. 2012, 5, 16–31. [Google Scholar] [CrossRef]
- Pilz, S.; Hariharan, A.; Günther, F.; Zimmermann, M.; Gebert, A. Influence of isothermal omega precipitation aging on deformation mechanisms and mechanical properties of a β-type Ti-Nb alloy. J. Alloys Compd. 2023, 930, 167309. [Google Scholar] [CrossRef]
- Zhao, X.; Niinomi, M.; Nakai, M.; Hieda, J. Beta type Ti–Mo alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2012, 8, 1990–1997. [Google Scholar] [CrossRef]
- Santos, R.F.M.d.; Kuroda, P.A.B.; Reis, C.N.; Afonso, C.R.M. Influence of Hot Rolling on β Ti-Nb-Zr(-Ta) Multiprincipal Alloys for Biomedical Application. Mater. Res. 2023, 26, e20230214. [Google Scholar] [CrossRef]
- Muthukumaran, V.; Selladurai, V.; Nandhakumar, S.; Senthilkumar, M. Experimental investigation on corrosion and hardness of ion implanted AISI 316L stainless steel. Mater. Des. 2010, 31, 2813–2817. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Reimann, Ł. Influence of Cr and Co on hardness and corrosion resistance CoCrMo alloys used on dentures. J. Achiev. Mater. Manuf. Eng. 2011, 49, 193–199. [Google Scholar]
- Kuroda, P.A.B.; Grandini, C.R.; Afonso, C.R.M. Structural Characterization of the Hot-rolled Ti-25Ta-xZr Alloys by Rietveld Method. Mater. Res. 2023, 26, e20220559. [Google Scholar] [CrossRef]
- Donato, T.A.G.; Sousa, K.d.S.J.; Kuroda, P.A.B.; Grandini, C.R. A α + β Ti-15Nb Alloy with Low Elastic Modulus: Characterization and In Vitro Evaluation on Osteogenic Phenotype. J. Funct. Biomater. 2023, 14, 452. [Google Scholar] [CrossRef]
- Stráský, J.; Preisler, D.; Seiner, H.; Bodnárová, L.; Janovská, M.; Košutová, T.; Harcuba, P.; Šalata, K.; Halmešová, K.; Džugan, J.; et al. Achieving high strength and low elastic modulus in interstitial biomedical Ti–Nb–Zr–O alloys through compositional optimization. Mater. Sci. Eng. A 2022, 839, 142833. [Google Scholar] [CrossRef]
- Tito Patricio, M.A.; Lustosa, C.J.R.; Chaves, J.A.M.; Marques, P.W.B.; Silva, P.S.; Almeida, A.; Vilar, R.; Florêncio, O. Relationship between microstructure, phase transformation, and mechanical behavior in Ti–40Ta alloys for biomedical applications. J. Mater. Res. Technol. 2021, 14, 210–219. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef]
- Liu, H.; Niinomi, M.; Nakai, M.; Cho, K. beta-Type titanium alloys for spinal fixation surgery with high Young’s modulus variability and good mechanical properties. Acta Biomater. 2015, 24, 361–369. [Google Scholar] [CrossRef]
- Karre, R.; Dey, S.R. Progress in Development of Beta Titanium Alloys for Biomedical Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]



| Element (Wt%) | Ti-50Nb | Ti-50Nb-3Mo | Ti-50Nb-7Mo | Ti-50Nb-12Mo |
|---|---|---|---|---|
| Ti | balance | balance | balance | balance |
| Nb | 54.5 ± 0.2 | 55.0 ± 0.3 | 54.9 ± 0.2 | 51.2 ± 0.2 |
| Mo | <0.01 | 2.9 ± 0.3 | 7.6 ± 0.3 | 12.7 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins Junior, J.R.S.; Kuroda, P.A.B.; Grandini, C.R. Investigation of the Chemical Composition, Microstructure, Density, Microhardness, and Elastic Modulus of the New β Ti-50Nb-xMo Alloys for Biomedical Applications. Materials 2024, 17, 250. https://doi.org/10.3390/ma17010250
Martins Junior JRS, Kuroda PAB, Grandini CR. Investigation of the Chemical Composition, Microstructure, Density, Microhardness, and Elastic Modulus of the New β Ti-50Nb-xMo Alloys for Biomedical Applications. Materials. 2024; 17(1):250. https://doi.org/10.3390/ma17010250
Chicago/Turabian StyleMartins Junior, José Roberto Severino, Pedro Akira Bazaglia Kuroda, and Carlos Roberto Grandini. 2024. "Investigation of the Chemical Composition, Microstructure, Density, Microhardness, and Elastic Modulus of the New β Ti-50Nb-xMo Alloys for Biomedical Applications" Materials 17, no. 1: 250. https://doi.org/10.3390/ma17010250
APA StyleMartins Junior, J. R. S., Kuroda, P. A. B., & Grandini, C. R. (2024). Investigation of the Chemical Composition, Microstructure, Density, Microhardness, and Elastic Modulus of the New β Ti-50Nb-xMo Alloys for Biomedical Applications. Materials, 17(1), 250. https://doi.org/10.3390/ma17010250







