Materials Nanoarchitectonics at Dynamic Interfaces: Structure Formation and Functional Manipulation
Abstract
1. Introduction
2. Molecular-Level Dynamic Process: Manipulation, Arrangement, and Assembly
3. Material Production: Dynamic Materials-Level Formation Process at Interfaces
4. Fullerene Assembly: Examples of Dynamic Formation Process from Zero-Dimensional Unit to Advanced Materials
5. Summary and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, P.; Fromme, P.; Witt, H.; Klukas, O.; Saenger, W.; Krauß, N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 2018, 30, 1706759. [Google Scholar] [CrossRef]
- Khosravi, N.; Pishavar, E.; Baradaran, B.; Oroojalian, F.; Mokhtarzadeh, A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: A promising biomimetic nanoplatforms for cancer theranostics. J. Control. Release 2022, 348, 706–722. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Saidul Islam, M.S.; Shudo, Y.; Hayami, S. Energy conversion and storage in fuel cells and super-capacitors from chemical modifications of carbon allotropes: State-of-art and prospect. Bull. Chem. Soc. Jpn. 2022, 95, 1–25. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered oxygen-deficient double perovskites as promising cathode materials for solid oxide fuel cells. Materials 2022, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takeiri, F.; Kobayashi, G.; Matsuishi, S.; Ogino, H.; Ida, S.; Mori, T.; Uchimoto, Y.; Tanabe, S.; Hasegawa, T.; et al. Recent progress on mixed-anion materials for energy applications. Bull. Chem. Soc. Jpn. 2022, 95, 26–37. [Google Scholar] [CrossRef]
- Jakhar, M.; Kumar, A.; Ahluwalia, P.K.; Tankeshwar, K.; Pandey, R. Engineering 2D materials for photocatalytic water-splitting from a theoretical perspective. Materials 2022, 15, 2221. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T. Highly efficient wideband solar energy conversion employing singlet-triplet transitions. Bull. Chem. Soc. Jpn. 2022, 95, 341–352. [Google Scholar] [CrossRef]
- Pastuszak, J.; Węgierek, P. Photovoltaic cell generations and current research directions for their development. Materials 2022, 15, 5542. [Google Scholar] [CrossRef]
- Murugan, S.; Lee, E.-C. Recent Advances in the synthesis and application of vacancy-ordered halide double perovskite materials for solar cells: A promising alternative to lead-based perovskites. Materials 2023, 16, 5275. [Google Scholar] [CrossRef]
- Desoky, M.M.H.; Caldera, F.; Brunella, V.; Ferrero, R.; Hoti, G.; Trotta, F. Cyclodextrins for lithium batteries applications. Materials 2023, 16, 5540. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Chapman, A.; Ertekin, E.; Kubota, M.; Nagao, A.; Bertsch, K.; Macadre, A.; Tsuchiyama, T.; Masamura, T.; Takaki, S.; Komoda, R.; et al. Achieving a carbon neutral future through advanced functional materials and technologies. Bull. Chem. Soc. Jpn. 2022, 95, 73–103. [Google Scholar] [CrossRef]
- Qaidi, S.; Najm, H.M.; Abed, S.M.; Özkılıç, Y.O.; Al Dughaishi, H.; Alosta, M.; Sabri, M.M.S.; Alkhatib, F.; Milad, A. Concrete containing waste glass as an environmentally friendly aggregate: A review on fresh and mechanical characteristics. Materials 2022, 15, 6222. [Google Scholar] [CrossRef]
- Yagyu, J.; Islam, M.S.; Yasutake, H.; Hirayama, H.; Zenno, H.; Sugimoto, A.; Takagi, S.; Sekine, Y.; Ohira, S.; Hayami, S. Insights and further understanding of radioactive cesium removal using zeolite, Prussian blue and graphene oxide as adsorbents. Bull. Chem. Soc. Jpn. 2022, 95, 862–870. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Zhang, Q.; Teng, F.; McLellan, B.C. A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Mamun, M.R.A.; Yusuf, M.A.; Bhuyan, M.M.; Bhuiyan, M.S.H.; Arafath, M.A.; Uddin, M.N.; Soeb, M.J.A.; Almahri, A.; Rahman, M.M.; Karim, M.R. Acidity controlled desulfurization of biogas by using iron (III) and ferrosoferric (II, III) oxide. Bull. Chem. Soc. Jpn. 2022, 95, 1234–1241. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and development of novel composites containing nickel ferrites supported on activated carbon derived from agricultural wastes and its application in water remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chong, H.L.H.; Moh, P.Y.; Albaqami, M.D.; Tighezza, A.M.; Qin, C.; Ni, X.; Cao, J.; Xu, X.; Yamauchi, Y. β-FeOOH nanospindles as chloride-capturing electrodes for electrochemical faradic deionization of saline water. Bull. Chem. Soc. Jpn. 2023, 96, 306–309. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Glukhova, O.E.; Plugin, I.A.; Kolosov, D.A.; Nagornov, I.A.; Simonenko, T.L.; Varezhnikov, A.S.; Simonenko, E.P.; Sysoev, V.V.; Kuznetsov, N.T. The Ti0.2V1.8C MXene ink-prepared chemiresistor: From theory to tests with humidity versus VOCs. Chemosensors 2023, 11, 7. [Google Scholar] [CrossRef]
- Chowdhury, R.; Al Biruni, M.T.; Afia, A.; Hasan, M.; Islam, M.R.; Ahmed, T. Medical waste incineration fly ash as a mineral filler in dense bituminous course in flexible pavements. Materials 2023, 16, 5612. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Fujita, Y.; Niizeki, T.; Fukumitsu, N.; Ariga, K.; Yamauchi, Y.; Malgras, V.; Kaneti, Y.V.; Liu, C.-H.; Hatano, K.; Suematsu, H.; et al. Mechanisms responsible for adsorption of molybdate ions on alumina for the production of medical radioisotopes. Bull. Chem. Soc. Jpn. 2022, 95, 129–137. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Pradipta, A.R.; Michiba, H.; Kubo, A.; Fujii, M.; Tanei, T.; Morimoto, K.; Shimazu, K.; Tanaka, K. The second-generation click-to-sense probe for intraoperative diagnosis of breast cancer tissues based on acrolein targeting. Bull. Chem. Soc. Jpn. 2022, 95, 421–426. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Soares, G.A.B.E.; Chopra, H.; Rahman, M.M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of phyto-nanotechnology for the treatment of neurodegenerative disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef] [PubMed]
- Sahayasheela, V.J.; Yu, Z.; Hirose, Y.; Pandian, G.N.; Bando, T.; Sugiyama, H. Inhibition of GLI-mediated transcription by cyclic pyrrole-imidazole polyamide in cancer stem cells. Bull. Chem. Soc. Jpn. 2022, 95, 693–699. [Google Scholar] [CrossRef]
- Quader, S.; Kataoka, K.; Cabral, H. Nanomedicine for brain cancer. Adv. Drug Deliv. Rev. 2022, 182, 114115. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M. Molecular mechanisms of the medicines for COVID-19. Bull. Chem. Soc. Jpn. 2022, 95, 1308–1317. [Google Scholar] [CrossRef]
- Larasati, L.; Lestari, W.W.; Firdaus, M. Dual-action Pt(IV) prodrugs and targeted delivery in metal-organic frameworks: Overcoming cisplatin resistance and improving anticancer activity. Bull. Chem. Soc. Jpn. 2022, 95, 1561–1577. [Google Scholar] [CrossRef]
- Niwa, T.; Tahara, T.; Chase, C.E.; Fang, F.G.; Nakaoka, T.; Irie, S.; Hayashinaka, E.; Wada, Y.; Mukai, H.; Masutomi, K.; et al. Synthesis of 11C-radiolabeled eribulin as a companion diagnostics PET tracer for brain glioblastoma. Bull. Chem. Soc. Jpn. 2023, 96, 283–290. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, Q.; Huang, J.; Liu, J.; Tan, G.; Sun, C.; Li, T.; Liu, S.; Li, Y.; Wang, H.; et al. Visually resolving the direct Z-scheme heterojunction in CdS@ZnIn2S4 hollow cubes for photocatalytic evolution of H2 and H2O2 from pure water. Appl. Catal. B Environ. 2021, 293, 120213. [Google Scholar] [CrossRef]
- Mori, H.; Yamada, Y.; Minagawa, Y.; Hasegawa, N.; Nishihara, Y. Effects of acyloxy groups in anthrabisthiadiazole-based semiconducting polymers on electronic properties, thin-film structure, and solar cell performance. Bull. Chem. Soc. Jpn. 2022, 95, 942–952. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, J.; Kong, Y.; Zhao, Y.; Chen, S.; Li, D.; Liu, W.; Chen, Y.; Xie, T.; Cui, J.; et al. Unraveling of cocatalysts photodeposited selectively on facets of BiVO4 to boost solar water splitting. Nat. Commun. 2022, 13, 484. [Google Scholar] [CrossRef]
- Charles-Blin, Y.; Kondo, T.; Wu, Y.; Bandow, S.; Awaga, K. Salt-assisted pyrolysis of covalent organic framework for controlled active nitrogen functionalities for oxygen reduction reaction. Bull. Chem. Soc. Jpn. 2022, 95, 972–977. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Matsuzawa, K.; Nagai, T.; Ikegami, K.; Kuroda, Y.; Monden, R.; Ishihara, A. Fe, N-Doped SrTiO3 synthesized using pyrazine carboxylic acid-metal complexes: Application as an oxygen reduction catalyst for polymer electrolyte fuel cell cathodes in acidic media. Bull. Chem. Soc. Jpn. 2023, 96, 175–177. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, J.; Vequizo, J.J.M.; Hisatomi, T.; Ma, Y.; Nakabayashi, M.; Takata, T.; Yamakata, A.; Shibata, N.; Domen, K. Overall water splitting by a SrTaO2N-based photocatalyst decorated with an Ir-promoted Ru-based cocatalyst. J. Am. Chem. Soc. 2023, 145, 3839–3843. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Suzuk, K.; Hashimoto, C.; Tsutsumi, C.; Hayase, N.; Kitani, A. Trial fabrication of NADH-dependent enzymatic ethanol biofuel cell providing H2 gas as well as Electricity. Bull. Chem. Soc. Jpn. 2023, 96, 331–338. [Google Scholar] [CrossRef]
- Huang, G.; Hu, M.; Xu, X.; Alothman, A.A.; Mushab, M.S.S.; Ma, S.; Shen, P.K.; Zhu, J.; Yamauchi, Y. Optimizing heterointerface of Co2P–CoxOy Nanoparticles within a porous carbon network for deciphering superior water splitting. Small Struct. 2023, 4, 2200235. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Sahoo, S.; Shim, J.-J.; Tan, W.K.; Matsuda, A.; Singh, R.K. An overview of recent progress in nanostructured carbon-based supercapacitor electrodes: From zero to bi-dimensional materials. Carbon 2022, 193, 298–338. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Joanni, E.; Sahoo, S.; Kawamura, G.; Matsuda, A. Microwave-assisted synthesis of iron oxide homogeneously dispersed on reduced graphene oxide for high-performance supercapacitor electrodes. J. Energy Storage 2022, 56, 105896. [Google Scholar] [CrossRef]
- Das, H.T.; Dutta, S.; Balaji, T.E.; Das, N.; Das, P.; Dheer, N.; Kanojia, R.; Ahuja, P.; Ujjain, S.K. Recent rrends in carbon nanotube electrodes for flexible supercapacitors: A review of smart energy storage device assembly and performance. Chemosensors 2022, 10, 223. [Google Scholar] [CrossRef]
- Zhang, G.; Qiuhong Bai, Q.; Wang, X.; Li, C.; Uyama, H.; Shen, Y. Preparation and mechanism investigation of walnut shell-based hierarchical porous carbon for supercapacitors. Bull. Chem. Soc. Jpn. 2023, 96, 190–197. [Google Scholar] [CrossRef]
- Arumugam, B.; Mayakrishnan, G.; Subburayan Manickavasagam, S.K.; Kim, S.C.; Vanaraj, R. An overview of active electrode materials for the efficient high-performance supercapacitor application. Crystals 2023, 13, 1118. [Google Scholar] [CrossRef]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-performance supercapacitors: A comprehensive review on paradigm shift of conventional energy storage devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Gnawali, C.L.; Manandha, S.; Shahi, S.; Shrestha, R.G.; Adhikari, M.P.; Rajbhandari, R.; Pokharel, B.P.; Ma, R.; Ariga, K.; Shrestha, L.K. Nanoporous carbon materials from terminalia bellirica seed for iodine and methylene blue adsorption and high-performance supercapacitor applications. Bull. Chem. Soc. Jpn. 2023, 96, 572–581. [Google Scholar] [CrossRef]
- Sasai, R.; Fujimura, T.; Sato, H.; Nii, E.; Sugata, M.; Nakayashiki, Y.; Hoashi, H.; Moriyoshi, C.; Oishi, E.; Fujii, Y.; et al. Origin of selective nitrate removal by Ni2+–Al3+ layered double hydroxides in aqueous media and its application potential in seawater purification. Bull. Chem. Soc. Jpn. 2022, 95, 802–812. [Google Scholar] [CrossRef]
- Li, S.-L.; Chang, G.; Huang, C.Y.; Kinooka, K.; Chen, K.Y.; Fu, W.; Gong, G.; Yoshioka, T.; McKeown, N.B.; Hu, Y. 2,2′-Biphenol-based ultrathin microporous nanofilms for highly efficient molecular sieving separation. Angew. Chem. Int. Ed. 2022, 61, e202212816. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; He, R.; Ren, J.; Tao, F.; Yang, H.; Lv, H.; Ju, X. A friendly UV-responsive fluorine-free superhydrophobic coating for oil-water separation and dye degradation. Bull. Chem. Soc. Jpn. 2022, 95, 1091–1099. [Google Scholar] [CrossRef]
- Kushida, W.; Gonzales, R.R.; Shintani, T.; Matsuoka, A.; Nakagawa, K.; Yoshioka, T.; Hideto Matsuyama, H. Organic solvent mixture separation using fluorine-incorporated thin film composite reverse osmosis membrane. J. Mater. Chem. A 2022, 10, 4146–4156. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Lu, J.; Teng, H.; Fukuda, H.; Qin, W.; Wei, T.; Liu, Y. Highly selective membrane for efficient separation of environmental determinands: Enhanced molecular imprinting in polydopamine-embedded porous sleeve. Chem. Eng. J. 2022, 449, 137825. [Google Scholar] [CrossRef]
- Kukobat, R.; Sakai, M.; Tanaka, H.; Otsuka, H.; Vallejos-Burgos, F.; Lastoskie, C.; Matsukata, M.; Sasaki, Y.; Yoshida, K.; Hayashi, T.; et al. Ultrapermeable 2D-channeled graphene-wrapped zeolite molecular sieving membranes for hydrogen separation. Sci. Adv. 2022, 8, eabl3521. [Google Scholar] [CrossRef]
- Zhang, T.; Cain, A.K.; Semenec, L.; Liu, L.; Hosokawa, Y.; Inglis, D.W.; Yalikun, Y.; Li, M. Microfluidic separation and enrichment of escherichia coli by size using viscoelastic flows. Anal. Chem. 2023, 95, 2561–2569. [Google Scholar] [CrossRef]
- Hung, H.-L.; Iizuka, T.; Deng, X.; Lyu, Q.; Hsu, C.-H.; Oe, N.; Lin, L.-C.; Hosono, N.; Kang, D.-Y. Engineering gas separation property of metal–organic framework membranes via polymer insertion. Sep. Purif. Technol. 2023, 310, 123115. [Google Scholar] [CrossRef]
- Salahuddin, B.; Masud, M.K.; Aziz, S.; Liu, C.-H.; Amiralian, N.; Ashok, A.; Hossain, M.A.; Park, H.; Wahab, M.A.; Amin, M.A.; et al. p-Carrageenan gel modified mesoporous gold chronocoulometric sensor for ultrasensitive detection of microRNA. Bull. Chem. Soc. Jpn. 2022, 95, 198–207. [Google Scholar] [CrossRef]
- Dong, L.; Wang, M.; Wu, J.; Zhu, C.; Shi, J.; Morikawa, H. Stretchable, adhesive, self-healable, and conductive hydrogel-based deformable triboelectric nanogenerator for energy harvesting and human motion sensing. ACS Appl. Mater. Interfaces 2022, 14, 9126–9137. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, S.; Liu, M.; Liu, L. A cucurbit[6]uril-based supramolecular assembly as a multifunctional material for the detection and removal of organic explosives and antibiotics. Bull. Chem. Soc. Jpn. 2022, 95, 1445–1452. [Google Scholar] [CrossRef]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuat. B Chem. 2022, 364, 131876. [Google Scholar] [CrossRef]
- Saito, S.; Haraga, T.; Marumo, K.; Sato, Y.; Nakano, Y.; Tasaki-Handa, Y.; Shibukawa, M. Americium(III)/curium(III) complete separation and sensitive fluorescence detection by capillary and gel electrophoresis using emissive hexadentate/octadentate polyaminocarboxylate ligands. Bull. Chem. Soc. Jpn. 2023, 96, 223–225. [Google Scholar] [CrossRef]
- Kalyana Sundaram, S.d.; Hossain, M.M.; Rezki, M.; Ariga, K.; Tsujimura, S. Enzyme cascade electrode reactions with nanomaterials and their applicability towards biosensor and biofuel cells. Biosensors 2023, 13, 1018. [Google Scholar] [CrossRef]
- Anggraini, L.E.; Rahmawati, H.; Nasution, M.A.F.; Jiwanti, P.K.; Einaga, Y.; Ivandini, T.A. Development of an acrylamide biosensor using guanine and adenine as biomarkers at boron-doped diamond electrodes: Integrated molecular docking and experimental studies. Bull. Chem. Soc. Jpn. 2023, 96, 420–428. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tsujimura, S.; Zelsmann, M.; Zebda, A. Hierarchical structure of gold and carbon electrode for Bilirubin oxidase-biocathode. Biosensors 2023, 13, 482. [Google Scholar] [CrossRef]
- Maeki, M.; Uno, S.; Niwa, A.; Okada, Y.; Tokeshi, M. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Control. Release 2022, 344, 80–96. [Google Scholar] [CrossRef]
- Tiburcius, S.; Krishnan, K.; Patel, V.; Netherton, J.; Sathish, C.I.; Weidenhofer, J.; Yang, J.-H.; Verrills, N.M.; Karakoti, A.; Vinu, A. Triple surfactant assisted synthesis of novel core-shell mesoporous silica nanoparticles with high surface area for drug delivery for prostate cancer. Bull. Chem. Soc. Jpn. 2022, 95, 331–340. [Google Scholar] [CrossRef]
- Yokoo, H.; Oba, M.; Uchida, S. Cell-penetrating peptides: Emerging tools for mRNA delivery. Pharmaceutics 2022, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Soendoro, A.; Okayama, S.; Rahmania, F.J.; Nagai, T.; Imae, T.; Tsutsumiuchi, K.; Kawai, N. Drug release stimulated by magnetic field and light on magnetite- and carbon dot-loaded carbon nanohorn. Bull. Chem. Soc. Jpn. 2022, 95, 582–594. [Google Scholar] [CrossRef]
- Zhao, Y.; Das, S.; Sekine, T.; Mabuchi, H.; Irie, T.; Sakai, J.; Wen, D.; Zhu, W.; Ben, T.; Negishi, Y. Record ultralarge-pores, low density three-dimensional covalent organic Framework for controlled drug delivery. Angew. Chem. Int. Ed. 2023, 62, e202300172. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.A.; Sato, Y.; Elewa, Y.H.A.; Kon, Y.; Harashima, H. Self-homing nanocarriers for mRNA delivery to the activated hepatic stellate cells in liver fibrosis. J. Control. Release 2023, 353, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Kumar, N.; Zhao, D.; Dai, X.; Kawamoto, K.; Matsumura, K. Polyampholyte-based polymer hydrogels for the long-term storage, protection and delivery of therapeutic proteins. Adv. Healthc. Mater. 2023, 12, 2203253. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Baskoy, S.A.; Yang, C.; Hong, J.; Chae, J.; Ha, H.; Lee, S.; Tanaka, M.; Choi, Y.; Choi, J. Lipid-based colloidal nanoparticles for applications in targeted vaccine delivery. Nanoscale Adv. 2023, 5, 1853–1869. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Segawa, Y.; Murakami, K.; Itami, K. Polycyclic arene synthesis by annulative π-extension. J. Am. Chem. Soc. 2019, 141, 3–10. [Google Scholar] [CrossRef]
- Fan, W.; Matsuno, T.; Han, Y.; Wang, X.; Zhou, Q.; Isobe, H.; Wu, J. Synthesis and chiral resolution of twisted carbon nanobelts. J. Am. Chem. Soc. 2021, 143, 15924–15929. [Google Scholar] [CrossRef]
- Tanaka, T. Synthesis of novel heteronanographenes via fold-in approach. Bull. Chem. Soc. Jpn. 2022, 95, 602–610. [Google Scholar] [CrossRef]
- Miera, G.G.; Matsubara, S.; Kono, H.; Murakami, K.; Itami, K. Synthesis of octagon-containing molecular nanocarbons. Chem. Sci. 2022, 13, 1848–1868. [Google Scholar] [CrossRef]
- Mori, K. C(sp3)–H Bond functionalization mediated by hydride a shift/cyclization system. Bull. Chem. Soc. Jpn. 2022, 95, 296–305. [Google Scholar] [CrossRef]
- Sugiyama, M.; Akiyama, M.; Yonezawa, Y.; Komaguchi, K.; Higashi, M.; Nozaki, K.; Okazoe, T. Electron in a cube: Synthesis and characterization of perfluorocubane as an electron acceptor. Science 2022, 377, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y. Nonplanar aromatic hydrocarbons: Design and synthesis of highly strained structures. Bull. Chem. Soc. Jpn. 2022, 95, 1600–1610. [Google Scholar] [CrossRef]
- Hirano, K. Copper-catalyzed electrophilic amination: An umpolung strategy for new C–N bond formations. Bull. Chem. Soc. Jpn. 2023, 96, 198–207. [Google Scholar] [CrossRef]
- Toya, M.; Omine, T.; Ishiwari, F.; Saeki, A.; Ito, H.; Itami, K. Expanded [2,1][n]carbohelicenes with 15- and 17-benzene rings. J. Am. Chem. Soc. 2023, 145, 11553–11565. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, N.; Wang, Y.; Yang, G.; He, Y. Rational design of novel reaction pathways and tailor-made catalysts for value-added chemicals synthesis from CO2 hydrogenation. Bull. Chem. Soc. Jpn. 2023, 96, 291–302. [Google Scholar] [CrossRef]
- Hossain, S.; Niihori, Y.; Nair, L.V.; Kumar, B.; Kurashige, W.; Negishi, Y. Alloy clusters: Precise synthesis and mixing effects. Acc. Chem. Res. 2018, 51, 3114–3124. [Google Scholar] [CrossRef]
- Tokoro, H.; Nakabayashi, K.; Nagashima, S.; Song, Q.; Yoshikiyo, M.; Ohkoshi, S. Optical properties of epsilon iron oxide nanoparticles in the millimeter- and terahertz-wave regions. Bull. Chem. Soc. Jpn. 2022, 95, 538–552. [Google Scholar] [CrossRef]
- Taniguchi, T.; Nurdiwijayanto, L.; Ma, R.; Sasaki, T. Chemically exfoliated inorganic nanosheets for nanoelectronics. Appl. Phys. Rev. 2022, 9, 021313. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Yadav, N.; Gaikwad, R.P.; Mishra, V.; Gawande, M.B. Synthesis and photocatalytic Applications of functionalized carbon quantum dots. Bull. Chem. Soc. Jpn. 2022, 95, 1638–1679. [Google Scholar] [CrossRef]
- Wu, D.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Kitagawa, H. Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 2020, 142, 13833–13838. [Google Scholar] [CrossRef] [PubMed]
- Peera, S.G.; Koutavarapu, R.; Chao, L.; Singh, L.; Murugadoss, G.; Rajeshkhanna, G. 2D MXene nanomaterials as electrocatalysts for hydrogen evolution reaction (HER): A review. Micromachines 2022, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, characterization and biomedical application of silver nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Minamihara, H.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Murakami, Y.; Matsumura, S.; Kumara, L.S.R.; Sakata, O.; Kawaguchi, S.; Kubota, Y.; et al. Continuous-flow chemical synthesis for sub-2 nm ultra-multielement alloy nanoparticles consisting of group IV to XV elements. J. Am. Chem. Soc. 2023, 145, 17136–17142. [Google Scholar] [CrossRef]
- Okamoto, K.; Imoto, H.; Naka, K. Silsesquioxane cage-fused siloxane rings as a novel class of inorganic-based host molecules. Bull. Chem. Soc. Jpn. 2023, 96, 84–89. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, G.; Yin, W.; Pang, H.; Xu, Q. Recent progress in Prussian blue/Prussian blue analogue-derived metallic compounds. Bull. Chem. Soc. Jpn. 2022, 95, 230–260. [Google Scholar] [CrossRef]
- Bieniek, A.; Terzyk, A.P.; Wiśniewski, M.; Roszek, K.; Kowalczyk, P.; Sarkisov, L.; Keskin, S.; Kaneko, K. MOF materials as therapeutic agents, drug carriers, imaging agents and biosensors in cancer biomedicine: Recent advances and perspectives. Prog. Mater. Sci. 2021, 117, 100743. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Cheng, Y.-H. Stimuli-responsive and switchable platinum(II) complexes and their applications in memory storage. Bull. Chem. Soc. Jpn. 2022, 95, 846–854. [Google Scholar] [CrossRef]
- Kim, M.; Xin, R.; Earnshaw, J.; Tang, J.; Hill, J.P.; Ashok, A.; Nanjundan, A.K.; Kim, J.; Young, C.; Sugahara, Y.; et al. MOF-derived nanoporous carbons with diverse tunable nanoarchitectures. Nat. Protoc. 2022, 17, 2990–3027. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, E.; Tsang, M.Y.; Troyano, J.; Craig, G.A.; Furukawa, S. Assembling metal–organic cages as porous materials. Chem. Soc. Rev. 2022, 51, 4876–4889. [Google Scholar] [CrossRef] [PubMed]
- Sarango-Ramírez, M.K.; Donoshita, M.; Yoshida, Y.; Lim, D.-W.; Kitagawa, H. Cooperative proton and Li-ion conduction in a 2D-layered MOF via mechanical insertion of lithium halides. Angew. Chem. Int. Ed. 2023, 62, e202301284. [Google Scholar] [CrossRef] [PubMed]
- Ohki, Y. Biomimetic transition metal cluster complexes: Synthetic studies and applications in the reduction of inert small molecules. Bull. Chem. Soc. Jpn. 2023, 96, 639–648. [Google Scholar] [CrossRef]
- Hayashi, R.; Tashiro, S.; Asakura, M.; Mitsui, S.; Shionoya, M. Effector-dependent structural transformation of a crystalline framework with allosteric effects on molecular recognition ability. Nat. Commun. 2023, 14, 4490. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Wang, J.; Hiraide, S.; Götz, A.; Miyahara, M.T.; Hartmann, M.; Apeleo Zubiri, B.; Spiecker, E.; Vogel, N.; Watanabe, S. Fast gas-adsorption kinetics in supraparticle-based MOF packings with hierarchical porosity. Adv. Mater. 2023, 35, 2305980. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Kato, Y.; Higashiharaguchi, S.; Aratsu, K.; Isobe, A.; Saito, T.; Prabhu, D.D.; Kitamoto, Y.; Hollamby, M.J.; Smith, A.J.; et al. Self-assembled poly-catenanes from supramolecular toroidal building blocks. Nature 2020, 583, 400–405. [Google Scholar] [CrossRef]
- Takezawa, H.; Shitozawa, K.; Fujita, M. Enhanced reactivity of twisted amides inside a molecular cage. Nat. Chem. 2020, 12, 574–578. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kubota, R.; Minami, T. Molecular self-assembled chemosensors and their arrays. Coord. Chem. Rev. 2021, 429, 213607. [Google Scholar] [CrossRef]
- Hamada, K.; Shimoyama, D.; Hirao, T.; Haino, T. Chiral supramolecular polymer formed via host-guest complexation of an octaphosphonate biscavitand and a chiral diammonium guest. Bull. Chem. Soc. Jpn. 2022, 95, 621–627. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Morishita, D.; Aoki, T.; Itoh, Y.; Yano, K.; Araoka, F.; Aida, T. Anomalous chiral transfer: Supramolecular polymerization in a chiral medium of a mesogenic molecule. Chem. Asian J. 2022, 17, e202200223. [Google Scholar] [CrossRef] [PubMed]
- Bezrukov, A.; Galyametdinov, Y. Activation and switching of supramolecular chemical signals in multi-output microfluidic devices. Micromachines 2022, 13, 1778. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Isobe, H. Trapped yet free inside the tube: Supramolecular chemistry of molecular peapods. Bull. Chem. Soc. Jpn. 2023, 96, 406–419. [Google Scholar] [CrossRef]
- Kubota, R. Supramolecular–polymer composite hydrogels: From In Situ network observation to functional properties. Bull. Chem. Soc. Jpn. 2023, 96, 802–812. [Google Scholar] [CrossRef]
- Otsuka, C.; Takahashi, S.; Isobe, A.; Saito, T.; Aizawa, T.; Tsuchida, R.; Yamashita, S.; Harano, K.; Hanayama, H.; Shimizu, N.; et al. Supramolecular polymer polymorphism: Spontaneous helix–helicoid transition through dislocation of hydrogen-bonded p-rosettes. J. Am. Chem. Soc. 2023, 145, 22563–22576. [Google Scholar] [CrossRef]
- Ogoshi, T. Supramolecular assemblies and polymer recognition based on polygonal and pillar-shaped macrocycles “pillar[n]arenes”. Polym. J. 2023, 55, 1247–1260. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef]
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wan, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.J.; Mori, T. Polymer based thermoelectric nanocomposite materials and devices: Fabrication and characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Ubukata, T.; Seki, T. Unconventional approaches to light-promoted dynamic surface morphing on polymer films. Bull. Chem. Soc. Jpn. 2022, 95, 138–162. [Google Scholar] [CrossRef]
- Zhang, W.; You, L.; Meng, X.; Wang, B.; Lin, D. Recent advances on conducting polymers based nanogenerators for energy harvesting. Micromachines 2021, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Tsuchii, Y.; Menda, T.; Hwang, S.; Yasuda, T. Photovoltaic properties of p-conjugated polymers based on fused cyclic Imide and amide skeletons. Bull. Chem. Soc. Jpn. 2023, 96, 90–94. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Oyaizu, K. Redox: Organic robust radicals and their polymers for energy conversion/storage devices. Chem. Rev. 2023, 123, 11336–11391. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, S.; Nagao, A.; Hashimoto, Y.; Matsune, A.; Okazoe, T.; Suzuki, C.; Wada, H.; Kakiuchi, T.; Tsuda, A. Non-Isocyanate polyurethane synthesis by polycondensation of alkylene and arylene bis(fluoroalkyl) bis(carbonate)s with diamines. Bull. Chem. Soc. Jpn. 2023, 96, 663–670. [Google Scholar] [CrossRef]
- Iizuka, T.; Sano, H.; Le Ouay, B.; Hosono, N.; Uemura, T. An approach to MOFaxanes by threading ultralong polymers through metal–organic framework microcrystals. Nat. Commun. 2023, 14, 3241. [Google Scholar] [CrossRef]
- Kawaguchi, D. Aggregation states, thermal molecular motion and carrier properties in functional polymer thin films. Polym. J. 2023, 55, 1237–1245. [Google Scholar] [CrossRef]
- Akiba, U.; Minaki, D.; Anzai, J. Host-guest chemistry in layer-by-layer assemblies containing calix[n]arenes and cucurbit[n]urils: A Review. Polymers 2018, 10, 130. [Google Scholar] [CrossRef]
- Minamiki, T.; Ichikawa, Y.; Kurita, R. The power of assemblies at interfaces: Nanosensor platforms based on synthetic receptor membranes. Sensors 2020, 20, 2228. [Google Scholar] [CrossRef]
- Yang, X.; Biswas, S.K.; Han, J.; Tanpichai, S.; Li, M.-C.; Chen, C.; Zhu, S.; Das, A.K.; Yano, H. Surface and interface engineering for nanocellulosic Advanced materials. Adv. Mater. 2021, 33, 2002264. [Google Scholar] [CrossRef]
- Zhu, H. Interfacial preparation of ferroelectric polymer nanostructures for electronic applications. Polym. J. 2021, 53, 877–886. [Google Scholar] [CrossRef]
- Miyabe, K.; Aoki, K. Moment analysis of solute permeation kinetics at an interface of mixed micelles of anionic and nonionic surfactants. Bull. Chem. Soc. Jpn. 2022, 95, 1715–1722. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, J.; Groll, J.; Matsusaki, M. Layer-by-layer assembly methods and their biomedical applications. Biomater. Sci. 2022, 10, 4077–4094. [Google Scholar] [CrossRef] [PubMed]
- Makiura, R. Creation of metal–organic framework nanosheets by the Langmuir-Blodgett technique. Coord. Chem. Rev. 2022, 469, 214650. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Nakagawa, M.; Ito, T.; Imura, Y.; Wang, K.-H.; Kawai, T. Chiral transcription from chiral Au nanowires to self-assembled monolayers of achiral azobenzene derivatives. Bull. Chem. Soc. Jpn. 2022, 95, 1006–1010. [Google Scholar] [CrossRef]
- Yao, M.S.; Otake, K.; Kitagawa, S. Interface chemistry of conductive crystalline porous thin films. Trends Chem. 2023, 5, 588–604. [Google Scholar] [CrossRef]
- Akamatsu, M.; Sakai, K.; Sakai, H. Dynamic control of interfacial properties and self-assembly with photoirradiation. Chem. Lett. 2023, 52, 573–581. [Google Scholar] [CrossRef]
- Mitsudo, K.; Kurimoto, Y.; Yoshioka, K.; Suga, S. Miniaturization and combinatorial approach in organic electrochemistry. Chem. Rev. 2018, 118, 5985–5999. [Google Scholar] [CrossRef]
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New horizon of nanoparticle and cluster catalysis with dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef]
- Pan, Z.-Z.; Lv, W.; Yang, Q.-H.; Nishihara, H. Aligned macroporous monoliths by ice-templating. Bull. Chem. Soc. Jpn. 2022, 95, 611–620. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Adschiri, T.; Takami, S.; Umetsu, M.; Ohara, S.; Naka, T.; Minami, K.; Hojo, D.; Togashi, T.; Arita, T.; Taguchi, M.; et al. Supercritical hydrothermal reactions for material synthesis. Bull. Chem. Soc. Jpn. 2023, 96, 133–147. [Google Scholar] [CrossRef]
- Zeybek, Ö.; Özkılıç, Y.O.; Karalar, M.; Çelik, A.İ.; Qaidi, S.; Ahmad, J.; Burduhos-Nergis, D.D.; Burduhos-Nergis, D.P. Influence of replacing cement with waste glass on mechanical properties of concrete. Materials 2022, 15, 7513. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yamato, M.; Shimizu, T.; Sekine, H.; Ohashi, K.; Kanzaki, M.; Ohki, T.; Nishida, K.; Okano, T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials 2007, 28, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Mijanović, O.; Pylaev, T.; Nikitkina, A.; Artyukhova, M.; Branković, A.; Peshkova, M.; Bikmulina, P.; Turk, B.; Bolevich, S.; Avetisov, S.; et al. Tissue engineering meets nanotechnology: Molecular mechanism modulations in cornea regeneration. Micromachines 2021, 12, 1336. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Inoue, M.; Yoshimitsu, Y.; Hashimoto, I.; Ohashi, N.; Tsumura, K.; Suzuki, K.; Watanabe, T.; Hohsaka, T. Chemical synthesis and cell-free expression of thiazoline ring-bridged cyclic Peptides and their properties on biomembrane permeability. Bull. Chem. Soc. Jpn. 2022, 95, 359–366. [Google Scholar] [CrossRef]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and gold nanoparticles for antimicrobial purposes against multi-drug resistance bacteria. Materials 2022, 15, 1799. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Nakamura, M.; Kanjo, M.; Gon, M.; Watanabe, H.; Kita, H.; Tanaka, K. Facile preparation of near infrared-luminescent protein complexes with conjugated polymers consisting of boron azobenzene units. Bull. Chem. Soc. Jpn. 2023, 96, 659–662. [Google Scholar] [CrossRef]
- Zhou, P.; Xing, R.; Li, Q.; Li, J.; Yuan, C.; Yan, X. Steering phase-separated droplets to control fibrillar network evolution of supramolecular peptide hydrogels. Matter 2023, 6, 1945–1963. [Google Scholar] [CrossRef]
- Miyagawa, A.; Hagiya, K.; Nagatomo, S.; Nakatani, K. Protein adsorption on carboxy-functionalized microparticles revealed by zeta potential and absorption spectroscopy measurements. Bull. Chem. Soc. Jpn. 2023, 96, 759–765. [Google Scholar] [CrossRef]
- Li, Z.; Yu, F.; Xu, X.; Wang, T.; Fei, J.; Hao, J.; Li, J. Photozyme-catalyzed ATP generation based on ATP synthase-reconstituted nanoarchitectonics. J. Am. Chem. Soc. 2023, 145, 20907–20912. [Google Scholar] [CrossRef]
- Hieda, M.; Tsujimura, K.; Kinoshita, M.; Matsumori, N. Formation of a tight complex between amphidinol and sterols in lipid bilayers revealed by short-range energy transfer. Bull. Chem. Soc. Jpn. 2022, 95, 1753–1759. [Google Scholar] [CrossRef]
- Inamasu, R.; Yamaguchi, H.; Arai, T.; Chang, J.; Kuramochi, M.; Mio, K.; Sasaki, Y.V. Observation of molecular motions in polymer thin films by laboratory grazing incidence diffracted X-ray blinking. Polym. J. 2023, 55, 703–709. [Google Scholar] [CrossRef]
- Liu, C.; Morimoto, N.; Jiang, L.; Kawahara, S.; Noritomi, T.; Yokoyama, H.; Mayumi, K.; Ito, K. Tough hydrogels with rapid self-reinforcement. Science 2021, 372, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, K.; Watanabe, M.; Hisaeda, Y.; Shimakoshi, H. Development of visible light-driven hybrid catalysts composed of earth abundant metal ion modified TiO2 and B12 complex. Bull. Chem. Soc. Jpn. 2022, 95, 1016–1024. [Google Scholar] [CrossRef]
- Uchida, J.; Soberats, B.; Gupta, M.; Kato, T. Advanced functional liquid crystals. Adv. Mater. 2022, 34, 2109063. [Google Scholar] [CrossRef] [PubMed]
- Roopsung, N.; An, T.L.H.; Sugawara, A.; Asoh, T.; Hsu, Y.-I.; Uyama, H. Switchable stiffness of composite hydrogels triggered by thermoresponsive phase-change particles. Bull. Chem. Soc. Jpn. 2023, 96, 636–638. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W.; Fujishima, A.; Terashima, C. Enhanced photocatalytic degradation activity using the V2O5/RGO Composite. Nanomaterials 2023, 13, 338. [Google Scholar] [CrossRef]
- Li, Z.; Nakamura, K.; Kobayashi, N. Solvent-free mechanochemical synthesis of strongly luminescent Eu(III) hybrid materials using tetramethylammonium salts. Bull. Chem. Soc. Jpn. 2023, 96, 816–823. [Google Scholar] [CrossRef]
- Liu, M.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. An anisotropic hydrogel with electrostatic repulsion between cofacially aligned nanosheets. Nature 2015, 517, 68–72. [Google Scholar] [CrossRef]
- Kasuya, N.; Tsurumi, J.; Okamoto, T.; Watanabe, S.; Takeya, J. Two-dimensional hole gas in organic semiconductors. Nat. Mater. 2021, 20, 1401–1406. [Google Scholar] [CrossRef]
- Nishijima, A.; Kametani, Y.; Uemura, T. Reciprocal regulation between MOFs and polymers. Coord. Chem. Rev. 2022, 466, 214601. [Google Scholar] [CrossRef]
- Jung, T.A.; Schlittler, R.R.; Gimzewski, J.K.; Tang, H.; Joachim, C. Controlled room-temperature positioning of individual molecules: Molecular flexure and motion. Science 1996, 271, 181–184. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Pou, P.; Abe, M.; Jelinek, P.; Pérez, R.; Morita, S.; Custance, Ó. Chemical identification of individual surface atoms by atomic force microscopy. Nature 2007, 446, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Soe, W.-H.; Srivastava, S.; Joachim, C. Train of single molecule-gears. J. Phys. Chem. Lett. 2019, 10, 6462–6467. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Krejčí, O.; Nishiuchi, T.; Sahara, K.; Kodama, T.; Pawlak, R.; Meyer, E.; Kubo, T.; Foster, A.S. Three-dimensional graphene nanoribbons as a framework for molecular assembly and local probe chemistry. Sci. Adv. 2020, 6, eaay8913. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Takeuchi, K.; Kamei, K.; Nakamuro, T.; Harano, K.; Nakamura, E. Atomic-number (Z)-correlated atomic sizes for deciphering electron microscopic molecular images. Proc. Natl. Acad. Sci. USA 2022, 119, e2114432119. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Hinuma, Y.; Ichikawa, S.; Tanaka, S. Investigation of the interaction between Au and brookite TiO2 using transmission electron microscopy and density functional theory. Bull. Chem. Soc. Jpn. 2023, 96, 373–380. [Google Scholar] [CrossRef]
- Kimura, K.; Miwa, K.; Imada, H.; Imai-Imada, M.; Kawahara, S.; Takeya, J.; Kawai, M.; Galperin, M.; Kim, Y. Selective triplet exciton formation in a single molecule. Nature 2019, 570, 210–213. [Google Scholar] [CrossRef]
- Kawai, S.; Sang, H.; Kantorovich, L.; Takahashi, K.; Nozaki, K.; Ito, S. An endergonic synthesis of single Sondheimer–Wong diyne by local probe chemistry. Angew. Chem. Int. Ed. 2020, 59, 10842. [Google Scholar] [CrossRef]
- Imahori, H. Molecular photoinduced charge separation: Fundamentals and application. Bull. Chem. Soc. Jpn. 2023, 96, 339–352. [Google Scholar] [CrossRef]
- Ito, S.; Akama, H.; Kojima, K.M.; McKenzie, I.; Kuwahata, K.; Tachikawa, M. Muon spin rotation (μSR) for characterizing radical addition to C=S in xanthene-9-thione and thioxanthene-9-thione. Bull. Chem. Soc. Jpn. 2023, 96, 461–464. [Google Scholar] [CrossRef]
- Liu, X.; Farahi, G.; Chiu, C.-L.; Papic, Z.; Watanabe, K.; Taniguchi, T.; Zaletel, M.P.; Yazdani, A. Visualizing broken symmetry and topological defects in a quantum Hall ferromagnet. Science 2022, 375, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Higa, S.; Yoshida, K.; Sumitani, K.; Kurisaki, T. Structure of aqueous scandium(III) nitrate solution by large-angle X-ray scattering combined with empirical potential refinement modeling, X-ray absorption fine structure, and discrete variational Xα calculations. Bull. Chem. Soc. Jpn. 2022, 95, 673–679. [Google Scholar] [CrossRef]
- Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a new era of SERS and TERS at the nanometer scale: From fundamentals to innovative applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y.; Arai, N.; Amo, Y.; Usuki, T.; Han, J.; Watanabe, H.; Umebayashi, Y.; Tsuzuki, S.; Ikeda, K.; Otomo, T. Neutron diffraction with 34S/natS isotopic substitution method on the solvation structure of S8 molecule in concentrated CS2 solutions. Bull. Chem. Soc. Jpn. 2022, 95, 1481–1485. [Google Scholar] [CrossRef]
- Lostao, A.; Lim, K.S.; Pallarés, M.C.; Ptak, A.; Marcuello, C. Recent advances in sensing the inter-biomolecular interactions at the nanoscale—A comprehensive review of AFM-based force spectroscopy. Int. J. Biol. Macromol. 2023, 238, 124089. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tsurumi, J.; Ohno, M.; Fujimoto, R.; Kumagai, S.; Kurosawa, T.; Okamoto, T.; Takeya, J.; Watanabe, S. Efficient molecular doping of polymeric semiconductors driven by anion exchange. Nature 2019, 572, 634–638. [Google Scholar] [CrossRef]
- Kobayashi, H.; Takeuchi, K.; Morinaga, Y.; Honda, H.; Yamamoto, M.; Odanaka, Y.; Inagaki, M. Inter-spin interactions of 1D chains of different-sized nitroxide radicals incorporated in the organic 1D nanochannels of tris(o-phenylenedioxy)cyclotriphosphazene. Bull. Chem. Soc. Jpn. 2022, 95, 981–988. [Google Scholar] [CrossRef]
- Tsuji, H. Carbon-bridged oligo(phenylenevinylene)s that reveal cryogenic phenomena at room temperature. Bull. Chem. Soc. Jpn. 2022, 95, 657–662. [Google Scholar] [CrossRef]
- Fan, L.-B.; Shu, C.-C.; Dong, D.; He, J.; Henriksen, N.E.; Nori, F. Quantum coherent control of a single molecular-polariton rotation. Phys. Rev. Lett. 2023, 130, 043604. [Google Scholar] [CrossRef]
- Wooten, B.L.; Iguchi, R.; Tang, P.; Kang, J.S.; Uchida, K.; Bauer, G.E.W.; Heremans, J.P. Electric field–dependent phonon spectrum and heat conduction in ferroelectrics. Sci. Adv. 2021, 9, eadd7194. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Li, M.; Richards, G.J.; Hill, J.P. Nanoarchitectonics: A conceptual paradigm for design and synthesis of dimension-conrolled functional nanomaterials. J. Nanosci. Nanotechnol. 2011, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnolog. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Y.; Parakhonskiy, B.; Skirtach, A.G. Nanoarchitectonics beyond perfect order—Not quite perfect but quite useful. Nanoscale 2022, 14, 15964–16002. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Varghese, B.S.; Suresh, M.; Panwar, C.; Gupta, T.K. Nanoarchitectonics: Functional nanomaterials and nanostructures—A review. J. Nanopart. Res. 2022, 24, 196. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s plenty of room at the bottom. Calif. Inst. Technol. J. Eng. Sci. 1960, 4, 23–36. [Google Scholar]
- Roukes, M. Plenty of room, indeed. Sci. Am. 2001, 285, 48–57. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.; Bando, Y.; Aono, M. Forming nanomaterials as layered functional structures toward materials nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What are the emerging concepts and challenges in NANO? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016, 48, 371–389. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: What’s coming next after nanotechnology? Nanoscale Horiz. 2021, 6, 364–378. [Google Scholar] [CrossRef]
- Ariga, K. Molecular nanoarchitectonics: Unification of nanotechnology and molecular/materials science. Beilstein J. Nanotechnol. 2023, 14, 434–453. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond self-assembly: Challenges to create bio-like hierarchic organization. Angew. Chem. Int. Ed. 2020, 59, 15424–15446. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Liu, R.; Kimura, H.; Li, J.; Jiang, H.; Zhang, X.; Yu, Z.; Sun, X.; Algadi, H.; Guo, Z.; et al. Supramolecular self-assembly synthesis of hemoglobin-like amorphous CoP@N, P-doped carbon composites enable ultralong stable cycling under high-current density for lithium-ion battery anodes. Adv. Compos. Hybrid Mater. 2023, 6, 23. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: A navigator from materials to life. Mater. Chem. Front. 2017, 1, 208–211. [Google Scholar] [CrossRef]
- Aono, M.; Ariga, K. The way to nanoarchitectonics and the way of nanoarchitectonics. Adv. Mater. 2016, 28, 989–992. [Google Scholar] [CrossRef]
- Ariga, K. Liquid interfacial nanoarchitectonics: Molecular machines, organic semiconductors, nanocarbons, stem cells, and others. Curr. Opin. Colloid Interface Sci. 2023, 63, 101656. [Google Scholar] [CrossRef]
- Shen, X.; Song, J.; Kawakami, K.; Ariga, K. Molecule-to-material-to-bio nanoarchitectonics with biomedical fullerene nanoparticles. Materials 2022, 15, 5404. [Google Scholar] [CrossRef]
- Ariga, K.; Fakhrullin, R. Materials nanoarchitectonics from atom to living cell: A method for everything. Bull. Chem. Soc. Jpn. 2022, 95, 774–795. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: Method for everything in material science. Bull. Chem. Soc. Jpn. 2024; in press. [Google Scholar] [CrossRef]
- Laughlin, R.B.; Pines, D. The theory of everything. Proc. Natl. Acad. Sci. USA 2000, 97, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-dimensional (2D) nanomaterials towards electrochemical nanoarchitectonics in energy-related applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Oaki, Y.; Sato, K. Nanoarchitectonics for conductive polymers using solid and vapor phases. Nanoscale Adv. 2022, 4, 2773–2781. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Lebastard, C.; Wilmet, M.; Dumait, N.; Renaud, A.; Cordier, S.; Ohashi, N.; Uchikoshi, T.; Grasset, F. A review on functional nanoarchitectonics nanocomposites based on octahedral metal atom clusters (Nb6, Mo6, Ta6, W6, Re6): Inorganic 0D and 2D powders and films. Sci. Technol. Adv. Mater. 2022, 23, 547–578. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.K.R. Exploring the self-cleaning facets of fluorinated graphene nanoarchitectonics: Progress and perspectives. ChemNanoMat 2023, 9, e202300135. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Xia, H. Nanoarchitectonics and applications of gallium-based liquid metal micro- and nanoparticles. ChemNanoMat 2023, 9, e202300078. [Google Scholar] [CrossRef]
- Hikichi, R.; Tokura, Y.; Igarashi, Y.; Imai, H.; Oaki, Y. Fluorine-free substrate-independent superhydrophobic Coatings by nanoarchitectonics of polydispersed 2D materials. Bull. Chem. Soc. Jpn. 2023, 96, 766–774. [Google Scholar] [CrossRef]
- Trifoi, A.R.; Matei, E.; Râpă, M.; Berbecaru, A.-C.; Panaitescu, C.; Banu, I.; Doukeh, R. Coprecipitation nanoarchitectonics for the synthesis of magnetite: A review of mechanism and characterization. React. Kinet. Mech. Catal. 2023, 136, 2835–2874. [Google Scholar] [CrossRef]
- Guan, X.; Li, Z.; Geng, X.; Lei, Z.; Karakoti, A.; Wu, T.; Kumar, P.; Yi, J.; Vinu, A. Emerging trends of carbon-based quantum dots: Nanoarchitectonics and applications. Small 2023, 19, 2207181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gao, J.; Zhang, X.; Guo, N. Composite micro-nanoarchitectonics of MMT-SiO2: Space charge characteristics under tensile state. Polymers 2021, 13, 4354. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, C.; Xue, N.; Li, Z.; Li, F.; Wan, X.; Guo, S.; Hao, J. High-frequency surface insulation strength with nanoarchitectonics of disiloxane modified polyimide films. Polymers 2022, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wu, D.; Ren, Y.; Lin, J. Nanoarchitectonics of illite-based materials: Effect of metal oxides lntercalation on the mechanical properties. Nanomaterials 2022, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, H. Nanoarchitectonics of carbon nanostructures: Sodium dodecyl sulfonate @ sodium chloride system. Nanomaterials 2022, 12, 1652. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.L.; Hanif, A.; Alam, T.; Islam, M.T.; Arshad, H.; Soliman, M.S.; Albadran, S.M.; Islam, M.S. Ultrawideband polarization-independent nanoarchitectonics: A prfect metamaterial absorber for visible and infrared optical window applications. Nanomaterials 2022, 12, 2849. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Lai, Y.-R.; Sung, H.-L.; Chung, T.-W.; Lin, K.-Y.A. Design of amine-modified Zr–Mg mixed oxide aerogel nanoarchitectonics with dual lewis acidic and basic sites for CO2/propylene oxide cycloaddition reactions. Nanomaterials 2022, 12, 3442. [Google Scholar] [CrossRef]
- Ling, L.; Wu, C.; Xing, F.; Memon, S.A.; Sun, H. Recycling nanoarchitectonics of graphene oxide from carbon fiber Reinforced polymer by the electrochemical method. Nanomaterials 2022, 12, 3657. [Google Scholar] [CrossRef]
- Qiu, Z.; Jinschek, J.R.; Gouma, P.-I. Two-step solvothermal process for nanoarchitectonics of metastable hexagonal WO3 nanoplates. Crystals 2023, 13, 690. [Google Scholar] [CrossRef]
- Kim, D.; Gu, M.; Park, M.; Kim, T.; Kim, B.-S. Layer-by-layer assembly for photoelectrochemical nanoarchitectonics. Mol. Syst. Des. Eng. 2019, 4, 65–77. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Shen, Z.; Liu, M. Chiral nanoarchitectonics: Towards the design, self-assembly, and function of nanoscale chiral twists and helices. Adv. Mater. 2016, 28, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Shionoya, M. Nanoarchitectonics for coordination asymmetry and related chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 839–859. [Google Scholar] [CrossRef]
- Marin, E.; Tapeinos, C.; Sarasua, J.R.; Larrañaga, A. Exploiting the layer-by-layer nanoarchitectonics for the fabrication of polymer capsules: A toolbox to provide multifunctional properties to target complex pathologies. Adv. Colloid Interface Sci. 2022, 304, 102680. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, C.; Lin, X.; Ouyang, G.; Liu, M. Stepwise solution-interfacial nanoarchitectonics for assembled film with full-color and white-light circularly polarized luminescence. ACS Appl. Mater. Interfaces 2023, 15, 31077–31086. [Google Scholar] [CrossRef] [PubMed]
- Parbat, D.; Jana, N.; Dhar, M.; Mann, U. Reactive multilayer coating as versatile nanoarchitectonics for customizing various bioinspired liquid wettabilities. ACS Appl. Mater. Interfaces 2022, 15, 25232–25247. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P. Role of graphitic carbon in g-C3N4 nanoarchitectonics towards efficient photocatalytic reaction kinetics: A review. Carbon 2023, 216, 118584. [Google Scholar] [CrossRef]
- Haketa, Y.; Yamasumi, K.; Maeda, H. p-Electronic ion pairs: Building blocks for supramolecular nanoarchitectonics via ip-ip interactions. Chem. Soc. Rev. 2023, 52, 7170–7196. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Hsieh, P.-Y.; Chung, C.-J.; Chou, C.-M.; He, J.-L. Nanoarchitectonics for ultrathin gold films deposited on collagen fabric by high-power impulse magnetron sputtering. Nanomaterials 2022, 12, 1627. [Google Scholar] [CrossRef]
- Béres, K.A.; Homonnay, Z.; Bereczki, L.; Dürvanger, Z.; Petruševski, V.M.; Farkas, A.; Kótai, L. Crystal nanoarchitectonics and characterization of the octahedral iron(III)–nitrate complexes with isomer dimethylurea ligands. Crystals 2023, 13, 1019. [Google Scholar] [CrossRef]
- Lahmidi, S.; Anouar, E.H.; Ettahiri, W.; El Hafi, M.; Lazrak, F.; Alanazi, M.M.; Alanazi, A.S.; Hefnawy, M.; Essassi, E.M.; Mague, J.T. Nanoarchitectonics and molecular docking of 4-(dimethylamino)pyridin-1-ium 2-3 methyl-4-oxo-pyri-do[1,2-a]pyrimidine-3-carboxylate. Crystals 2023, 13, 1333. [Google Scholar] [CrossRef]
- Hecht, S. Welding, organizing, and planting organic molecules on substrate surfaces—Promising approaches towards nanoarchitectonics from the bottom up. Angew. Chem. Int. Ed. 2003, 42, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, D.; Ouyang, G.; Liu, M. Double helical p-aggregate nanoarchitectonics for amplified circularly polarized luminescence. Nat. Commun. 2022, 13, 1710. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, M. Inner and interfacial environmental nanoarchitectonics of supramolecular assemblies formed by amphiphiles: From emergence to application. J. Oleo Sci. 2023, 72, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pahal, S.; Boranna, R.; Tripathy, A.; Goudar, V.S.; Veetil, V.T.; Kurapati, R.; Prashanth, G.R.; Vemula, P.K. Nanoarchitectonics for free-standing polyelectrolyte multilayers films: Exploring the flipped surfaces. ChemNanoMat 2023, 9, e202200462. [Google Scholar] [CrossRef]
- Huang, L.; Yang, J.; Asakura, Y.; Shuai, Q.; Yamauchi, Y. Nanoarchitectonics of hollow covalent organic frameworks: Synthesis and applications. ACS Nano 2023, 17, 8918–8934. [Google Scholar] [CrossRef] [PubMed]
- Deepak, D.; Soin, N.; Roy, S.S. Optimizing the efficiency of triboelectric nanogenerators by surface nanoarchitectonics of graphene-based electrodes: A review. Mater. Today Commun. 2023, 34, 105412. [Google Scholar] [CrossRef]
- Musa, A.; Hakim, M.L.; Alam, T.; Islam, M.T.; Alshammari, A.S.; Mat, K.; Almalki, S.H.; Islam, M.S. Polarization independent metamaterial absorber with anti-reflection coating nanoarchitectonics for visible and infrared window applications. Materials 2022, 15, 3733. [Google Scholar] [CrossRef]
- Conti Nibali, V.; D’Angelo, G.; Arena, A.; Ciofi, C.; Scandurra, G.; Branca, C. TiO2 Nanoparticles dispersion in block-copolymer aqueous solutions: Nanoarchitectonics for self-assembly and aggregation. J. Funct. Biomater. 2022, 13, 39. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Z.; Huang, J.; Wang, X.; Guo, H.; Li, W.-H. Anti-fouling performance of hydrophobic hydrogels with unique surface hydrophobicity and nanoarchitectonics. Gels 2022, 8, 407. [Google Scholar] [CrossRef]
- Nayak, A.; Unayama, S.; Tai, S.; Tsuruoka, T.; Waser, R.; Aono, M.; Valov, I.; Hasegawa, T. Nanoarchitectonics for controlling the number of dopant atoms in solid electrolyte nanodots. Adv. Mater. 2018, 30, 1703261. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, M.; Nugraha, A.S.; Rowan, A.E.; Shapter, J.; Yamauchi, Y. Adsorchromism: Molecular nanoarchitectonics at 2D nanosheets—Old chemistry for advanced chromism. Adv. Sci. 2021, 8, 2100539. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Sun, H.; He, Y.; Wang, S.; Liu, X. Recent advances in the photoreactions triggered by porphyrin-based triplet–triplet annihilation upconversion systems: Molecular innovations and nanoarchitectonics. Int. J. Mol. Sci. 2022, 23, 8041. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Z.; Tian, Y.; Pan, F.; Gould, T.; Zhang, S. Nanoarchitectonics of two-dimensional electrochromic materials: Achievements and future challenges. Adv. Mater. Technol. 2023, 8, 2200917. [Google Scholar] [CrossRef]
- Ogawa, S. Aqueous sugar-based amphiphile systems: Recent advances in phase behavior and nanoarchitectonics. J. Oleo Sci. 2023, 72, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, X.; Tang, X.; Hao, Q.; Chen, M. Micro spectrometers based on materials nanoarchitectonics. Materials 2023, 16, 2253. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P. CsPbX3 (X = Cl, Br, and I) Nanocrystals in substrates toward stable photoluminescence: Nanoarchitectonics, properties, and applications. Langmuir 2023, 39, 11188–11212. [Google Scholar] [CrossRef]
- Lun-Fu, A.V.; Bubenchikov, A.M.; Bubenchikov, M.A.; Ovchinnikov, V.A. Numerical simulation of interaction between Kr+ ion and rotating C60 fullerene towards for nanoarchitectonics of fullerene materials. Crystals 2021, 11, 1204. [Google Scholar] [CrossRef]
- Li, X.; Weng, L.; Wang, H.; Wang, X. Nanoarchitectonics of BN/AgNWs/epoxy composites with high thermal conductivity and electrical insulation. Polymers 2021, 13, 4417. [Google Scholar] [CrossRef]
- Yang, K.; Qin, G.; Wang, L.; Zhao, M.; Lu, C. Theoretical nanoarchitectonics of GaN nanowires for ultraviolet irradiation-dependent electromechanical properties. Materials 2023, 16, 1080. [Google Scholar] [CrossRef]
- Tang, R.; Li, G.; Hu, X.; Gao, N.; Li, J.; Huang, K.; Kang, J.; Zhang, R. Micro-nanoarchitectonics of Ga2O3/GaN core-shell rod arrays for high-performance broadband ultraviolet photodetection. Crystals 2023, 13, 366. [Google Scholar] [CrossRef]
- Gao, K.; Wu, N.; Ji, B.; Liu, J. A film electrode upon nanoarchitectonics of bacterial cellulose and conductive fabric for forehead electroencephalogram measurement. Sensors 2023, 23, 7887. [Google Scholar] [CrossRef] [PubMed]
- Howorka, S. DNA Nanoarchitectonics: Assembled DNA at interfaces. Langmuir 2013, 29, 7344–7353. [Google Scholar] [CrossRef] [PubMed]
- Pandeeswar, M.; Senanayak, S.P.; Govindaraju, T. Nanoarchitectonics of small molecule and DNA for ultrasensitive detection of mercury. ACS Appl. Mater. Interfaces 2016, 8, 30362–30371. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Liu, K.; Abbas, M.; Yan, X. Peptide-modulated self-assembly of chromophores toward biomimetic light-harvesting nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Stulz, E. Nanoarchitectonics with porphyrin functionalized DNA. Acc. Chem. Res. 2017, 50, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Chemistry can make strict and fuzzy controls for bio-systems: DNA Nanoarchitectonics and cell-macromolecular nanoarchitectonics. Bull. Chem. Soc. Jpn. 2017, 90, 967–1004. [Google Scholar] [CrossRef]
- Jia, Y.; Yan, X.; Li, J. Schiff base mediated dipeptide assembly toward nanoarchitectonics. Angew. Chem. Int. Ed. 2022, 61, e202207752. [Google Scholar] [CrossRef]
- Jia, X.; Chen, J.; Lv, W.; Li, H.; Ariga, K. Engineering dynamic and interactive biomaterials using material nanoarchitectonics for modulation of cellular behaviors. Cell Rep. Phys. Sci. 2023, 4, 101251. [Google Scholar] [CrossRef]
- Chang, R.; Zhao, L.; Xing, R.; Li, J.; Yan, X. Functional chromopeptide nanoarchitectonics: Molecular design, self-assembly and biological applications. Chem. Soc. Rev. 2023, 52, 2688–2712. [Google Scholar] [CrossRef]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Yu, B.; Meng, Z.; Zhang, X.; Li, J.; Zheng, L. DNA-based dissipative assembly toward nanoarchitectonics. Adv. Funct. Mater. 2022, 32, 2201196. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Park, S.; Park, H.; Tae, H.; Jackman, J.A.; Cho, N.-J. Lipid nanoparticle technologies for nucleic acid delivery: A nanoarchitectonics perspective. Adv. Funct. Mater. 2022, 32, 2203669. [Google Scholar] [CrossRef]
- Komiyama, M. Cyclodextrins as eminent constituents in nanoarchitectonics for drug delivery systems. Beilstein J. Nanotechnol. 2023, 14, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, J.; Sevencan, C.; Leong, D.T.; Ariga, K. Bio-interactive nanoarchitectonics with two-dimensional materials and environments. Sci. Technol. Adv. Mater. 2022, 23, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Shi, J.; Lv, W.; Jia, X.; Ariga, K. Regulation of stem cell fate and function by using bioactive materials with nanoarchitectonics for regenerative medicine. Sci. Technol. Adv. Mater. 2022, 23, 393–412. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; Wang, X.; Zeng, H. Recent advances in antimicrobial surfaces via tunable molecular interactions: Nanoarchitectonics and bioengineering applications. Curr. Opin. Colloid Interface Sci. 2023, 66, 101707. [Google Scholar] [CrossRef]
- Piorecka, K.; Kurjata, J.; Stanczyk, W.A. Nanoarchitectonics: Complexes and conjugates of platinum drugs with silicon containing nanocarriers. An overview. Int. J. Mol. Sci. 2021, 22, 9264. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Yang, G. DNA–lysozyme nanoarchitectonics: Quantitative investigation on charge inversion and compaction. Polymers 2022, 14, 1377. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J.; Prochoń, M.; Masek, A. Nanoarchitectonics for biodegradable superabsorbent based on carboxymethyl starch and chitosan cross-linked with vanillin. Int. J. Mol. Sci. 2022, 23, 5386. [Google Scholar] [CrossRef]
- Kalinova, R.; Mladenova, K.; Petrova, S.; Doumanov, J.; Dimitrov, I. Nanoarchitectonics of spherical nucleic acids with biodegradable polymer cores: Synthesis and evaluation. Materials 2022, 15, 8917. [Google Scholar] [CrossRef] [PubMed]
- Osetrov, K.; Uspenskaya, M.; Zaripova, F.; Olekhnovich, R. Nanoarchitectonics of a skin-adhesive hydrogel based on the gelatin resuscitation fluid Gelatinol®. Gels 2023, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Na, J.; Yao, Y.; Azhar, A.; Yan, X.; Qi, J.; Yamauchi, Y.; Li, J. 0D–1D hybrid nanoarchitectonics: Tailored design of FeCo@N–C yolk–shell nanoreactors with dual sites for excellent Fenton-like catalysis. Chem. Sci. 2021, 12, 15418–15422. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, P.; Luo, Y.; Ruan, Q.; Liu, L.; Wu, Y.; Li, Q.; Xu, Y.; Liu, R.; Chu, P.K. Recent progress and perspective of cobalt-based catalysts for water splitting: Design and nanoarchitectonics. Mater. Today Energy 2022, 23, 100911. [Google Scholar] [CrossRef]
- Arulraj, A.; Murugesan, P.K.; Rajkumar, C.; Zamorano, A.T.; Mangalaraja, R.V. Nanoarchitectonics of layered metal chalcogenides-based ternary electrocatalyst for water splitting. Energies 2023, 16, 1669. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Chhabra, T.; Kaur, H.; Kumar, A.; Qamar, M.; Krishnan, V. Frontier nanoarchitectonics of graphitic carbon nitride based plasmonic photocatalysts and photoelectrocatalysts for energy, environment and organic reactions. Mater. Chem. Front. 2023, 7, 1197–1247. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, Y.; Sun, F.; Wang, S.; Kang, Y.; Xu, X.; Zhao, J.; You, J.; Eguchi, M.; Yamauch, Y.; et al. Nanoarchitectonics of metallene materials for electrocatalysis. ACS Nano 2023, 17, 13017–13043. [Google Scholar] [CrossRef]
- Lee, G.; Hossain, M.A.; Yoon, M.; Jhung, S.H. Nanoarchitectonics of metal–organic frameworks having hydroxy group for adsorption, catalysis, and sensing. J. Ind. Eng. Chem. 2023, 119, 181–192. [Google Scholar] [CrossRef]
- Sharma, D.; Choudhary, P.; Kumar, S.; Krishnan, V. Transition metal phosphide nanoarchitectonics for versatile organic catalysis. Small 2023, 19, 2207053. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; He, S.; Lu, J.; Liu, J.; Lu, H.; Song, D.; Luo, Y. Nanoarchitectonics of Ni/CeO2 catalysts: The effect of pretreatment on the low-temperature steam reforming of glycerol. Nanomaterials 2022, 12, 816. [Google Scholar] [CrossRef]
- Cao, H.; Li, H.; Liu, L.; Xue, K.; Niu, X.; Hou, J.; Chen, L. Salt-templated nanoarchitectonics of CoSe2-NC nanosheets as an efficient bifunctional oxygen electrocatalyst for water splitting. Int. J. Mol. Sci. 2022, 23, 5239. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Lin, L.; Huang, J.; Jing, X.; Chen, S.; Li, Q. Microorganism-templated nanoarchitectonics of hollow TiO2-SiO2 microspheres with enhanced photocatalytic activity for degradation of methyl orange. Nanomaterials 2022, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hil, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Mori, T.; Ariga, K. Molecular imprinting: Materials nanoarchitectonics with molecular information. Bull. Chem. Soc. Jpn. 2018, 91, 1075–1111. [Google Scholar] [CrossRef]
- Jadhav, R.W.; Khobrekar, P.P.; Bugde, S.T.; Bhosale, S.V. Nanoarchitectonics of neomycin-derived fluorescent carbon dots for selective detection of Fe3+ ions. Anal. Methods 2022, 14, 3289–3298. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Nishat, Z.S.; Hossain, T.; Islam, M.N.; Phan, H.-P.; Wahab, M.A.; Moni, M.A.; Salomon, C.; Amin, M.A.; Sina, A.A.I.; Hossain, M.S.A.; et al. Hydrogel nanoarchitectonics: An evolving paradigm for ultrasensitive biosensing. Small 2022, 18, 2107571. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Pumera, M. Wearable sensors for telehealth based on emerging materials and nanoarchitectonics. npj Flex. Electron. 2023, 7, 26. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.U.; Jeon, M.J.; Kim, S.-K.; Hwang, S.-H.; Honge, M.E.; Sim, S.J. Bio-conjugated nanoarchitectonics with dual-labeled nanoparticles for a colorimetric and fluorescent dual-mode serological lateral flow immunoassay sensor in detection of SARS-CoV-2 in clinical samples. RSC Adv. 2023, 13, 27225–27232. [Google Scholar] [CrossRef]
- Joshi, V.; Hussain, S.; Dua, S.; Arora, N.; Mir, S.H.; Rydzek, G.; Senthilkumar, T. Oligomer sensor nanoarchitectonics for “turn-on” fluorescence detection of cholesterol at the nanomolar level. Molecules 2022, 27, 2856. [Google Scholar] [CrossRef]
- Singh, V.; Thamizhanban, A.; Lalitha, K.; Subbiah, D.K.; Rachamalla, A.K.; Rebaka, V.P.; Banoo, T.; Kumar, Y.; Sridharan, V.; Ahmad, A.; et al. Self-assembling nanoarchitectonics of twisted nanofibers of fluorescent amphiphiles as chemo-resistive sensor for methanol detection. Gels 2023, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J.P. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef] [PubMed]
- Vuk, D.; Radovanović-Perić, F.; Mandić, V.; Lovrinčević, V.; Rath, T.; Panžić, I.; Le-Cunff, J. Synthesis and nanoarchitectonics of novel squaraine derivatives for organic photovoltaic devices. Nanomaterials 2022, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Nakayama, T.; Ariga, K. Nanoarchitectonics intelligence with atomic switch and neuromorphic network system. Appl. Phys. Express 2022, 15, 100101. [Google Scholar] [CrossRef]
- Baek, S.; Kim, S.; Han, S.A.; Kim, Y.H.; Kim, S.; Kim, J.H. Synthesis strategies and nanoarchitectonics for high-performance transition metal dichalcogenide thin film field-effect transistors. ChemNanoMat 2023, 9, e202300104. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, D.-Q.; Wang, Y.-C.; Li, Z.-X.; Hu, S.; Huang, L.; Zhang, X.-W.; Jin, D.; Sheng, C.-X.; Xu, C.-X.; et al. Hierarchical nanoarchitectonics of ultrathin 2D organic nanosheets for aqueous processed electroluminescent devices. Small 2023, 19, 2208174. [Google Scholar] [CrossRef] [PubMed]
- Azzaroni, O.; Piccinini, E.; Fenoy, G.; Marmisollé, W.; Ariga, K. Field-effect transistors engineered via solution-based layer-by-layer nanoarchitectonics. Nanotechnology 2023, 34, 472001. [Google Scholar] [CrossRef]
- Halder, S.; Chakraborty, C. Fe(II)–Pt(II) based metallo-supramolecular macrocycle nanoarchitectonics for high-performance gel-state electrochromic device. Dye. Pigment. 2023, 212, 111131. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, Y.; Fu, F.; Liu, L.; Luo, Z. Thickness nanoarchitectonics with edge-enhanced Raman, polarization Raman, optoelectronic properties of GaS nanosheets devices. Crystals 2023, 13, 1506. [Google Scholar] [CrossRef]
- Chen, G.; Singh, S.K.; Takeyasu, K.; Hill, J.P.; Nakamura, J.; Ariga, K. Versatile nanoarchitectonics of Pt with morphology control of oxygen reduction reaction catalysts. Sci. Technol. Adv. Mater. 2022, 23, 413–423. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Xu, X.; Kang, Y.; Henzie, J.; Que, W.; Yamauchi, Y. MXene nanoarchitectonics: Defect-engineered 2D MXenes towards enhanced electrochemical water splitting. Adv. Energy Mater. 2022, 12, 2103867. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Xue, Y.; Fan, J.; Shen, S.; Hossain, M.S.A.; Amin, M.A.; Pan, L.; Xu, X.; Yamauchi, Y. Nanoarchitectonics of MXene/semiconductor heterojunctions toward artificial photosynthesis via photocatalytic CO2 reduction. Coord. Chem. Rev. 2022, 459, 214440. [Google Scholar] [CrossRef]
- Feng, J.-C.; Xia, H. Application of nanoarchitectonics in moist-electric generation. Beilstein J. Nanotechnol. 2022, 13, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, P. g-C3N4 Nanosheet nanoarchitectonics: H2 Generation and CO2 reduction. ChemNanoMat 2023, 9, e202300041. [Google Scholar] [CrossRef]
- Ravipati, M.; Badhulika, S. Solvothermal synthesis of hybrid nanoarchitectonics nickel-metal organic framework modified nickel foam as a bifunctional electrocatalyst for direct urea and nitrate fuel cell. Adv. Powder Technol. 2023, 34, 104087. [Google Scholar] [CrossRef]
- Zhang, X.; Matras-Postolek, K.; Yang, P.; Jiang, S.P. Z-scheme WOx/Cu-g-C3N4 heterojunction nanoarchitectonics with promoted charge separation and transfer towards efficient full solar-spectrum photocatalysis. J. Colloid Interface Sci. 2023, 636, 646–656. [Google Scholar] [CrossRef]
- Kumar, A.V.N.; Yin, S.; Wang, Z.; Qian, X.; Yang, D.; Xu, Y.; Li, X.; Wang, H.; Wang, L. Direct fabrication of bimetallic AuPt nanobrick spherical nanoarchitectonics for the oxygen reduction reaction. New J. Chem. 2019, 43, 9628–9633. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- Ezika, A.C.; Sadiku, E.R.; Ray, S.S.; Hamam, Y.; Folorunso, O.; Adekoya, C.J. Emerging advancements in polypyrrole MXene hybrid nanoarchitectonics for capacitive energy storage applications. J. Inorg. Organomet. Polym. 2022, 32, 1521–1540. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Wang, X.; Xu, K.; Zhong, J. Biomass-derived activated carbon nanoarchitectonics with hibiscus flowers for high-performance supercapacitor electrode applications. Chem. Eng. Technol. 2022, 45, 649–657. [Google Scholar] [CrossRef]
- Ramadass, K.; Sathish, C.; Singh, G.; Ruban, S.M.; Ruban, A.M.; Bahadur, R.; Kothandam, G.; Belperio, T.; Marsh, J.; Karakoti, A.; et al. Morphologically tunable nanoarchitectonics of mixed kaolin-halloysite derived nitrogen-doped activated nanoporous carbons for supercapacitor and CO2 capture applications. Carbon 2022, 192, 133–144. [Google Scholar] [CrossRef]
- Olivares, R.D.O.; Lobato-Peralta, D.R.; Arias, D.M.; Okolie, J.A.; Cuentas-Gallegos, A.K.; Sebastian, P.J.; Mayer, A.R.; Okoye, P.U. Production of nanoarchitectonics corncob activated carbon as electrode material for enhanced supercapacitor performance. J. Energy Storage 2022, 55, 105447. [Google Scholar] [CrossRef]
- Shinde, P.A.; Chodankar, N.R.; Kim, H.-J.; Abdelkareem, M.A.; Ghaferi, A.A.; Han, Y.K.; Olabi, A.G.; Ariga, K. Ultrastable 1T-2H WS2 heterostructures by nanoarchitectonics of phosphorus-triggered phase transition for hybrid supercapacitors. ACS Energy Lett. 2023, 8, 4474–4487. [Google Scholar] [CrossRef]
- Liu, S.; Wei, C.; Wang, H.; Yang, W.; Zhang, J.; Wang, Z.; Zhao, W.; Lee, P.S.; Ai, G. Processable nanoarchitectonics of two-dimensional metallo-supramolecular polymer for electrochromic energy storage devices with high coloration efficiency and stability. Nano Energy 2023, 110, 108337. [Google Scholar] [CrossRef]
- Li, T.; Dong, H.; Shi, Z.; Yue, H.; Yin, Y.; Li, X.; Zhang, H.; Wu, X.; Li, B.; Yang, S. Composite nanoarchitectonics with CoS2 nanoparticles embedded in graphene sheets for an anode for lithium-ion batteries. Nanomaterials 2022, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Kalidass, J.; Anandan, S.; Sivasankar, T. Sonoelectrochemical nanoarchitectonics of crystalline mesoporous magnetite @ manganese oxide nanocomposite as an alternate anode material for energy-storage applications. Crystals 2023, 13, 557. [Google Scholar] [CrossRef]
- Shin, M.; Awasthi, G.P.; Sharma, K.P.; Pandey, P.; Park, M.; Ojha, G.P.; Yu, C. Nanoarchitectonics of three-dimensional carbon nanofiber-supported hollow copper sulfide spheres for asymmetric supercapacitor applications. Int. J. Mol. Sci. 2023, 24, 9685. [Google Scholar] [CrossRef]
- Ali, N.; Funmilayo, O.R.; Khan, A.; Ali, F.; Bilal, M.; Yang, Y.; Akhter, M.S.; Zhou, C.; Wenjie, Y.; Iqbal, H.M.N. Nanoarchitectonics: Porous hydrogel as bio-sorbent for effective remediation of hazardous contaminants. J. Inorg. Organomet. Polym. 2022, 32, 3301–3320. [Google Scholar] [CrossRef]
- Ismail, K.S.I.K.; Tajudin, A.A.; Ikeno, S.; Hamzah, A.S.A. Heteroligand nanoarchitectonics of functionalized gold nanoparticle for Hg2+ detection. J. Nanopart. Res. 2022, 24, 253. [Google Scholar] [CrossRef]
- Salehipour, M.; Rezaei, S.; Asadi Khalili, H.F.; Motaharian, A.; Manzari, M.M. Nanoarchitectonics of enzyme/metal–organic framework composites for wastewater treatment. J. Inorg. Organomet. Polym. 2022, 32, 3321–3338. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, X.; Ye, B.; Zhang, W.; Zhao, Z. Biomass-derived mesoporous nanoarchitectonics with magnetic MoS2 and activated carbon for enhanced adsorption of industrial cationic dye and tetracycline contaminants. Nano 2022, 17, 2250085. [Google Scholar] [CrossRef]
- Maimaitizi, H.; Abulizi, A.; Talifu, D.; Tursun, Y. Nanoarchitectonics of chlorophyll and Mg co-modified hierarchical BiOCl microsphere as an efficient photocatalyst for CO2 reduction and ciprofloxacin degradation. Adv. Powder Technol. 2022, 33, 103562. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Shrestha, L.K.; Ariga, K. Porous carbon nanoarchitectonics for the environment: Detection and adsorption. CrystEngComm 2022, 24, 6804–6824. [Google Scholar] [CrossRef]
- Barreca, D.; Maccato, C. Nanoarchitectonics of metal oxide materials for sustainable technologies and environmental applications. CrystEngComm 2023, 25, 3968–3987. [Google Scholar] [CrossRef]
- Deng, G.; Xie, L.; Xu, S.; Kang, X.; Ma, J. Fiber nanoarchitectonics for pre-treatments in facile detection of short-chain fatty acids in waste water and faecal samples. Polymers 2021, 13, 3906. [Google Scholar] [CrossRef]
- Si, R.; Chen, Y.; Wang, D.; Yu, D.; Ding, Q.; Li, R.; Wu, C. Nanoarchitectonics for high adsorption capacity carboxymethyl cellulose nanofibrils-based adsorbents for efficient Cu2+ removal. Nanomaterials 2022, 12, 160. [Google Scholar] [CrossRef]
- Ashraf, I.; Li, R.; Chen, B.; Al-Ansari, N.; Aslam, M.R.; Altaf, A.R.; Elbeltagi, A. Nanoarchitectonics and kinetics insights into fluoride removal from drinking water using magnetic tea biochar. Int. J. Environ. Res. Public Health 2022, 19, 13092. [Google Scholar] [CrossRef]
- Shao, Y.; Xiang, L.; Zhang, W.; Chen, Y. Responsive shape-shifting nanoarchitectonics and its application in tumor diagnosis and therapy. J. Control. Release 2022, 352, 600–618. [Google Scholar] [CrossRef]
- Kumbhar, P.; Kolekar, K.; Khot, C.; Dabhole, S.; Salawi, A.; Sabei, F.A.; Mohite, A.; Kole, K.; Mhatre, S.; Jha, N.K.; et al. Co-crystal nanoarchitectonics as an emerging strategy in attenuating cancer: Fundamentals and applications. J. Control. Release 2023, 353, 1150–1170. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J.; Guo, S.; Li, A.; Wan, J.-B. Macromolecule-based hydrogels nanoarchitectonics with mesenchymal stem cells for regenerative medicine: A review. Int. J. Biol. Macromol. 2023, 243, 125161. [Google Scholar] [CrossRef]
- Aziz, T.; Nadeem, A.A.; Sarwar, A.; Perveen, I.; Hussain, N.; Khan, A.A.; Daudzai, Z.; Cui, H.; Lin, L. Particle nanoarchitectonics for nanomedicine and nanotherapeutic drugs with special emphasis on nasal drugs and aging. Biomedicines 2023, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Sutrisno, L.; Ariga, K. Pore-engineered nanoarchitectonics for cancer therapy. NPG Asia Mater. 2023, 15, 21. [Google Scholar] [CrossRef]
- Li, B.; Huang, Y.; Zou, Q. Peptide-based nanoarchitectonics for the treatment of liver fibrosis. ChemBioChem 2023, 24, e202300002. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Baek, S.; Sluyter, R.; Konstantinov, K.; Kim, J.H.; Kim, S.; Kim, Y.H. Wearable and implantable bioelectronics as eco-friendly and patient-friendly integrated nanoarchitectonics for next-generation smart healthcare technology. EcoMat 2023, 5, e12356. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Xu, Y.; Zhang, X.; Cui, Y.; Liang, Q.; Liu, C.; Wang, X.; Wu, S.; Yang, R. Single Nano-sized metal–organic framework for bio-nanoarchitectonics with in vivo fluorescence imaging and chemo-photodynamic therapy. Nanomaterials 2022, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, L.; Shi, Z.; Chen, J. Micro/nanoarchitectonics of 3D printed scaffolds with excellent biocompatibility prepared using femtosecond laser two-photon polymerization for tissue engineering applications. Nanomaterials 2022, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Carrara, S.; Rouvier, F.; Auditto, S.; Brunel, F.; Jeanneau, C.; Camplo, M.; Sergent, M.; About, I.; Bolla, J.-M.; Raimundo, J.-M. Nanoarchitectonics of electrically activable phosphonium self-assembled monolayers to efficiently kill and tackle bacterial infections on demand. Int. J. Mol. Sci. 2022, 23, 2183. [Google Scholar] [CrossRef]
- Kulikov, O.A.; Zharkov, M.N.; Ageev, V.P.; Yakobson, D.E.; Shlyapkina, V.I.; Zaborovskiy, A.V.; Inchina, V.I.; Balykova, L.A.; Tishin, A.M.; Sukhorukov, G.B.; et al. Magnetic Hyperthermia Nanoarchitectonics via iron oxide nanoparticles stabilised by oleic acid: Anti-tumour efficiency and safety evaluation in animals with transplanted carcinoma. Int. J. Mol. Sci. 2022, 23, 4234. [Google Scholar] [CrossRef]
- Do, T.T.A.; Wicaksono, K.; Soendoro, A.; Imae, T.; Garcia-Celma, M.J.; Grijalvo, S. Complexation nanoarchitectonics of carbon dots with doxorubicin toward photodynamic anti-cancer therapy. J. Funct. Biomater. 2022, 13, 219. [Google Scholar] [CrossRef]
- Jayachandran, P.; Ilango, S.; Suseela, V.; Nirmaladevi, R.; Shaik, M.R.; Khan, M.; Khan, M.; Shaik, B. Green synthesized silver nanoparticle-loaded liposome-based nanoarchitectonics for cancer management: In Vitro drug release analysis. Biomedicines 2023, 11, 217. [Google Scholar] [CrossRef]
- Papadopoulou-Fermeli, N.; Lagopati, N.; Pippa, N.; Sakellis, E.; Boukos, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Composite nanoarchitectonics of photoactivated titania-based materials with anticancer properties. Pharmaceutics 2023, 15, 135. [Google Scholar] [CrossRef]
- Osada, M.; Sasaki, T. Nanoarchitectonics in dielectric/ferroelectric layered perovskites: From bulk 3D systems to 2D nanosheets. Dalton Trans. 2018, 47, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, C.; Lambricht, L.; Préat, V.; Cornu, O.; Dupont-Gillain, C.; van der Straeten, A. Layer-by-layer nanoarchitectonics using protein–polyelectrolyte complexes toward a generalizable tool for protein surface immobilization. Langmuir 2022, 38, 5579–5589. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. Chemistry of materials nanoarchitectonics for two-dimensional films: Langmuir–Blodgett, layer-by-layer assembly, and newcomers. Chem. Mater. 2023, 35, 5233–5254. [Google Scholar] [CrossRef]
- Banszerus, L.; Schmitz, M.; Engels, S.; Dauber, J.; Oellers, M.; Haupt, F.; Watanabe, K.; Taniguchi, T.; Beschoten, B.; Stampfer, C. Ultrahigh-mobility graphene devices from chemical vapor deposition on reusable copper. Sci. Adv. 2015, 1, e1500222. [Google Scholar] [CrossRef]
- Zhou, X.; Gan, L.; Tian, W.; Zhang, Q.; Jin, S.; Li, H.; Bando, Y.; Golberg, D.; Zhai, T. Ultrathin SnSe2 flakes grown by chemical vapor deposition for high-performance photodetectors. Adv. Mater. 2015, 27, 8035–8041. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Uno, K.; Jinno, R.; Fujita, S. Prospects for phase engineering of semi-stable Ga2O3 semiconductor thin films using mist chemical vapor deposition. J. Appl. Phys. 2022, 131, 090902. [Google Scholar] [CrossRef]
- Murata, T.; Minami, K.; Yamazaki, T.; Sato, T.; Koinuma, H.; Ariga, K.; Matsuki, N. Nanometer-flat DNA-featured thin films prepared via laser molecular beam deposition under high-vacuum for selective methanol sensing. Bull. Chem. Soc. Jpn. 2023, 96, 29–34. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Rydzek, G.; Ji, Q.; Li, M.; Schaaf, P.; Hill, J.P.; Boulmedais, F.; Ariga, K. Electrochemical nanoarchitectonics and layer-by-layer assembly: From basics to future. Nano Today 2015, 10, 138–167. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Park, M.J.; Yu, H.; Matsuyama, H.; Drioli, E.; Shon, H.K. Recent advances of nanocomposite membranes using layer-by-layer assembly. J. Membr. Sci. 2022, 661, 120926. [Google Scholar] [CrossRef]
- Ariga, K.; Lvov, Y.; Decher, G. There is still plenty of room for layer-by-layer assembly for constructing nanoarchitectonics-based materials and devices. Phys. Chem. Chem. Phys. 2022, 24, 4097–4115. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-W.; Kim, J.-S.; Kim, T.-H.; Hong, Y.J.; Kang, Y.C.; Lee, J.-H. A new strategy for humidity independent oxide chemiresistors: Dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small 2016, 12, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, L.; Li, H.; Wang, T.; Ren, X.; Cheng, Y.; Li, Y.; Huang, Q. Layer-by-layer assembled smart antibacterial coatings via mussel-inspired polymerization and dynamic covalent chemistry. Adv. Healthc. Mater. 2022, 11, 2200112. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Ohshiro, K.; Watanabe, T.; Hyeon-Deuk, K.; Kim, D. Temperature-dependent exciton dynamics in CdTe quantum dot superlattices fabricated via layer-by-layer assembly. Adv. Opt. Mater. 2022, 10, 2102781. [Google Scholar] [CrossRef]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999, 71, 779–819. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J.P. 25th Anniversary article: What can be done with the Langmuir-Blodgett method? Recent developments and its critical role in materials science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef]
- Ariga, K. Don’t forget Langmuir–Blodgett films 2020: Interfacial nanoarchitectonics with molecules, materials, and living objects. Langmuir 2020, 36, 7158–7180. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir–Blodgett films. Nat. Nanotechnol. 2008, 3, 538–542. [Google Scholar] [CrossRef]
- Fang, C.; Yoon, I.; Hubble, D.; Tran, T.-N.; Kostecki, R.; Liu, G. Recent applications of Langmuir–Blodgett technique in battery research. ACS Appl. Mater. Interfaces 2022, 14, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.N., Jr.; Caseli, L.; Ariga, K. The Past and the future of Langmuir and Langmuir–Blodgett films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Kasuya, Y.; Ji, Q.; Sathish, M.; Shrestha, L.K.; Ishihara, S.; Minami, K.; Morita, H.; Yamazaki, T.; Hanagata, N.; et al. Vortex-aligned fullerene nanowhiskers as a scaffold for orienting cell growth. ACS Appl. Mater. Interfaces 2015, 7, 15667–15673. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yamashita, Y.; Tsuneda, Y.; Mori, T.; Takeya, J.; Watanabe, S.; Ariga, K. 100 °C-Langmuir–Blodgett method for fabricating highly oriented, ultrathin films of polymeric semiconductors. ACS Appl. Mater. Interfaces 2020, 12, 56522–56529. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yamashita, Y.; Mori, T.; Chiba, M.; Futae, T.; Takeya, J.; Watanabe, S.; Ariga, K. Hyper 100 °C Langmuir–Blodgett (Langmuir–Schaefer) technique for organized ultrathin film of polymeric semiconductors. Langmuir 2022, 38, 5237–5247. [Google Scholar] [CrossRef]
- Ariga, K. Materials nanoarchitectonics in a two-dimensional world within a nanoscale distance from the liquid phase. Nanoscale 2022, 14, 10610–10629. [Google Scholar] [CrossRef]
- Ariga, K. Liquid–liquid interfacial nanoarchitectonics. Small 2023, 2305636. [Google Scholar] [CrossRef]
- Negi, S.; Hamori, M.; Kitagishi, H.; Kano, K. Highly ordered monolayers of an optically active amphiphilic pyrene derivative at the air–water interface. Bull. Chem. Soc. Jpn. 2022, 95, 1537–1545. [Google Scholar] [CrossRef]
- Negi, S.; Hamori, M.; Kubo, Y.; Kitagishi, H.; Kano, K. Monolayer formation and chiral recognition of binaphthyl amphiphiles at the air–water interface. Bull. Chem. Soc. Jpn. 2023, 96, 48–56. [Google Scholar] [CrossRef]
- Takase, S.; Aritsu, T.; Sakamoto, Y.; Sakuno, Y.; Shimizu, Y. Preparation of highly conductive phthalocyaninato-cobalt iodide at the interface between aqueous KI solution and organic solvent and catalytic properties for electrochemical reduction of CO2. Bull. Chem. Soc. Jpn. 2023, 96, 649–653. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Ishihara, S.; Kawakami, K.; Hill, J.P. Bridging the difference to the billionth-of-a-meter length scale: How to operate nanoscopic machines and nanomaterials by using macroscopic actions. Chem. Mater. 2014, 26, 519–532. [Google Scholar] [CrossRef]
- Adachi, J.; Naito, M.; Sugiura, S.; Le, N.H.-T.; Nishimura, S.; Huang, S.; Suzuki, S.; Kawamorita, S.; Komiya, N.; Hill, J.P.; et al. Coordination amphiphile: Design of planar-coordinated platinum complexes for monolayer formation at an air-water interface based on ligand characteristics and molecular topology. Bull. Chem. Soc. Jpn. 2022, 95, 889–897. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Hill, J.P. Mechanical control of nanomaterials and nanosystems. Adv. Mater. 2012, 24, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. Molecular machines and microrobots: Nanoarchitectonics developments and on-water performances. Micromachines 2023, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Mori, T.; Inoue, R.; Naito, M.; Ngoc Le, N.H.-T.; Kawamorita, S.; Hill, J.P.; Naota, T.; Ariga, K. Emission control by molecular manipulation of double-paddled binuclear PtII complexes at the air-water interface. Chem. Asian J. 2020, 15, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Mori, T.; Ikeshita, M.; Ma, S.C.; Muller, G.; Ariga, K.; Naota, T. Vortex flow-controlled circularly polarized luminescence of achiral Pt(II) complex aggregates assembled at the air-water interface. Small Methods 2022, 6, 2200936. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.-G.; Fink, Z.; Chen, Z.; Emrick, T.; Russell, T.P. Bottlebrush polymers at liquid interfaces: Assembly dynamics, mechanical properties, and all-liquid printed constructs. ACS Nano 2023, 17, 14731–14741. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.C.; Valentine, M.J.; Baiz, C.R. Ultrafast dynamics at lipid–water interfaces. Acc. Chem. Res. 2020, 53, 1860–1868. [Google Scholar] [CrossRef]
- Hammel, M.; Tainer, J.A. X-ray scattering reveals disordered linkers and dynamic interfaces in complexes and mechanisms for DNA double-strand break repair impacting cell and cancer biology. Protein Sci. 2021, 30, 1735–1756. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Roeters, S.J.; Bonn, M.; Peukert, W.; Woutersen, S.; Weidner, T. Structure and dynamics of interfacial peptides and proteins from vibrational sum-frequency generation spectroscopy. Chem. Rev. 2020, 120, 3420–3465. [Google Scholar] [CrossRef]
- Agashe, C.; Varshney, R.; Sangwan, R.; Gill, A.K.; Alam, M.; Patra, D. Anisotropic compartmentalization of the liquid–liquid interface using dynamic imine chemistry. Langmuir 2022, 38, 8296–8303. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Shao, F.; Szczerbiński, J.; McCaffrey, R.; Zenobi, R.; Jin, Y.; Schlüter, A.D.; Zhang, W. Synthesis of a two-dimensional covalent organic monolayer through dynamic imine chemistry at the air/water interface. Angew. Chem. Int. Ed. 2016, 55, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Makiura, R.; Motoyama, S.; Umemura, Y.; Yamanaka, H.; Sakata, O.; Kitagawa, H. Surface nano-architecture of a metal–organic framework. Nat. Mater. 2010, 9, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Sakamoto, R.; Kusamoto, T.; Pal, T.; Fukui, N.; Hoshiko, K.; Shimojima, T.; Wang, Z.; Hirahara, T.; Ishizaka, K.; et al. Redox control and high conductivity of nickel bis(dithiolene) complex p-nanosheet: A potential organic two-dimensional topological insulator. J. Am. Chem. Soc. 2014, 136, 14357–14360. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Pal, M.; Rou, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wang, C.; Dong, Y.; Guo, P.; Cheng, P.; Fu, Y.; Liu, D.; He, D.; Das, S.; Negishi, Y. Ion-selective covalent organic framework membranes as a catalytic polysulfide trap to arrest the redox shuttle effect in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2022, 14, 4079–4090. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, Q.; He, W.; Liu, L.; Song, Z.; Yao, A.; Ma, J.; Cao, D.; Hassan, S.U.; Guan, J.; et al. A 2D soft covalent organic framework membrane prepared via a molecular bridge. Adv. Mater. 2023, 35, 2300975. [Google Scholar] [CrossRef]
- Ohata, T.; Tachimoto, K.; Takeno, K.J.; Nomoto, A.; Watanabe, T.; Hirosawa, I.; Makiura, R. Influence of the solvent on the assembly of Ni3(hexaiminotriphenylene)2 metal–organic framework nanosheets at the air/liquid interface. Bull. Chem. Soc. Jpn. 2023, 96, 274–282. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotecnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Song, J.; Murata, T.; Tsai, K.-C.; Jia, X.; Sciortino, F.; Ma, R.; Yamauchi, Y.; Hill, J.P.; Shrestha, L.K.; Ariga, K. Fullerphene nanosheets: A bottom-Up 2D material for single-carbon-atom-level molecular discrimination. Adv. Mater. Interfaces 2022, 9, 2102241. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Lei, Y.; Yu, H.; Lin, Y.; Terrones, M. Biomimetic construction of ferrite quantum dot/graphene heterostructure for enhancing ion/charge transfer in supercapacitors. Adv. Mater. 2023, 35, 2300940. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Tanaka, H.; Dalui, A.; Mitoma, N.; Suzuki, K.; Matsumoto, M.; Aggarwal, N.; Patnaik, A.; Acharya, S.; Shrestha, L.K.; et al. Carbon nanosheets by morphology-retained carbonization of two-dimensional assembled anisotropic carbon nanorings. Angew. Chem. Int. Ed. 2018, 57, 9679–9683. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, L.; Chen, R.; You, J.; Ma, Y.; Wang, J.; Wu, Y.; Liu, B.; Zhao, K.; Chen, J.; et al. Spontaneous interface healing by a dynamic liquid-crystal transition for high-performance perovskite solar cells. Adv. Mater. 2022, 34, 2207362. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.; Kim, J.; Hosono†, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A. The lithium-ion battery: Two breakthroughs in development and two reasons for the Nobel Prize. Bull. Chem. Soc. Jpn. 2022, 95, 195–197. [Google Scholar] [CrossRef]
- Kondo, Y.; Abe, T.; Yamada, Y. Kinetics of interfacial ion transfer in lithium-ion batteries: Mechanism understanding and improvement strategies. ACS Appl. Mater. Interfaces 2022, 14, 22706–22718. [Google Scholar] [CrossRef]
- Qiu, Y.-F.; Murayama, H.; Fujitomo, C.; Kawai, S.; Haruta, A.; Hiasa, T.; Mita, H.; Motohashi, K.; Yamamoto, E.; Tokunaga, M. Oxidative decomposition mechanism of ethylene carbonate on positive electrodes in lithium-ion batteries. Bull. Chem. Soc. Jpn. 2023, 96, 444–451. [Google Scholar] [CrossRef]
- Matsumoto, F.; Yamada, M.; Tsuta, M.; Nakamura, S.; Ando, N.; Soma, N. Review of the structure and performance of through-holed anodes and cathodes prepared with a picosecond pulsed laser for lithium-ion batteries. Int. J. Extrem. Manuf. 2023, 5, 012001. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Hosaka, T.; Komaba, S. Development of nonaqueous electrolytes for high-voltage K-ion batteries. Bull. Chem. Soc. Jpn. 2022, 95, 569–581. [Google Scholar] [CrossRef]
- Kim, E.J.; Kumar, P.R.; Gossage, Z.T.; Kubota, K.; Hosaka, T.; Tatara, R.; Komaba, S. Active material and interphase structures governing performance in sodium and potassium ion batteries. Chem. Sci. 2022, 13, 6121–6158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Muramatsu, T.; Kheawhom, S.; Wattanakit, C.; Yonezawa, T. Impact of morphology and transition metal doping of vanadate nanowires without surface modification on the performance of aqueous zinc-ion batteries. Bull. Chem. Soc. Jpn. 2022, 95, 728–734. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Y.; Yang, H.; Zhou, H. Regulating water activity for rechargeable zinc-ion batteries: Progress and perspective. ACS Energy Lett. 2022, 7, 2515–2530. [Google Scholar] [CrossRef]
- Ganesan, P.; Ishihara, A.; Staykov, A.; Nakashima, N. Recent advances in nanocarbon-based nonprecious metal catalysts for oxygen/hydrogen reduction/evolution reactions and Zn-air battery. Bull. Chem. Soc. Jpn. 2023, 96, 429–443. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Ganesan, S.; Nguyen, M.T.; Yonezawa, T.; Praserthdam, S.; Pornprasertsuk, R.; Kheawhom, S. Critical roles of metal–organic frameworks in improving the Zn anode in aqueous zinc-ion batteries. Chem. Eng. J. 2023, 457, 141334. [Google Scholar] [CrossRef]
- Guo, Z.; Fan, L.; Zhao, C.; Chen, A.; Liu, N.; Zhang, Y.; Zhang, N. A dynamic and self-adapting interface coating for stable Zn-metal anodes. Adv. Mater. 2022, 34, 2105133. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis of fullerene nanowhiskers using the liquid–liquid interfacial precipitation method and their mechanical, electrical and superconducting properties. Sci. Technol. Adv. Mater. 2015, 16, 013502. [Google Scholar] [CrossRef]
- Miyazawa, K.; Kuwasaki, Y.; Obayashi, A.; Kuwabara, M. C60 nanowhiskers formed by the liquid–liquid interfacial precipitation method. J. Mater. Res. 2002, 17, 83–88. [Google Scholar] [CrossRef]
- Koya, I.; Yokoyama, Y.; Sakka, T.; Nishi, N. Formation of Au nanofiber/fullerene nanowhisker 1D/1D composites via reductive deposition at the interface between an ionic liquid and water. Chem. Lett. 2022, 51, 643–645. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K. Size-tunable hexagonal fullerene (C60) nanosheets at the liquid−liquid interface. J. Am. Chem. Soc. 2007, 129, 13816–13817. [Google Scholar] [CrossRef]
- Chen, G.; Shrestha, L.K.; Ariga, K. Zero-to-two nanoarchitectonics: Fabrication of two-dimensional materials from zero-dimensional fullerene. Molecules 2021, 26, 4636. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yoon, E.; Kawano, M.; Joo, T.; Choi, H.C. Self-crystallization of C70 cubes and remarkable enhancement of photoluminescence. Angew. Chem. Int. Ed. 2010, 49, 9670–9675. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.; Choi, H.C. Selective growth of a C70 crystal in a mixed solvent system: From cube to tube. Chem. Mater. 2015, 27, 2408–2413. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics for hierarchical fullerene nanomaterials. Nanomaterials 2021, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-M.; Hu, J.-S.; Guo, Y.-G.; Zheng, S.-F.; Zhong, L.-S.; Song, W.-G.; Wan, L.-J. Tin-nanoparticles encapsulated in elastic hollow carbon spheres for high-performance anode material in lithium-ion batteries. Adv. Mater. 2008, 20, 1160–1165. [Google Scholar] [CrossRef]
- Tan, H.; Li, Y.; Kim, J.; Takei, T.; Wang, Z.; Xu, X.; Wang, J.; Bando, Y.; Kang, Y.-M.; Tang, J.; et al. Sub-50 nm iron–nitrogen-doped hollow carbon sphere-encapsulated iron carbide nanoparticles as efficient oxygen reduction catalysts. Adv. Sci. 2018, 5, 1800120. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. PdAg Alloy nanoparticles encapsulated in N-doped microporous hollow carbon spheres for hydrogenation of CO2 to formate. Appl. Catal. B Environ. 2021, 283, 119628. [Google Scholar] [CrossRef]
- Chen, G.; Sciortino, F.; Takeyasu, K.; Nakamura, J.; Hill, J.P.; Shrestha, L.K.; Ariga, K. Hollow spherical fullerene obtained by kinetically controlled liquid-liquid interfacial precipitation. Chem. Asian J. 2022, 17, e20220075. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Hsu, S.-h.; Maji, S.; Chahal, M.K.; Song, J.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Post-assembly dimension-dependent face-selective etching of fullerene crystals. Mater. Horiz. 2020, 7, 787–795. [Google Scholar] [CrossRef]
- Chen, G.; Bhadra, B.N.; Sutrisno, L.; Shrestha, L.K.; Ariga, K. Fullerene rosette: Two-dimensional interactive nanoarchitectonics and selective vapor sensing. Int. J. Mol. Sci. 2022, 23, 5454. [Google Scholar] [CrossRef]
- Wei, Z.; Song, J.; Ma, R.; Ariga, K.; Shrestha, L.K. Self-assembled corn-husk-shaped fullerene crystals as excellent acid vapor sensors. Chemosensors 2022, 10, 16. [Google Scholar] [CrossRef]
- Tang, Q.; Bairi, P.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Zeng, H.; Ji, Q.; Shrestha, L.K. Quasi 2D mesoporous carbon microbelts derived from fullerene crystals as an electrode material for electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 44458–44465. [Google Scholar] [CrossRef] [PubMed]
- Samukawa, S. Ultimate top-down etching processes for future nanoscale devices: Advanced neutral-beam etching. Jpn. J. Appl. Phys. 2006, 45, 2395. [Google Scholar] [CrossRef]
- Pennelli, G. Top down fabrication of long silicon nanowire devices by means of lateral oxidation. Microelectron. Eng. 2009, 86, 2139–2143. [Google Scholar] [CrossRef]
- Urs, K.M.B.; Sahoo, K.; Bhat, N.; Kamble, V. Complementary metal oxide semiconductor-compatible top-down fabrication of a Ni/NiO nanobeam room temperature hydrogen sensor device. ACS Appl. Electron. Mater. 2022, 4, 87–91. [Google Scholar] [CrossRef]
- Balzani, V.; Credi, A.; Venturi, M. The bottom-up approach to molecular-level devices and machines. Chem. Eur. J. 2022, 8, 5524–5532. [Google Scholar] [CrossRef]
- Li, C.; Huixia Feng, H.; Xu, H.; Chen, B.; Yang, T. An intelligent superhydrophilic/underwater superoleophobic temperature sensitive switch device with excellent targeted oil-water separation performance. Bull. Chem. Soc. Jpn. 2022, 95, 532–537. [Google Scholar] [CrossRef]
- Arjmand, T.; Legallais, M.; Nguyen, T.T.T.; Serre, P.; Vallejo-Perez, M.; Morisot, F.; Salem, B.; Ternon, C. Functional devices from bottom-up silicon nanowires: A Review. Nanomaterials 2022, 12, 1043. [Google Scholar] [CrossRef]
- Saito, Y.; Sasabe, H.; Tsuneyama, H.; Abe, S.; Matsuya, M.; Kawano, T.; Kori, Y.; Hanayama, T.; Kido, J. Quinoline-modified phenanthroline electron-transporters as n-type exciplex partners for highly efficient and stable deep-red OLEDs. Bull. Chem. Soc. Jpn. 2023, 96, 24–28. [Google Scholar] [CrossRef]
- Matsuya, M.; Sasabe, H.; Sumikoshi, S.; Hoshi, K.; Nakao, K.; Kumada, K.; Sugiyama, R.; Sato, R.; Kido, J. Highly luminescent aluminum complex with β-diketone ligands exhibiting near-unity photoluminescence quantum yield, thermally activated delayed fluorescence, and rapid radiative decay rate properties in solution-processed organic light-emitting devices. Bull. Chem. Soc. Jpn. 2023, 96, 183–189. [Google Scholar] [CrossRef]
- Ishii, M.; Yamashita, Y.; Watanabe, S.; Ariga, K.; Takeya, J. Doping of molecular semiconductors through proton-coupled electron transfer. Nature 2023, 622, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nakano, S.; Shigeta, Y. Dynamical interaction analysis of proteins by a random forest-fragment molecular orbital (RF-FMO) method and application to Src tyrosine kinase. Bull. Chem. Soc. Jpn. 2023, 96, 42–47. [Google Scholar] [CrossRef]
- Mahmood, A.; Sandali, Y.; Wang, J.-L. Easy and fast prediction of green solvents for small molecule donor-based organic solar cells through machine learning. Phys. Chem. Chem. Phys. 2023, 25, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiao, C.; Zhou, P.; Li, W.; Zang, Y.; Liu, Y.; Yang, G.; Liu, L.; Cheng, J.; Liang, G.; et al. Highly efficient perovskite solar cells with light management of surface antireflection. Bull. Chem. Soc. Jpn. 2023, 96, 148–155. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, D.; Wang, D.; Luan, H.; Chen, X.; Yan, W. In Situ polymerized MXene/polypyrrole/hydroxyethyl cellulose-based flexible strain sensor enabled by machine learning for handwriting recognition. ACS Appl. Mater. Interfaces 2023, 15, 5811–5821. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Nawachi, A.; Kondo, Y.; Choi, J.; Morimoto, H.; Ohshima, T. Functional group evaluation kit for digitalization of information on the functional group compatibility and chemoselectivity of organic reactions. Bull. Chem. Soc. Jpn. 2023, 96, 465–474. [Google Scholar] [CrossRef]
- Ramprasad, R.; Batra, R.; Pilania, G.; Mannodi-Kanakkithodi, A.; Kim, C. Machine learning in materials informatics: Recent applications and prospects. npj Comput. Mater. 2017, 3, 54. [Google Scholar] [CrossRef]
- Agrawala, A.; Choudhary, A. Perspective: Materials informatics and big data: Realization of the “fourth paradigm” of science in materials science. APL Mater. 2016, 4, 053208. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Umeki, M.; Adachi, H.; Kuwata, N.; Hasegawa, G.; Oyaizu, K. Exploration of organic superionic glassy conductors by process and materials informatics with lossless graph database. npj Comput. Mater. 2022, 8, 170. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Yamauchi, Y.; Ariga, K. Material evolution with nanotechnology, nanoarchitectonics, and materials informatics: What will be the next paradigm shift in nanoporous materials? Adv. Mater. 2022, 34, 2107212. [Google Scholar] [CrossRef]
- Oviedo, L.R.; Oviedo, V.R.; Martins, M.O.; Fagan, S.B.; da Silva, W.L. Nanoarchitectonics: The role of artificial intelligence in the design and application of nanoarchitectures. J. Nanopart. Res. 2022, 24, 157. [Google Scholar] [CrossRef]









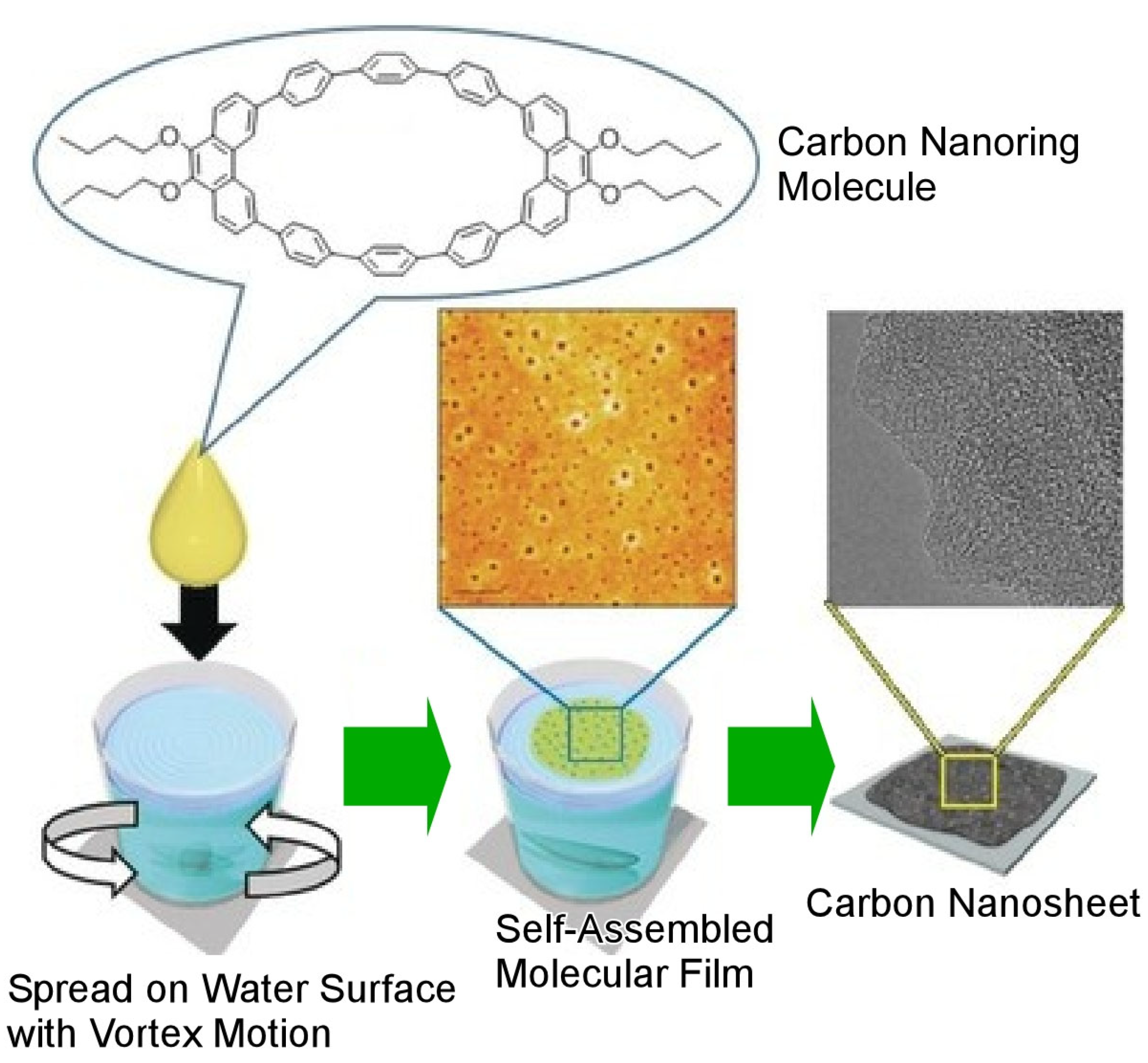






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariga, K. Materials Nanoarchitectonics at Dynamic Interfaces: Structure Formation and Functional Manipulation. Materials 2024, 17, 271. https://doi.org/10.3390/ma17010271
Ariga K. Materials Nanoarchitectonics at Dynamic Interfaces: Structure Formation and Functional Manipulation. Materials. 2024; 17(1):271. https://doi.org/10.3390/ma17010271
Chicago/Turabian StyleAriga, Katsuhiko. 2024. "Materials Nanoarchitectonics at Dynamic Interfaces: Structure Formation and Functional Manipulation" Materials 17, no. 1: 271. https://doi.org/10.3390/ma17010271
APA StyleAriga, K. (2024). Materials Nanoarchitectonics at Dynamic Interfaces: Structure Formation and Functional Manipulation. Materials, 17(1), 271. https://doi.org/10.3390/ma17010271





