Recycle Option for Municipal Solid Waste Incineration Fly Ash (MSWIFA) as a Partial Replacement for Cement in Mortars Containing Calcium Sulfoaluminate Cement (CSA) and Portland Cement to Save the Environment and Natural Resources
Abstract
:1. Introduction
1.1. Municipal Solid Waste Incineration Fly Ash Challenges
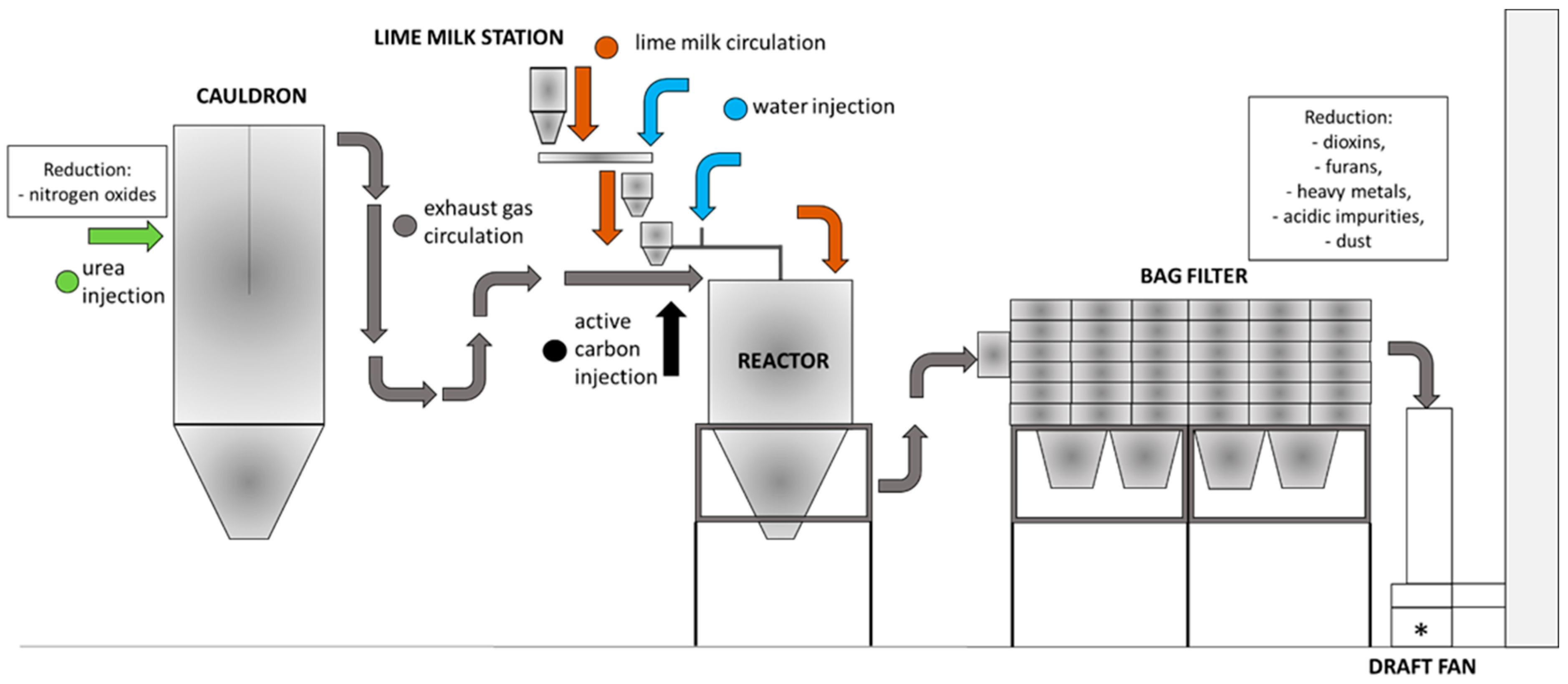
1.2. Place of Arising and Composition of Fly Ash in the Waste Incineration Plant and Flue Gas Cleaning System
- -
- Acid pollution reduction systems;
- -
- Heavy metal, dioxin, and furan reduction systems;
- -
- Nitrogen oxide removal systems;
- -
- Exhaust gas dedusting system, which may consist of electrostatic precipitators, fabric filters, and cyclones.
1.3. Concrete with MSWIFA as an Eco-Product
2. Materials
3. Methods
4. Results
4.1. MSWIFA Heavy Metal Content Determination
4.2. MSWIFA Leachability Tests
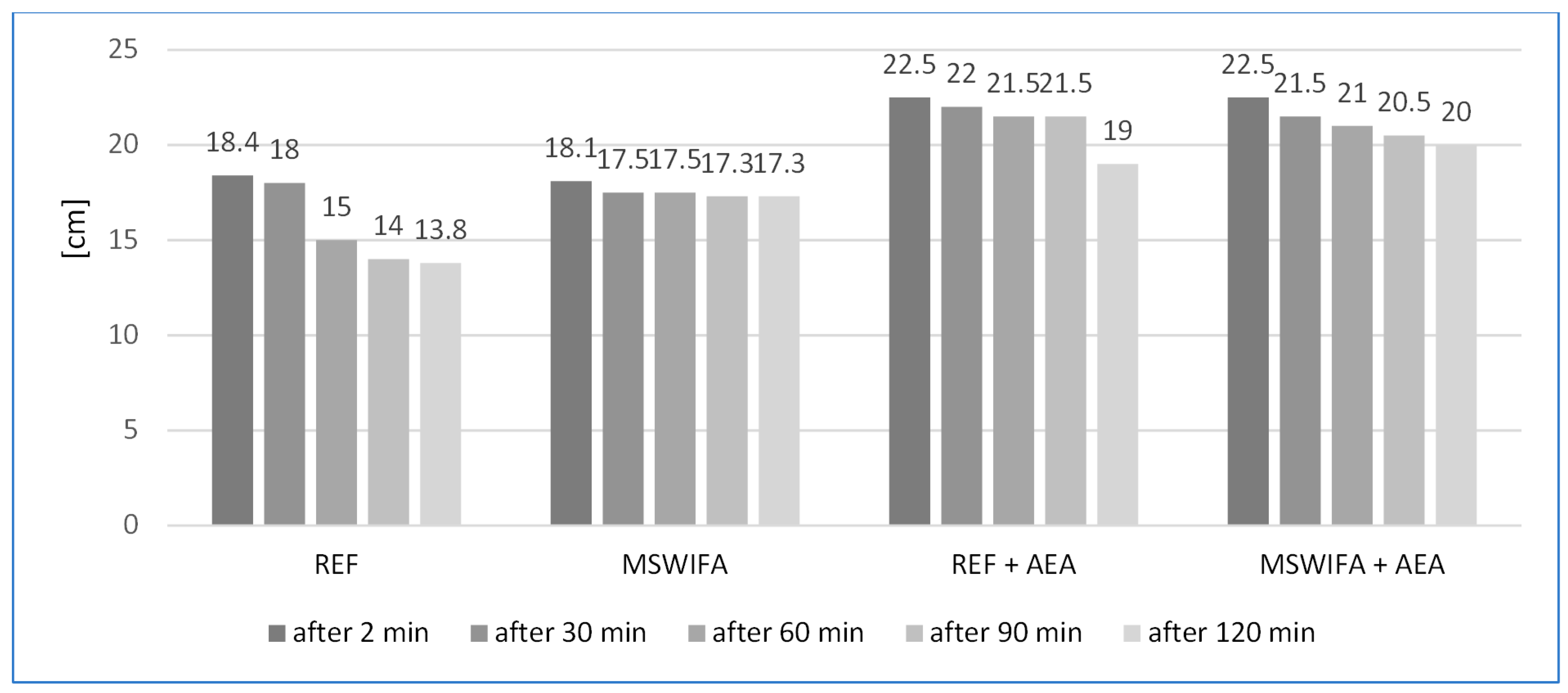
4.3. Consistency of Mortars and Its Maintenance over Time
4.4. Air Content and Its Maintenance over Time
4.5. Initial Setting Time
4.6. Water Demand
4.7. Soundness
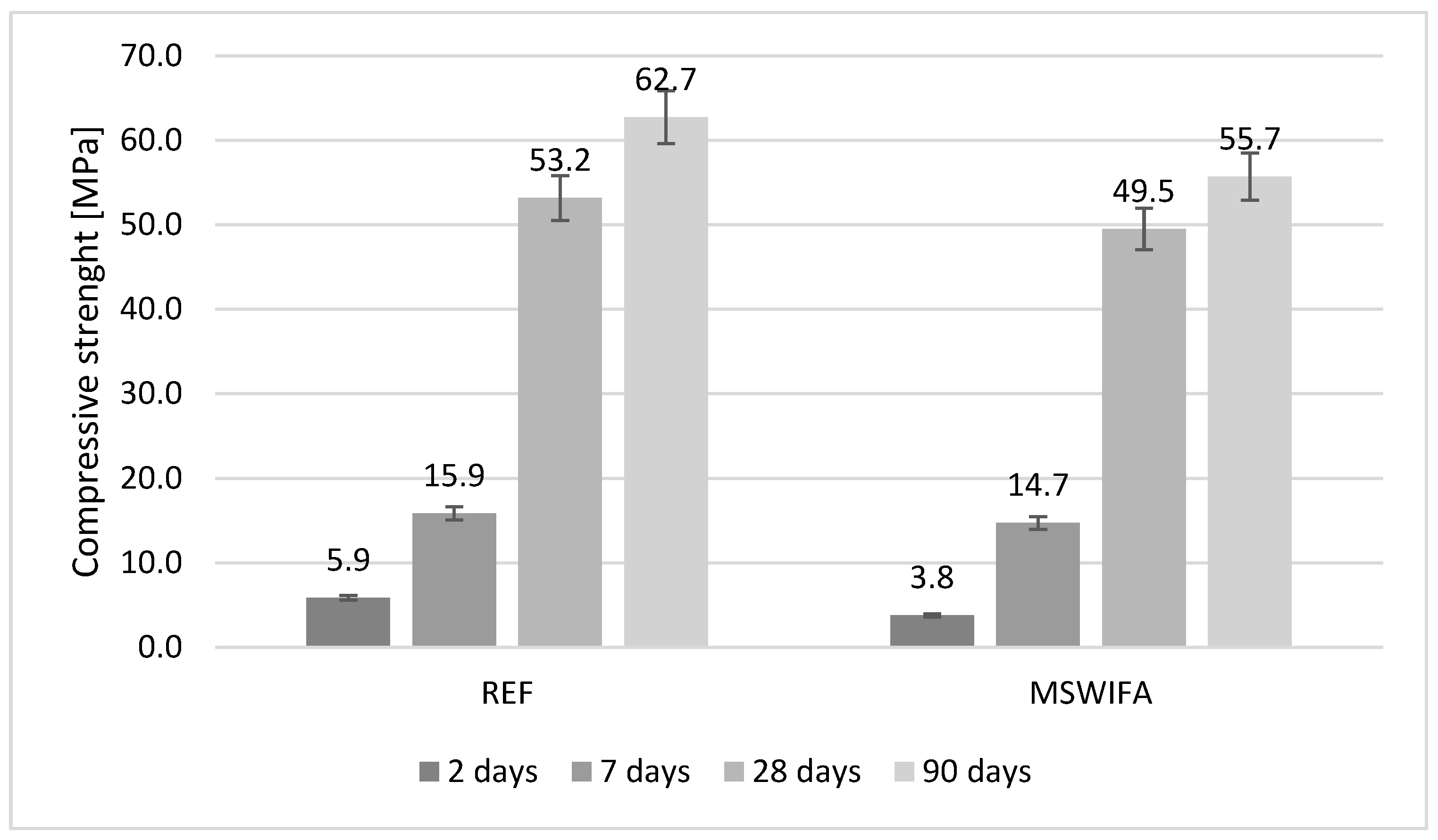
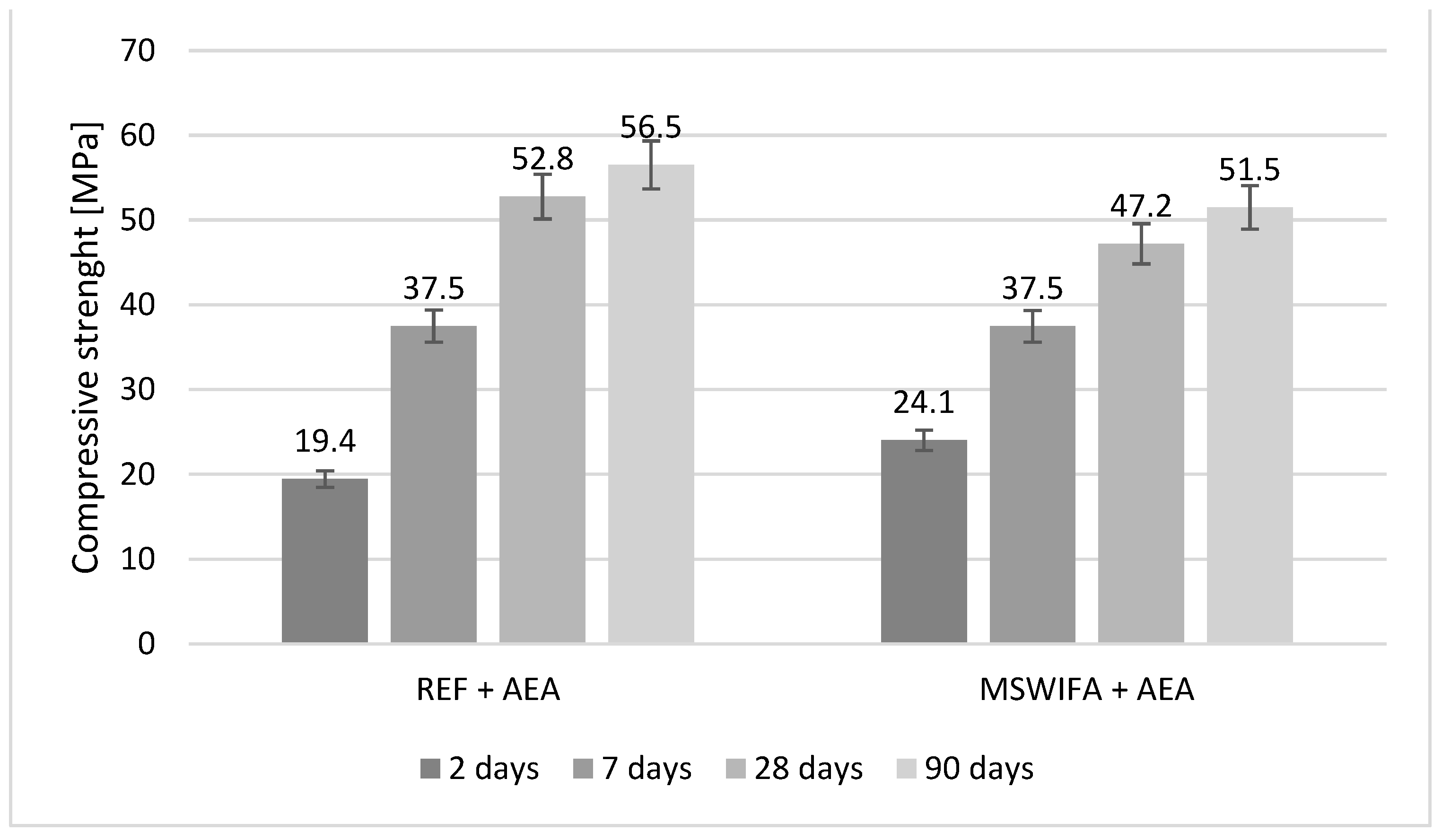
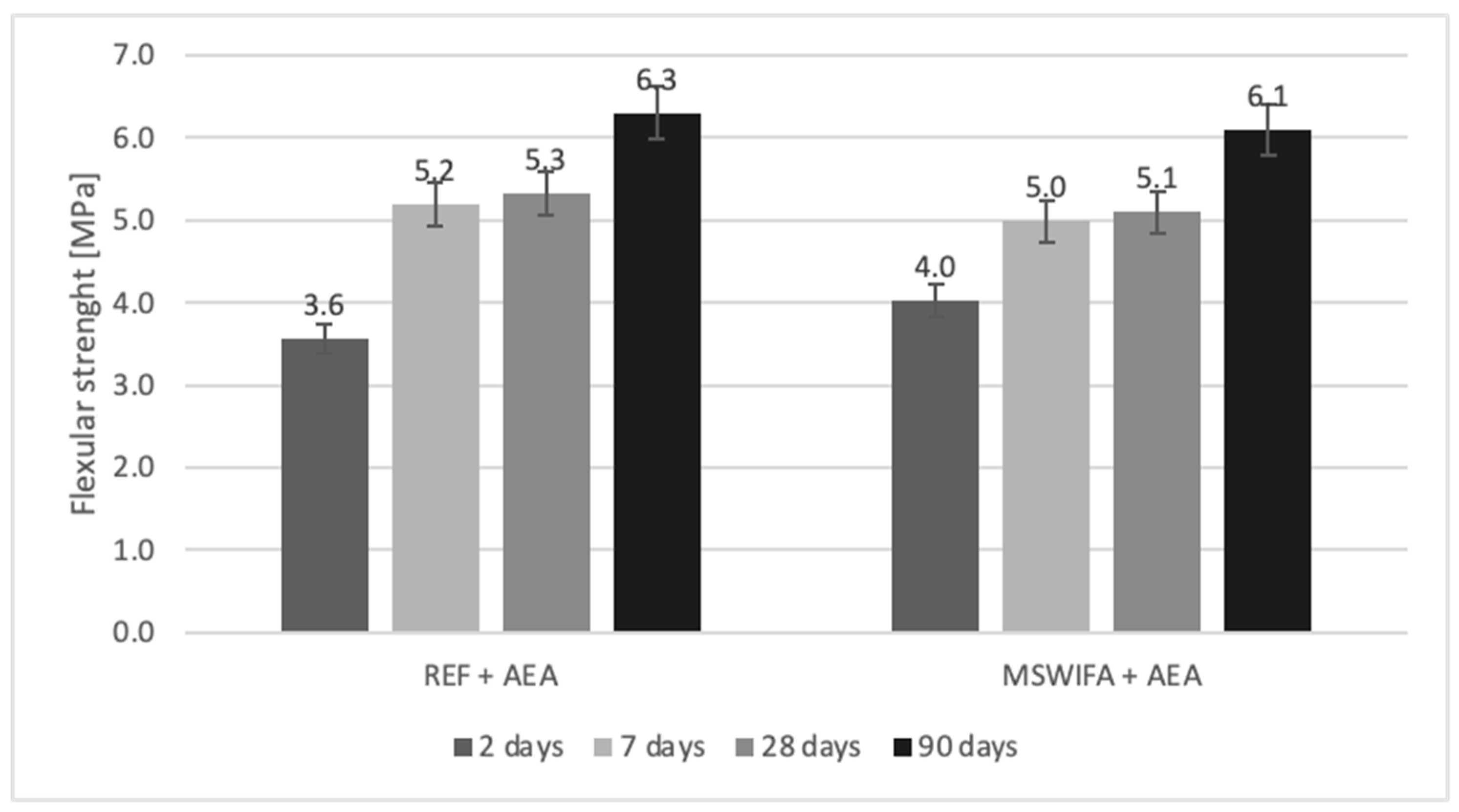
4.8. Compressive and Flexural Strength
4.9. Shrinkage
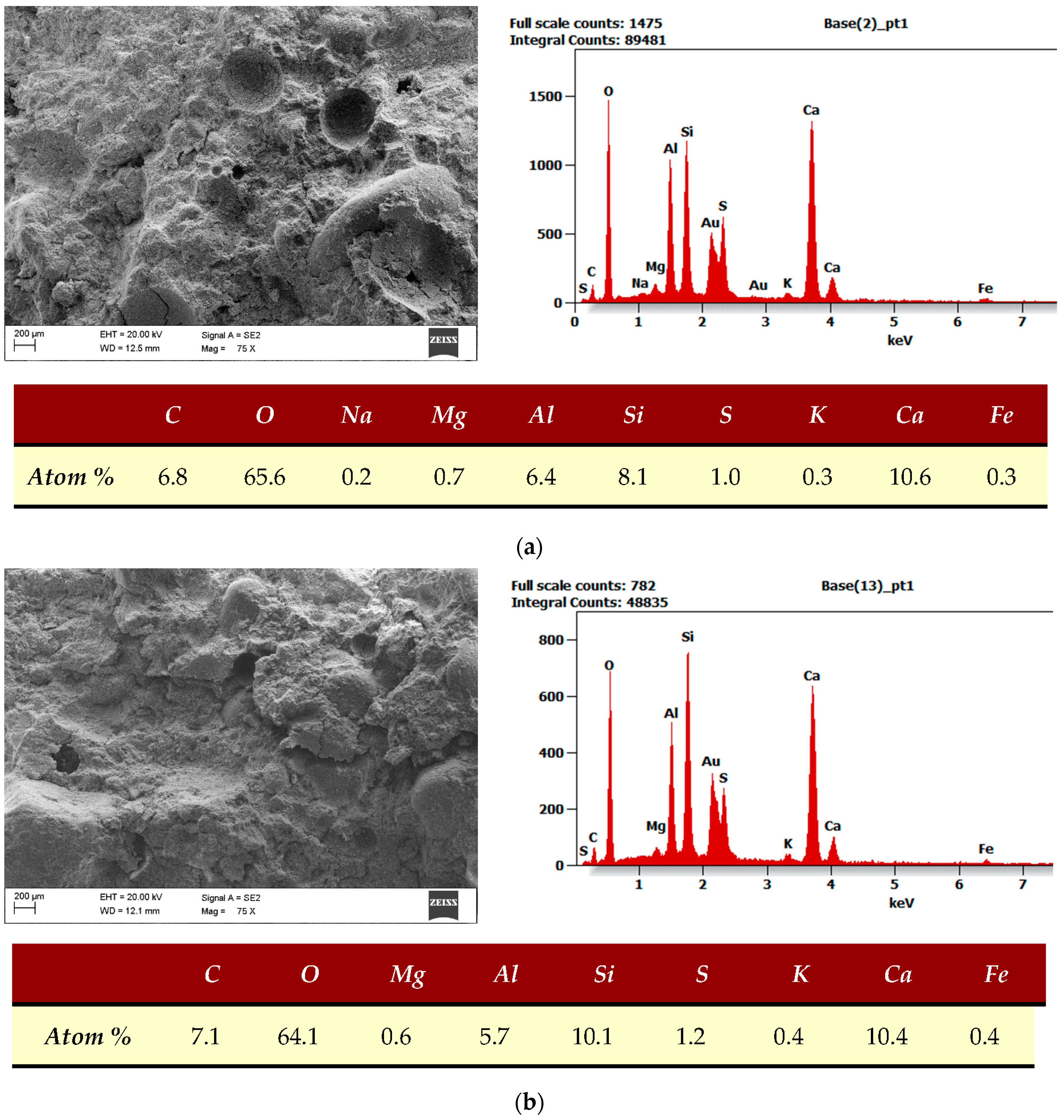
4.10. SEM with EDS
5. Conclusions
5.1. Market and Environmental Conclusions
5.2. Summary of the Construction Findings of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU Green Deal—European Compost Network. Available online: https://www.compostnetwork.info/eu-green-deal/ (accessed on 19 September 2021).
- Czajkowski, A.; Remiorz, L.; Pawlak, S.; Remiorz, E.; Szyguła, J.; Marek, D.; Paszkuta, M.; Drabik, G.; Baron, G.; Paduch, J.; et al. Global Water Crisis: Concept of a New Interactive Shower Panel Based on IoT and Cloud Computing for Rational Water Consumption. Appl. Sci. 2021, 11, 4081. [Google Scholar] [CrossRef]
- The European Green Deal. Available online: https://ec.europa.eu/info/sites/default/files/european-green-deal-communication_en.pdf (accessed on 19 September 2021).
- The 17 Goals of the Sustainable Development. United Nations. Available online: https://sdgs.un.org/goals (accessed on 16 November 2021).
- United Nations. Kyoto Protocol to the United Nations Framework Convention on Climate Change; United Nations: Kyoto, Japan, 1998. [Google Scholar]
- Directive 2008/98/EC on Waste (Waste Framework Directive)-Environment-European Commission. Available online: https://ec.europa.eu/environment/waste/framework/ (accessed on 12 September 2020).
- European Commission. Regulation of the European Parliament and of the Council. Establishing the Framework for Achieving Climate Neutrality and Amending Regulation (EU) 2018/1999 (European Climate Law). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1588581905912&uri=CELEX%3A52020PC0080&fbclid=IwAR1QjYzmS7WNyVgr1IcRxrJhglSH3_K0moNbSm1OQR5X7PxoyP6k9z-q-c8 (accessed on 20 August 2021).
- Climate Change-United Nations Sustainable Development. Goal 13: Take Urgent Action to Combat Climate Change and Its Impacts. Available online: https://www.un.org/sustainabledevelopment/climate-change/?fbclid=IwAR1nC2m2YioXkEvzdXQRa_AL5hPkL-chZe2QIVRNQz4TOHwPEsRGLHwCMkg (accessed on 20 August 2021).
- Çetin, S.; de Wolf, C.; Bocken, N. Circular Digital Built Environment: An Emerging Framework. Sustainability 2021, 13, 6348. [Google Scholar] [CrossRef]
- Pikoń, K.; Poranek, N.; Czajkowski, A.; Łaźniewska-Piekarczyk, B. Poland’s Proposal for a Safe Solution of Waste Treatment during the COVID-19 Pandemic and Circular Economy Connection. Appl. Sci. 2021, 11, 3939. [Google Scholar] [CrossRef]
- Closing the Loop—An EU Action Plan for the Circular Economy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1453384154337&uri=CELEX:52015DC0614 (accessed on 19 September 2021).
- Poranek, N.; Łaźniewska-Piekarczyk, B.; Czajkowski, A.; Pikoń, K. Circular Economy for Municipal Solid Waste Incineration Bottom Ash (MSWIBA) Management in Mortars with CSA and CEM I, MSWIBA Glassy Phase, and DTG. Energies 2022, 15, 135. [Google Scholar] [CrossRef]
- The Circular Economy Package: New EU Targets for Recycling. European Parliament. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20170120STO59356/the-circular-economy-package-new-eu-targets-for-recycling (accessed on 19 September 2021).
- Patel, A.K.; Wajda, A.; Brociek, R.; Pleszczy, M. Optimization of Energy Recovery from Hazardous Waste in a Waste Incineration Plant with the Use of an Application. Processes 2022, 10, 462. [Google Scholar] [CrossRef]
- Kajda-Szcześniak, M.; Jaworski, T.; Wajda, A. Theoretical and experimental model of the combustion process in a layer on the grate of the waste thermal treatment installation, International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management. SGEM 2019, 19, 759–766. [Google Scholar] [CrossRef]
- The Industrial Emissions Directive-Environment-European Commission. Available online: https://ec.europa.eu/environment/industry/stationary/ied/legislation.htm (accessed on 19 September 2021).
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. The Circular Economy and Organic Fraction of Municipal Solid Waste Recycling Strategies. Energies 2020, 13, 4366. [Google Scholar] [CrossRef]
- Purchase, C.K.; Manna, D.; Zulayq, A.; O’brien, B.T.; Kowalewski, M.J.; Berenjian, A.; Tarighaleslami, A.H.; Seifan, M. Circular Economy of Construction and Demolition Waste: A Literature Review on Lessons, Challenges, and Benefits. Materials 2022, 15, 76. [Google Scholar] [CrossRef]
- Wajda, A. Management of wastes from energy industry in the frame of circular economy on the example of microspheres, International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management. SGEM 2018, 18, 71–78. [Google Scholar] [CrossRef]
- Magdalena, B.; Nikolina, P.; Beata, Ł.-P.; Krzysztof, P. Removal of Pollutants from Secondary Waste from an Incineration Plant: The Review of Methods. Energies 2020, 13, 6322. [Google Scholar]
- Li, J. Municipal Solid Waste Incineration Ash-Incorporated Concrete: One Step towards Environmental Justice. Buildings 2021, 11, 495. [Google Scholar] [CrossRef]
- Incineration (Heat Recovery) Plant|Kawasaki Heavy Industries. Available online: https://global.kawasaki.com/en/industrial_equipment/environment_recycling/waste/heat.html (accessed on 2 April 2022).
- Rémond, S.; Pimienta, P.; Bentz, D.P. Effects of the incorporation of Municipal Solid Waste Incineration fly ash in cement pastes and mortars: I. Experimental study. Cem. Concr. Res. 2002, 32, 303–311. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Yang, E.H. Alkali-activated ground granulated blast-furnace slag incorporating incinerator fly ash as a potential binder. Constr. Build. Mater. 2016, 112, 1005–1012. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Kuo, J.J.; Lin, S.H. Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Manag. 2008, 28, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Jing, Z.; Pan, L.; Zhou, L.; Pan, X.; Lu, L. Hydrothermal solidification of municipal solid waste incineration fly ash. Res. Chem. Intermed. 2011, 37, 551–565. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Q.W.; Zhang, S.G. Integrated utilization of municipal solid waste incineration fly ash and bottom ash for preparation of foam glass-ceramics. Rare Met. 2019, 38, 914–921. [Google Scholar] [CrossRef]
- Tang, J.; Steenari, B.M. Leaching optimization of municipal solid waste incineration ash for resource recovery: A case study of Cu, Zn, Pb and Cd. Waste Manag. 2016, 48, 315–322. [Google Scholar] [CrossRef]
- Wang, F.H.; Zhang, F.; Chen, Y.J.; Gao, J.; Zhao, B. A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash. J. Hazard Mater. 2015, 300, 451–458. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, C.; Wang, W.; Shi, Y.; Gao, X. Immobilization of MSWI fly ash through geopolymerization: Effects of water-wash. Waste Manag. 2011, 31, 311–317. [Google Scholar] [CrossRef]
- Wen, Z.; Di, J.; Liu, S.; Han, J.; Lee, J.C.K. Evaluation of flue-gas treatment technologies for municipal waste incineration: A case study in Changzhou, China. J. Clean. Prod. 2018, 184, 912–920. [Google Scholar] [CrossRef]
- Kurdowski, W.; Szeląg, H. Korozja betonu wywołana opóźnionym powstawaniem ettringitu. Concrete destruction caused by delayed ettringite formation, Awarie Budowlane. Constr. Fail. 2011, 14, 1119–1126. [Google Scholar]
- Xuan, D.; Poon, C.S. Removal of metallic Al and Al/Zn alloys in MSWI bottom ash by alkaline treatment. J. Hazard Mater. 2018, 344, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ye, H.; Yuan, Q.; Li, B.; Li, Y.; Zhou, D. Factors Influencing the Hydration, Dimensional Stability, and Strength Development of the OPC-CSA-Anhydrite Ternary System. Materials 2021, 14, 7001. [Google Scholar] [CrossRef] [PubMed]
- EN 12457-2:2006; Waste Characterization—Leachability. European Committee for Standardization: Brussels, Belgium, 2006. Available online: https://sklep.pkn.pl/pn-en-12457-2-2006p.html (accessed on 12 December 2023).
- EN 16174:2012; Sewage Sludge, Treated Bio-Waste and Soil. European Committee for Standardization: Brussels, Belgium, 2012. Available online: https://sklep.pkn.pl/pn-en-16174-2012e.html (accessed on 12 December 2023).
- EN ISO 11885:2009; Water Quality. ISO: Geneva, Switzerland, 2009. Available online: https://sklep.pkn.pl/pn-en-iso-11885-2009e.html (accessed on 12 December 2023).
- PN-EN 1015-3:2000/A2:2007; Test Methods for Mortars for Walls. Comite Europeen de Normalisation: Warsaw, Poland, 2007. Available online: https://sklep.pkn.pl/pn-en-1015-3-2000-a2-2007e.html (accessed on 12 December 2023).
- EN 1015-7:1998; Methods of Testing Mortar for Masonry—Part 7: Determination of Air Content of Fresh Mortar. European Committee for Standardization: Brussels, Belgium, 1998. Available online: https://standards.iteh.ai/catalog/standards/cen/3c66138f-1eb7-41a2-99c6-92ed26a7bdd1/en-1015-7-1998 (accessed on 12 December 2023).
- EN 196-3:2018; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. European Committee for Standardization: Brussels, Belgium, 2018. Available online: https://standards.iteh.ai/catalog/standards/cen/e4921eca-8101-4261-b066-25d19b9b8e8a/en-196-3-2016 (accessed on 12 December 2023).
- EN 196-1:2018; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2018. Available online: https://standards.iteh.ai/catalog/standards/cen/37b8816e-4085-4dcc-a642-a383d9bddd6c/en-196-1-2016 (accessed on 12 December 2023).
- Lijuan, S.; Guosheng, F.; Bing, L.; Qi, S.; Zhang, X.; Zhen, S. Working performance and microscopic mechanistic analyses of municipal solid waste incineration (MSWI) fly ash-based self-foaming filling materials. Constr. Build. Mater. 2022, 361, 129647. [Google Scholar]
- Mondal, S.K.; Welz, A.; Clinton, C.; Khayat, K.; Kumar, A.; Okoronkwo, M.U. Quantifying the Workability of Calcium Sulfoaluminate Cement Paste Using Time-Dependent Rheology. Materials 2022, 15, 5775. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoaluminate cement at various temperature by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- Marinković, S.; Dragaš, J. Fly ash. In Waste and Supplementary Cementitious Materials in Concrete: Characterisation; Woodhead Publishing: Cambridge, UK, 2018; pp. 325–360. [Google Scholar] [CrossRef]
- Puthipad, N.; Ouchi, M.; Rath, S.; Attachaiyawuth, A. Enhancement in self-compactability and stability in volume of entrained air in self-compacting concrete with high volume fly ash. Constr. Build. Mater. 2016, 128, 349–360. [Google Scholar] [CrossRef]
- Benaicha, M.; Jalbaud, O.; Alaoui, A.H.; Burtschell, Y. Porosity effects on rheological and mechanical behavior of self-compacting concrete. J. Build. Eng. 2022, 48, 103964. [Google Scholar] [CrossRef]
- Cao, L.; Shi, F.; Qiu, M.; Chen, W.; Cao, P.; Zhou, C. Effects of air entraining agent on the rheological properties and electrochemical parameters of cement mortar. Constr. Build. Mater. 2022, 344, 128233. [Google Scholar] [CrossRef]
- Şahin, Y.; Akkaya, Y.; Boylu, F.; Taşdemir, M.A. Characterization of air entraining admixtures in concrete using surface tension measurements. Cem. Concr. Compos. 2017, 28, 95–104. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 64, 15–20. [Google Scholar] [CrossRef]
- Park, S.; Jeong, Y.; Moon, J.; Lee, N. Hydration characteristics of calcium sulfoaluminate (CSA) cement/portland cement blended pastes. J. Build. Eng. 2021, 34, 101880. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Łaźniewska-Piekarczyk, B.; Szwabowski, J. Stability of Air-content in the Case of Innovative Air-entraining Portland Multicomponent Cement. Procedia Eng. 2015, 108, 559–567. [Google Scholar] [CrossRef]
- Piasta, W.; Marczewska, J. The effect of air entraining admixtures and cement types on the properties of fresh mortar (Wpływ domieszki napowietrzającej i rodzaju cementu na właściwości świeżej zaprawy). Struct. Environ. 2013, 5, 11–17. [Google Scholar]
- EN 206-1 2003; Concrete—Production and Compliance Requirements. European Committee for Standardization: Brussels, Belgium, 2003. Available online: https://www.studocu.com/pl/document/politechnika-swietokrzyska-w-kielcach/budownictwo-i-konstrukcje-inzynierskie/pn-en-206-1-2003-beton-wymagania-wlasciwosci-produkcja-i-zgodnosc/55725570 (accessed on 12 December 2023).
- Liu, Q.; Chen, Z.; Yang, Y. Effect of fly ash on the air void size distribution entrained by selected anionic, cationic and nonionic surfactants in hardened cement mortars. Cem. Concr. Compos. 2021, 124, 104253. [Google Scholar] [CrossRef]
- Johnson, A.; Catalan, L.J.J.; Kinrade, S.D. Characterization and evaluation of fly-ash from co-combustion of lignite and wood pellets for use as cement admixture. Fuel 2010, 89, 3042–3050. [Google Scholar] [CrossRef]
- Tunstall, L.E.; Scherer, G.W.; Prud’homme, R.K. A new hypothesis for air loss in cement systems containing fly ash. Cem. Concr. Res. 2021, 142, 106352. [Google Scholar] [CrossRef]
- Trezza, M.A. Hydration study of ordinary portland cement in the presence of zinc ions. Mater. Res. 2007, 10, 331–334. [Google Scholar] [CrossRef]
- Gawlicki, M.; Czamarska, D. Effect of ZnO on the hydration of Portland cement. J. Therm. Anal. 1992, 38, 2157–2161. [Google Scholar] [CrossRef]
- Antoniazzi, J.P.; Mohamad, G.; Casali, J.M. Influence of cement type, air-entrained admixture and hydration stabilizing admixture on mortars’ setting time. Rev. IBRACON Estrut. Mater. 2020, 14, e14114. [Google Scholar] [CrossRef]
- Nowak-Michta, A. Impact analysis of air-entraining and superplasticizing admixtures on concrete compressive strength. Procedia Struct. Integr. 2019, 23, 77–82. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]






| Parameter | CaO | SiO2 | MgO | Al2O3 | FeO3 | P2O5 | Na2O | K2O | Cl | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| MSWIFA 19 01 07 * | 16.4 | 27.2 | 2.5 | 11.7 | 1.8 | 0.3 | 5.9 | 5.8 | 7.2 | [23] |
| 47.4 | 2.2 | 1.6 | 1.0 | 0.8 | 0.7 | 4.9 | 6.7 | 25.0 | [24] | |
| 45.4 | 13.6 | 3.2 | 0.9 | 3.8 | 1.7 | 4.2 | 3.9 | 9.7 | [25] | |
| 46.8 | 8.8 | 1.6 | 2.6 | 0.9 | 1.2 | 4.4 | 4.9 | N.P. | [26] | |
| 46.3 | 8.4 | 2.7 | 1.8 | 1.3 | 0.7 | 6.2 | 5.2 | N.P. | [27] |
| Symbol | Zn | Ba | Cu | Pb | Mn | Ni | Cr | Ref. |
|---|---|---|---|---|---|---|---|---|
| Parameter | Zinc | Bar | Copper | Lead | Manganese | Nickel | Chrome | |
| MSWIFA 19 01 07 * | 17,000 | 140 | 840 | 3000 | 1100 | 220 | 490 | [28] |
| 37,384 | N.P. | 3081 | 1356 | N.P. | 1584 | 566 | [29] | |
| 3692 | N.P. | 2817 | 826 | N.P. | 78 | 1369 | [30] | |
| N.P. | 123 | 405 | 908 | 787 | 46 | 80 | o.r. ** |
| Clinker | Composition [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C3S (Alite) | C2S (Belite) | C3A (Celite) | C4AF (Brown Millerite) | C4A3Ŝ (Ye’Elimite) | CŜ (Anhydrite) | 3C2S·3CŜ·CaF2 (Fluorellastidite) | MgO (Periclase) | C3MS2 (Merwinite) | |
| CSA | - | 10.4 | - | 1.2 | 64.9 | 2.6 | 9.4 | 4.9 | 0.8 |
| OPC | 69 | 9.6 | 9.4 | 9 | - | - | - | 0.15 | - |
| Reference Sample | Municipal Solid Waste Incineration Plant Fly Ash Sample | Reference Sample + Air-Entraining Admixture | Municipal Solid Waste Incineration Plant Fly Ash Sample + Air-Entraining Admixture | |
|---|---|---|---|---|
| CEM I 42.5 R | 108 | 100 | 108 | 100 |
| CSA | 284 | 270.5 | 284 | 270.5 |
| Zeolite | 60 | 60 | 60 | 60 |
| MSWIFA | - | 21.5 | - | 21.5 |
| Sand * | 1350 | 1350 | 1350 | 1350 |
| Water | 190 | 190 | 190 | 190 |
| SPF | 13.5 | 13.5 | 13.5 | 13.5 |
| Retarder | 13.56 (10.30) ** | 13.56 (10.30) ** | 10.3 | 10.3 |
| air-entraining admixture (AEA) | - | - | 0.04 | 0.1 |
| Property | Standard/Method |
|---|---|
| Water extract | Water extract was prepared according to EN 12457-2:2006 [35]. Sample was shaken in water for 24 h in a 1:10 proportion (sample/water). |
| Heavy metal content | Heavy metal content was determined according to EN 16174:2012 [36] and EN ISO 11885:2009 [37]. |
| Consistency | EN 1015-3:2000/A2:2007 [38] Methods of testing mortar for masonry—Part 3: determination of the consistency of fresh mortar (by flow table). |
| The consistency was tested just after mixing and after 30, 60, 90, and 120 min. Before each test, the mortar was mixed for 15 s in the mixer. | |
| Air content | EN 1015-7:1998 [39] Methods of testing mortar for masonry—Part 7: Determination of air content of fresh mortar. |
| The air content was tested just after mixing and the after 30 and 60 min | |
| Water demand | EN 196-3:2018 [40] Methods of testing cement—Part 3: Determination of setting times and soundness. |
| Initial setting time | |
| Soundness | |
| Flexural and compressive strength | EN 196-1:2018 [41] Methods of testing cement—Part 1: Determination of strength. |
| The samples in the form of prisms measuring 160 × 40 × 40 mm were molded and kept in a humidity of 90% and a temperature of 20 ± 1 °C for 24 h. Then, samples were demolded and placed in the water at 20 ± 1 °C. Both flexural and compressive strengths were tested after 2, 7, 28, and 90 days. | |
| Shrinkage | The samples in the form of prisms measuring 160 × 40 × 40 mm, equipped with metal tips on both ends, were molded and kept in a relative humidity of 90% and a temperature of 20 ± 1 °C for 24 h. Then samples were demolded and put in the climatic chamber at 20 ± 1 °C and 60% relative humidity. The elongation/shortening of the samples was tested. The first measurement was conducted after 48 h, and it was used as a base for further measurements after 7, 28, and 90 days. |
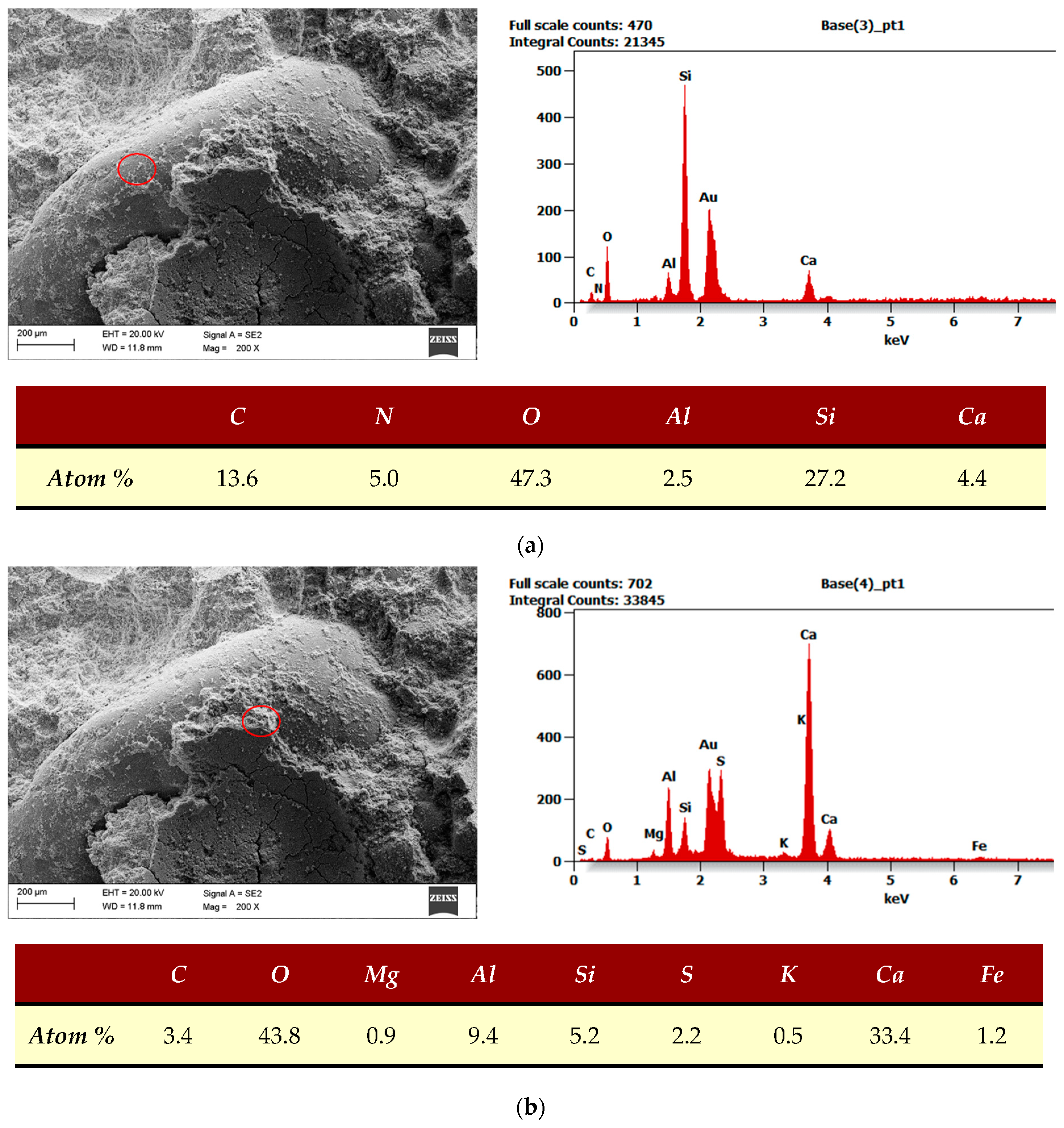
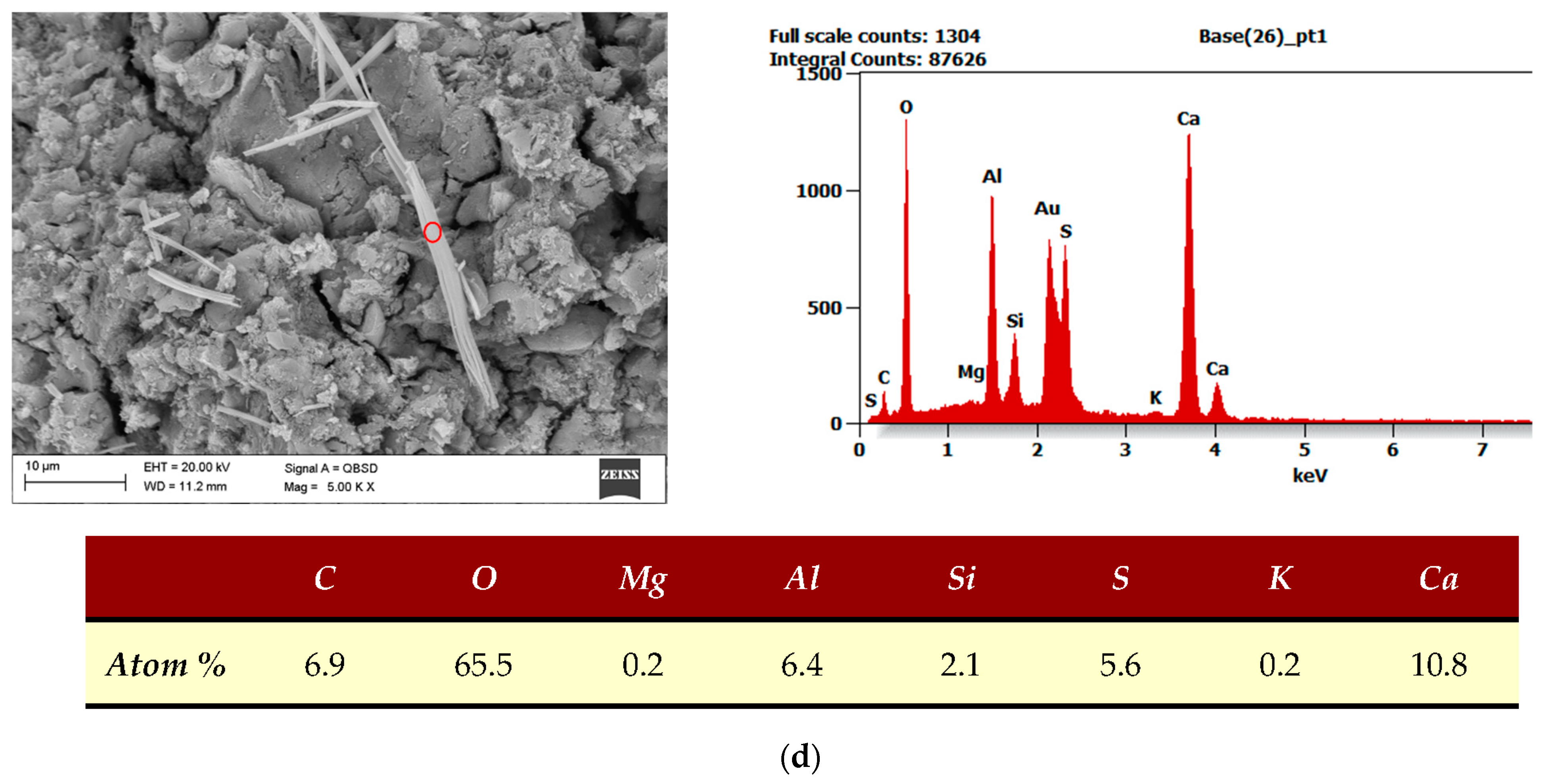
| SEM, EDS | The examination was performed using a scanning microscope (Supra35 Zeiss with EDS detectors and WDS–EDAX from EDAX Company, Mahwah, NJ, USA). The measurement was based on the measurement of the characteristic X-ray energy spectrum. The research was done in The Silesian University of Technology in Gliwice. |
| Symbol | Ba | Cu | Pb | Mn | Ni | Cr | Cd | Co |
|---|---|---|---|---|---|---|---|---|
| Parameter | Barium | Copper | Lead | Manganese | Nickel | Chromium | Cadmium | Cobalt |
| Unit | mg/kg | |||||||
| Results | 123.10 | 404.82 | 907.83 | 787.10 | 46.47 | 80.13 | 98.81 | 10.04 |
| Material | Cadmium | Chrome | Copper | Iron | Manganese | Nickel | Lead | Zinc |
|---|---|---|---|---|---|---|---|---|
| Symbol | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn |
| Unit | µg/mL | µg/mL | µg/mL | µg/mL | µg/mL | µg/mL | µg/mL | µg/mL |
| MSWIFA 19 01 07 * | 0.02189 | 0.77382 | BLQ | BLQ | BLQ | 0.18530 | 1.42056 | 45.8850 |
| Element | Symbol | Unit | MSWIFA 19 01 07 * |
|---|---|---|---|
| Sodium | Na | mg/dm3 | 14,820.0 |
| Potassium | K | mg/dm3 | 1129.0 |
| Lithium | Li | mg/dm3 | 10.0 |
| Calcium | Ca | mg/dm3 | 10,410.0 |
| Barium | Ba | mg/dm3 | 596.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poranek, N.; Pizoń, J.; Łaźniewska-Piekarczyk, B.; Czajkowski, A.; Lagashkin, R. Recycle Option for Municipal Solid Waste Incineration Fly Ash (MSWIFA) as a Partial Replacement for Cement in Mortars Containing Calcium Sulfoaluminate Cement (CSA) and Portland Cement to Save the Environment and Natural Resources. Materials 2024, 17, 39. https://doi.org/10.3390/ma17010039
Poranek N, Pizoń J, Łaźniewska-Piekarczyk B, Czajkowski A, Lagashkin R. Recycle Option for Municipal Solid Waste Incineration Fly Ash (MSWIFA) as a Partial Replacement for Cement in Mortars Containing Calcium Sulfoaluminate Cement (CSA) and Portland Cement to Save the Environment and Natural Resources. Materials. 2024; 17(1):39. https://doi.org/10.3390/ma17010039
Chicago/Turabian StylePoranek, Nikolina, Jan Pizoń, Beata Łaźniewska-Piekarczyk, Adrian Czajkowski, and Ruslan Lagashkin. 2024. "Recycle Option for Municipal Solid Waste Incineration Fly Ash (MSWIFA) as a Partial Replacement for Cement in Mortars Containing Calcium Sulfoaluminate Cement (CSA) and Portland Cement to Save the Environment and Natural Resources" Materials 17, no. 1: 39. https://doi.org/10.3390/ma17010039








