Synthesis of Multicolor Carbon Dots Catalyzed by Inorganic Salts with Tunable Nonlinear Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
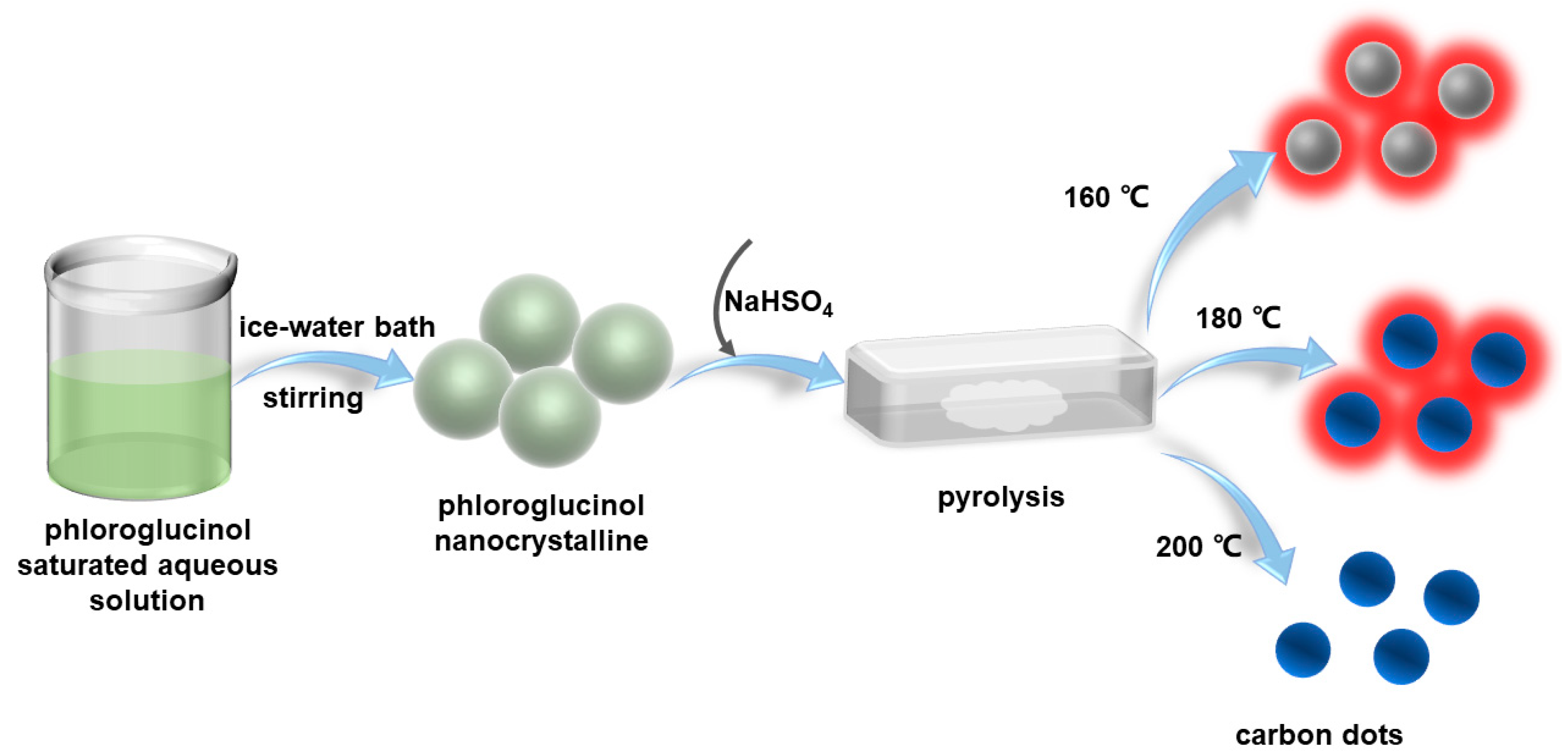
2.1. Preparation of Carbon Dots
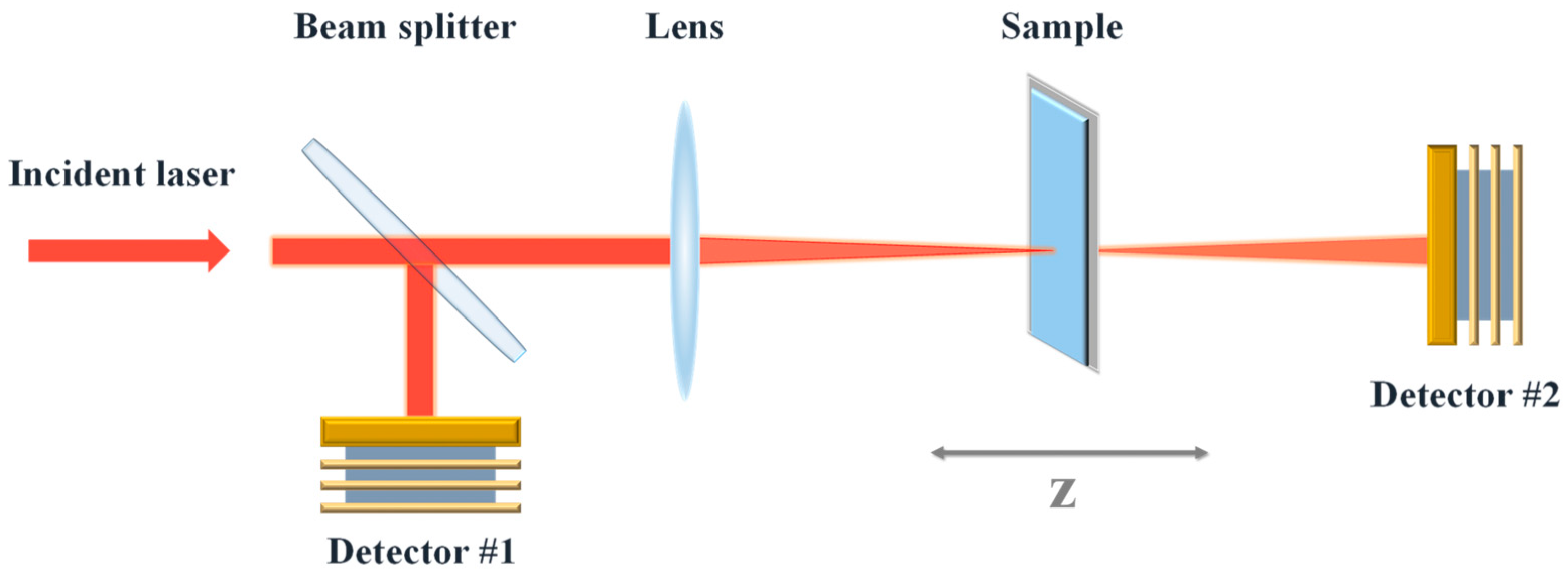
2.2. Nonlinear Optical Measurement of Carbon Dots
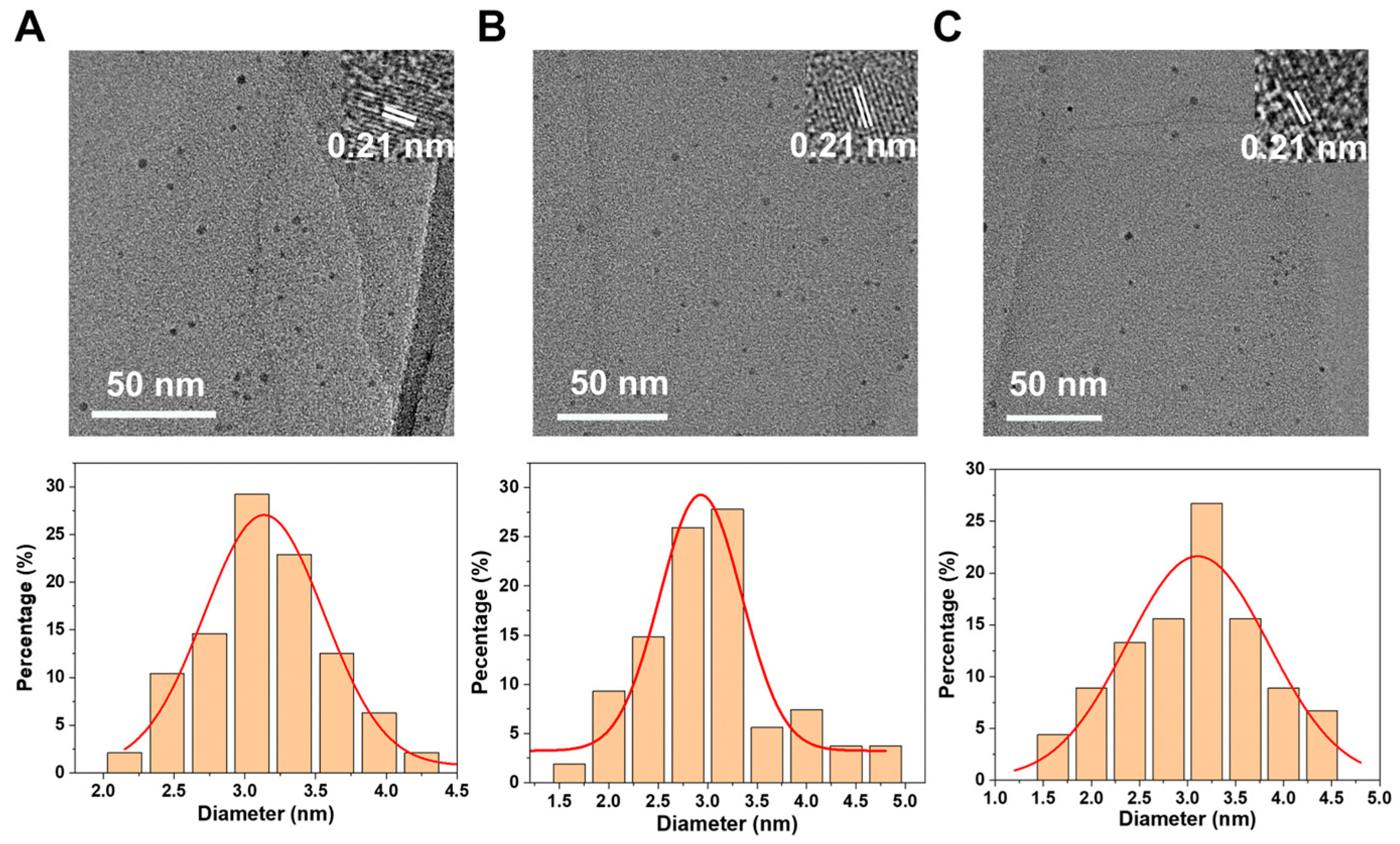
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, L.; Lu, S. Precise carbon dots synthesis: Building bridges between organic chemistry and inorganic chemistry. Sci. Bull. 2022, 67, 2369–2371. [Google Scholar] [CrossRef] [PubMed]
- Suner, S.S.; Bhethanabotla, V.R.; Ayyala, R.S.; Sahiner, N. Rapid Pathogen Purge by Photosensitive Arginine-Riboflavin Carbon Dots without Toxicity. Materials 2023, 16, 6512. [Google Scholar] [CrossRef] [PubMed]
- Hakiem, A.F.A.; Urriza-Arsuaga, I.; Urraca, J.L. Development of a Screening Method for Fluoroquinolones in Meat Samples Using Molecularly Imprinted Carbon Dots. Biosensors 2023, 13, 972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tan, Z.A. Fluorescent Carbon Dots: Fantastic Electroluminescent Materials for Light-Emitting Diodes. Adv. Sci. 2021, 8, 2001977. [Google Scholar] [CrossRef] [PubMed]
- Dhenadhayalan, N.; Lin, K.-C.; Saleh, T.A. Recent Advances in Functionalized Carbon Dots toward the Design of Efficient Materials for Sensing and Catalysis Applications. Small 2020, 16, 1905767. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhao, R.; Zhang, Z.-H.; Yang, J.-J.; Wang, Z.; Xiao, L.-L.; Li, X.-H.; He, X.-J.; Xiong, H.-M. Kilogram-scale synthesis of carbon dots with high-efficiency full-color solid-state fluorescence using an aggregation-induced emission strategy. Chem. Eng. J. 2023, 476, 146405. [Google Scholar] [CrossRef]
- Chung, Y.J.; Kim, J.; Park, C.B. Photonic Carbon Dots as an Emerging Nanoagent for Biomedical and Healthcare Applications. ACS Nano 2020, 14, 6470–6497. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, W.; Zhao, B.; Yang, B. Spectroscopic studies of the optical properties of carbon dots: Recent advances and future prospects. Mater. Chem. Front. 2020, 4, 472–488. [Google Scholar] [CrossRef]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.-H.; Sain, M. Future Perspectives and Review on Organic Carbon Dots in Electronic Applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Zhao, Q.-L.; Zhang, Z.-L.; Huang, B.-H.; Peng, J.; Zhang, M.; Pang, D.-W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 41, 5116–5118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chi, Y.; Dong, Y.; Lin, J.; Wang, B. Electrochemiluminescence of Water-Soluble Carbon Nanocrystals Released Electrochemically from Graphite. J. Am. Chem. Soc. 2009, 131, 4564–4565. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, T.; Mao, C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Travas-Sejdic, J. Simple Aqueous Solution Route to Luminescent Carbogenic Dots from Carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Egorova, M.; Tomskaya, A.; Smagulova, S.A. Optical Properties of Carbon Dots Synthesized by the Hydrothermal Method. Materials 2023, 16, 4018. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-color fluorescent carbon quantum dots. Sci. Adv. 2020, 6, eabb6772. [Google Scholar] [CrossRef]
- Niu, X.; Zheng, W.; Song, T.; Huang, Z.; Yang, C.; Zhang, L.; Li, W.; Xiong, H. Pyrolysis of single carbon sources in SBA-15: A recyclable solid phase synthesis to obtain uniform carbon dots with tunable luminescence. Chin. Chem. Lett. 2023, 34, 107560. [Google Scholar] [CrossRef]
- Ibarra-Prieto, H.D.; Garcia-Garcia, A.; Aguilera-Granja, F.; Navarro-Ibarra, D.C.; Rivero-Espejel, I. One-Pot, Optimized Microwave-Assisted Synthesis of Difunctionalized and B–N Co-Doped Carbon Dots: Structural Characterization. Nanomaterials 2023, 13, 2753. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Zheng, J.; Yang, Y.; Liu, X.; Xu, B. N, B-Codoping Induces High-Efficiency Solid-State Fluorescence and Dual Emission of Yellow/Orange Carbon Dots. ACS Sustain. Chem. Eng. 2021, 9, 2224–2236. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, R.; Ling, L.; Li, Q.; Chen, S. Rapid and Large-Scale Production of Multi-Fluorescence Carbon Dots via Magnetic Hyperthermia Method. Angew. Chem. Int. Ed. 2020, 59, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.-F.; Chen, S. Amphiphilic Egg-Derived Carbon Dots: Rapid Plasma Fabrication, Pyrolysis Process, and Multicolor Printing Patterns. Angew. Chem. 2012, 124, 9431–9435. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-Y.; Liu, K.-K.; Wei, J.-Y.; Lou, Q.; Shang, Y.; Shan, C.-X. Deep-Ultraviolet Emissive Carbon Nanodots. Nano Lett. 2019, 19, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Liang, T.; Ushakova, E.V.; Wang, B.; Zhang, B.; Zhou, H.; Xing, G.; Wang, C.; Tang, Z.; Qu, S.; et al. Enhanced Near-Infrared Emission from Carbon Dots by Surface Deprotonation. J. Phys. Chem. Lett. 2021, 12, 604–611. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Xu, M.; Hu, X.; Li, S.; Zhang, M.; Zhang, Q.; Wang, Q.; Lu, S.; Tian, Y.; et al. UV–Vis–NIR Full-Range Responsive Carbon Dots with Large Multiphoton Absorption Cross Sections and Deep-Red Fluorescence at Nucleoli and In Vivo. Small 2020, 16, 2000680. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Lu, S.; Sui, L.; Liu, J.; Zhu, S.; Chen, A.; Jin, M.; Yang, B. Near-Infrared Photoluminescent Polymer–Carbon Nanodots with Two-Photon Fluorescence. Adv. Mater. 2017, 29, 1603443. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Qiao, S.; Fang, Y.; Tian, J.; McLeod, J.; Song, Y.; Huang, H.; Liu, Y.; Kang, Z. Third-order nonlinear optical properties of carboxyl group dominant carbon nanodots. J. Mater. Chem. C 2016, 4, 8490–8495. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Shi, W.; Sun, D.; Chu, S.; Sun, L.; Zhai, J.; Pei, J.; Wang, L.; Ruan, S.; et al. Synthesis of carbon nanodots in zeolite SAPO-46 channels for Q-switched fiber laser generation. J. Alloys Compd. 2019, 782, 837–844. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Trivizas, G.; Karakassides, M.A.; Baikousi, M.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Hola, K.; Kozak, O.; Zboril, R.; et al. Green and simple route toward boron doped carbon dots with significantly enhanced non-linear optical properties. Carbon 2015, 83, 173–179. [Google Scholar] [CrossRef]
- Ma, L.; Xiang, W.; Gao, H.; Wang, J.; Ni, Y.; Liang, X. Facile synthesis of tunable fluorescent carbon dots and their third-order nonlinear optical properties. Dye. Pigment. 2016, 128, 1–7. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Karakassides, M.A.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Papagiannouli, I.; Aloukos, P.; Couris, S.; Hola, K.; Zboril, R.; et al. Synthesis, characterization and non-linear optical response of organophilic carbon dots. Carbon 2013, 61, 640–643. [Google Scholar] [CrossRef]
- Aloukos, P.; Papagiannouli, I.; Bourlinos, A.B.; Zboril, R.; Couris, S. Third-order nonlinear optical response and optical limiting of colloidal carbon dots. Opt. Express 2014, 22, 12013–12027. [Google Scholar] [CrossRef]
- Tan, D.; Yamada, Y.; Zhou, S.; Shimotsuma, Y.; Miura, K.; Qiu, J. Carbon nanodots with strong nonlinear optical response. Carbon 2014, 69, 638–640. [Google Scholar] [CrossRef]
- Koutsioukis, A.; Akouros, A.; Zboril, R.; Georgakilas, V.J.N. Solid phase extraction for the purification of violet, blue, green and yellow emitting carbon dots. Nanoscale 2018, 10, 11293–11296. [Google Scholar] [CrossRef]
- Wei, S.-M.; Feng, K.; Li, C.; Xie, N.; Wang, Y.; Yang, X.-L.; Chen, B.; Tung, C.-H.; Wu, L.-Z. ZnCl2 Enabled Synthesis of Highly Crystalline and Emissive Carbon Dots with Exceptional Capability to Generate O2–. Matter 2020, 2, 495–506. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.A.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Dong, Y.; Chen, Y.; Lu, C.-H.; Chi, Y.; Yang, H.-H.; Yu, T. Luminescence origin of carbon based dots obtained from citric acid and amino group-containing molecules. Carbon 2017, 118, 319–326. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Yuan, K.; Lu, Z.; Cheng, Y.; Li, L.; Zhang, X.; Jin, P.; Meng, F.; Liu, H. Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions. Nanomaterials 2018, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.-L.; Tian, Z.-Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.-W. Electrochemical Tuning of Luminescent Carbon Nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C.; Suresh, R.; Ramamurthy, P. Unravelling the Multiple Emissive States in Citric-Acid-Derived Carbon Dots. J. Phys. Chem. C 2016, 120, 1252–1261. [Google Scholar] [CrossRef]
- Liu, C.; Bao, L.; Yang, M.L.; Zhang, S.; Zhou, M.M.; Tang, B.; Wang, B.S.; Liu, Y.F.; Zhang, Z.L.; Zhang, B.; et al. Surface Sensitive Photoluminescence of Carbon Nanodots: Coupling between the Carbonyl Group and π-Electron System. J. Phys. Chem. Lett. 2019, 10, 3621–3629. [Google Scholar] [CrossRef] [PubMed]
- Ehrat, F.; Bhattacharyya, S.; Schneider, J.; Löf, A.; Wyrwich, R.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Tracking the Source of Carbon Dot Photoluminescence: Aromatic Domains versus Molecular Fluorophores. Nano Lett. 2017, 17, 7710–7716. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, A.; Cayuela, A.; Soriano, M.L.; Gelardi, F.M.; Cannas, M.; Valcarcel, M.; Messina, F. Different natures of surface electronic transitions of carbon nanoparticles. PCCP 2017, 19, 22670–22677. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Yang, H.; Qian, S. Femtosecond nonlinear optical properties of carbon nanoparticles. Appl. Phys. Lett. 2002, 81, 2088–2090. [Google Scholar] [CrossRef]
- Li, Z.; He, C.; Wang, Z.; Gao, Y.; Dong, Y.; Zhao, C.; Chen, Z.; Wu, Y.; Song, W. Ethylenediamine-modified graphene oxide covalently functionalized with a tetracarboxylic Zn(ii) phthalocyanine hybrid for enhanced nonlinear optical properties. Photochem. Photobiol. Sci. 2016, 15, 910–919. [Google Scholar] [CrossRef]
- Fu, L.; Fang, Y.; Guan, Z.; Wei, Z.; Yang, R.; Shan, N.; Liu, F.; Zhao, Y.; Zhang, M.; Huang, Z.; et al. Dramatic femtosecond nonlinear absorption at a strongly coupled porphyrin-graphene nanoconjugate. Nano Res. 2023, 16, 5900–5908. [Google Scholar] [CrossRef]

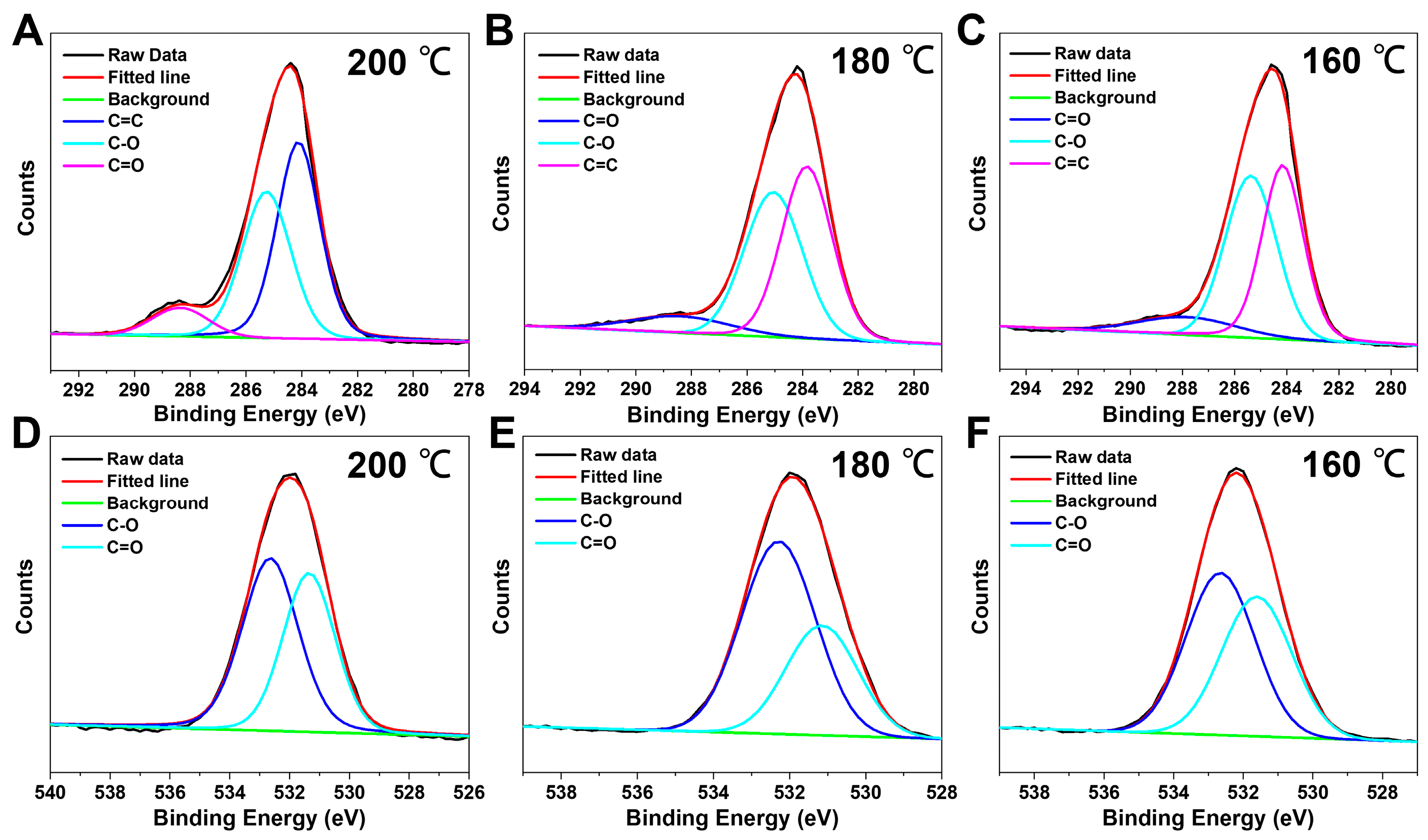
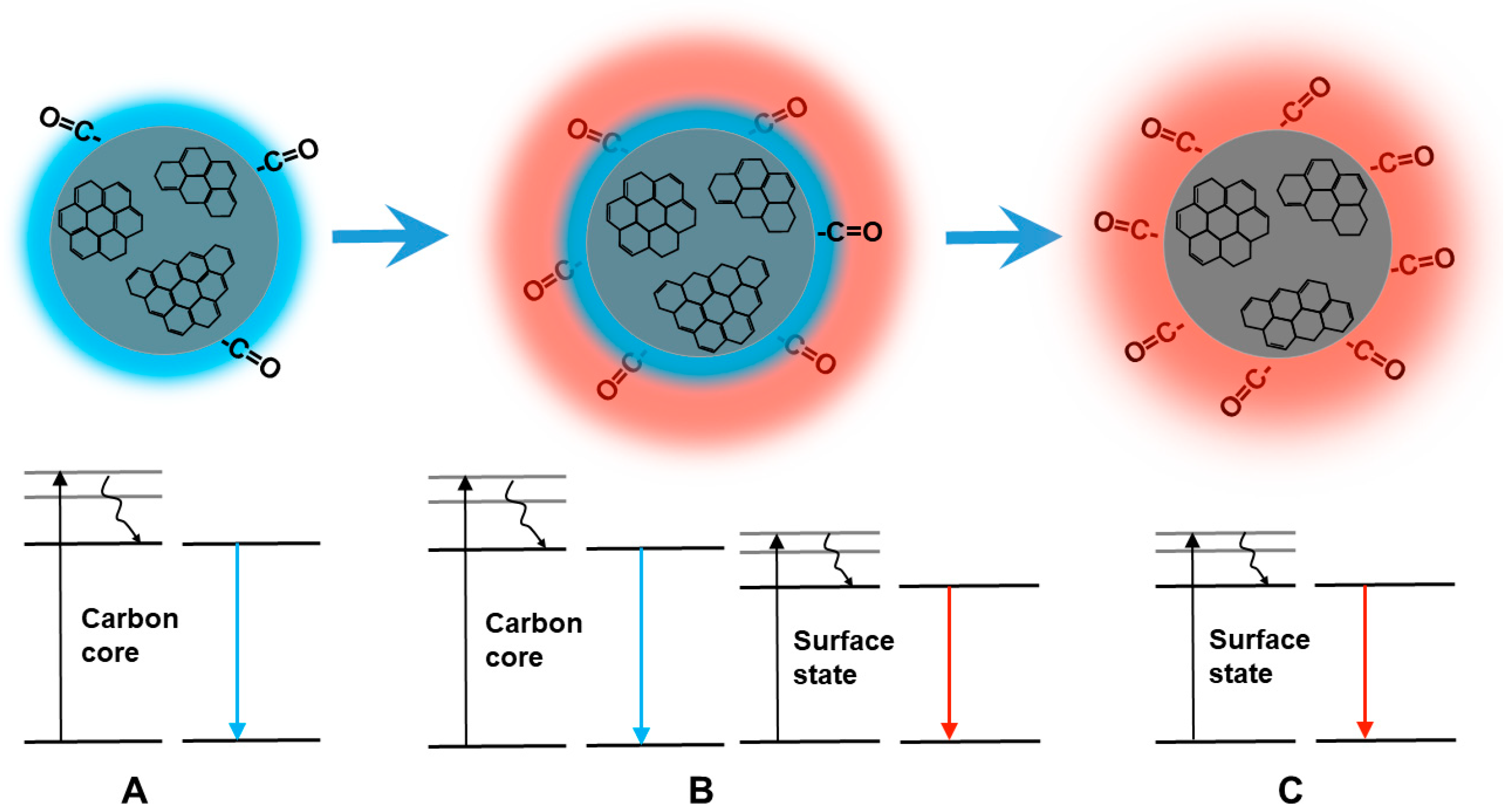
| Sample | C | O | C=C | C-O | C=O |
|---|---|---|---|---|---|
| A | 73.10% | 26.90% | 50.38% | 40.86% | 8.73% |
| B | 71.64% | 28.36% | 47.09% | 44.17% | 8.74% |
| C | 71.90% | 28.10% | 43.35% | 46.87% | 9.78% |
| Sample | τ1 (ns) | A1 (%) | τ2 (ns) | A2 (%) | τave (ns) | R2 |
|---|---|---|---|---|---|---|
| A | 1.03 | 40.71 | 5.05 | 59.29 | 4.56 | 0.999 |
| B | 2.65 | 65.88 | 5.80 | 34.12 | 4.32 | 0.999 |
| C | 3.02 | 72.93 | 6.01 | 27.07 | 4.29 | 0.999 |
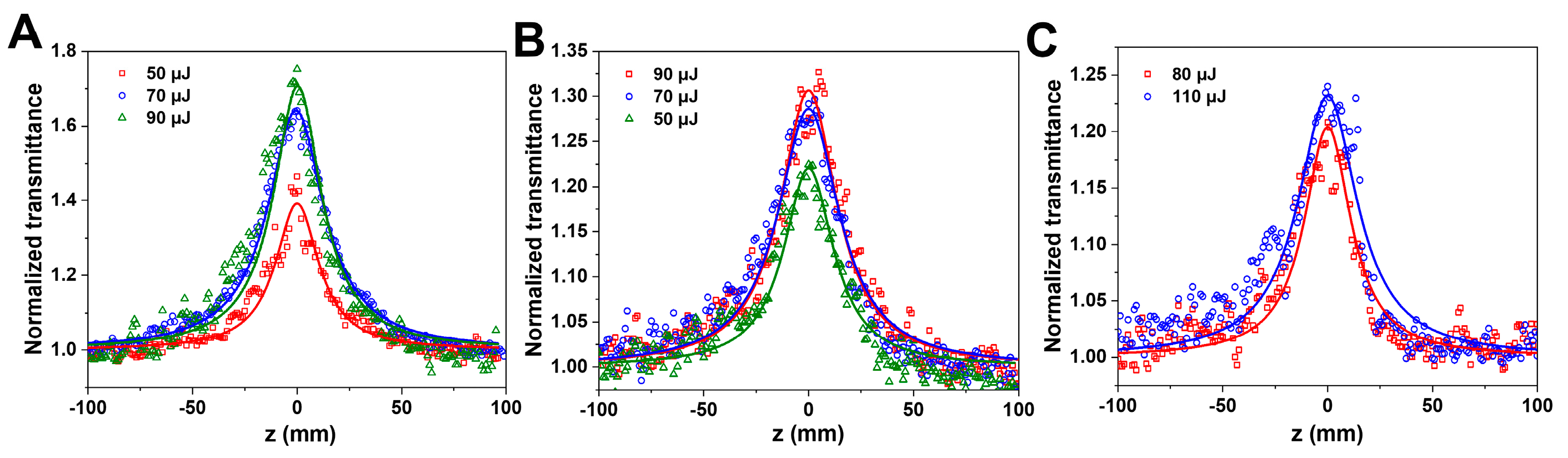
| Sample | I (MW cm−2) | βeff (cm GW−1) | Imχ(3) (esu) | FOM (esu cm) |
|---|---|---|---|---|
| A | 27.09 | −183.98 | −6.97 × 10−11 | 2.84 × 10−12 |
| 37.88 | −246.55 | −9.34 × 10−11 | 3.80 × 10−12 | |
| B | 27.09 | −82.02 | −3.11 × 10−11 | 2.57 × 10−12 |
| 37.88 | −90.96 | −3.45 × 10−11 | 2.85 × 10−12 | |
| C | 43.34 | −46.26 | −1.75 × 10−11 | 1.36 × 10−12 |
| 59.58 | −47.90 | −1.81 × 10−11 | 1.41 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Hou, R.; Zhang, L.; Gao, H.; Hu, J. Synthesis of Multicolor Carbon Dots Catalyzed by Inorganic Salts with Tunable Nonlinear Optical Properties. Materials 2024, 17, 42. https://doi.org/10.3390/ma17010042
Niu X, Hou R, Zhang L, Gao H, Hu J. Synthesis of Multicolor Carbon Dots Catalyzed by Inorganic Salts with Tunable Nonlinear Optical Properties. Materials. 2024; 17(1):42. https://doi.org/10.3390/ma17010042
Chicago/Turabian StyleNiu, Xiaoqing, Ruipeng Hou, Luo Zhang, Hongli Gao, and Junzhou Hu. 2024. "Synthesis of Multicolor Carbon Dots Catalyzed by Inorganic Salts with Tunable Nonlinear Optical Properties" Materials 17, no. 1: 42. https://doi.org/10.3390/ma17010042
APA StyleNiu, X., Hou, R., Zhang, L., Gao, H., & Hu, J. (2024). Synthesis of Multicolor Carbon Dots Catalyzed by Inorganic Salts with Tunable Nonlinear Optical Properties. Materials, 17(1), 42. https://doi.org/10.3390/ma17010042




