Biocompatible Hydrogel-Based Liquid Marbles with Magnetosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particles and Liquids
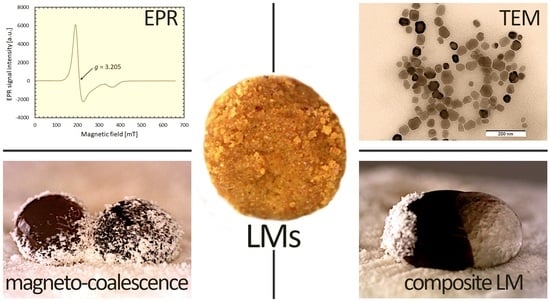
2.2. Preparation of Magnetosomes
2.3. Preparation of Magnetosome-Based Liquid Marbles with Aqueous and Hydrogel Cores
2.4. Characterization of Particles and Liquid Marbles
3. Results and Discussion
3.1. Properties of Magnetosomes and Stabilizing Particles
3.2. Behavior of Turmeric-Based Liquid Marbles
3.3. Behavior of Liquid Marbles Stabilized with Lycopodium Pollen
3.4. Multi-Step Functionalization of Hydrogel-Based Liquid Marbles with Magnetosomes
3.5. Controlled Transport of Hydrogel-Based Liquid Marbles with Magnetosomes
3.6. Magnetically-Assisted Manipulation of Liquid Marbles for the Fabrication of Composite Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahadevan, L.; Pomeau, Y. Rolling droplets. Phys. Fluids 1999, 11, 2449–2453. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Liquid marbles. Nature 2001, 411, 924–927. [Google Scholar] [CrossRef] [PubMed]
- McHale, G.; Newton, M.I. Liquid marbles: Principles and applications. Soft Matter 2011, 7, 5473–5481. [Google Scholar] [CrossRef]
- Asaumi, Y.; Rey, M.; Oyama, K.; Vogel, N.; Hirai, T.; Nakamura, Y.; Fujii, S. Effect of Stabilizing Particle Size on the Structure and Properties of Liquid Marbles. Langmuir 2020, 36, 13274–13284. [Google Scholar] [CrossRef] [PubMed]
- Mohammadrashidi, M.; Bijarchi, M.A.; Shafii, M.B.; Taghipoor, M. Experimental and Theoretical Investigation on the Dynamic Response of Ferrofluid Liquid Marbles to Steady and Pulsating Magnetic Fields. Langmuir 2023, 39, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Ettelaie, R.; Lishchuk, S.V.; Cheng, H.; Zhao, N.; Xiao, F.; Cheng, F.; Yang, H. Liquid marble-derived solid-liquid hybrid superparticles for CO2 capture. Nat. Commun. 2019, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, B.S.; Varanakkottu, S.N. Janus liquid marbles: Fabrication techniques, recent developments, and applications. Droplet 2023, 2, e44. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Niu, H.; Wang, X.; Lin, T. Magnetic liquid marbles: Toward “lab in a droplet”. Adv. Funct. Mater. 2015, 25, 437–444. [Google Scholar] [CrossRef]
- Nguyen, N.-K.; Tran, D.T.; Chuang, A.; Singha, P.; Kijanka, G.; Burford, M.; Ooi, C.H.; Nguyen, N.-T. Liquid marble–a high-yield micro-photobioreactor platform. React. Chem. Eng. 2023, 8, 2710–2716. [Google Scholar] [CrossRef]
- Basit, A.; Wang, J.; Guo, F.; Niu, W.; Jiang, W. Improved methods for mass production of magnetosomes and applications: A review. Microb. Cell Factories 2020, 19, 197. [Google Scholar] [CrossRef]
- Mickoleit, F.; Borkner, C.B.; Toro-Nahuelpan, M.; Herold, H.M.; Maier, D.S.; Plitzko, J.R.M.; Scheibel, T.; Schüler, D. In vivo coating of bacterial magnetic nanoparticles by magnetosome expression of spider silk-inspired peptides. Biomacromolecules 2018, 19, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, Y.; Zhang, Y.; Wang, X.; Liu, J.; Basit, A.; Miao, T.; Liu, W.; Jiang, W. Bacterial magnetosomes loaded with doxorubicin and transferrin improve targeted therapy of hepatocellular carcinoma. Nanotheranostics 2019, 3, 284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Fan, D.; Tian, J.; Xiang, Z.; Fang, Q. Self-Confirming Magnetosomes for Tumor-Targeted T1/T2 Dual-Mode MRI and MRI-Guided Photothermal Therapy. Adv. Healthc. Mater. 2022, 11, 2200841. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhu, Y.; Lu, Z.; Deng, J.; Guo, J.; Ding, X.; Wang, J.; Cao, R.; Chen, A.; Huang, Z. pH-Responsive doxorubicin-loaded magnetosomes for magnetic resonance-guided focused ultrasound real-time monitoring and ablation of breast cancer. Biomater. Sci. 2023, 11, 7158–7168. [Google Scholar] [CrossRef] [PubMed]
- Kuzajewska, D.; Wszołek, A.; Żwierełło, W.; Kirczuk, L.; Maruszewska, A. Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer? Biology 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.M.; Reis, R.L.; Mano, J.F. The Potential of Liquid Marbles for Biomedical Applications: A Critical Review. Adv. Healthc. Mater. 2017, 6, 1700192. [Google Scholar] [CrossRef]
- Fullarton, C.; Draper, T.C.; Phillips, N.; Mayne, R.; de Lacy Costello, B.P.J.; Adamatzky, A. Evaporation, Lifetime, and Robustness Studies of Liquid Marbles for Collision-Based Computing. Langmuir 2018, 34, 2573–2580. [Google Scholar] [CrossRef]
- Nguyen, N.-K.; Singha, P.; Dai, Y.; Rajan Sreejith, K.; Tran, D.T.; Phan, H.-P.; Nguyen, N.-T.; Ooi, C.H. Controllable high-performance liquid marble micromixer. Lab A Chip 2022, 22, 1508–1518. [Google Scholar] [CrossRef]
- Kaushal, A.; Shoval, S.; Binks, B.P.; Bormashenko, E. Universality of Scaling Laws Governing Contact and Spreading Time Spans of Bouncing Liquid Marbles and its Physical Origin. Langmuir 2023, 39, 12488–12496. [Google Scholar] [CrossRef]
- Nguyen, N.-K.; Singha, P.; An, H.; Phan, H.-P.; Nguyen, N.-T.; Ooi, C.H. Electrostatically excited liquid marble as a micromixer. React. Chem. Eng. 2021, 6, 1386–1394. [Google Scholar] [CrossRef]
- Kumar, N.; Arakeri, J.H. Heat and mass transfer from a system of closely packed capillaries—Possible choice for wicks. Int. J. Therm. Sci. 2020, 148, 106151. [Google Scholar] [CrossRef]
- Roy, P.K.; Shoval, S.; Fujii, S.; Bormashenko, E. Interfacial crystallization in the polyhedral liquid marbles. J. Colloid Interface Sci. 2023, 630, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, T. Polymeric capsules and micelles as promising carriers of anticancer drugs. Polym. Med. 2022, 52, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wanyan, H.; Lu, S.; Xiao, H.; Zhang, M.; Liu, K.; Li, X.; Du, B.; Huang, L.; Chen, L.; et al. Robust cellulose-based hydrogel marbles with excellent stability for gas sensing. Carbohydr. Polym. 2023, 306, 120617. [Google Scholar] [CrossRef]
- Leite, Á.J.; Oliveira, N.M.; Song, W.; Mano, J.F. Bioactive Hydrogel Marbles. Sci. Rep. 2018, 8, 15215. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.M.; Zhang, Y.S.; Ju, J.; Chen, A.-Z.; Chen, Y.; Sonkusale, S.R.; Dokmeci, M.R.; Reis, R.L.; Mano, J.F.; Khademhosseini, A. Hydrophobic Hydrogels: Toward Construction of Floating (Bio)microdevices. Chem. Mater. 2016, 28, 3641–3648. [Google Scholar] [CrossRef]
- Vadivelu, R.K.; Kamble, H.; Munaz, A.; Nguyen, N.-T. Liquid Marble as Bioreactor for Engineering Three-Dimensional Toroid Tissues. Sci. Rep. 2017, 7, 12388. [Google Scholar] [CrossRef]
- Liu, M.; Chen, C.; Yu, J.; Zhang, H.; Liang, L.; Guo, B.; Qiu, Y.; Yao, F.; Zhang, H.; Li, J. The gelatin-based liquid marbles for cell cryopreservation. Mater. Today Bio 2022, 17, 100477. [Google Scholar] [CrossRef]
- Bielas, R.; Kubiak, T.; Kopčanský, P.; Šafařík, I.; Józefczak, A. Tunable particle shells of thermo-responsive liquid marbles under alternating magnetic field. J. Mol. Liq. 2023, 391, 123283. [Google Scholar] [CrossRef]
- Mosiniewicz-Szablewska, E.; Safarikova, M.; Safarik, I. Magnetic studies of ferrofluid-modified spruce sawdust. J. Phys. D Appl. Phys. 2007, 21, 6490. [Google Scholar] [CrossRef]
- Molcan, M.; Gojzewski, H.; Skumiel, A.; Dutz, S.; Kovac, J.; Kubovcikova, M.; Kopcansky, P.; Vekas, L.; Timko, M. Energy losses in mechanically modified bacterial magnetosomes. J. Phys. D Appl. Phys. 2016, 49, 365002. [Google Scholar] [CrossRef]
- Musiał, J.; Skumiel, A.; Bielas, R. Study of the calorimetric effect in ferrogels subjected to the high-frequency rotating magnetic field. J. Magn. Magn. Mater. 2023, 588, 171462. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Hornowski, T.; Dobosz, B.; Józefczak, A. Influence of magnetic nanoparticles on the focused ultrasound hyperthermia. Materials 2018, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-F.; Potroz, M.G.; Tan, E.-L.; Park, J.H.; Miyako, E.; Cho, N.-J. Human blood plasma catalyses the degradation of Lycopodium plant sporoderm microcapsules. Sci. Rep. 2019, 9, 2944. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Akutagawa, S.; Sawada, K.; Iwasa, T.; Shimoyama, Y. A ferromagnetic resonance study of iron complexes as biologically synthesized in magnetic bacteria. Mater. Trans. 2009, 50, 2187–2191. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kirilenko, D.A.; Kosterov, A.; Koziaeva, V.V.; Levitskii, V.S.; Multhoff, G.; Nepomnyashchaya, E.K.; Nikitin, A.V. Magnetic properties of bacterial magnetosomes produced by magnetospirillum caucaseum so-1. Microorganisms 2021, 9, 1854. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Kurczewska, J.; Schroeder, G. The influence of surface modification, coating agents and pH value of aqueous solutions on physical properties of magnetite nanoparticles investigated by ESR method. J. Magn. Magn. Mater. 2017, 429, 203–210. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Kurczewska, J.; Schroeder, G. The dynamics of functionalized magnetite nanoparticles in various solutions studied by ESR method. Mater. Chem. Phys. 2017, 198, 297–302. [Google Scholar] [CrossRef]
- Kubiak, T.; Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J.; Hałupka-Bryl, M. A study of magnetite nanoparticles in whole human blood by means of electron paramagnetic resonance. Acta Bio-Opt. Et Inform. Medica. Biomed. Eng. 2015, 21, 9–15. [Google Scholar]
- Krzyminiewski, R.; Kubiak, T.; Dobosz, B.; Schroeder, G.; Kurczewska, J. EPR spectroscopy and imaging of TEMPO-labeled magnetite nanoparticles. Curr. Appl. Phys. 2014, 14, 798–804. [Google Scholar] [CrossRef]
- Noginova, N.; Chen, F.; Weaver, T.; Giannelis, E.; Bourlinos, A.; Atsarkin, V. Magnetic resonance in nanoparticles: Between ferro-and paramagnetism. J. Phys. Condens. Matter 2007, 19, 246208. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, T.; Zubko, M.; Józefczak, A. The impact of ultrasound on Janus capsules at gel-liquid interface. Curr. Appl. Phys. 2022, 38, 22–29. [Google Scholar] [CrossRef]
- Kubiak, T.; Zubko, M.; Józefczak, A. Ultrasound-triggered directional release from turmeric capsules. Particuology 2021, 57, 19–27. [Google Scholar] [CrossRef]
- Takei, T.; Tomimatsu, R.; Matsumoto, T.; Sreejith, K.R.; Nguyen, N.-T.; Yoshida, M. Hydrophobically Modified Gelatin Particles for Production of Liquid Marbles. Polymers 2022, 14, 4849. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tavares, F.; Das, R.; Mishra, H. How particle–particle and liquid–particle interactions govern the fate of evaporating liquid marbles. Soft Matter 2021, 17, 7628–7644. [Google Scholar] [CrossRef]
- Khaw, M.K.; Ooi, C.H.; Mohd-Yasin, F.; Nguyen, A.V.; Evans, G.M.; Nguyen, N.-T. Dynamic behaviour of a magnetically actuated floating liquid marble. Microfluid. Nanofluidics 2017, 21, 110. [Google Scholar] [CrossRef]
- Nguyen, N.-T. Deformation of Ferrofluid Marbles in the Presence of a Permanent Magnet. Langmuir 2013, 29, 13982–13989. [Google Scholar] [CrossRef]
- Tian, J.; Arbatan, T.; Li, X.; Shen, W. Liquid marble for gas sensing. Chem. Commun. 2010, 46, 4734–4736. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Yuan, S.; Yao, X.; Chao, Y.; Cao, Y.; Song, Q.; Sauret, A.; Binks, B.P.; Shum, H.C. Effects of particle size on the electrocoalescence dynamics and arrested morphology of liquid marbles. J. Colloid Interface Sci. 2022, 608, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Molcan, M.; Petrenko, V.I.; Avdeev, M.V.; Ivankov, O.I.; Garamus, V.M.; Skumiel, A.; Jozefczak, A.; Kubovcikova, M.; Kopcansky, P.; Timko, M. Structure characterization of the magnetosome solutions for hyperthermia study. J. Mol. Liq. 2017, 235, 11–16. [Google Scholar] [CrossRef]
- Molcan, M.; Skumiel, A.; Timko, M.; Safarik, I.; Zolochevska, K.; Kopcansky, P. Tuning of Magnetic Hyperthermia Response in the Systems Containing Magnetosomes. Molecules 2022, 27, 5605. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.O.; Neves, B.M.; Rezk, A.R.; Eshtiaghi, N.; Yeo, L.Y. Continuous Production of Janus and Composite Liquid Marbles with Tunable Coverage. ACS Appl. Mater. Interfaces 2016, 8, 17751–17756. [Google Scholar] [CrossRef] [PubMed]
- Krov, M.; Rychecký, O.; Prachár, M.; Zadražil, A.; Šrámek, R.; Štěpánek, F. Operating limits and parametric sensitivity of laboratory device for continuous production of liquid marbles. Powder Technol. 2022, 411, 117944. [Google Scholar] [CrossRef]
- Tenjimbayashi, M. Production of small powder-stabilized droplets using superhydrophobic mesh. Appl. Phys. Lett. 2023, 122, 251604. [Google Scholar]
- Azizian, P.; Mohammadrashidi, M.; Abbas Azimi, A.; Bijarchi, M.A.; Shafii, M.B.; Nasiri, R. Magnetically driven manipulation of nonmagnetic liquid marbles: Billiards with liquid marbles. Micromachines 2022, 14, 49. [Google Scholar] [CrossRef]
- Draper, T.C.; Fullarton, C.; Mayne, R.; Phillips, N.; Canciani, G.E.; de Lacy Costello, B.P.J.; Adamatzky, A. Mapping outcomes of liquid marble collisions. Soft Matter 2019, 15, 3541–3551. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, X.; Binks, B.P.; Shum, H.C. Coalescence of electrically charged liquid marbles. Soft Matter 2017, 13, 119–124. [Google Scholar] [CrossRef]
- Rozynek, Z.; Bielas, R.; Józefczak, A. Efficient formation of oil-in-oil Pickering emulsions with narrow size distributions by using electric fields. Soft Matter 2018, 14, 5140–5149. [Google Scholar] [CrossRef]
- Bielas, R.; Rozynek, Z.; Hornowski, T.; Józefczak, A. Ultrasound control of oil-in-oil Pickering emulsions preparation. J. Phys. D Appl. Phys. 2019, 53, 085301. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Dao, D.V.; Nguyen, N.-T. Coalescence Processes of Droplets and Liquid Marbles. Micromachines 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Binks, B.P.; Bormashenko, E.; Legchenkova, I.; Fujii, S.; Shoval, S. Manufacture and properties of composite liquid marbles. J. Colloid Interface Sci. 2020, 575, 35–41. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, S.; Zhao, Y.; Wu, P.; Liu, C.; Jiang, W. Preparation of Phase Change Melt Marbles with High Thermal Stability by Spontaneous Shrinkage and Encapsulation. Langmuir 2022, 38, 12644–12656. [Google Scholar] [CrossRef]







| Sample | g-Value | [mT] | Asymmetry Parameter |
|---|---|---|---|
| IONP powder | 2.412 ± 0.013 | 136.6 ± 1.1 | 1.295 ± 0.001 |
| sMAG dispersed in water | 2.401 ± 0.007 | 55.3 ± 1.1 | 1.490 ± 0.001 |
| MTs dispersed in water | 3.205 ± 0.012 | 36.9 ±1.1 | 2.694 ± 0.001 |
| MTs dispersed in HEPES | 3.326 ± 0.013 | 21.6 ± 1.1 | 3.444 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielas, R.; Kubiak, T.; Molcan, M.; Dobosz, B.; Rajnak, M.; Józefczak, A. Biocompatible Hydrogel-Based Liquid Marbles with Magnetosomes. Materials 2024, 17, 99. https://doi.org/10.3390/ma17010099
Bielas R, Kubiak T, Molcan M, Dobosz B, Rajnak M, Józefczak A. Biocompatible Hydrogel-Based Liquid Marbles with Magnetosomes. Materials. 2024; 17(1):99. https://doi.org/10.3390/ma17010099
Chicago/Turabian StyleBielas, Rafał, Tomasz Kubiak, Matus Molcan, Bernadeta Dobosz, Michal Rajnak, and Arkadiusz Józefczak. 2024. "Biocompatible Hydrogel-Based Liquid Marbles with Magnetosomes" Materials 17, no. 1: 99. https://doi.org/10.3390/ma17010099
APA StyleBielas, R., Kubiak, T., Molcan, M., Dobosz, B., Rajnak, M., & Józefczak, A. (2024). Biocompatible Hydrogel-Based Liquid Marbles with Magnetosomes. Materials, 17(1), 99. https://doi.org/10.3390/ma17010099