Temperature Evolution of Composition, Thermal, Electrical and Magnetic Properties of Ti3C2Tx-MXene
Abstract
:1. Introduction
2. Materials and Methods
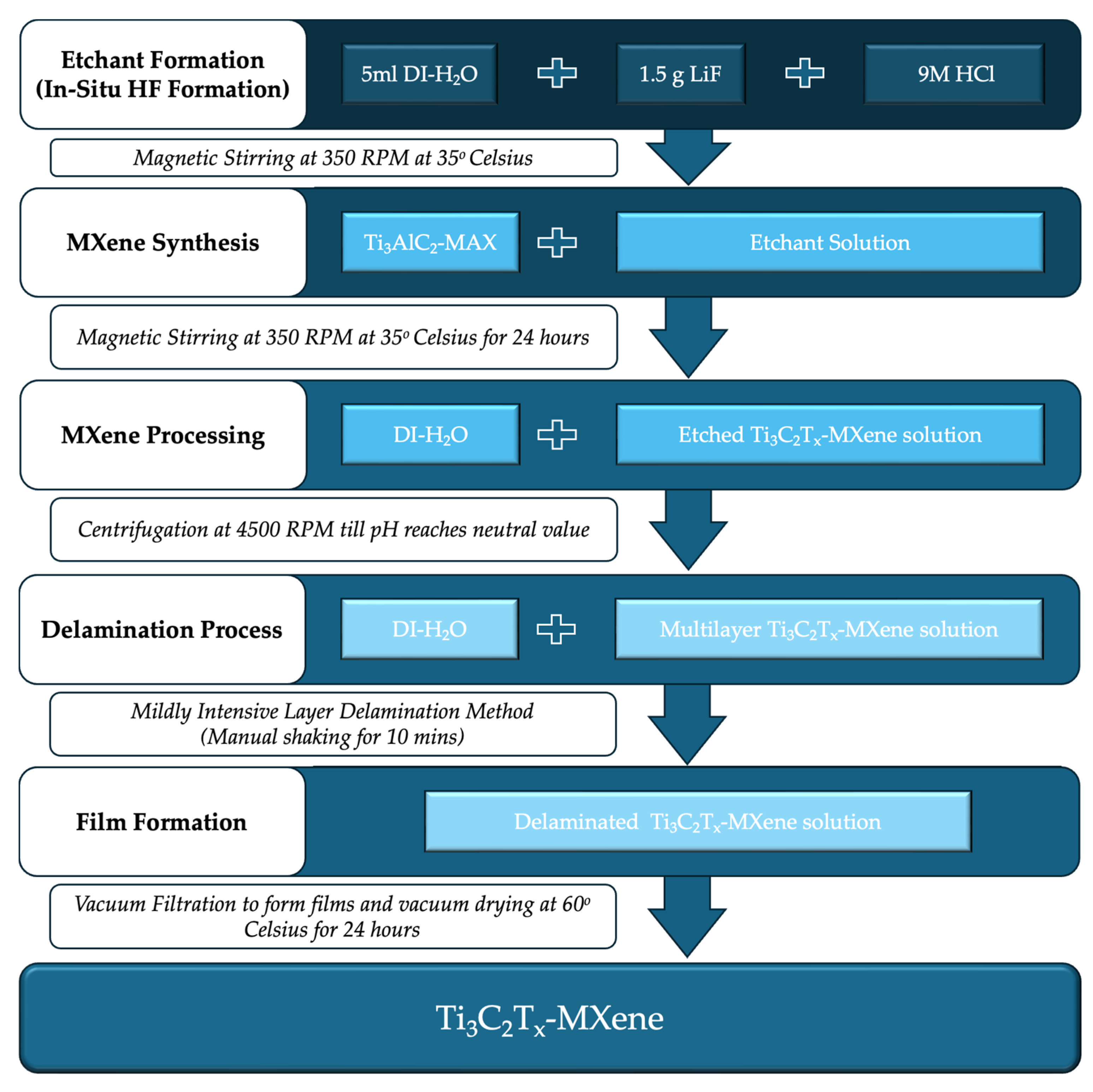
2.1. Ti3C2Tx-MXene Synthesis and Processing
2.2. X-ray Diffraction Study
2.3. Specific Heat Measurements
2.4. Thermal Analysis Experiments
2.5. Electrical Resistivity and Magnetoresistance Measurements
2.6. Magnetometric Measurements
3. Results
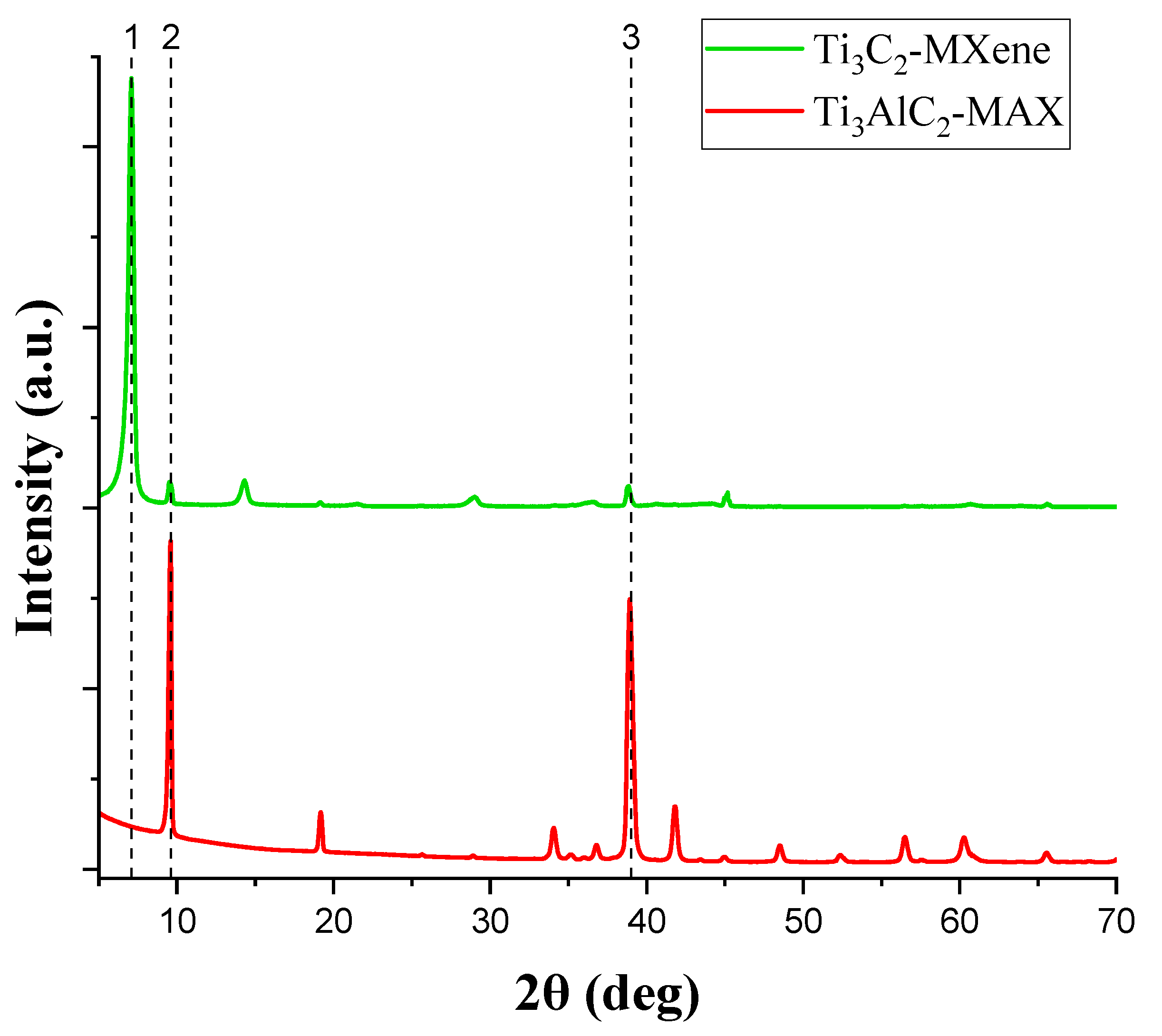
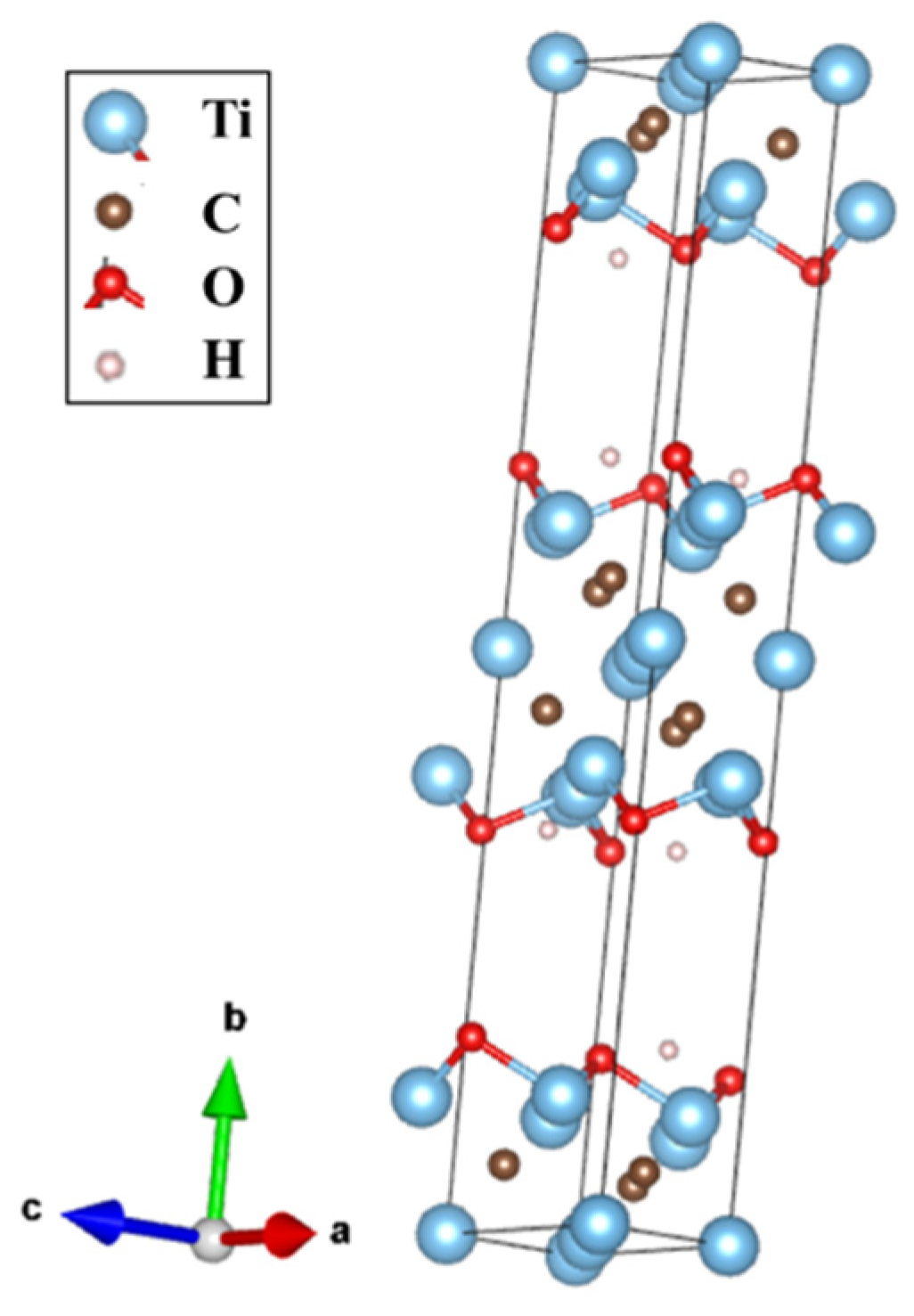
3.1. X-ray Diffraction Study
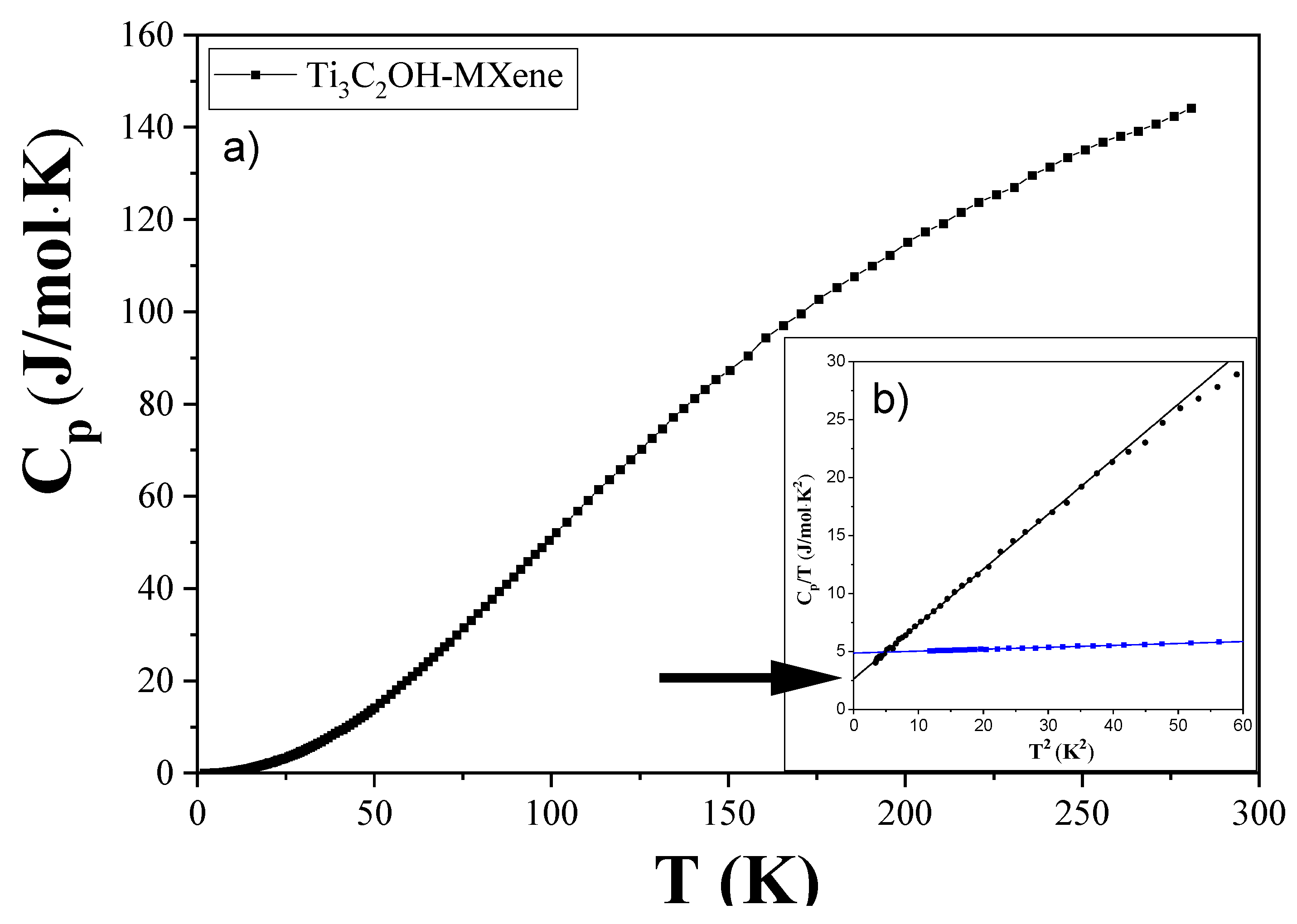
3.2. Specific Heat Measurements
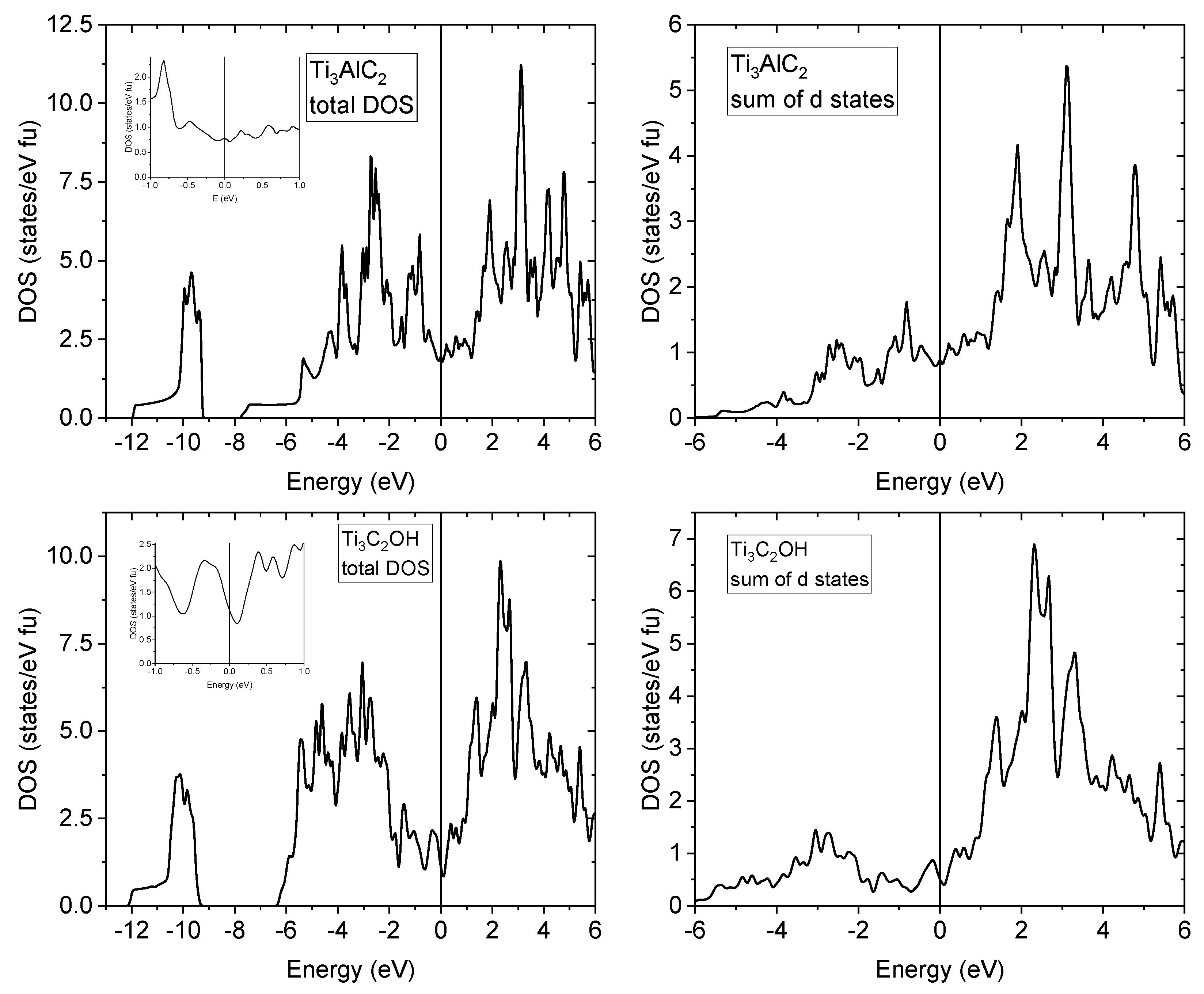
3.3. Density of States Calculations
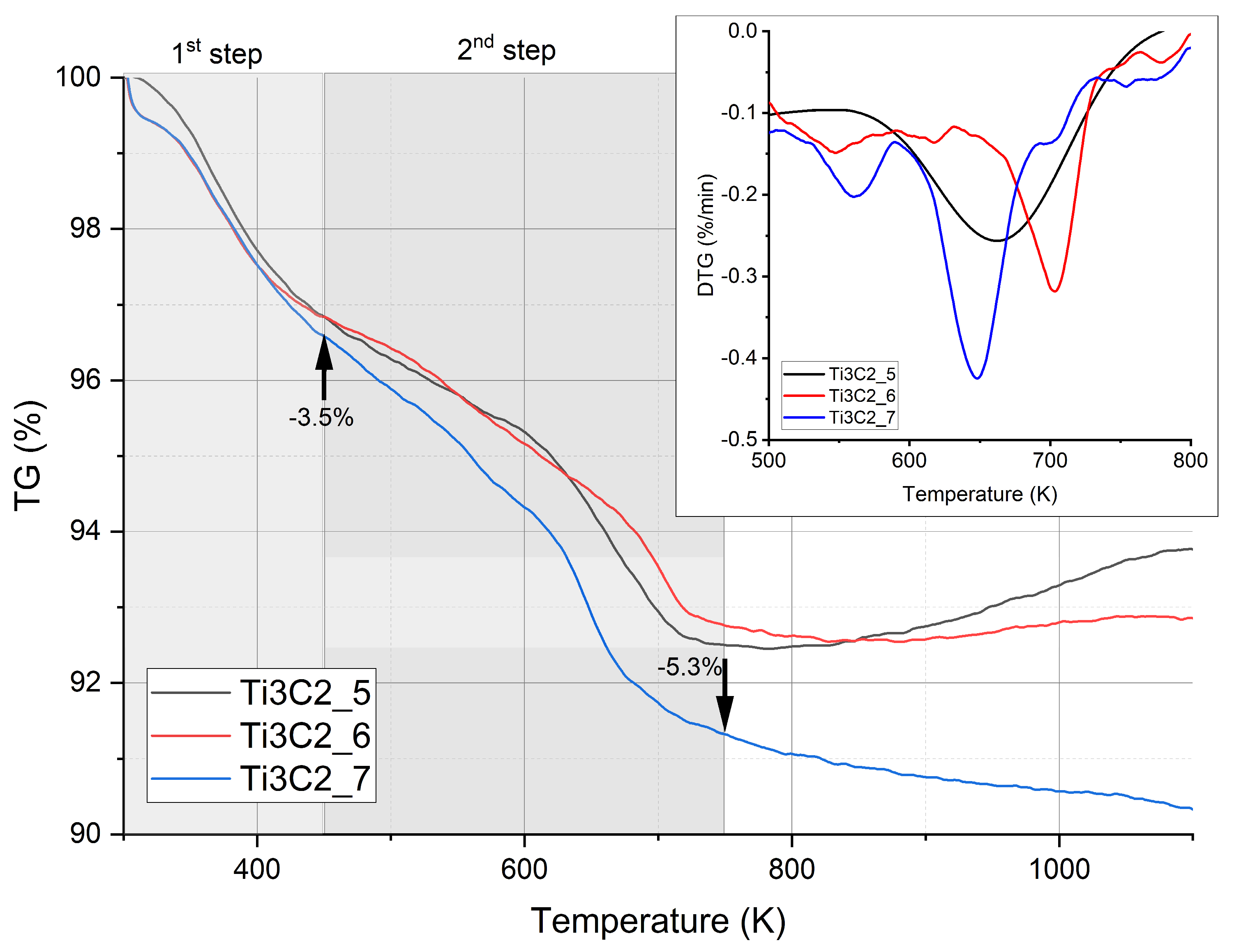
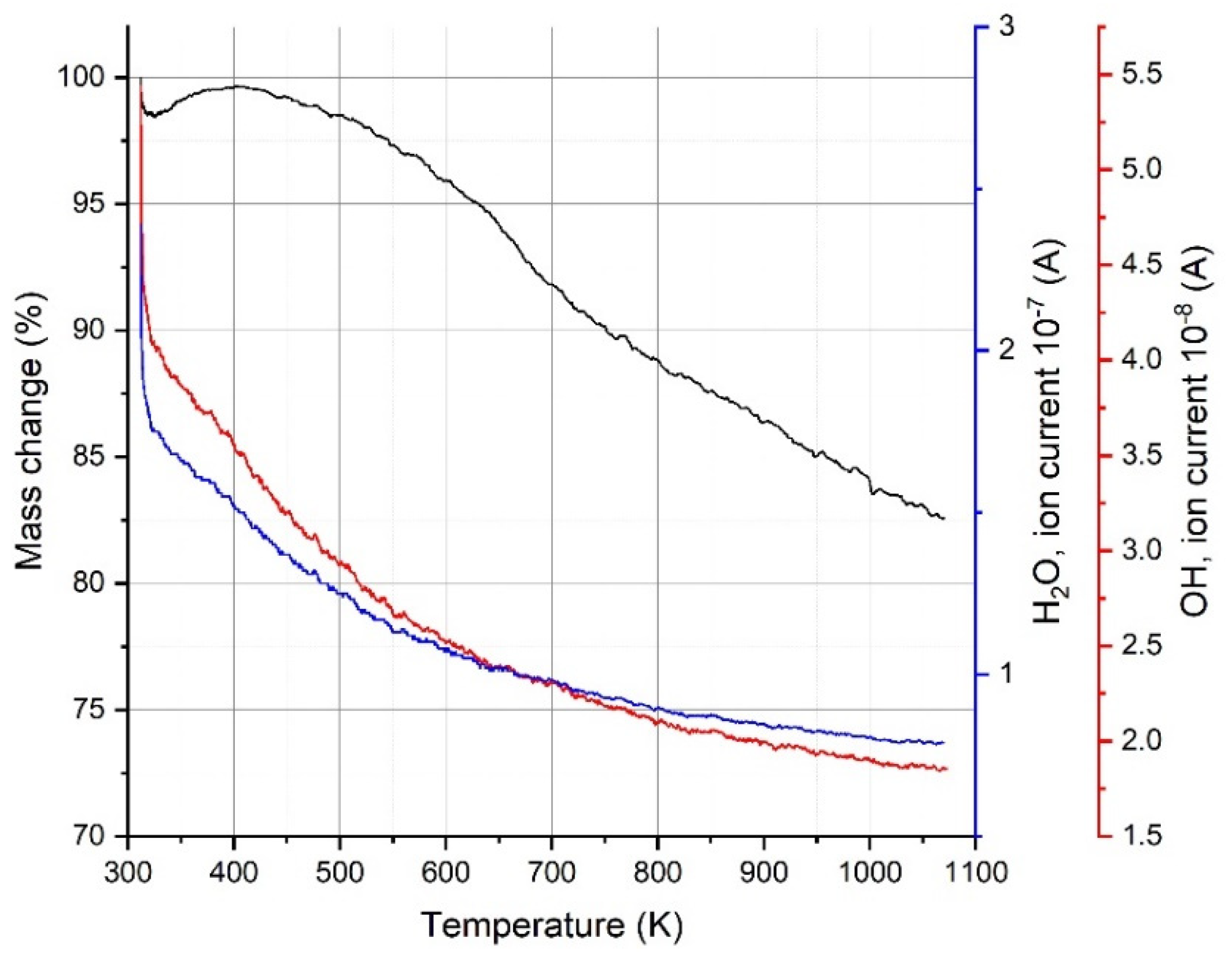
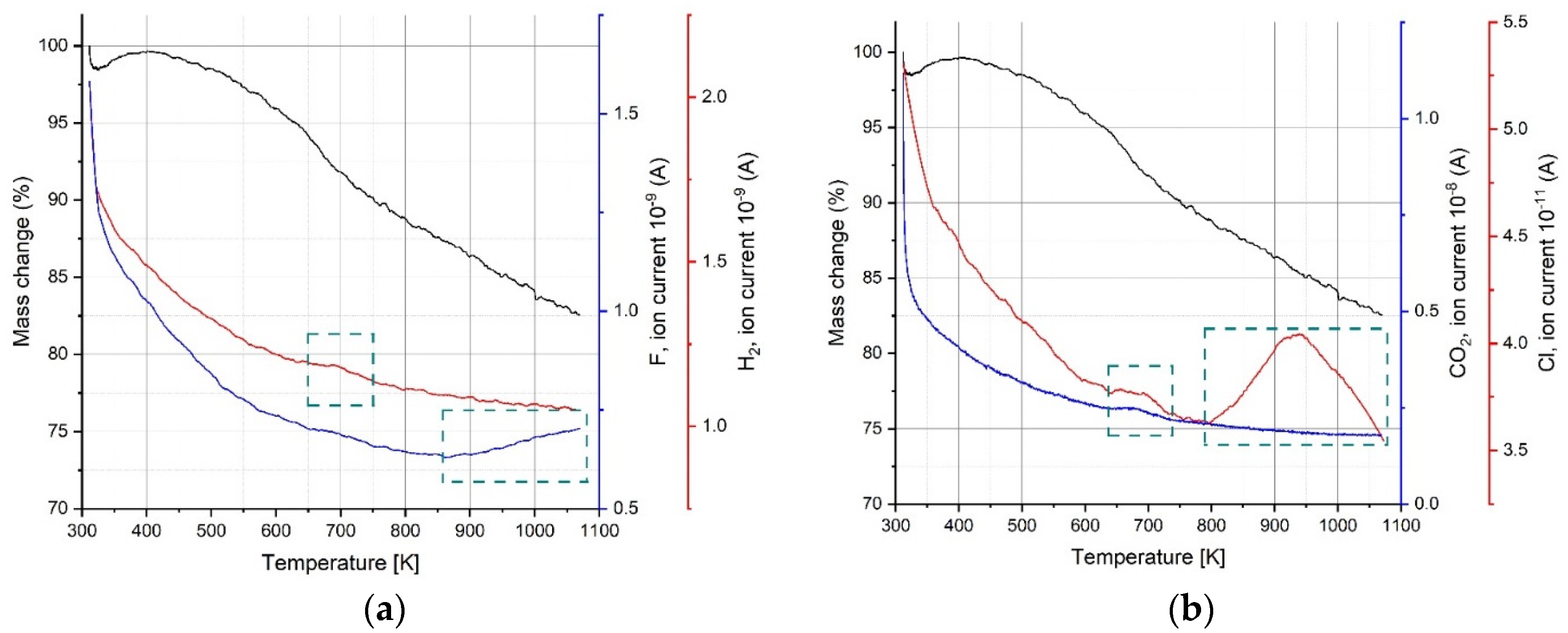
3.4. Thermal Analysis
3.5. Electrical Resistivity Measurements
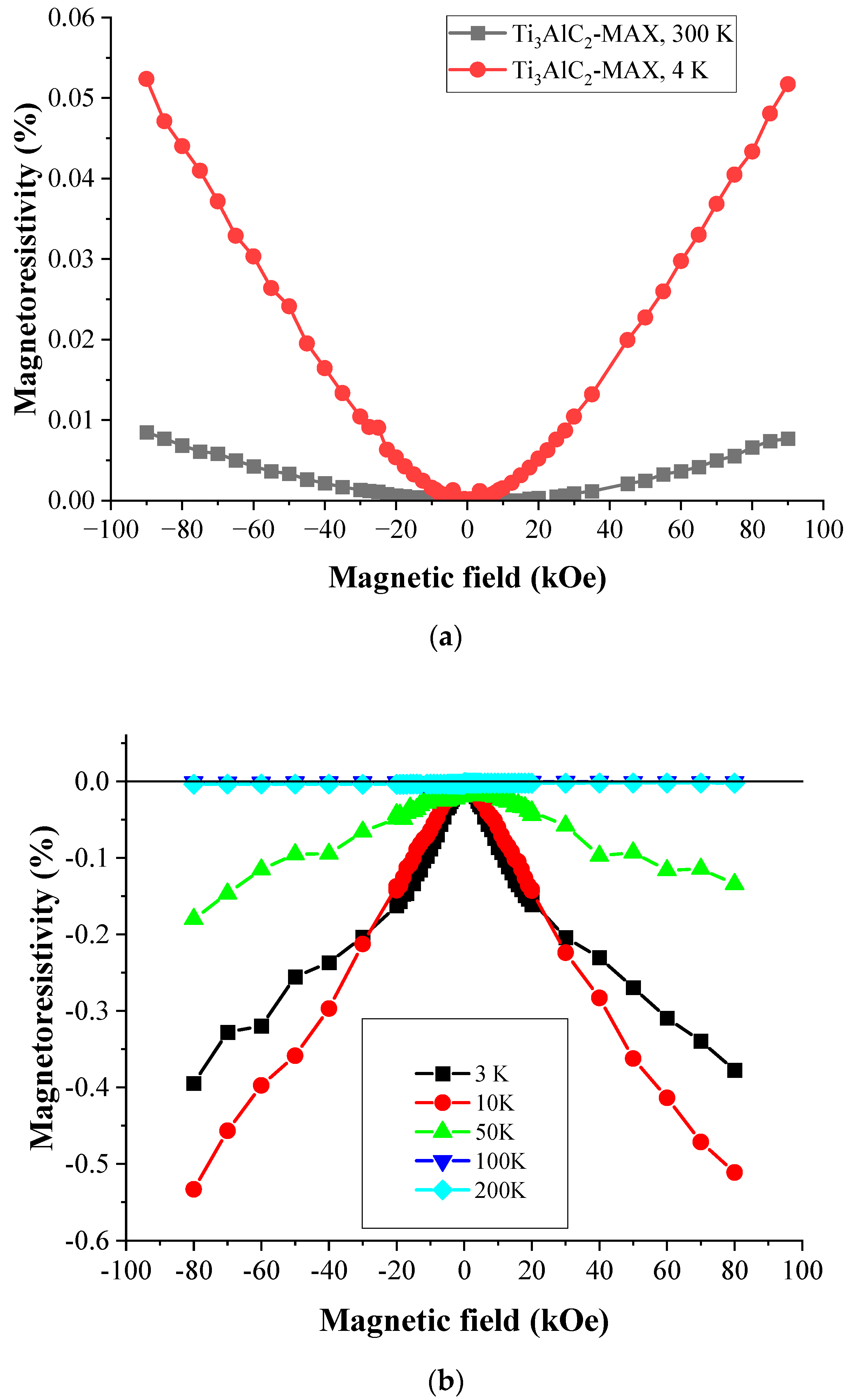
3.6. Magnetoresistivity and Magnetization Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Terrones, M.; Park, C.R.; Mukherjee, R.; Monthioux, M.; Koratkar, N.; Kim, Y.S.; Hurt, R.; Frackowiak, E.; Enoki, T.; et al. Carbon science in 2016: Status, challenges and perspectives. Carbon 2016, 98, 708–732. [Google Scholar] [CrossRef]
- Barney, C.R.D.; Bauman, J.J.; Feinberg, L.D.; Mccleese, D.J.; Singh, U.N.; Stahl, H.P. NASA Science Instruments, Observatories, & Sensor Systems Technology Area Roadmap—TA08 2010. Available online: https://nap.nationalacademies.org/read/13354/chapter/18 (accessed on 1 January 2024).
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, A.; Lu, H.; Alhabeb, M.; Anasori, B.; Gruverman, A.; Gogotsi, Y.; Sinitskii, A. Elastic properties of 2D Ti3C2Tx MXene monolayers and bilayers. Sci. Adv. 2018, 4, eaat0491. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, A.; Goad, A.; Loes, M.J.; Vorobeva, N.S.; Abourahma, J.; Gogotsi, Y.; Sinitskii, A. High electrical conductivity and breakdown current density of individual monolayer Ti3C2T MXene flakes. Matter 2021, 4, 1413–1427. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed]
- Srivatsa, S.; Belthangadi, P.; Ekambaram, S.; Pai, M.; Sen, P.; Uhl, T.; Kumar, S.; Grabowski, K.; Nayak, M.M. Dynamic response study of Ti3C2-MXene films to shockwave and impact forces. RSC Adv. 2020, 10, 29147–29155. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Sinha, A.; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Rastin, H.; Zhang, B.; Mazinani, A.; Hassan, K.; Bi, J.; Tung, T.T.; Losic, D. 3D bioprinting of cell-laden electroconductive MXene nanocomposite bioinks. Nanoscale 2020, 12, 16069–16080. [Google Scholar] [CrossRef]
- Han, M.; Zhang, D.; Shuck, C.E.; Gogotsi, Y. Ultralow and Selective Infrared Emission from MXenes. arXiv 2021. [Google Scholar] [CrossRef]
- Grabowski, K.; Srivatsa, S.; Vashisth, A.; Mishnaevsky, L.; Uhl, T. Recent advances in MXene-based sensors for Structural Health Monitoring applications: A Review. Measurement 2022, 189, 110575. [Google Scholar] [CrossRef]
- Kedambaimoole, V.; Garwal, K.H.; Konandur, R.; Sen, P.; Nayak, M.M.; Kumar, S. MXene Wearables: Properties, Fabrication Strategies, Sensing Mechanism and Applications. Mater. Adv. 2022, 3, 3784–3808. [Google Scholar] [CrossRef]
- Driscoll, N.; Erickson, B.; Murphy, B.B.; Richardson, A.G.; Robbins, G.; Nicholas, V.; Mathis, T.; Hantanasirisakul, K.; Bagga, P.; Gullbrand, S.E.; et al. MXtrodes: MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci. Transl. Med. 2021, 13, eabf8629. [Google Scholar] [CrossRef]
- Carey, M.; Barsoum, M.W. MXene polymer nanocomposites: A review. Mater. Today Adv. 2021, 9, 10012. [Google Scholar] [CrossRef]
- Sarycheva, A.; Polemi, A.; Liu, Y.; Dandekar, K.; Anasori, B.; Gogotsi, Y. 2D titanium carbide (MXene) for wireless communication. Sci. Adv. 2018, 4, eaau0920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, T.; Tian, Z.; Bibi, K.; Zhang, Y.; Alshareef, H.N. MXenes for Energy Harvesting. Adv. Mater. 2022, 34, 2108560. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, Z.; Alshareef, H.N. MXetronics: Electronic and photonic applications of MXenes. Nano Energy 2019, 60, 179–197. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, X.; Alshareef, H.N. Large Dielectric Constant Enhancement in MXene Percolative Polymer Composites. ACS Nano. 2018, 12, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, J.; Simon, P.; Xu, B. Two-dimensional MXenes for electrochemical capacitor applications: Progress, challenges and perspectives. Energy Storage Mater. 2021, 35, 630–660. [Google Scholar] [CrossRef]
- Pathak, I.; Acharya, D.; Chhetri, K.; Lohani, P.C.; Ko, T.H.; Muthurasu, A.; Subedi, S.; Kim, T.; Saidin, S.; Dahal, B.; et al. Ti3C2Tx MXene integrated hollow carbon nanofibers with polypyrrole layers for MOF-derived freestanding electrodes of flexible asymmetric supercapacitors. Chem. Eng. J. 2023, 469, 143388. [Google Scholar] [CrossRef]
- Pathak, I.; Dahal, B.; Chhetri, K.; Muthurasu, A.; Rosyara, Y.R.; Kim, T.; Saidin, S.; Ko, T.H.; Kim, H.Y. Integrating V-doped CoP on Ti3C2Tx MXene-incorporated hollow carbon nanofibers as a freestanding positrode and MOF-derived carbon nanotube negatrode for flexible supercapacitors. Chem. Eng. J. 2023, 475, 146351. [Google Scholar] [CrossRef]
- Ding, G.; Yang, B.; Chen, R.-S.; Zhou, K.; Han, S.-T.; Zhou, Y. MXenes for memristive and tactile sensory systems. Appl. Phys. Rev. 2021, 8, 011316. [Google Scholar] [CrossRef]
- Dong, Y.; Mallineni, S.S.K.; Maleski, K.; Behlow, H.; Mochalin, V.N.; Rao, A.M.; Gogotsi, Y.; Podila, R. Metallic MXenes: A new family of materials for flexible triboelectric nanogenerators. Nano Energy 2018, 44, 103–110. [Google Scholar] [CrossRef]
- Tan, D.; Jiang, C.; Sun, N.; Huang, J.; Zhang, Z.; Zhang, Q.; Bu, J.; Bi, S.; Guo, Q.; Song, J. Piezoelectricity in monolayer MXene for nanogenerators and piezotronics. Nano Energy 2021, 90, 106528. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2020, 120, 100757. [Google Scholar] [CrossRef]
- Shuck, C.E.; Sarycheva, A.; Anayee, M.; Levitt, A.; Zhu, Y.; Uzun, S.; Balitskiy, V.; Zahorodna, V.; Gogotsi, O.; Gogotsi, Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater 2020, 22, 1901241. [Google Scholar] [CrossRef]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk During Synthesis. ACS Chem. Health Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
- Célérier, S.; Hurand, S.; Garnero, C.; Morisset, S.; Benchakar, M.; Habrioux, A.; Chartier, P.; Mauchamp, V.; Findling, N.; Lanson, B.; et al. Hydration of Ti3C2Tx MXene: An Interstratification Process with Major Implications on Physical Properties. Chem. Mater. 2019, 31, 454–461. [Google Scholar] [CrossRef]
- Wu, F.; Luo, K.; Huang, C.; Wu, W.; Meng, P.; Liu, Y.; Kan, E. Theoretical understanding of magnetic and electronic structures of Ti3C2 monolayer and its derivatives. Solid State Commun. 2015, 222, 9–13. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Goc, K.; Przewoźnik, J.; Witulska, K.; Chlubny, L.; Tokarz, W.; Straczek, T.; Michalik, J.M.; Jurczyk, J.; Utke, I.; Lis, J. Structure, morphology, heat capacity, and electrical transport properties of Ti3(Al,Si)C2 materials. Materials 2021, 14, 3222. [Google Scholar] [CrossRef] [PubMed]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef]
- Mann, S.; Rani, P.; Kumar, R.; Dubey, G.S.; Jindal, V.K. Thermodynamic properties of pure and doped (B, N) graphene. RSC Adv. 2016, 6, 12158–12168. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.; Kvasnicka, D.; Luitz, J.; Laskowski, R.; Tran, F.; Marks, L.D. WIEN2k—An Augmented Plane Wave Local Orbitals Program for Calculating Crystal Properties. 2021. Available online: http://www.wien2k.at/reg_user/textbooks/usersguide.pdf (accessed on 1 January 2024).
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Jiang, F.; Wang, Y.; Mia, L.; Yu, Y.; Song, L. Fabrication and thermal stability of NH4HF2-etched Ti3C2 MXene. Ceram. Int. 2017, 43, 6322–6328. [Google Scholar] [CrossRef]
- Liu, N.; Li, Q.; Wan, H.; Chang, L.; Wang, H.; Fang, J.; Ding, T.; Wen, Q.; Zhou, L.; Xiao, X. High-temperature stability on air of Ti3C2Tx MXene-nased composite with extracted bentonite. Nat. Commun. 2022, 13, 5551. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Anasori, B.; Shi, C.; Moon, E.J.; Xie, Y.; Voigt, C.A.; Kent, P.R.C.; May, S.J.; Billinge, S.J.L.; Barsoum, M.W.; Gogotsi, Y. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. 2016, 1, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Pinto, D.; Pivak, Y.; van Omme, J.T.; May, S.J.; Gogotsi, Y.; Taheri, M.L. Control of MXenes’ electronic properties through termination and intercalation. Nat. Commun. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.; Xu, K.; Ren, G.; Mitchell, A.; Ou, J.Z. Machine Learning-Enabled Smart Sensor Systems. Adv. Intell. Syst. 2020, 2, 2000063. [Google Scholar] [CrossRef]
- Rodriguez-Lujan, I.; Fonollosa, J.; Vergara, A.; Homer, M.; Huerta, R. On the calibration of sensor arrays for pattern recognition using the minimal number of experiments. Chemom. Intell. Lab. Syst. 2014, 130, 123–134. [Google Scholar] [CrossRef]
- Fonollosa, J.; Vergara, A.; Huerta, R. Algorithmic mitigation of sensor failure: Is sensor replacement really necessary? Sens. Actuators B Chem. 2013, 183, 211–221. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1804779. [Google Scholar] [CrossRef]
- Goc, K.; Prendota, W.; Chlubny, L.; Strączek, T.; Tokarz, W.; Chachlowska, P.B.; Chabior, K.W.; Bućko, M.M.; Przewoźnik, J.; Lis, J. Structure, morphology and electrical transport properties of the Ti3AlC2 materials. Ceram. Int. 2018, 44, 18322–18328. [Google Scholar] [CrossRef]
- Yoon, Y.; Le, T.A.; Tiwari, A.P.; Kim, I.; Barsoum, M.W.; Lee, H. Low temperature solution synthesis of reduced two dimensional Ti3C2 MXenes with paramagnetic behaviour. Nanoscale 2018, 10, 22429–22438. [Google Scholar] [CrossRef]
- Zhang, K.; Di, M.; Fu, L.; Deng, Y.; Du, Y.; Tang, N. Enhancing the magnetism of 2D carbide MXene Ti3C2Tx by H2 annealing. Carbon 2020, 157, 90–96. [Google Scholar] [CrossRef]
- Teng, J.W.; Nergui, D.; Tzintzarov, G.N.; Ringel, B.L.; Brumbach, Z.R.; Heimerl, J.P.; Mensah, Y.A.; Moody, J.P.; Thorbourn, D.O.; Del Castillo, L.; et al. Cryogenic Total-Ionizing-Dose Response of 4th-Generation SiGe HBTs using 1-MeV Electrons for Europa-Surface Applications. IEEE Trans. Nucl. Sci. 2023, 70, 611–619. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivatsa, S.; Tokarz, W.; Przewoźnik, J.; Strączek, T.; Grabowski, K.; Rutkowski, P.; Uhl, T.; Kulawik, J.; Kata, D.; Madej, D.; et al. Temperature Evolution of Composition, Thermal, Electrical and Magnetic Properties of Ti3C2Tx-MXene. Materials 2024, 17, 2199. https://doi.org/10.3390/ma17102199
Srivatsa S, Tokarz W, Przewoźnik J, Strączek T, Grabowski K, Rutkowski P, Uhl T, Kulawik J, Kata D, Madej D, et al. Temperature Evolution of Composition, Thermal, Electrical and Magnetic Properties of Ti3C2Tx-MXene. Materials. 2024; 17(10):2199. https://doi.org/10.3390/ma17102199
Chicago/Turabian StyleSrivatsa, Shreyas, Waldemar Tokarz, Janusz Przewoźnik, Tomasz Strączek, Krzysztof Grabowski, Paweł Rutkowski, Tadeusz Uhl, Jan Kulawik, Dariusz Kata, Dominika Madej, and et al. 2024. "Temperature Evolution of Composition, Thermal, Electrical and Magnetic Properties of Ti3C2Tx-MXene" Materials 17, no. 10: 2199. https://doi.org/10.3390/ma17102199
APA StyleSrivatsa, S., Tokarz, W., Przewoźnik, J., Strączek, T., Grabowski, K., Rutkowski, P., Uhl, T., Kulawik, J., Kata, D., Madej, D., Lis, J., & Kapusta, C. (2024). Temperature Evolution of Composition, Thermal, Electrical and Magnetic Properties of Ti3C2Tx-MXene. Materials, 17(10), 2199. https://doi.org/10.3390/ma17102199









