Comparison of MAF-32 and a One-Pot Synthesized Superparamagnetic Iron Oxide/MAF-32 Composite for the Adsorption of Diclofenac
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
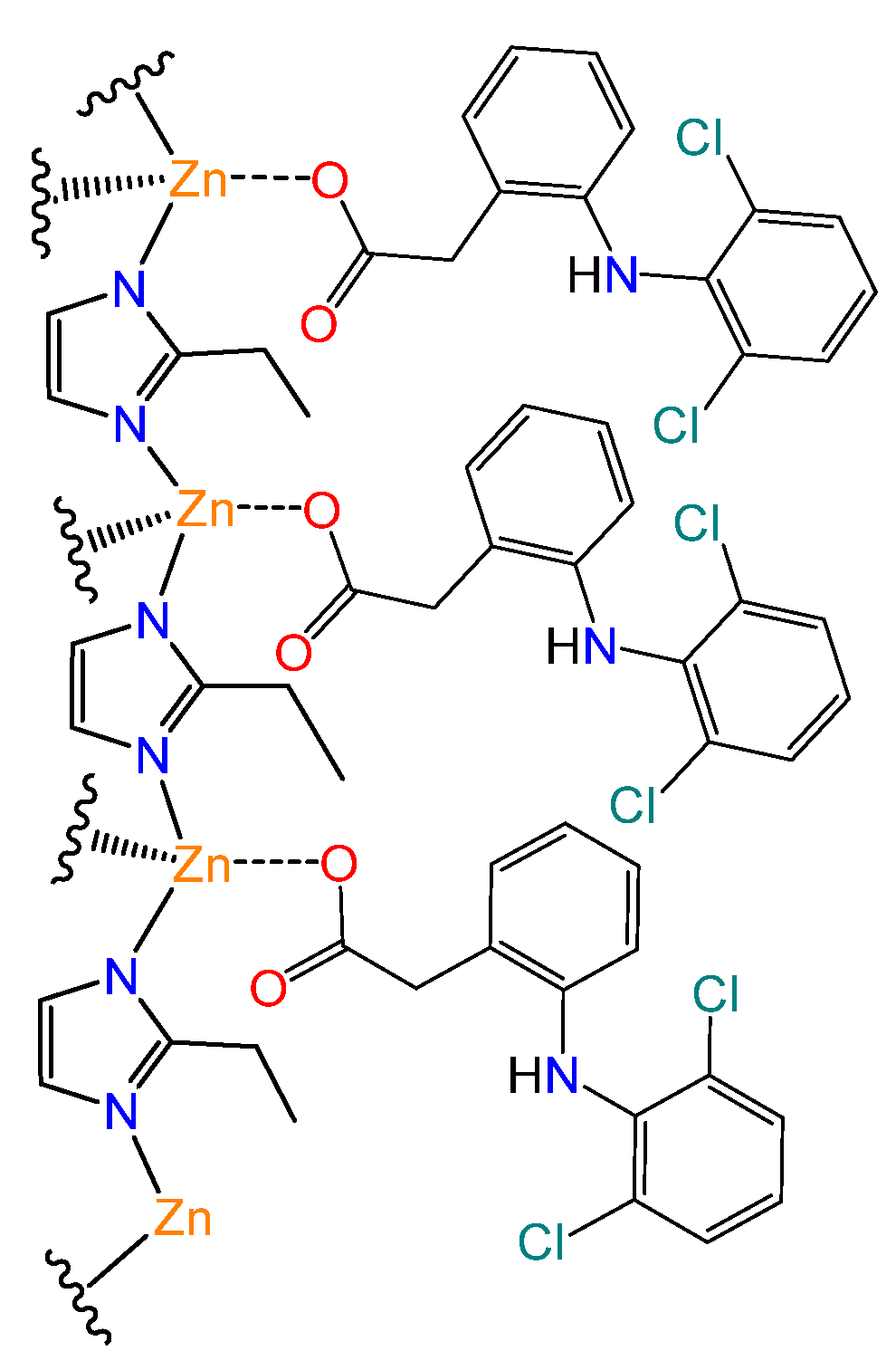
2.2. Preparation of MAF-32
2.3. One-Pot Preparation of Composite FexOy/MAF-32
2.4. Characterization
2.5. Adsorption Measurements
3. Results and Discussion
3.1. Compositional and Topological Identification
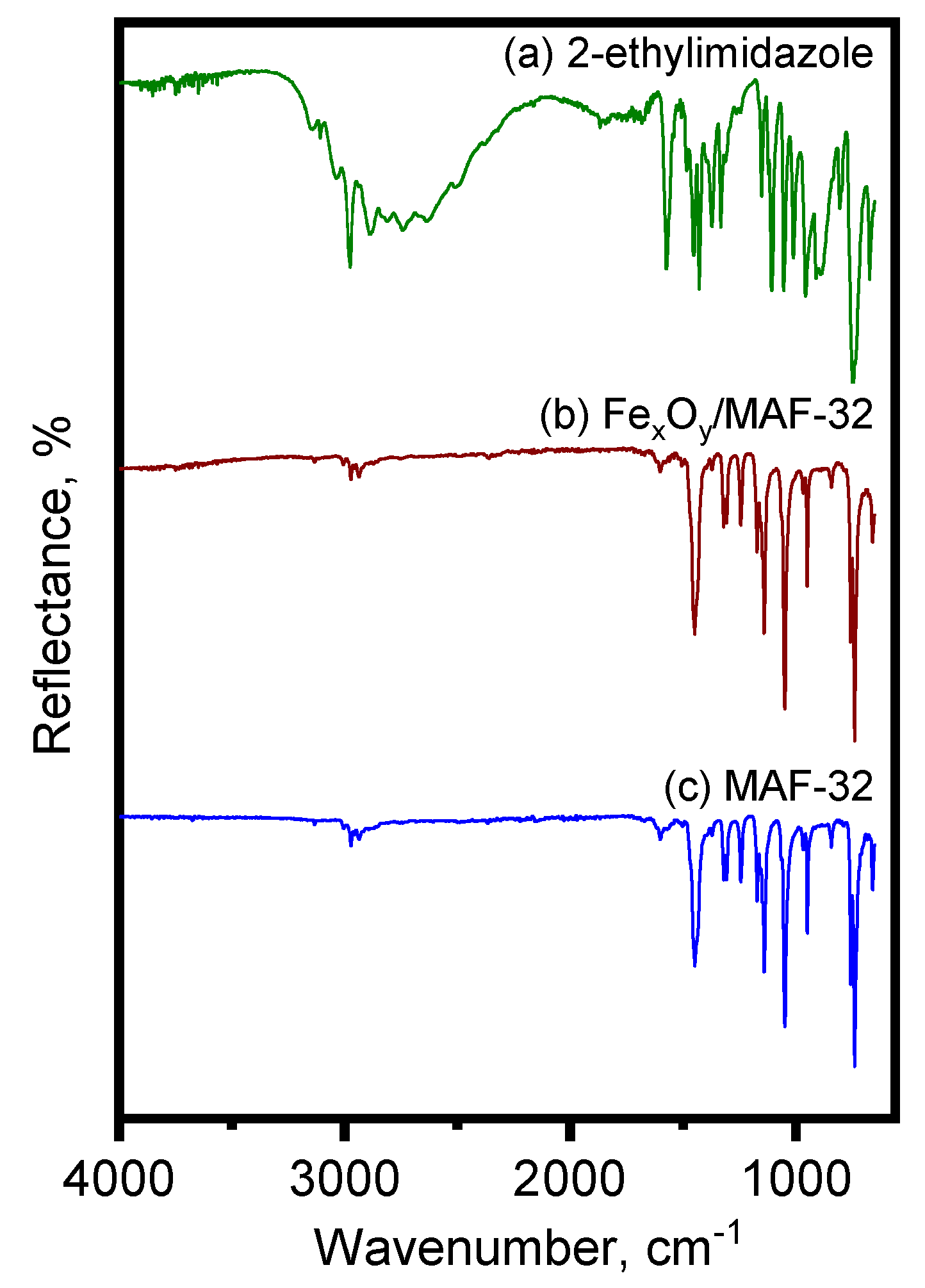
3.1.1. Vibrational Spectroscopy
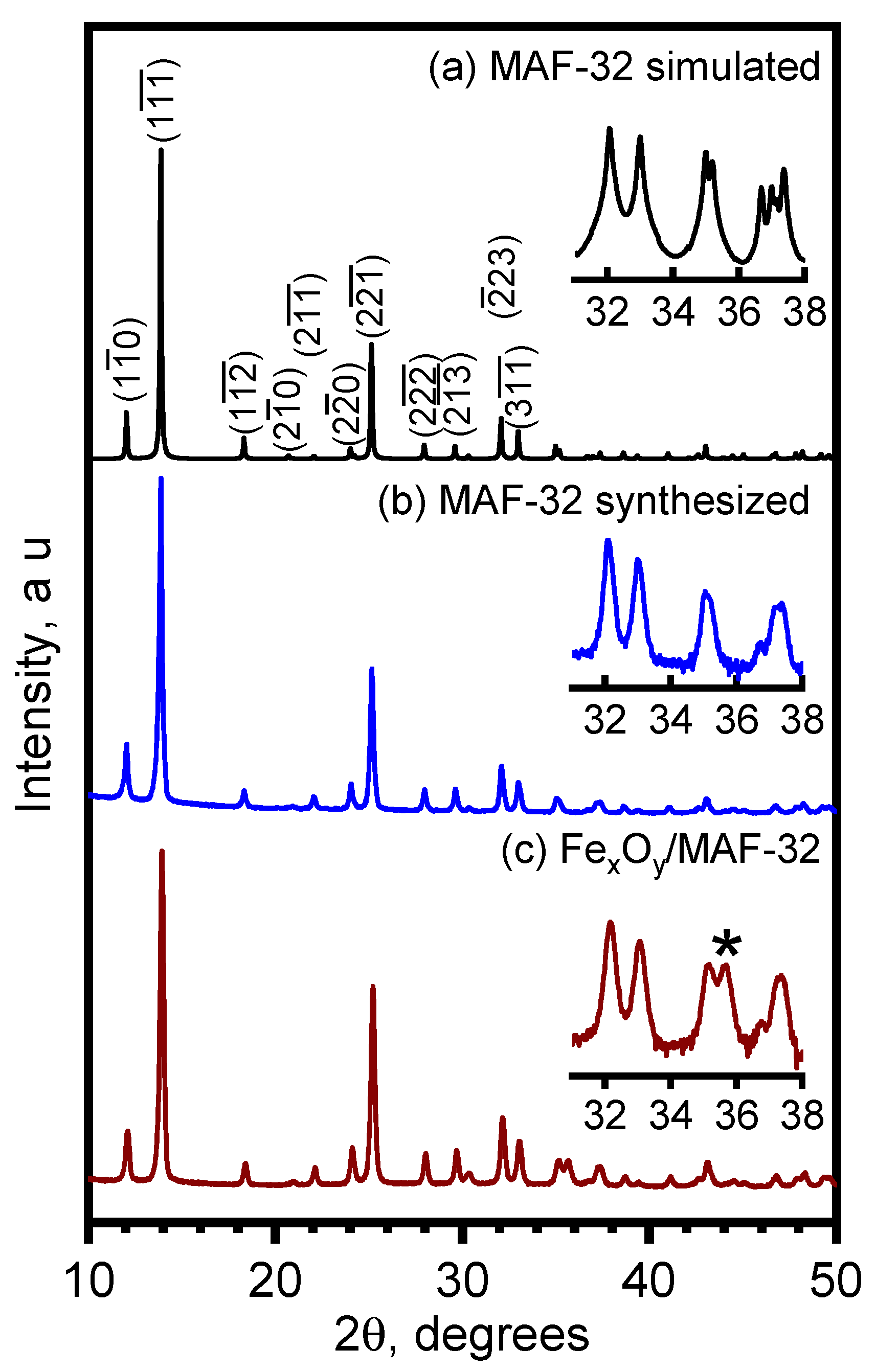
3.1.2. Topology and Crystallinity Analyzed by Powder X-ray Diffraction
3.2. Iron Oxide Phase Characterization and Magnetic Properties of the Composite
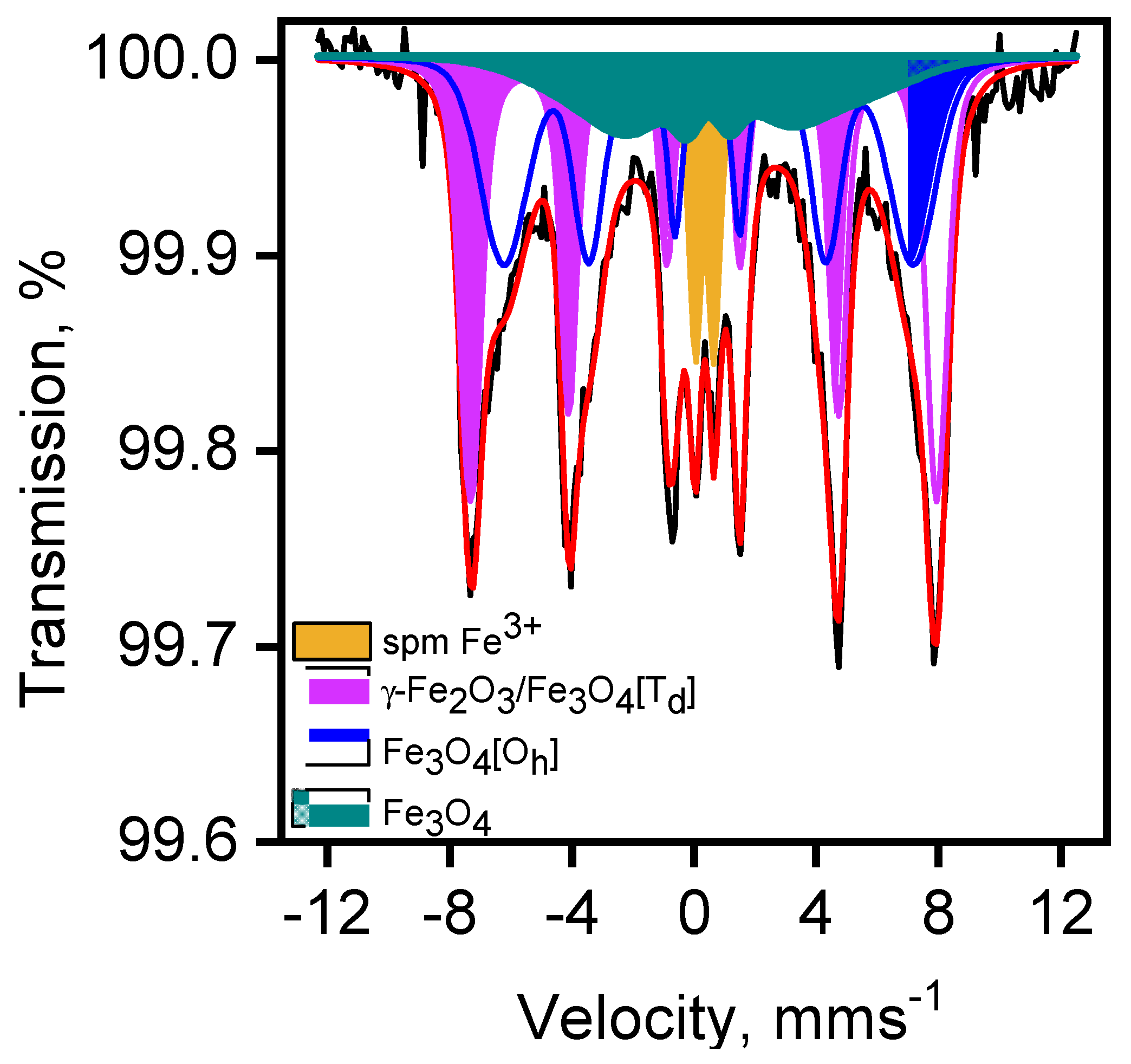
3.2.1. Magnetite/Maghemite Identification by Mössbauer Spectroscopy
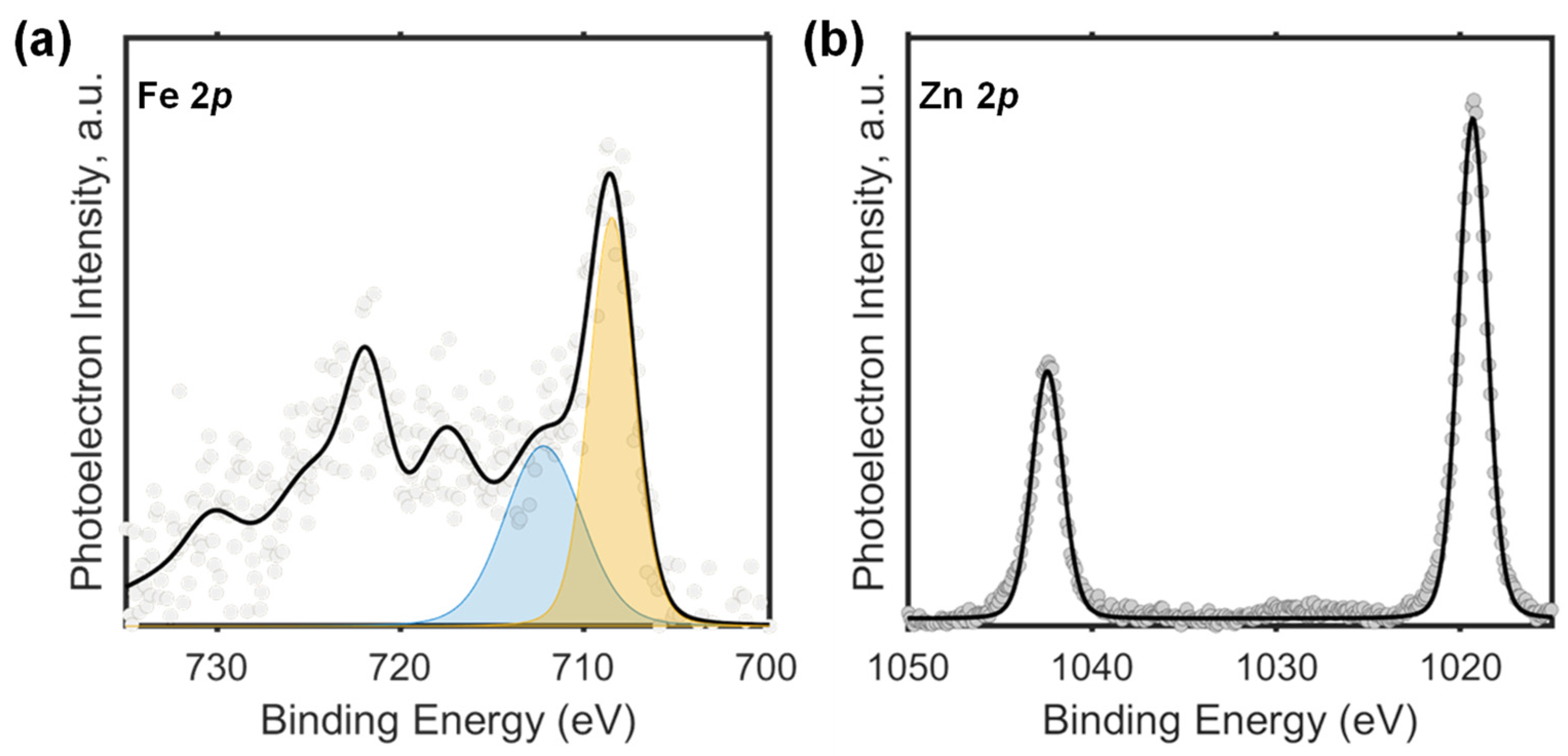
3.2.2. X-ray Photoelectron Spectroscopy Studies
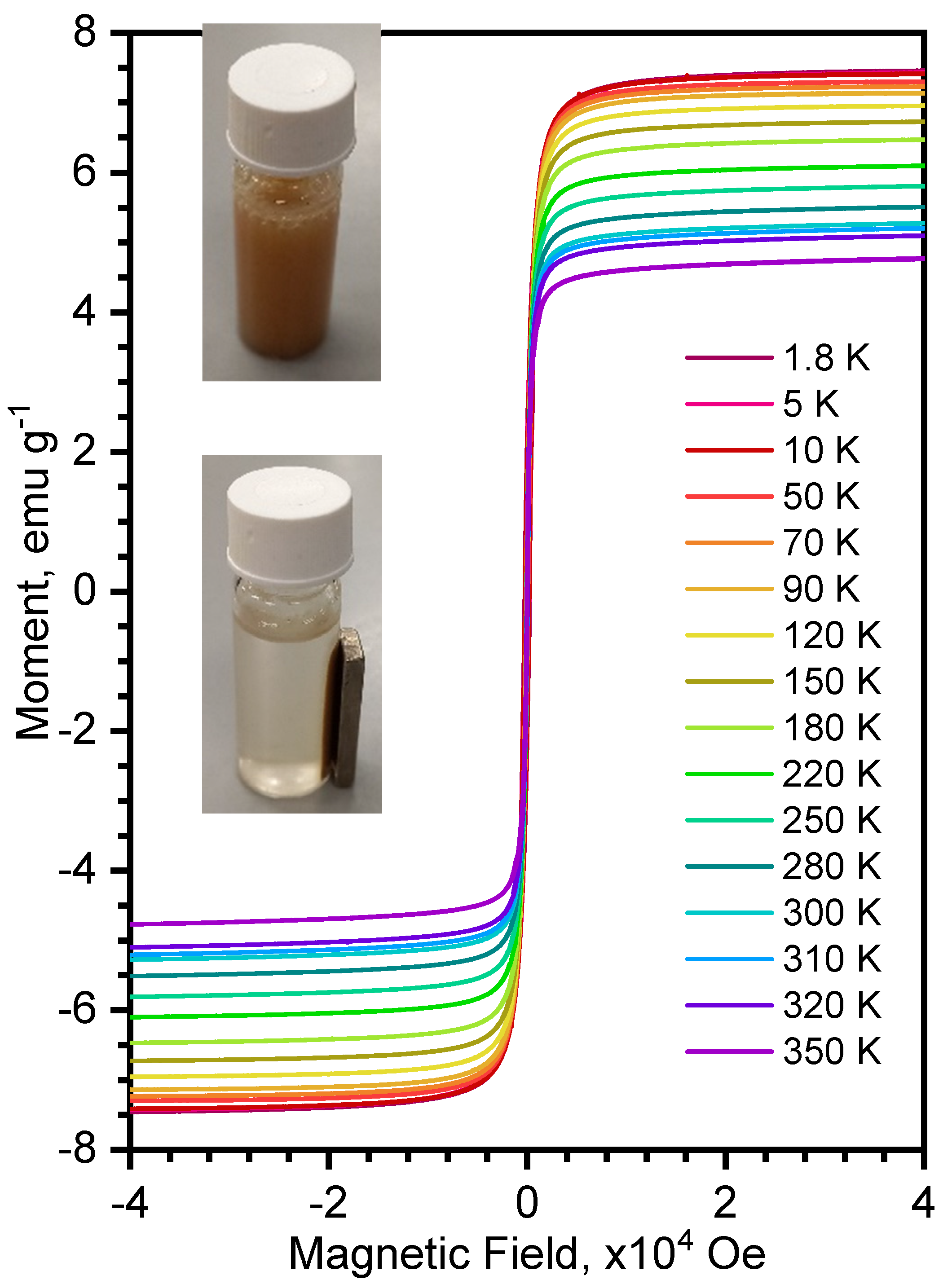
3.2.3. Magnetic Properties of the Composite FexOy/MAF-32
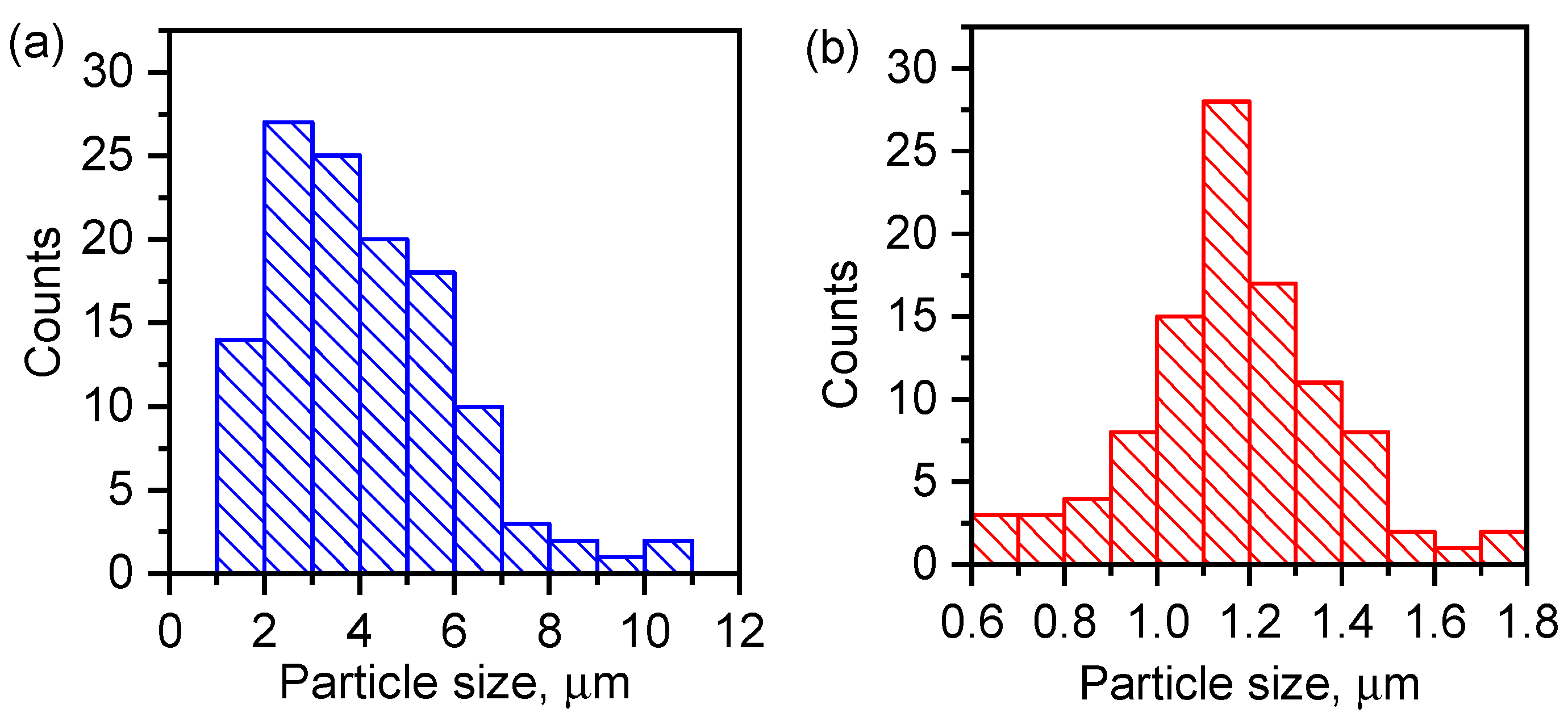
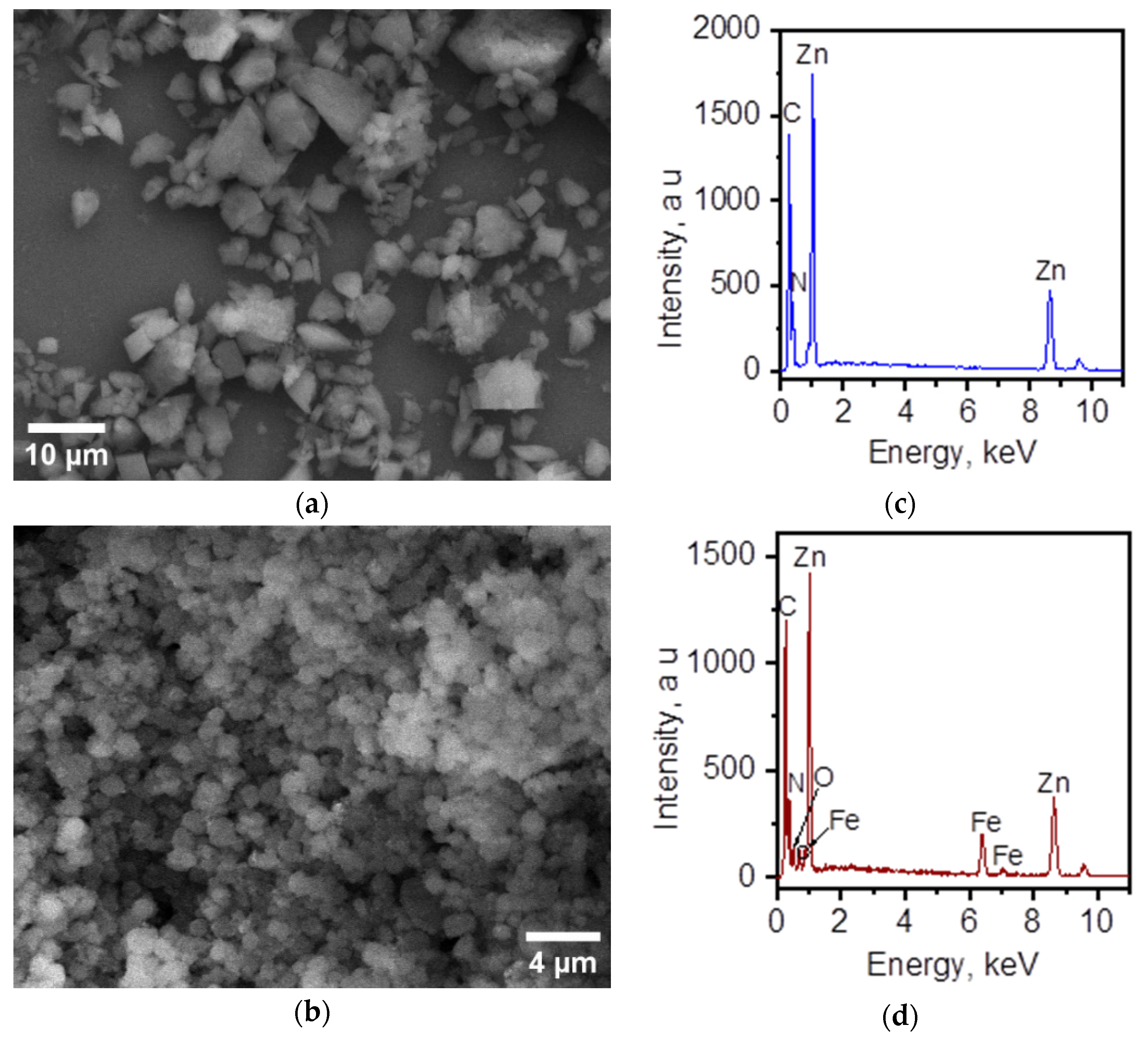
3.3. Morphology and Elemental Composition of the Surface
3.4. Optical Properties: MAF vs. MAF Composite
3.5. Textural Properties
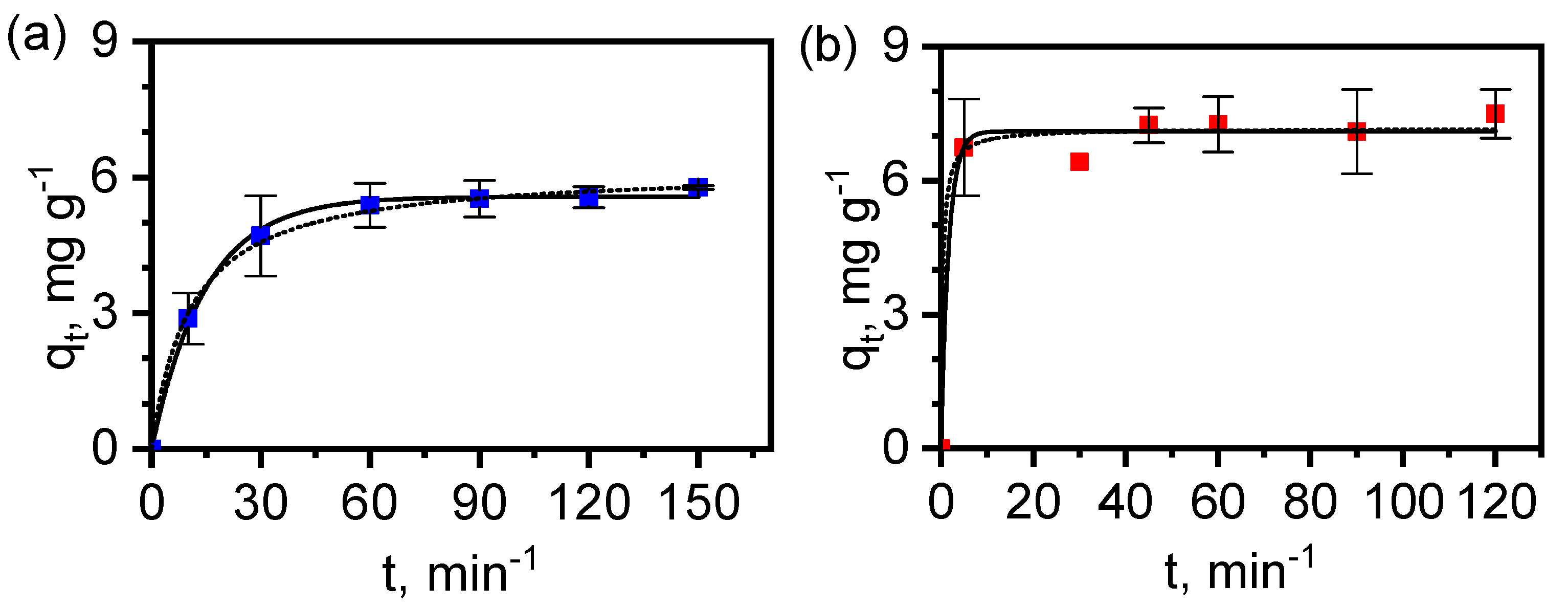
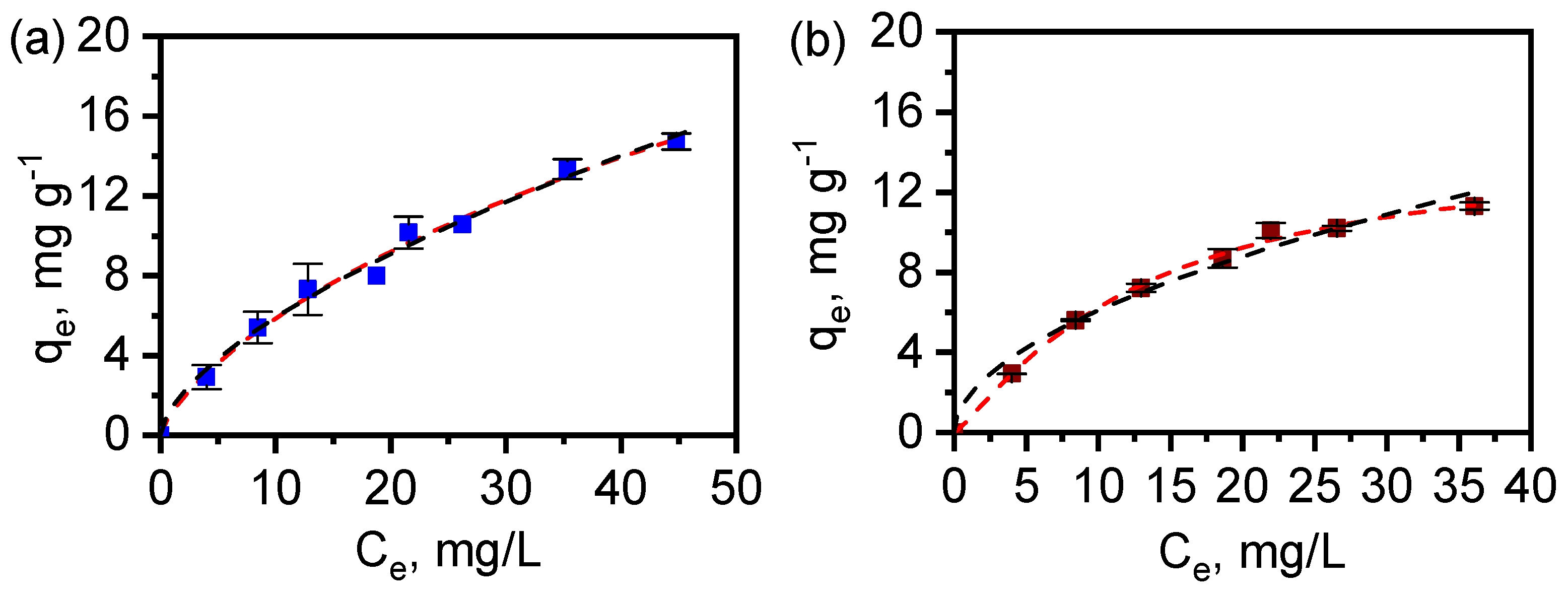
3.6. Adsorption of Diclofenac by MAF-32 and FexOy/MAF-32
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 20 September 2023).
- Orive, G.; Lertxundi, U.; Brodin, T.; Manning, P. Greening the Pharmacy. Science 2022, 377, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO2)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the Marine Environment: A Review of Its Occurrence and Effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and Its Transformation Products: Environmental Occurrence and Toxicity—A Review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Mirqasemi, M.S.; Homayoonfal, M.; Rezakazemi, M. Zeolitic Imidazolate Framework Membranes for Gas and Water Purification. Environ. Chem. Lett. 2020, 18, 1–52. [Google Scholar] [CrossRef]
- Thanh, M.T.; Thien, T.V.; Du, P.D.; Hung, N.P.; Khieu, D.Q. Iron Doped Zeolitic Imidazolate Framework (Fe-ZIF-8): Synthesis and Photocatalytic Degradation of RDB Dye in Fe-ZIF-8. J. Porous Mater. 2018, 25, 857–869. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Rangabhashiyam, S.; Adeyanju, C.A.; Ogunniyi, S.; Adeniyi, A.G.; Igwegbe, C.A. Zeolitic Imidazolate Frameworks (ZIFs) for Aqueous Phase Adsorption—A Review. J. Ind. Eng. Chem. 2022, 105, 34–48. [Google Scholar] [CrossRef]
- Andrew Lin, K.-Y.; Yang, H.; Lee, W.-D. Enhanced Removal of Diclofenac from Water Using a Zeolitic Imidazole Framework Functionalized with Cetyltrimethylammonium Bromide (CTAB). RSC Adv. 2015, 5, 81330–81340. [Google Scholar] [CrossRef]
- Guan, W.; Gao, X.; Ji, G.; Xing, Y.; Du, C.; Liu, Z. Fabrication of a Magnetic Nanocomposite Photocatalysts Fe3O4@ZIF-67 for Degradation of Dyes in Water under Visible Light Irradiation. J. Solid State Chem. 2017, 255, 150–156. [Google Scholar] [CrossRef]
- Manning, J.R.H.; Donval, G.; Tolladay, M.; Underwood, T.L.; Parker, S.C.; Düren, T. Identifying Pathways to Metal-Organic Framework Collapse during Solvent Activation with Molecular Simulations. J. Mater. Chem. A 2023, 11, 25929–25937. [Google Scholar] [CrossRef]
- Dai, H.; Yuan, X.; Jiang, L.; Wang, H.; Zhang, J.; Zhang, J.; Xiong, T. Recent Advances on ZIF-8 Composites for Adsorption and Photocatalytic Wastewater Pollutant Removal: Fabrication, Applications and Perspective. Coord. Chem. Rev. 2021, 441, 213985. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, S.; Jia, J.; Xu, F.; Long, Z.; Hou, X. Ultrasensitive Determination of Inorganic Arsenic by Hydride Generation-Atomic Fluorescence Spectrometry Using Fe3O4@ZIF-8 Nanoparticles for Preconcentration. Microchem. J. 2016, 124, 578–583. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.Y.; Liu, L.L.; Qiu, L.G.; Jiang, X. Fe3O4 Embedded ZIF-8 Nanocrystals with Ultra-High Adsorption Capacity towards Hydroquinone. J. Alloys Compd. 2015, 646, 1075–1082. [Google Scholar] [CrossRef]
- Mphuthi, L.E.; Erasmus, E.; Langner, E.H.G. Metal Exchange of ZIF-8 and ZIF-67 Nanoparticles with Fe(II) for Enhanced Photocatalytic Performance. ACS Omega 2021, 6, 31632–31645. [Google Scholar] [CrossRef]
- Archana, K.; Pillai, N.G.; Rhee, K.Y.; Asif, A. Super Paramagnetic ZIF-67 Metal Organic Framework Nanocomposite. Compos. B Eng. 2019, 158, 384–389. [Google Scholar] [CrossRef]
- Wang, X.L.; Sun, Y.Y.; Xiao, Y.; Chen, X.X.; Huang, X.C.; Zhou, H.L. Facile Solution-Refluxing Synthesis and Photocatalytic Dye Degradation of a Dynamic Covalent Organic Framework. Molecules 2022, 27, 8002. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.F.D.; Marrucho, I.M.; Freire, M.G. Removal of Nonsteroidal Anti-Inflammatory Drugs from Aqueous Environments with Reusable Ionic-Liquid-Based Systems. ACS Sustain. Chem. Eng. 2017, 5, 2428–2436. [Google Scholar] [CrossRef]
- Bergmann, J.; Friedel, P.; Kleeberg, R. BGMN—A New Fundamental Parameters Based Rietveld Program for Laboratory X-Ray Sources, It’s Use in Quantitative Analysis and Structure Investigations. Comm. Powder Diffr. Newslett. 1998, 20, 5–8. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid Room-Temperature Synthesis of Zeolitic Imidazolate Frameworks by Using Mechanochemistry. Angew. Chem. Int. Ed. 2010, 49, 9640–9643. [Google Scholar] [CrossRef]
- Wechsler, B.A.; Lindsley, D.H.; Prewitt, C.T. Crystal Structure and Cation Distribution in Titanomagnetites (Fe3-XTixO4). Am. Min. 1984, 69, 754–770. [Google Scholar]
- Pecharromán, C.; González-Carreño, T.; Iglesias, J.E. The Infrared Dielectric Properties of Maghemite, Gama-Fe2O3, from Reflectance Measurement on Pressed Powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Ramasamy, R. Vibrational Spectroscopic Studies of Imidazole. Armen. J. Phys. 2015, 8, 51–55. [Google Scholar]
- Ortiz, A.U.; Freitas, A.P.; Boutin, A.; Fuchs, A.H.; Coudert, F.X. What Makes Zeolitic Imidazolate Frameworks Hydrophobic or Hydrophilic? The Impact of Geometry and Functionalization on Water Adsorption. Phys. Chem. Chem. Phys. 2014, 16, 9940–9949. [Google Scholar] [CrossRef]
- He, C.T.; Jiang, L.; Ye, Z.M.; Krishna, R.; Zhong, Z.S.; Liao, P.Q.; Xu, J.; Ouyang, G.; Zhang, J.P.; Chen, X.M. Exceptional Hydrophobicity of a Large-Pore Metal-Organic Zeolite. J. Am. Chem. Soc. 2015, 137, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, A.B.; Sirotinkin, V.P.; Fedotov, M.A.; Korneyev, V.P.; Shamray, B.F.; Kovalenko, L.V. Quantitative Determination of Content of Magnetite and Maghemite in Their Mixtures by X-Ray Diffraction Methods. Inorg. Mater. Appl. Res. 2016, 7, 130–136. [Google Scholar] [CrossRef]
- Bergman, J.; Taut, T. Manual—Rietveld Analysis Program BGMN, 4th ed.; Dresden, Germany, 2005; pp. 33–34. Available online: http://www.bgmn.de/BGMN_manual_2005.pdf (accessed on 29 April 2024).
- Murad, E.; Johnston, J.H. Iron Oxides and Oxyhydroxides in Mössbauer Spectroscopy Applied to Inorganic Chemistry, 1st ed.; Long, G.J., Ed.; Plenum Press: New York, NY, USA; London, UK, 1987; Volume 2. [Google Scholar]
- Mørup, S. Mössbauer Spectroscopy Studies of Suspensions of Fe3O4 Microcrystals. J. Magn. Magn. Mater. 1983, 39, 45–47. [Google Scholar] [CrossRef]
- Roca, A.G.; Marco, J.F.; Del Puerto Morales, M.; Serna, C.J. Effect of Nature and Particle Size on Properties of Uniform Magnetite and Maghemite Nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Barrero, C.A.; Da Costa, G.M.; Van San, E.; De Grave, E. Mössbauer Characterization of Iron Oxides and (Oxy)Hydroxides: The Present State of the Art. Hyperfine Interact. 2000, 126, 247–259. [Google Scholar] [CrossRef]
- Mørup, S.; Topsøe, H.; Lipka, J. Modified Theory for Mössbauer Spectra of Superparamagnetic Particles: Application to Fe3O4. J. Phys. Colloq. 1976, 37, C6-287–C6-290. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.T.; Hendon, C.H.; Walsh, A. Designing Porous Electronic Thin-Film Devices: Band Offsets and Heteroepitaxy. Faraday Discuss. 2017, 201, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Grau-Crespo, R.; Aziz, A.; Collins, A.W.; Crespo-Otero, R.; Hernández, N.C.; Rodriguez-Albelo, L.M.; Ruiz-Salvador, A.R.; Calero, S.; Hamad, S. Modelling a Linker Mix-and-Match Approach for Controlling the Optical Excitation Gaps and Band Alignment of Zeolitic Imidazolate Frameworks. Angew. Chem. Int. Ed. 2016, 55, 16012–16016. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Katz, J.E.; Denlinger, J.D.; Yin, Y.; Falcone, R.; Waychunas, G.A. Soft X-Ray Spectroscopy Study of the Electronic Structure of Oxidized and Partially Oxidized Magnetite Nanoparticles. J. Phys. Chem. C 2010, 114, 21994–22001. [Google Scholar] [CrossRef]
- Ghazanfari, M.; Yazdani, A. Influence of MWCNTs on the Formation, Structure and Magnetic Properties of Magnetite. Mater. Sci. Semicond. Process. 2015, 40, 152–157. [Google Scholar] [CrossRef]
- Grimmett, M.R. Advances in Imidazole Chemistry. Adv. Heterocycl. Chem. 1970, 12, 103–183. [Google Scholar]
- Li, J.; Wu, Y.; Li, Z.; Zhang, B.; Zhu, M.; Hu, X.; Zhang, Y.; Li, F. Zeolitic Imidazolate Framework-8 with High Efficiency in Trace Arsenate Adsorption and Removal from Water. J. Phys. Chem. C 2014, 118, 27382–27387. [Google Scholar] [CrossRef]
- Chemicalize developed by ChemAxon. Available online: https://chemicalize.com/app/calculation (accessed on 4 March 2024).
- Arabkhani, P.; Javadian, H.; Asfaram, A.; Ateia, M. Decorating Graphene Oxide with Zeolitic Imidazolate Framework (ZIF-8) and Pseudo-Boehmite Offers Ultra-High Adsorption Capacity of Diclofenac in Hospital Effluents. Chemosphere 2021, 271, 129610. [Google Scholar] [CrossRef]
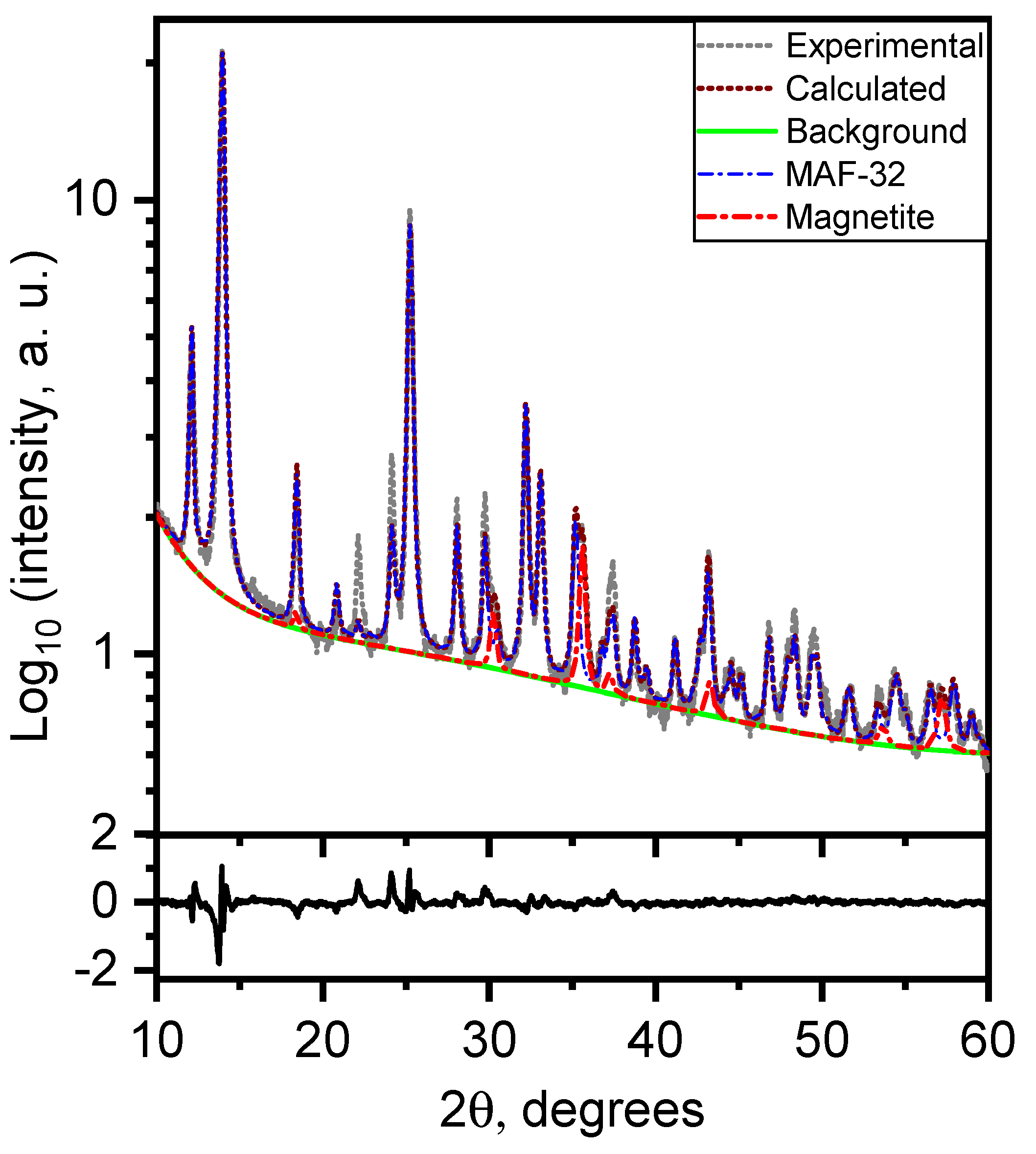
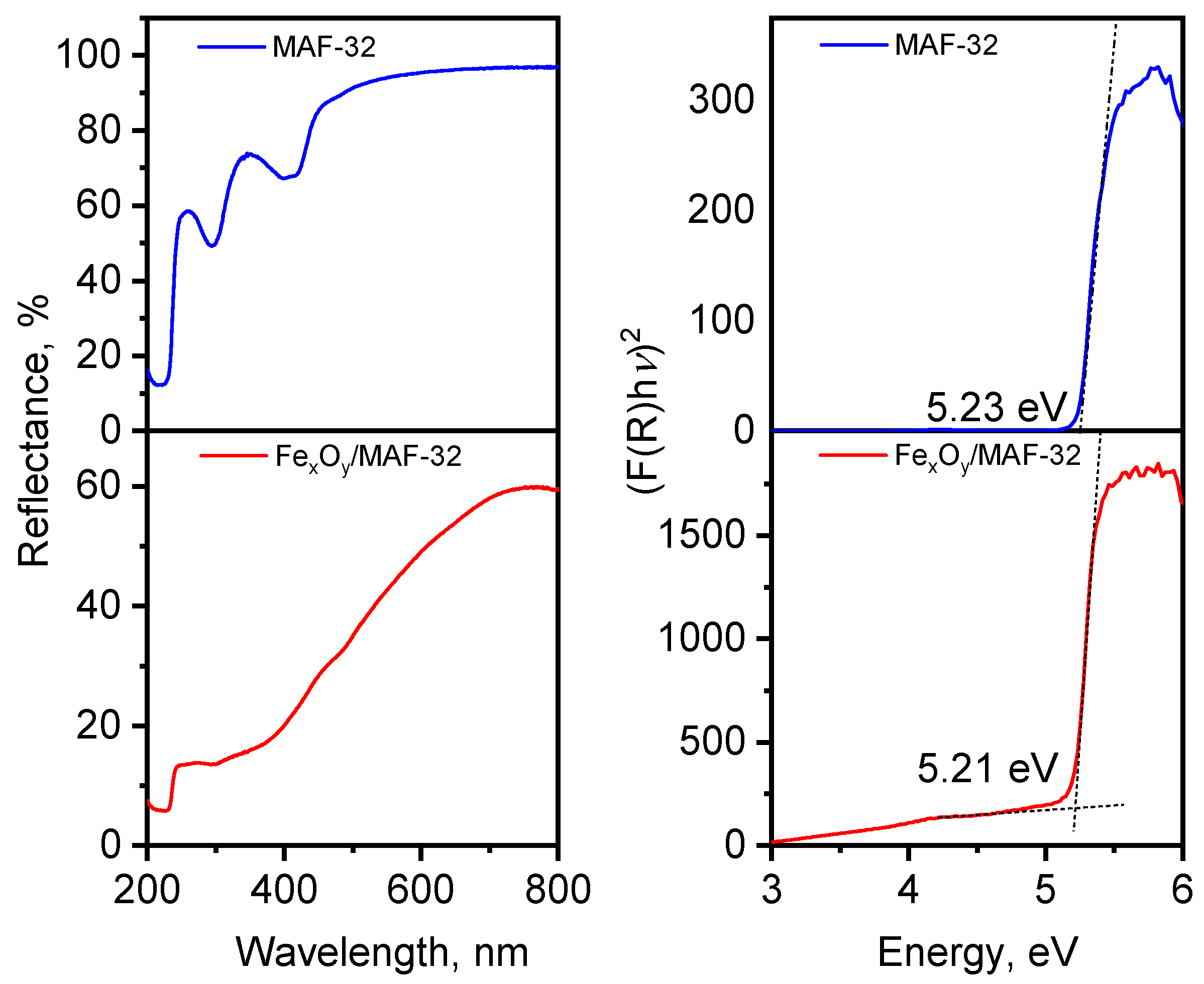
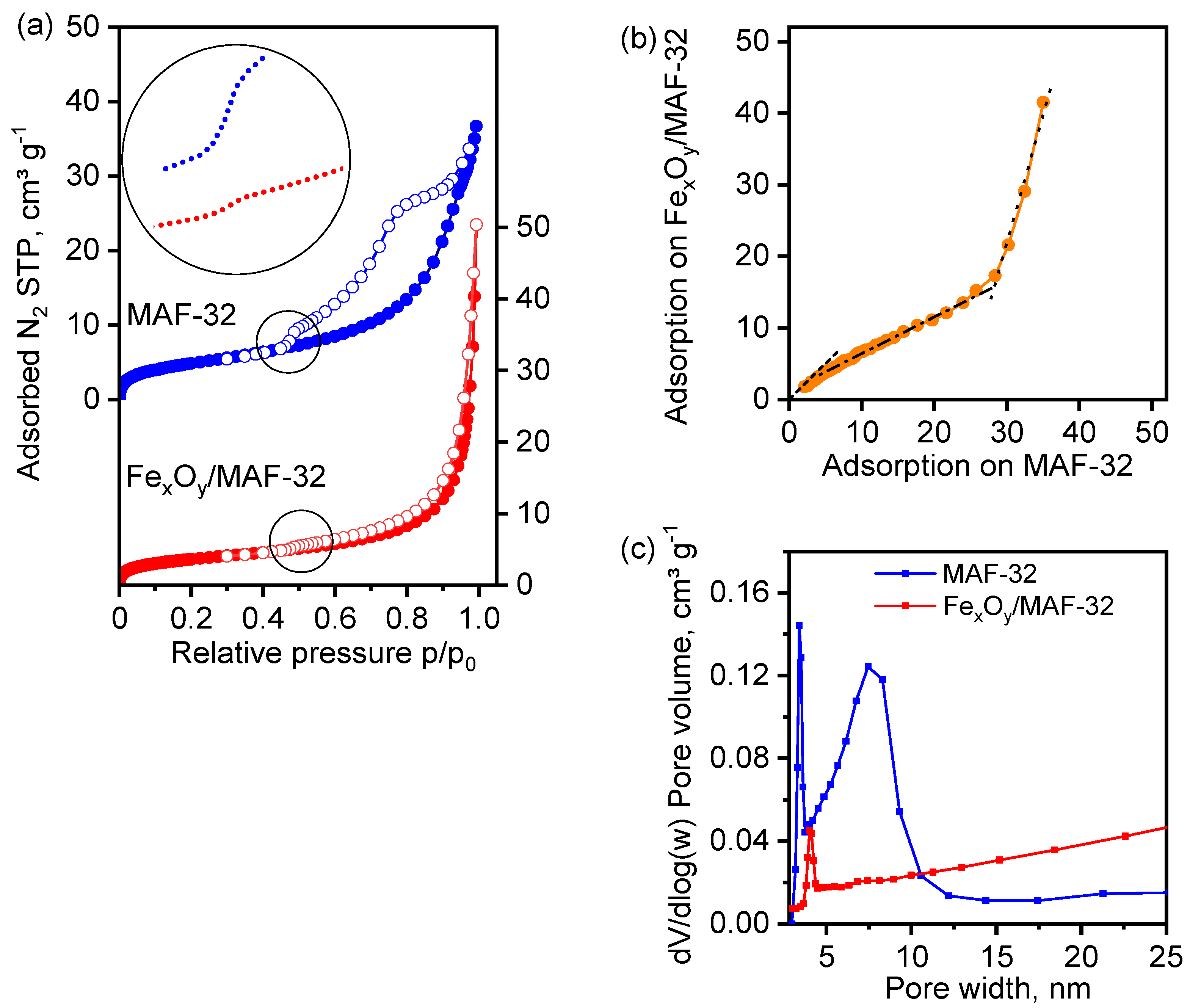
| Parameters | MAF-32 | Composite FexOy/MAF-32 | Composite FexOy/MAF-32 | ||
|---|---|---|---|---|---|
| MAF-32 | Magnetite Fe3O4 | MAF-32 | Maghemite Fe2O3 | ||
| Space group | P64 | P64 | 2/m | P64 | P4132 |
| Cell parameters | |||||
| a (nm) | 0.8483 (2) | 0.8497 (4) | 0.8395 (4) | 0.8498 (4) | 0.8397 (4) |
| c (nm) | 1.2852 (3) | 1.2858 (6) | – | 1.2859 (6) | – |
| k1 | 0 | 1 | 1 | 1 | 1 |
| Crystallite size (nm) | |||||
| <100> | 93 (3) | 48.3 (9) | 24 (1) | 48.3 (9) | 23 (1) |
| <010> | 93 (3) | 48.3 (9) | 24 (1) | 48.3 (9) | 23 (1) |
| <001> | 126 (11) | 77 (8) | 24 (1) | 78 (8) | 23 (1) |
| Composition (%) | 95.2 (2) | 4.8 (2) | 94.3 (3) | 5.7 (3) | |
| Rwp | 9.03 | 7.56 | 7.50 | ||
| Rexp | 4.53 | 2.87 | 2.87 | ||
| GoF | 1.99 | 2.63 | 2.61 | ||
| Site/Species | δ (mms−1) | Δ or 2ε (mms−1) | H (T) | Area (%) |
|---|---|---|---|---|
| spm Fe3+ (Doublet) | 0.34 | 0.63 | -- | 8 |
| γ-Fe2O3/Fe3O4[Td] (Sextet 1) | 0.32 | −0.01 | 47.3 | 39 |
| Fe3O4[Oh] (Sextet 2) | 0.45 | 0.04 | 41.5 | 36 |
| Fe3O4 (Sextet 3) | 0.66 | 0.39 | 26.2 | 17 |
| MAF-32 | FexOy/MAF-32 | |
|---|---|---|
| Pseudo-first-order | ||
| qe,exper (mg g−1) | 5.8 | 7.5 |
| qe,calc (mg g−1) | 5.6 ± 0.1 | 7.1 ± 0.2 |
| k1 × 103 (s−1) | 1.1 ± 0.1 | 9.9 ± 4.0 |
| R2 | 0.997 | 0.985 |
| Pseudo-second-order | ||
| qe,calc (mg g−1) | 6.2 ± 0.1 | 7.2 ± 0.2 |
| k2 × 103 (g mg−1 s−1) | 0.2 ± 0.02 | 6.2 ± 5.5 |
| R2 | 0.998 | 0.986 |
| MAF-32 | FexOy/MAF-32 | |
|---|---|---|
| Langmuir-Freundlich | ||
| qm (mg g−1) | 55.5 ± 60.9 | 14.7 ± 1.4 |
| kLF (mg g−1) | 0.02 ± 0.02 | 0.05 ± 0.01 |
| n | 0.75 ± 0.17 | 1.19 ± 0.14 |
| RLF2 | 0.992 | 0.997 |
| Freundlich | ||
| kF (mg g−1) (L mg−1)1/n | 1.4 ± 0.2 | 1.8 ± 0.3 |
| n | 0.62 ± 0.03 | 0.53 ± 0.05 |
| RF2 | 0.991 | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, E.; Carmona-Pérez, D.; Marco, J.F.; Sanchez-Lievanos, K.R.; Sabinas-Hernández, S.A.; Knowles, K.E.; Elizalde-González, M.P. Comparison of MAF-32 and a One-Pot Synthesized Superparamagnetic Iron Oxide/MAF-32 Composite for the Adsorption of Diclofenac. Materials 2024, 17, 2269. https://doi.org/10.3390/ma17102269
Ramírez E, Carmona-Pérez D, Marco JF, Sanchez-Lievanos KR, Sabinas-Hernández SA, Knowles KE, Elizalde-González MP. Comparison of MAF-32 and a One-Pot Synthesized Superparamagnetic Iron Oxide/MAF-32 Composite for the Adsorption of Diclofenac. Materials. 2024; 17(10):2269. https://doi.org/10.3390/ma17102269
Chicago/Turabian StyleRamírez, Erick, Daniela Carmona-Pérez, J. F. Marco, Karla R. Sanchez-Lievanos, Sergio A. Sabinas-Hernández, Kathryn E. Knowles, and María P. Elizalde-González. 2024. "Comparison of MAF-32 and a One-Pot Synthesized Superparamagnetic Iron Oxide/MAF-32 Composite for the Adsorption of Diclofenac" Materials 17, no. 10: 2269. https://doi.org/10.3390/ma17102269
APA StyleRamírez, E., Carmona-Pérez, D., Marco, J. F., Sanchez-Lievanos, K. R., Sabinas-Hernández, S. A., Knowles, K. E., & Elizalde-González, M. P. (2024). Comparison of MAF-32 and a One-Pot Synthesized Superparamagnetic Iron Oxide/MAF-32 Composite for the Adsorption of Diclofenac. Materials, 17(10), 2269. https://doi.org/10.3390/ma17102269







