Improved Synthesis of Cu2O NPs and Ascorbic Acid-Modified Derivatives for Adsorption of Brilliant Cresyl Blue: Surface and Reusability Studies
Abstract
1. Introduction
2. Method and Materials
2.1. Materials
2.2. Synthesis of Cu2O NPs
2.3. Ascorbic Acid-Mediated Cu2O Nanoparticles
2.4. Characterization of Cu2O NPs and Cu2O/Ascorbic Acid NPs
2.5. Photocatalytic Activity
3. Results and Discussion
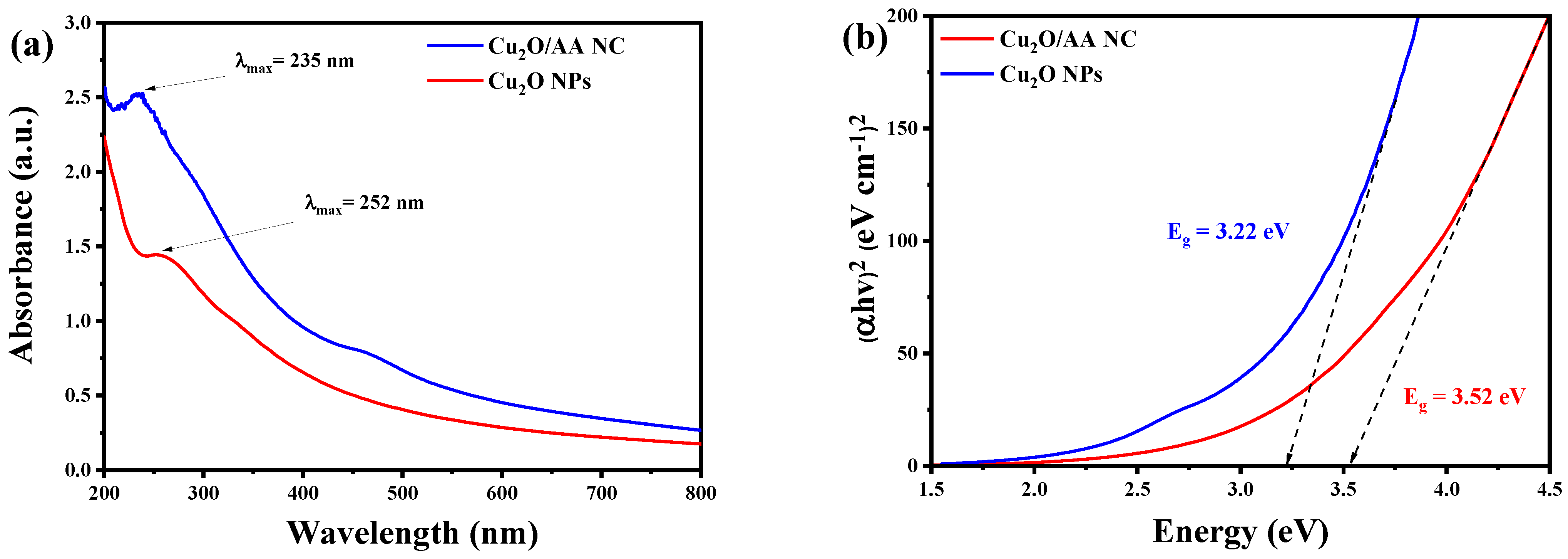
3.1. UV-Vis Analysis
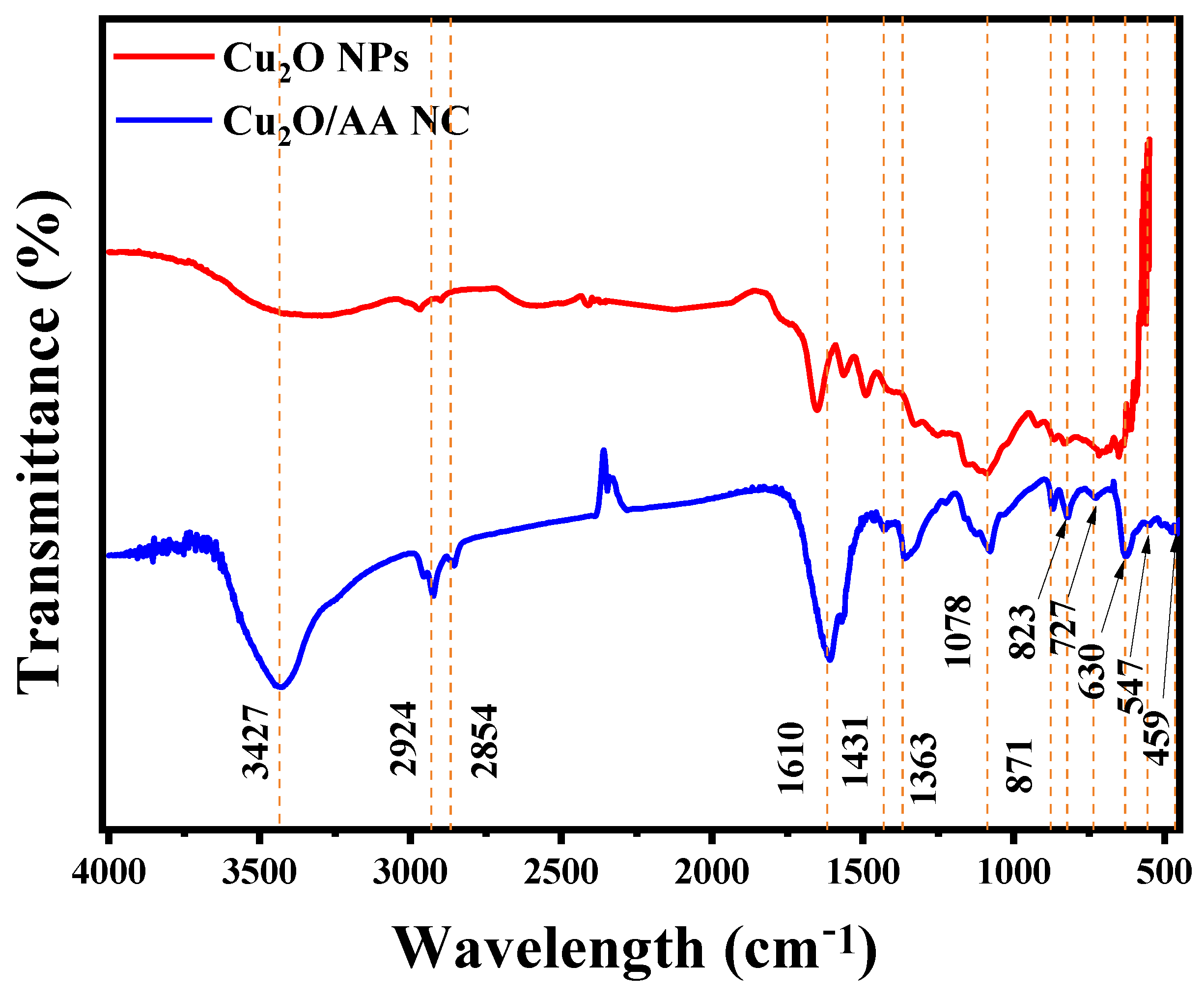
3.2. FTIR Analysis
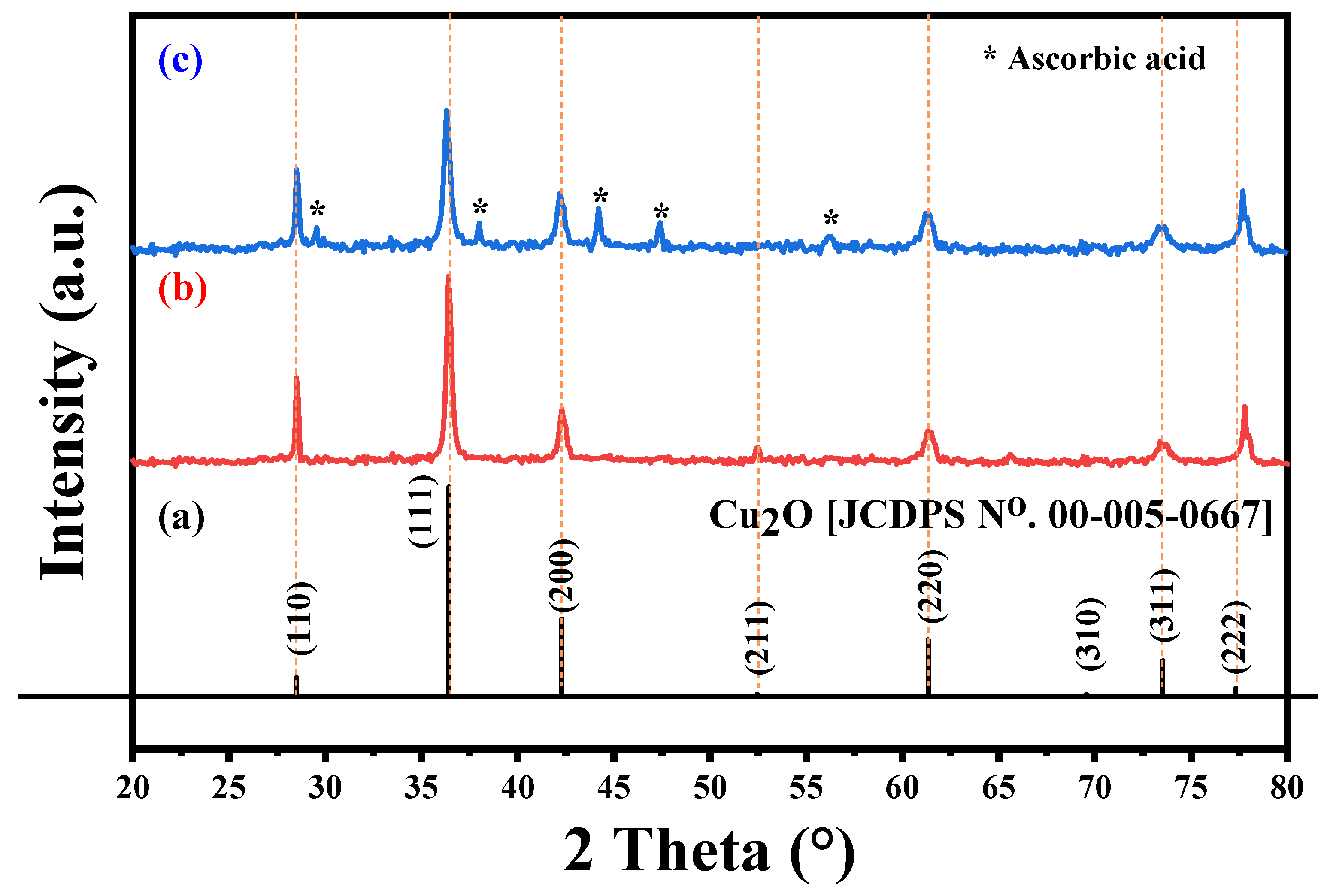
3.3. XRD Pattern Analysis
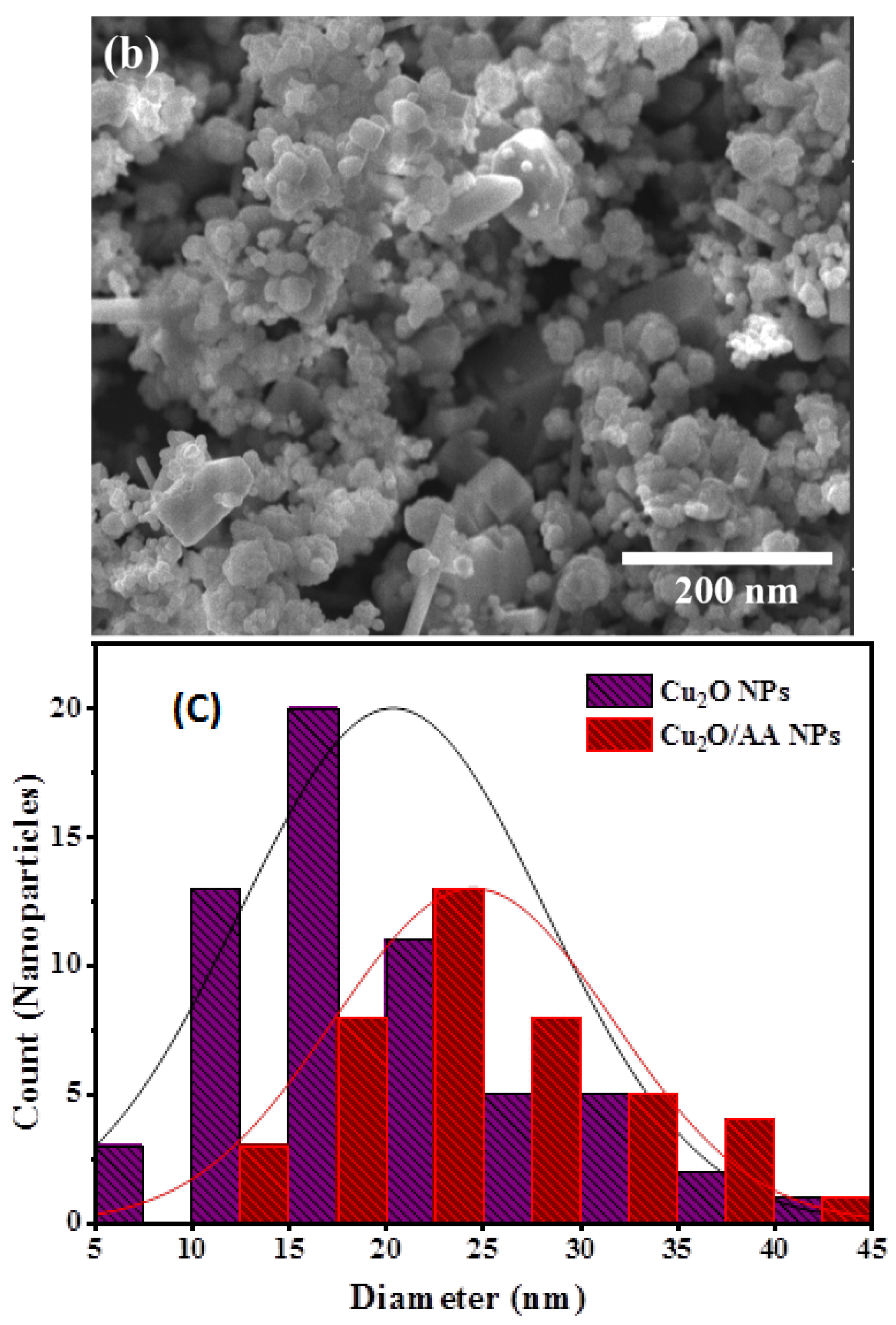
3.4. SEM Analysis
3.5. Zeta Potential Measurements
3.6. BET Analysis
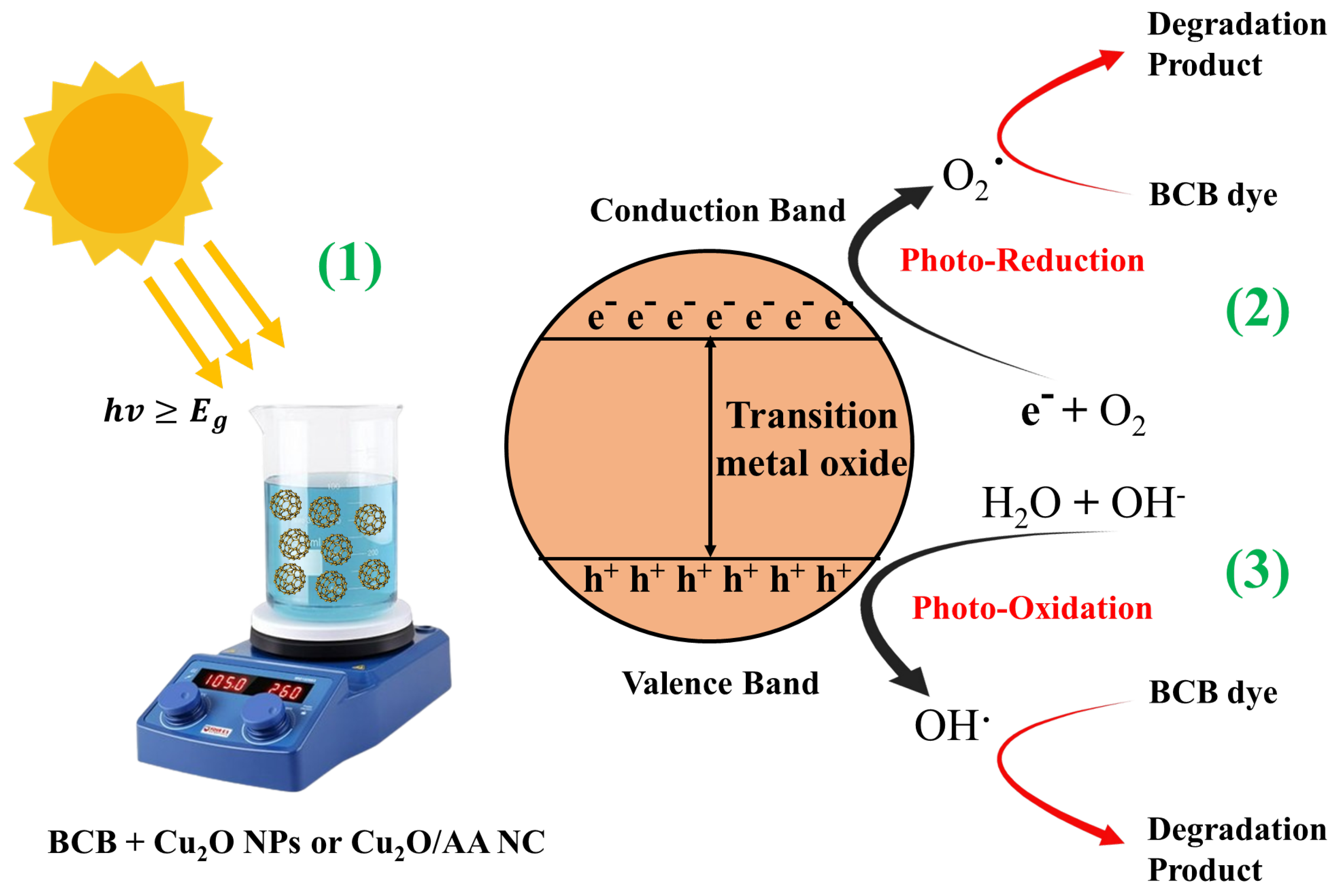
3.7. Photocatalytic Degradation of Brilliant Cresyl Blue
- Generation of electron–hole pairs: ;
- Water oxidation and hydroxyl radical formation: ;
- Superoxide radical formation: ;
- Additional hydroxyl radical formation: ;
- Degradation of BCB dye by ROS:
- Degradation of BCB dye by ROS:
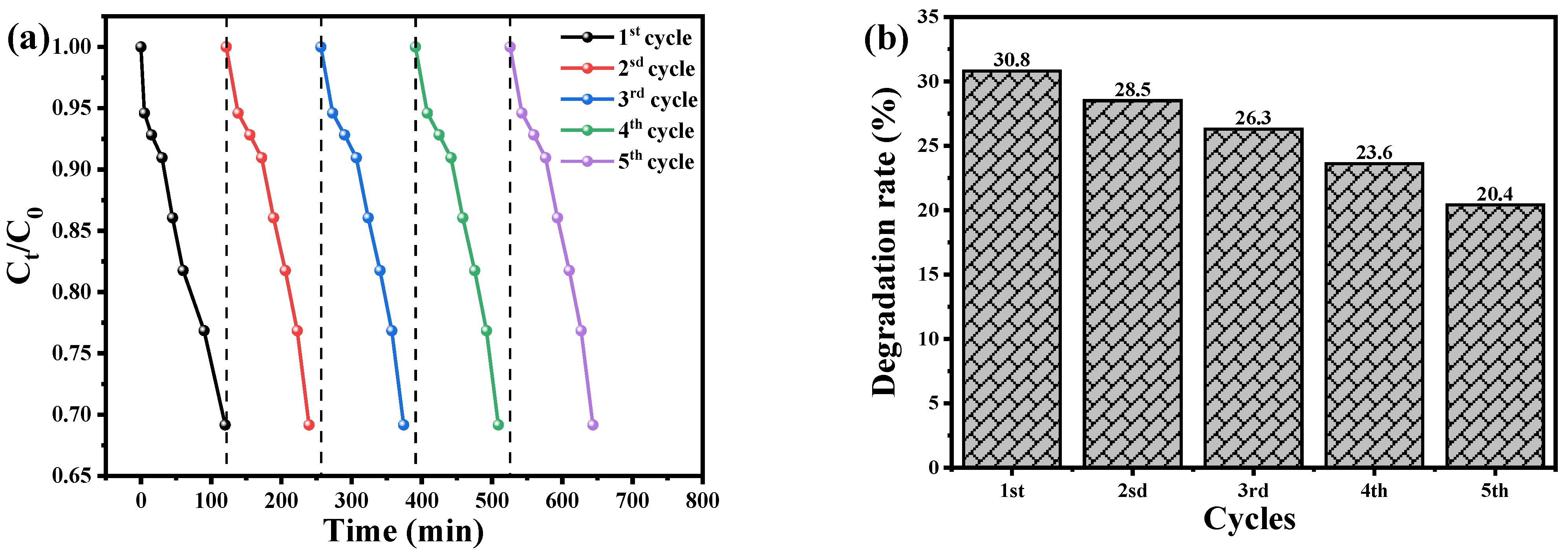
3.8. Recyclability and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maponya, T.C.; Hato, M.J.; Makhado, E.; Makgopa, K.; Khanuja, M.; Modibane, K.D. Photocatalytic Degradation of Dyes in Wastewater Using Metal Organic Frameworks. In Metal, Metal-Oxides and Metal-Organic Frameworks for Environmental Remediation; Springer International Publishing: Cham, Switzerland, 2021; pp. 261–285. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, S.; Ismail, A.M. Electrical and Optical Properties of Novel Brilliant Cresyl Blue Dye-doped Poly (Methyl Methacrylate) as Selective Cut-off Laser Filters. Polym. Int. 2020, 69, 1308–1318. [Google Scholar] [CrossRef]
- Roy, C.; Dutta, A.; Mahapatra, M.; Karmakar, M.; Roy, J.S.D.; Mitra, M.; Chattopadhyay, P.K.; Singha, N.R. Collagenic Waste and Rubber Based Resin-Cured Biocomposite Adsorbent for High-Performance Removal (s) of Hg (II), Safranine, and Brilliant Cresyl Blue: A Cost-Friendly Waste Management Approach. J. Hazard. Mater. 2019, 369, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Okoye, C.O.; Ezeorba, T.P.C.; Mao, G.; Chen, Y.; Xu, H.; Song, C.; Feng, W.; Wu, X. Emerging bio-dispersant and bioremediation technologies as environmentally friendly management responses toward marine oil spill: A comprehensive review. J. Environ. Manag. 2022, 322, 116123. [Google Scholar] [CrossRef] [PubMed]
- Gamelas, S.R.D.; Tomé, J.P.C.; Tomé, A.C.; Lourenço, L.M.O. Advances in Photocatalytic Degradation of Organic Pollutants in Wastewaters: Harnessing the Power of Phthalocyanines and Phthalocyanine-Containing Materials. RSC Adv. 2023, 13, 33957–33993. [Google Scholar] [CrossRef] [PubMed]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used for Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Franco, J.H.; Castañeda Cárdenas, S.D.; Zea Ramírez, H.R. Photocatalytic Degradation of Organic Dyes from Clinical Laboratory Wastewater. Water 2023, 15, 1238. [Google Scholar] [CrossRef]
- Aroob, S.; Carabineiro, S.A.C.; Taj, M.B.; Bibi, I.; Raheel, A.; Javed, T.; Yahya, R.; Alelwani, W.; Verpoort, F.; Kamwilaisak, K.; et al. Green Synthesis and Photocatalytic Dye Degradation Activity of CuO Nanoparticles. Catalysts 2023, 13, 502. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Stephen, A. Basic Principles, Mechanism, and Challenges of Photocatalysis BT. In Nanocomposites for Visible Light-Induced Photocatalysis; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–40. ISBN 978-3-319-62446-4. [Google Scholar]
- Gurylev, V. Photocatalysis: Basic Principles. In Advancement of Metal Oxide Materials for Photocatalytic Application; Gurylev, V., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–39. ISBN 978-3-031-20553-8. [Google Scholar]
- Ahmadian, A.; Ahmadi, S.; Goharrizi, B.A. Roles of Reactive Species in Photocatalysis: Effect of Scavengers and Inorganic Ions on Dye Removal from Wastewater. Int. J. Environ. Sci. Technol. 2023, 20, 6433–6448. [Google Scholar] [CrossRef]
- Náfrádi, M.; Veréb, G.; Firak, D.S.; Alapi, T. Photocatalysis: Introduction, Mechanism, and Effective Parameters. In Green Photocatalytic Semiconductors: Recent Advances and Applications; Garg, S., Chandra, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–31. ISBN 978-3-030-77371-7. [Google Scholar]
- Maheshwari, K.; Agrawal, M.; Gupta, A.B. Dye Pollution in Water and Wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–25. [Google Scholar]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of Copper Oxide Nanoparticles by Chemical and Biogenic Methods: Photocatalytic Degradation and in Vitro Antioxidant Activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, Selectivity, and Stability of Earth-Abundant CuO/Cu2O/Cu0-Based Photocatalysts toward CO2 Reduction. Chem. Eng. J. 2022, 429, 131579. [Google Scholar] [CrossRef]
- Nwanna, E.C.; Jen, T.-C. CuxOy Nanoparticle Fabrication: Synthesis, Characterization, and Applications. Mater. Sci. Eng. B 2024, 303, 117333. [Google Scholar] [CrossRef]
- Kavitha, S.; Jayamani, N.; Barathi, D. A Study on Preparation of Unique TiO2/Cu2O Nanocomposite with Highly Efficient Photocatalytic Reactivity under Visible-Light Irradiation. Mater. Technol. 2021, 36, 670–683. [Google Scholar] [CrossRef]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Mhamad, S.A.; Aziz, M. Review of Various Strategies to Boost the Photocatalytic Activity of the Cuprous Oxide-Based Photocatalyst. J. Environ. Chem. Eng. 2021, 9, 105138. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-Step Green Synthesis of Gold and Silver Nanoparticles with Ascorbic Acid and Their Versatile Surface Post-Functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Kader, D.A.; Rashid, S.O.; Omer, K.M. Green Nanocomposite: Fabrication, Characterization, and Photocatalytic Application of Vitamin C Adduct-Conjugated ZnO Nanoparticles. RSC Adv. 2023, 13, 9963–9977. [Google Scholar] [CrossRef] [PubMed]
- Bajić, V.; Spremo-Potparević, B.; Živković, L.; Čabarkapa, A.; Kotur-Stevuljević, J.; Isenović, E.; Sredojević, D.; Vukoje, I.; Lazić, V.; Ahrenkiel, S.P. Surface-Modified TiO2 Nanoparticles with Ascorbic Acid: Antioxidant Properties and Efficiency against DNA Damage in Vitro. Colloids Surf. B Biointerfaces 2017, 155, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Khan, A.A.P.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.-H. Engineering Nanostructures of CuO-Based Photocatalysts for Water Treatment: Current Progress and Future Challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-Edge Advances in Tailoring Size, Shape, and Functionality of Nanoparticles and Nanostructures: A Review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Díaz-García, Á.; Law, J.Y.; Romero, A.; Franco, V.; Guerrero, A. Quantifying the Structure and Properties of Nanomagnetic Iron Oxide Particles for Enhanced Functionality through Chemical Synthesis. Nanomaterials 2023, 13, 2242. [Google Scholar] [CrossRef] [PubMed]
- Vivas, L.; Chi-Duran, I.; Enríquez, J.; Barraza, N.; Singh, D.P. Ascorbic Acid Based Controlled Growth of Various Cu and Cu2O Nanostructures. Mater. Res. Express 2019, 6, 65033. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Jiang, L.; Zang, Z. Preparation of Cubic Cu2O Nanoparticles Wrapped by Reduced Graphene Oxide for the Efficient Removal of Rhodamine B. J. Alloys Compd. 2017, 718, 112–115. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Ascorbic Acid-Mediated Synthesis and Characterisation of Iron Oxide/Gold Core–Shell Nanoparticles. J. Exp. Nanosci. 2016, 11, 370–382. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eddine, L.S.; Hasan, G.G.; Meneceur, S.; Salmi, C.; Abdullah, J.A.A.; Abdullah, M.; Menaa, F. Efficient Removal of Heavy Metals, Dyes, and Contaminants from Industrial Wastewater Using Chitosan-Coated Fe3O4 Nanocomposites: Biosynthesis, Characterizations, and Performance Evaluation. Biomass Convers. Biorefinery 2024, 1–16. [Google Scholar] [CrossRef]
- Terea, H.; Selloum, D.; Rebiai, A.; Bouafia, A.; Ben Mya, O. Preparation and Characterization of Cellulose/ZnO Nanoparticles Extracted from Peanut Shells: Effects on Antibacterial and Antifungal Activities. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Allag, N.; Bouafia, A.; Chemsa, B.; Ben Mya, O.; Chala, A.; Siad, C.; Alam, M.W. Effect of Precursors on Structural, Optical and Surface Properties of ZnO Thin Film Prepared by Spray Pyrolysis Method: Efficient Removal of Cu (II) from Wastewater. Transit. Met. Chem. 2023, 49, 39–51. [Google Scholar] [CrossRef]
- Kir, I.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Mohammed, H.A.M. Biosynthesis and Characterization of Novel Nanocomposite ZnO/BaMg2 Efficiency for High-Speed Adsorption of AZO Dye. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Tedjani, M.L.; Khelef, A.; Laouini, S.E.; Bouafia, A.; Albalawi, N. Optimizing the Antibacterial Activity of Iron Oxide Nanoparticles Using Central Composite Design. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3564–3584. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Melo, E.I.; Alves, V.N.; Coelho, N.M.M. Moringa Oleifera Lam. Seeds as a Natural Solid Adsorbent for Removal of AgI in Aqueous Solutions. J. Braz. Chem. Soc. 2010, 21, 1727–1732. [Google Scholar] [CrossRef]
- Sakura, G.B.; Leung, A.Y.T. Experimental Study of Particle Collection Efficiency of Cylindrical Inlet Type Cyclone Separator. Int. J. Environ. Sci. Dev. 2015, 6, 160–164. [Google Scholar] [CrossRef]
- Zohra, R.; Meneceur, S.; Mohammed, H.A.; Hasan, G.G.; Bouafia, A.; Abdullah, J.A.A.; Alharthi, F.; Eddine, L.S. Enhanced Photocatalytic Degradation of Dyes and Antibiotics with Biosynthesized FeMn2O4 Nanocomposite under Sunlight Irradiation: Isotherm and Kinetic Study. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Laid, T.M.; Abdelhamid, K.; Eddine, L.S.; Abderrhmane, B. Optimizing the Biosynthesis Parameters of Iron Oxide Nanoparticles Using Central Composite Design. J. Mol. Struct. 2021, 1229, 129497. [Google Scholar] [CrossRef]
- Anber, A.A.; Essa, M.S.; Kadhim, G.A.; Hashim, S.S. Preparation of Nanoparticles Copper Oxide Using an Atmospheric-Pressure Plasma Jet. J. Phys. Conf. Ser. 2018, 1032, 12009. [Google Scholar] [CrossRef]
- Aloui, M.; Mentar, L.; Beniaiche, A.; Azizi, A. NH4Cl and KCl Effect on the Structural, Morphological, Optical and Electrochemical Properties of Cu2O Nanoparticles and Cu2O Nanostructures, Galvanostatically Electrodeposited. J. Solid State Chem. 2022, 315, 123435. [Google Scholar] [CrossRef]
- Ilie, A.; Ghiţulică, C.; Andronescu, E.; Cucuruz, A.; Ficai, A. New Composite Materials Based on Alginate and Hydroxyapatite as Potential Carriers for Ascorbic Acid. Int. J. Pharm. 2016, 510, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Bestimmung der Größe und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen. Math. Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Meneceur, S.; Bouafia, A.; Laouini, S.E.; Mohammed, H.A.; Daoudi, H.; Chami, S.; Hasan, G.G.; Abdullah, J.A.A.; Salmi, C. Removal Efficiency of Heavy Metals, Oily in Water, Total Suspended Solids, and Chemical Oxygen Demand from Industrial Petroleum Wastewater by Modern Green Nanocomposite Methods. J. Environ. Chem. Eng. 2023, 11, 111209. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Methods in Molecular Biology; Springer International Publishing: Cham, Switzerland, 2011; Volume 697, pp. 63–70. [Google Scholar]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Zidane, Y.; Laouini, S.E.; Bouafia, A.; Meneceur, S.; Tedjani, M.L.; Alshareef, S.A.; Almukhlifi, H.A.; Al-Essa, K.; Al-Essa, E.M.; Rahman, M.M.; et al. Green Synthesis of Multifunctional MgO@AgO/Ag2O Nanocomposite for Photocatalytic Degradation of Methylene Blue and Toluidine Blue. Front. Chem. 2022, 10, 1083596. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, J.L.; Muñoz Villegas, L.F.; Sánchez, V.I.Y.; Perez, F.A.N.; Rodriguez, J.M.Z. Adsorption of Brilliant Cresyl Blue from Aqueous Solution by Silver Nanoparticles on Zinc Oxide. MRS Adv. 2023. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K. Fabrication of Zno/Cuo Hybrid Nanocomposite for Photocatalytic Degradation of Brilliant Cresyl Blue (Bcb) Dye in Aqueous Solutions. J. Water Environ. Nanotechnol. 2021, 6, 196–211. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of Cobalt and Cobalt Oxide Nanoparticles as Nanocatalyst towards Dyes Degradation in Wastewater. Nano Struct. Nano Objects 2020, 21, 100405. [Google Scholar] [CrossRef]
- Meneceur, S.; Hemmami, H.; Bouafia, A.; Laouini, S.E.; Tedjani, M.L.; Berra, D.; Mahboub, M.S. Photocatalytic Activity of Iron Oxide Nanoparticles Synthesized by Different Plant Extracts for the Degradation of Diazo Dyes Evans Blue and Congo Red. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Zohra, R.; Meneceur, S.; Eddine, L.S.; Bouafia, A.; Mohammed, H.A.; Hasan, G.G. Biosynthesis and Characterization of MnO2 and Zn/Mn2O4 NPs Using Ziziphus Spina-Christi Aqueous Leaves Extract: Effect of Decoration on Photodegradation Activity against Various Organic Dyes. Inorg. Chem. Commun. 2023, 156, 111304. [Google Scholar] [CrossRef]
- Asif, S.A.B.; Khan, S.B.; Asiri, A.M. Efficient Solar Photocatalyst Based on Cobalt Oxide/Iron Oxide Composite Nanofibers for the Detoxification of Organic Pollutants. Nanoscale Res. Lett. 2014, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Messai, R.; Ferhat, M.F.; Belmekki, B.; Alam, M.W.; Al-Othoum, M.A.S.; Sadaf, S. GAD Plasma-Assisted Synthesis of ZnO Nanoparticles and Their Photocatalytic Activity. Mater. Res. Express 2024, 11, 015006. [Google Scholar] [CrossRef]
- Singh, P.; MAbdullah, M.; Sagadevan, S.; Kaur, C.; Ikram, S. Highly Sensitive Ethanol Sensor Based on TiO2 Nanoparticles and Its Photocatalyst Activity. Optik 2019, 182, 512–518. [Google Scholar] [CrossRef]
- Baig, A.; Baig, A.; Rathinam, V.; Ramya, V. Facile Fabrication of Zn-Doped SnO2 Nanoparticles for Enhanced Photocatalytic Dye Degradation Performance under Visible Light Exposure. Adv. Compos. Hybrid Mater. 2021, 4, 114–126. [Google Scholar] [CrossRef]
- El-Berry, M.F.; Sadeek, S.A.; Abdalla, A.M.; Nassar, M.Y. Microwave-Assisted Fabrication of Copper Nanoparticles Utilizing Different Counter Ions: An Efficient Photocatalyst for Photocatalytic Degradation of Safranin Dye from Aqueous Media. Mater. Res. Bull. 2021, 133, 111048. [Google Scholar] [CrossRef]


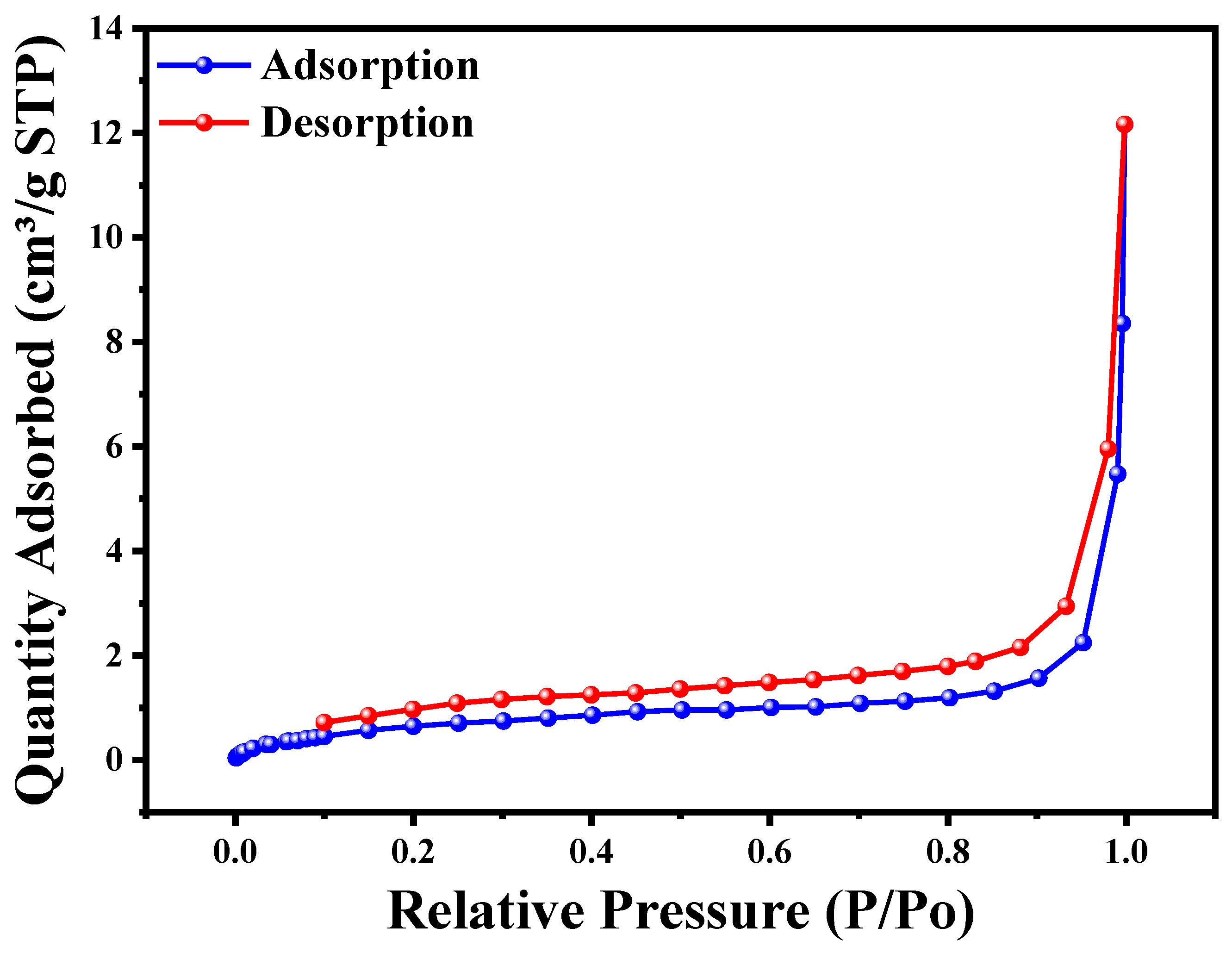


| Samples | Cu2O/AA NC | |
|---|---|---|
| Surface Area | BET Surface Area | 2.72 m2/g |
| Langmuir Surface Area | 10.75 m2/g | |
| t-Plot external surface area | 4.036 m2/g | |
| Pore Volume | Single point adsorption total pore volume of pores less than 40.3122 nm diameter at p/p° = 0.95 | 0.0034 cm3/g |
| Single point desorption total pore volume of pores less than 40.3122 nm diameter at p/p° = 0.95 | 0.0045 cm3/g | |
| Pore Size | BJH Adsorption average pore diameter (4 V/A) | 1.47 nm |
| BJH Desorption average pore diameter (4 V/A) | 1.07 nm | |
| Nanoparticle Size | Average Particle Size | 22.02 nm |
| Sample Name | Synthesis Method | Dye | Experimental Conditions | Catalysis Dose g/L | Deg Efficiency /Time | Ref. |
|---|---|---|---|---|---|---|
| g-C3N4/ZnO | Co-precipitation method | BCB | V = 50 mL, 25 ppm, pH = 10 Visible Light (250 w) Dark Room 30 min | 0.5 | 99.51%/1 h | [5] |
| Co3O4/Fe2O3 | - | BCB | V = 100 mL, 38.6 ppm, pH = 10 Sun-light | 1 | 97% 3 h | [57] |
| ZnO/CuO | Precipitation method | BCB | V = 100 mL, 15 ppm, Visible Light (250 w) Dark Room 30 min | 0.15 | 97.30% 1.66 h | [53] |
| ZnO | Cold Plasma GAD | BCB | V = 25 mL, 7 ppm, T° = 20 °C, pH = 7 UV Light (λ = 365 nm—30 W) Dark Room 30 min | 1 | 92.92% /2.5 h | [58] |
| TiO2 | Commercial | BCB | V = 100 mL, 3 ppm, UV Light (200 W) Dark Room 20 min | 2.6 | 74% /2 h | [59] |
| Al2O3 | Commercial | BCB | V = 100 mL, 50 ppm, pH = 10 (8.44 mW/cm2 light intensity) Dark Room 30 min | 1.7 | 92.87%/1 h Al2O3 + UV + 10 cm3/min air bubble | [6] |
| Ag/ZnO | Turkevich | BCB | V = 100 mL, 100 ppm, pH = 7.5 UV Light (λ = 625 nm) Dark Room 30 min | 0.1 | 91%/200 min | [6] |
| Cu2O/AA | Co-precipitation method | BCB | V = 200 mL, 50 ppm, pH = 7 UV Light (λ = 625 nm) Sun-light | 1 | 73.12%/2 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeghdi, S.; Laouini, S.E.; Mohammed, H.A.; Bouafia, A.; Tedjani, M.L.; Abdullah, M.M.S.; Trzepieciński, T. Improved Synthesis of Cu2O NPs and Ascorbic Acid-Modified Derivatives for Adsorption of Brilliant Cresyl Blue: Surface and Reusability Studies. Materials 2024, 17, 2358. https://doi.org/10.3390/ma17102358
Zeghdi S, Laouini SE, Mohammed HA, Bouafia A, Tedjani ML, Abdullah MMS, Trzepieciński T. Improved Synthesis of Cu2O NPs and Ascorbic Acid-Modified Derivatives for Adsorption of Brilliant Cresyl Blue: Surface and Reusability Studies. Materials. 2024; 17(10):2358. https://doi.org/10.3390/ma17102358
Chicago/Turabian StyleZeghdi, Saad, Salah Eddine Laouini, Hamdi Ali Mohammed, Abderrhmane Bouafia, Mohammed Laid Tedjani, Mahmood M. S. Abdullah, and Tomasz Trzepieciński. 2024. "Improved Synthesis of Cu2O NPs and Ascorbic Acid-Modified Derivatives for Adsorption of Brilliant Cresyl Blue: Surface and Reusability Studies" Materials 17, no. 10: 2358. https://doi.org/10.3390/ma17102358
APA StyleZeghdi, S., Laouini, S. E., Mohammed, H. A., Bouafia, A., Tedjani, M. L., Abdullah, M. M. S., & Trzepieciński, T. (2024). Improved Synthesis of Cu2O NPs and Ascorbic Acid-Modified Derivatives for Adsorption of Brilliant Cresyl Blue: Surface and Reusability Studies. Materials, 17(10), 2358. https://doi.org/10.3390/ma17102358










