Abstract
This study addresses the critical need for efficient and recyclable photocatalysts for water treatment applications by presenting a novel approach for the synthesis and characterization of copper (I) oxide (Cu2O) nanoparticles modified with ascorbic acid (Cu2O/AA). The motivation for this research stems from the increasing concern about environmental pollution caused by organic pollutants, such as Brilliant Cresyl Blue (BCB), and the necessity for sustainable solutions to mitigate this issue. Through comprehensive characterization techniques including Ultraviolet–Visible spectroscopy (UV-Vis), Fourier Transform Infrared spectroscopy (FTIR), X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), zeta potential measurements, and Brunauer–Emmett–Teller (BET) analysis, we demonstrate a significant modification to the electronic structure, enhancing the photocatalytic activity of Cu2O/AA. BET analysis revealed a mesoporous structure with a specific surface area of 2.7247 m2/g for Cu2O/AA, further emphasizing its potential for enhanced catalytic performance. The photocatalytic degradation studies showcased remarkable efficiency improvements, with degradation coefficients of 30.8% and 73.12% for Cu2O NPs and Cu2O/AA NC, respectively, within a 120 min timeframe. Additionally, recyclability experiments indicated sustained efficiency over five consecutive cycles, with both catalysts retaining crystalline integrity. These findings underscore the promising potential of Cu2O/AA nanoparticles as highly efficient and recyclable photocatalysts for the degradation of organic pollutants, offering superior performance compared to pure Cu2O NPs and addressing the pressing need for sustainable water treatment solutions.
1. Introduction
Industrialization has brought about immense progress, but it also carries a significant environmental cost, particularly in the form of water pollution [1,2]. Various industries generate organic dyes that are toxic to aquatic life, posing a severe threat to fragile ecosystems. Among the various contaminants, synthetic dyes represent a considerable portion, with Brilliant Cresyl Blue (BCB) being one of the commonly used dyes in the textile industries [3,4]. Due to its complex aromatic structure and persistence, BCB poses a significant threat to aquatic ecosystems and human health, necessitating effective remediation strategies [5]. Researchers have been actively seeking effective methods to remove these harmful dyes from wastewater, and one of the most promising approaches is the use of photocatalysts. Photocatalytic degradation, which employs semiconductor photocatalysts, has emerged as a cost-effective and environmentally friendly solution for breaking down these dyes [6,7]. By harnessing the power of light and catalytic materials, this method offers a sustainable and efficient way to mitigate the detrimental effects of industrial dye effluents on water bodies, paving the way for a cleaner and more sustainable future [8,9].
Semiconductor photocatalysts, such as copper oxide nanoparticles (Cu2O NPs), are capable of generating reactive oxygen species (ROS) upon exposure to light. This process begins with the absorption of photons by the photocatalyst, which is sufficient to excite electrons to a higher energy level, thereby creating electron-hole pairs in the semiconductor’s conduction and valence bands [10,11]. These charge carriers can then interact with adsorbed water and oxygen molecules on the photocatalyst’s surface, initiating redox reactions [12].
The ROS produced through these redox reactions, including hydroxyl radicals and superoxide ions , play a crucial role in the degradation of organic pollutants [13]. Les radicals are particularly reactive and can oxidize a wide range of organic compounds, leading to their decomposition into smaller, less toxic molecules [14]. This ability to decompose pollutants makes photocatalytic degradation a promising and effective method for water treatment.
Photocatalytic degradation has emerged as a promising approach for the removal of organic pollutants from water. This process harnesses the light-induced activity of semiconductor materials to generate reactive oxygen species (ROS), which effectively degrade organic molecules into harmless byproducts [15,16,17]. Among the various semiconductor materials investigated for photocatalytic applications, cuprous oxide nanoparticles (Cu2O NPs) have garnered significant attention due to their favorable bandgap energy, abundant availability, low cost, and environmentally friendly nature [18,19]. Cu2O NPs exhibit excellent photocatalytic properties, making them suitable candidates for the degradation of organic pollutants [20]. However, the practical application of Cu2O NPs in wastewater treatment is often hindered by challenges such as limited stability, rapid recombination of photogenerated charge carriers, and inefficient utilization of visible light [21,22].
To address these limitations and enhance the photocatalytic performance of Cu2O NPs, surface modification techniques have been explored to tailor their properties and improve their efficiency in dye degradation processes. Ascorbic acid (AA), a naturally occurring antioxidant and reducing agent, has emerged as a promising modifier for Cu2O NPs due to its ability to stabilize nanoparticles, inhibit electron–hole recombination, and enhance light absorption properties. The incorporation of ascorbic acid onto the surface of Cu2O NPs can potentially lead to synergistic effects, resulting in improved photocatalytic activity and stability [23]. Kader and coworkers investigated the efficacy of L-ascorbic acid adduct-conjugated ZnONPs in the degradation of Congo red, yielding promising results [24,25]. By functionalizing the nanoparticle surface with ascorbic acid, researchers can enhance charge separation efficiency, mitigate surface defects, and facilitate the generation of ROS, thereby augmenting the photocatalytic degradation capabilities of the nanoparticles [23,26].
The synthesis of nanoparticles through chemical processes stands as a cornerstone in the realm of nanomaterial fabrication, offering precise control over their size, morphology, and surface properties. Chemical methods provide a versatile platform for tailoring the characteristics of nanoparticles, allowing researchers to manipulate parameters such as precursor composition, reaction conditions, and stabilizing agents to achieve desired material properties [27,28]. In particular, the chemical synthesis of Cu2O NPs has garnered significant attention due to its simplicity, scalability, and potential for precise control over nanoparticle attributes [29,30]. By judiciously selecting reactants and optimizing reaction parameters, researchers can dictate the nucleation and growth kinetics, leading to the formation of Cu2O NPs with tailored sizes and morphologies. Moreover, chemical synthesis routes facilitate the incorporation of surface modifiers and functional groups, further enhancing the nanoparticles’ stability and reactivity [31,32]. Thus, leveraging chemical processes for the synthesis of Cu2O NPs holds immense promise for advancing various applications, including catalysis, sensing, and environmental remediation [33].
In this study, we present a novel approach by synthesizing and characterizing cuprous oxide (Cu2O) nanoparticles modified with ascorbic acid (Cu2O/AA) for enhanced photocatalytic degradation of Brilliant Cresyl Blue (BCB). Through chemical synthesis and detailed characterization techniques including UV-Vis spectroscopy, FTIR, XRD, SEM, zeta potential measurements, and Brunauer–Emmett–Teller (BET) analysis, this research elucidates the synergistic effects of nanoparticle composition and surface modification on catalytic performance. The primary application lies in advancing water treatment technologies by efficiently removing organic pollutants from aqueous environments. By uncovering the mechanisms of photocatalytic degradation and assessing practical applicability, this study aims to contribute to environmental remediation strategies with potential implications for wastewater treatment, catalysis, and sensing.
2. Method and Materials
2.1. Materials
Copper chloride (CuCl2, 99%), sodium hydroxide (NaOH), ascorbic acid (C6H8O6), Brilliant Cresyl Blue (BCB) dye were procured from Sigma-Aldrich, Darmstadt, Germany.
2.2. Synthesis of Cu2O NPs
The method of synthesizing Cu2O NPs was described in a previous research paper, with slight modifications implemented for this particular study [28]. In brief, a reducing agent, 1 M NaOH, was incrementally introduced into a solution containing 100 mL of 2 M copper chloride (CuCl2) while it was stirred for a duration of 5 min at 65 °C. Following this, the pH of the mixture was meticulously adjusted to 12.5 using a 2 M NaOH solution. Subsequently, the resulting solution was subjected to agitation for 2 h, leading to a discernible alteration in color to a dark violet blue. The subsequent isolation of the precipitate was accomplished through centrifugation at 3000 rpm for 5 min, accompanied by successive washings with distilled water (DW) to eliminate any impurities. The resultant precipitate underwent a drying process at 80 °C for approximately 15 h. A critical step in the synthesis protocol involved the annealing of the dried powder in an oven at 500 °C for 4 h, which played a pivotal role in both initiating and stabilizing the desired nanoparticle. This comprehensive procedure ensured the successful fabrication of Cu2O NPs.
2.3. Ascorbic Acid-Mediated Cu2O Nanoparticles
The obtained Cu2O NPs surfaces were modified with ascorbic acid according to previous study with slight modifications [29]. Initially, 0.5 g of Cu2O NPs was dispersed in 50 mL of distilled water and stirred at ambient temperature for 10 min. Subsequently, ultrasonic dispersion of the Cu2O NPs solution was conducted at 50 °C for 2 h. The pH of the solution was regulated to 6.5 by the addition of an ascorbic acid stock solution (0.1 g/mL). The reaction mixture was maintained under constant stirring at 70 °C for 2 h. Following the reaction, the resulting suspension was subjected to drying in a vacuum oven at 50 °C for 5 h, yielding ascorbic acid-stabilized Cu2O NPs (Cu2O/AA).
2.4. Characterization of Cu2O NPs and Cu2O/Ascorbic Acid NPs
The characterization of Cu2O NPs and Cu2O/ascorbic acid (AA) NPs was conducted using a range of analytical techniques. UV–visible spectrophotometry (SECOMAM, model 9600, Alès, France) was employed to analyze the optical properties of the synthesized NPs. X-ray diffraction (XRD) analysis (Proto Manufacturing Company’s Benchtop model, Maple Plain, MN, USA) was utilized to determine the crystallite size employing the Scherrer formula [34], where a prominent peak with the highest intensity was selected. Fourier transform infrared spectroscopy (FTIR) (Thermo Fisher Scientific, Nicolet iS5 model, Waltham, MA, USA) was employed to investigate the chemical composition and functional groups present on the surface of the NPs. Scanning electron microscopy (SEM) (TESCAN, VEGA3 model, Warrendale, PA, USA) provided insight into the morphology and size distribution of the NPs. Zeta potential measurements were carried out using the Entegris company’s Nicomp Nano Z3000 model (Billerica, MA, USA) with Dynamic Light Scattering (DLS) sizing capability to assess the surface charge and stability of the NPs. Additionally, the Brunauer-Emmett-Teller (BET) method (Model Nova 2000e, Quantachrome Instruments Limited, Boynton Beach, FL, USA) was employed to determine the specific surface area and pore size distribution of the synthesized NPs. These comprehensive characterization techniques facilitated a thorough understanding of the structural, optical, morphological, and surface properties of both Cu2O and Cu2O/AA NPs, crucial for evaluating their potential applications in various fields [35,36].
2.5. Photocatalytic Activity
The investigation assessed the photocatalytic capabilities of Cu2O NPs and Cu2O/ASA NPs in a solution containing Brilliant Cresyl Blue (BCB) dye. A solution was prepared, comprising 200 mL of BCB with a concentration of 50 parts per million (PPM). The impact of contact time was methodically evaluated at various intervals (5, 15, 30, 45, 60, 90, and 120 min) utilizing Cu2O NPs and Cu2O/ASA NPs at a concentration of 5 mg/5 mL. After the treatment, the nanoparticle samples were separated by centrifugation, and the UV-visible absorbance spectra of the BCB dye solution were analyzed using a spectrophotometer across the wavelength range of 200–800 nm. The degradation efficiency of BCB dye was quantified according to the provided equation [37].
where D represents the efficiency of degradation rate, while A0 and At signify the initial absorbance and the absorbance at a given time point, respectively.
3. Results and Discussion
3.1. UV-Vis Analysis
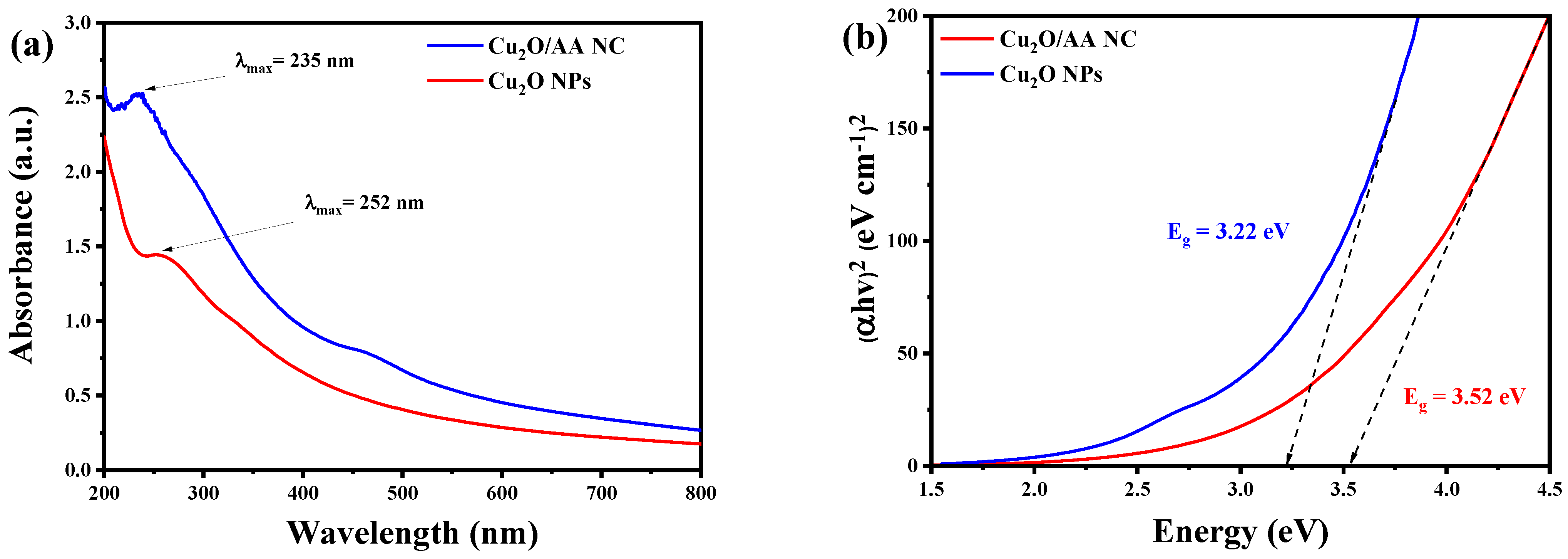
In Figure 1a, the UV-Vis absorption spectra of Cu2O NPs and Cu2O/AA NPs are presented. As seen, the Cu2O NPs exhibit a lower intensity absorbance at 252 nm. In contrast, the Cu2O/AA NPs display a higher intensity absorbance at a slightly shorter wavelength of 235 nm. The shift in wavelength suggests a change in the electronic structure or environment of Cu2O upon interaction with ascorbic acid, potentially leading to surface modification or electronic interactions between the two components. The increased absorbance intensity in the presence of ascorbic acid indicates enhanced light absorption or a change in the band structure.

Figure 1.
(a) UV-Vis spectra of Pure Cu2O and Cu2O/AA NPs; (b) band gap energy of Pure Cu2O and Cu2O/AA NPs. The arrows are did designed to determine the gap energy for each sample in the row X.
To further elucidate the optical properties, the band gap energy for both of Cu2O and Cu2O/AA NPs was determined utilizing Tauc’s relation [38]. By plotting (hv)2 against energy (eV), the band gap energy was computed, yielding values of 3.52 eV and 3.22 eV, respectively, as shown in Figure 1b. These values provide insights into the energy required for electronic transitions within the materials. The lower band gap energy required by Cu2O/AA NPs compared to pure Cu2O suggests a modification in the electronic structure due to the presence of ascorbic acid. This alteration in band gap energy further corroborates the UV-Vis absorption results, indicating changes in the optical properties of Cu2O upon interaction with ascorbic acid.
3.2. FTIR Analysis
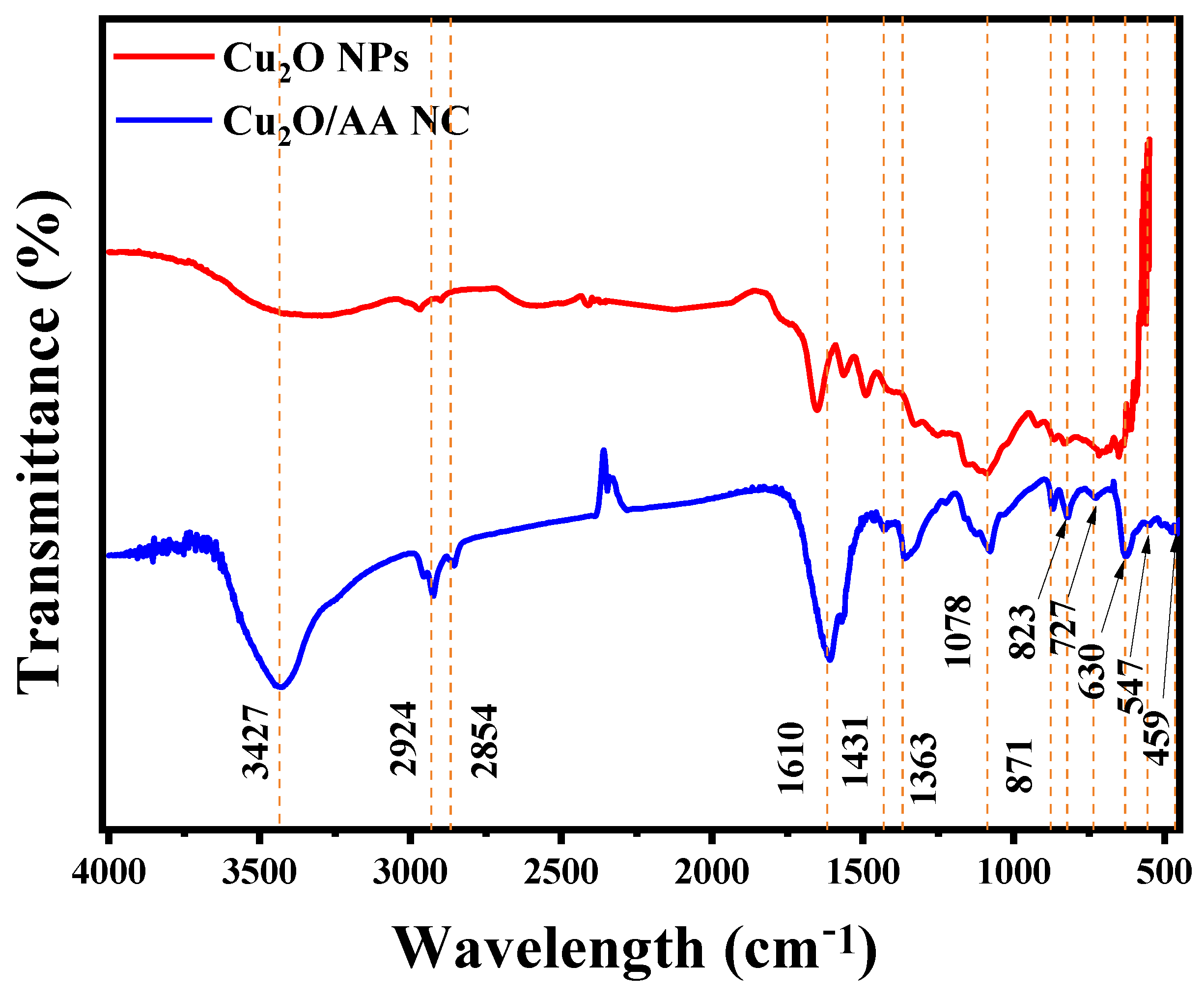
Figure 2 illustrates the FTIR spectrum of the Cu2O/AA NPs, displaying distinctive peaks associated with the identified constituents. These peaks offer valuable insights into the molecular composition of the sample. The prominent peak observed at 3427.51 cm−1 signifies O-H stretching vibrations [39], likely arising from hydroxyl groups present in both ascorbic acid and potentially residual water. Additionally, the peaks detected at 2924.09 cm−1 and 2854.65 cm−1 correspond to C-H stretching vibrations, suggesting the presence of aliphatic compounds, potentially originating from the organic ligands in the nanoparticles and ascorbic acid [40,41]. Additionally, the peak at 1610.56 cm−1 indicates C=C stretching vibrations, which are characteristic of aromatic compounds, and are likely associated with ascorbic acid or the organic ligands in the nanocomposite [42]. These findings collectively underscore the presence of organic constituents within the sample. Furthermore, the peaks at 1460.11 cm−1, 1431.18 cm−1, and 1363.67 cm−1 are indicative of C-H bending vibrations, commonly found in alkanes, potentially originating from the organic constituents of the nanocomposite [43]. The presence of peaks at 1078.21 cm−1 and 871.82 cm−1, attributed to C-O stretching vibrations, typical in alcohols, ethers, and esters, further supports the presence of ascorbic acid. Finally, the peaks observed at 823.60 cm−1, 727.16 cm−1, 630.72 cm−1, 547.78 cm−1, and 459.06 cm−1 are likely associated with metal-oxygen (Cu=O) in the Cu2O/AA NPs [44].

Figure 2.
FTIR spectra of Cu2O and Cu2O/ascorbic acid.
Upon comparing the FTIR spectra of pure Cu2O NPs with those of the Cu2O/AA NPs samples, a notable decrease in O-H peak intensity is observed in pure Cu2O NPs compared with Cu2O/AA NPs, suggesting an alteration in the composition of or interactions between functional groups upon the incorporation of ascorbic acid.
3.3. XRD Pattern Analysis
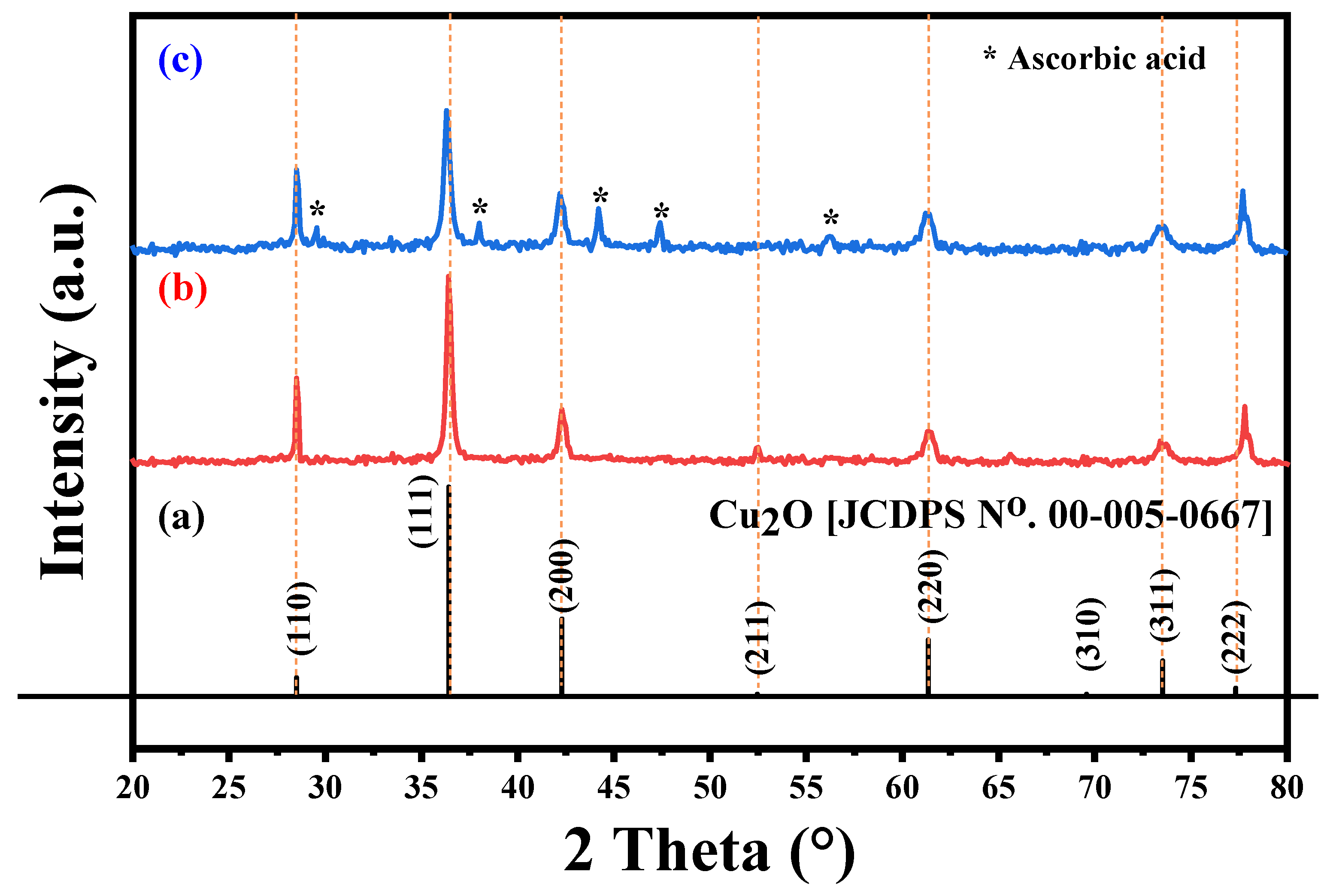
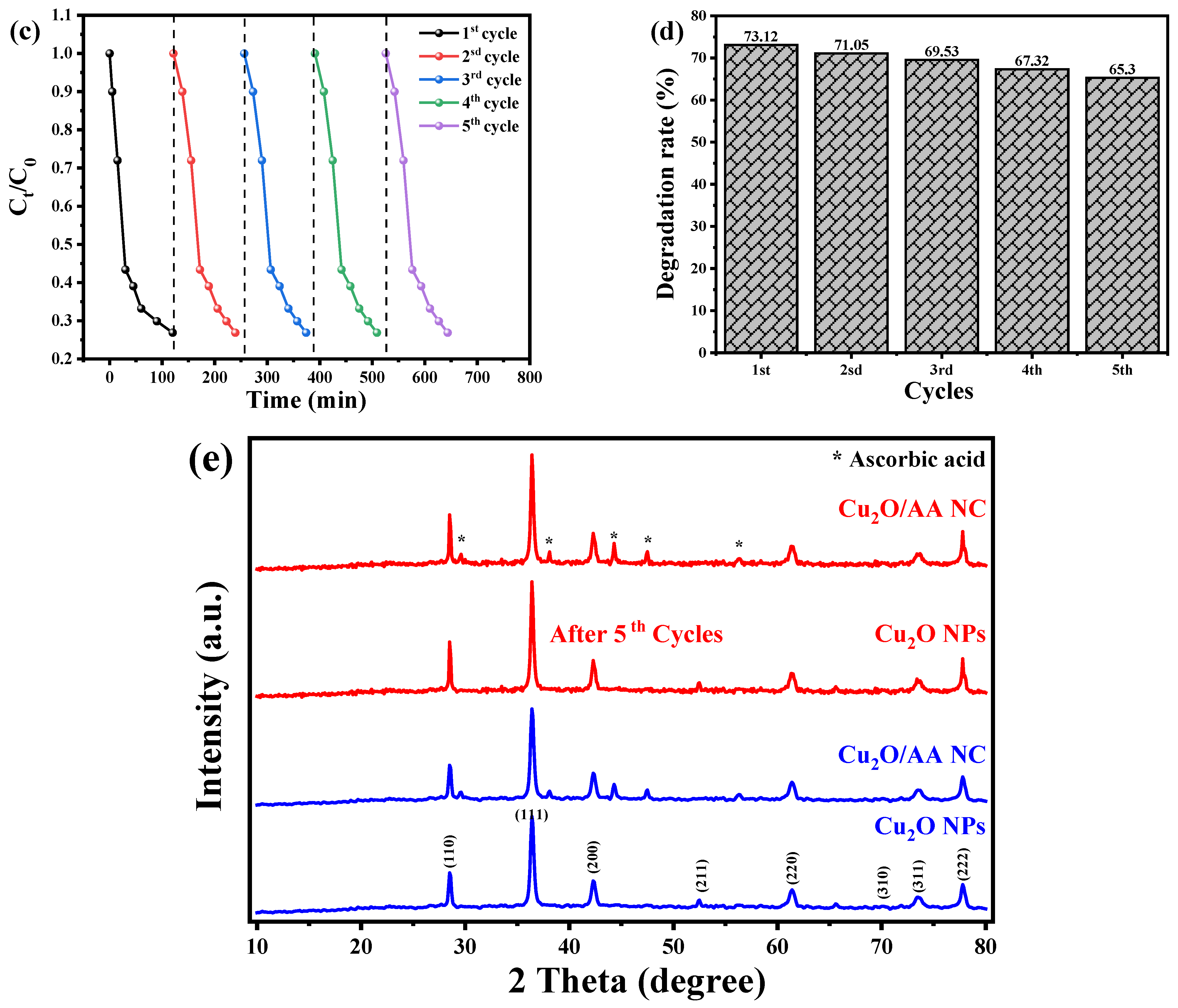
The X-ray diffraction patterns of Cu2O NPs and Cu2O/AA NPs are illustrated in Figure 3a. The pure Cu2O sample (Figure 3b) exhibited sharp peaks located at two-theta positions at 29.555°, 36.419°, 42.298°, 52.455°, 61.345°, 69.571°, 73.528°, and 77.326° correspond to crystal planes 110, 111, 200, 211, 220, 310, 311, and 222 of Cu2O, respectively, which is supported by JCPDS card number [00-005-0667] [45], confirming the cubic crystal system of the Cu2O phase. Upon modification of the Cu2O NPs with ascorbic acid to form Cu2O/AA NPs (Figure 3c), the XRD pattern displayed the same peaks as pure Cu2O NPs, with additional peaks at 29.58°, 38°, 44.16°, 47.33°, and 56.2°. The additional peaks corresponding to the presence of the AA phase indicate the successful coating of Cu2O NPs with ascorbic acid [46], without significant alteration of the Cu2O crystal structure.

Figure 3.
XRD patterns of (a) Cu2O standard XRD pattern [JCDPS no. 00-005-0667], (b) pure Cu2O, and (c) Cu2O/AA NPs.
By employing Scherrer’s equation, which relates crystallite size () to various parameters including the form factor (), wavelength (), Full Width at Half Maximum () denoted by , and diffraction angle () [47], the crystallite sizes were calculated as 22.04 nm and 28.08 nm for Cu2O/AA NPs and pure Cu2O NPs, respectively. This decrease in crystallite size could be attributed to the presence of ascorbic acid on the surface of Cu2O NPs, which might inhibit the growth of crystalline domains or induce some level of structural disorder.
3.4. SEM Analysis
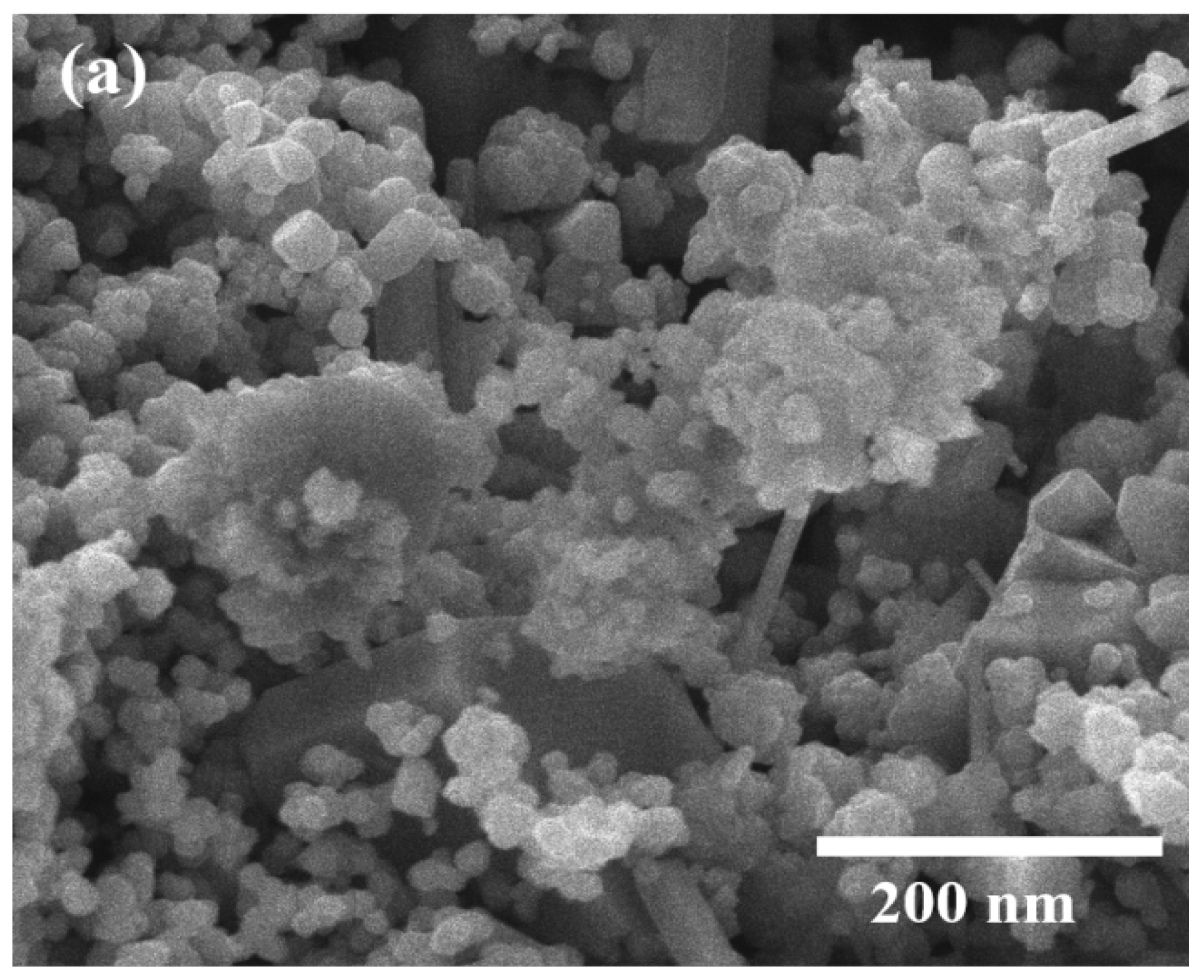
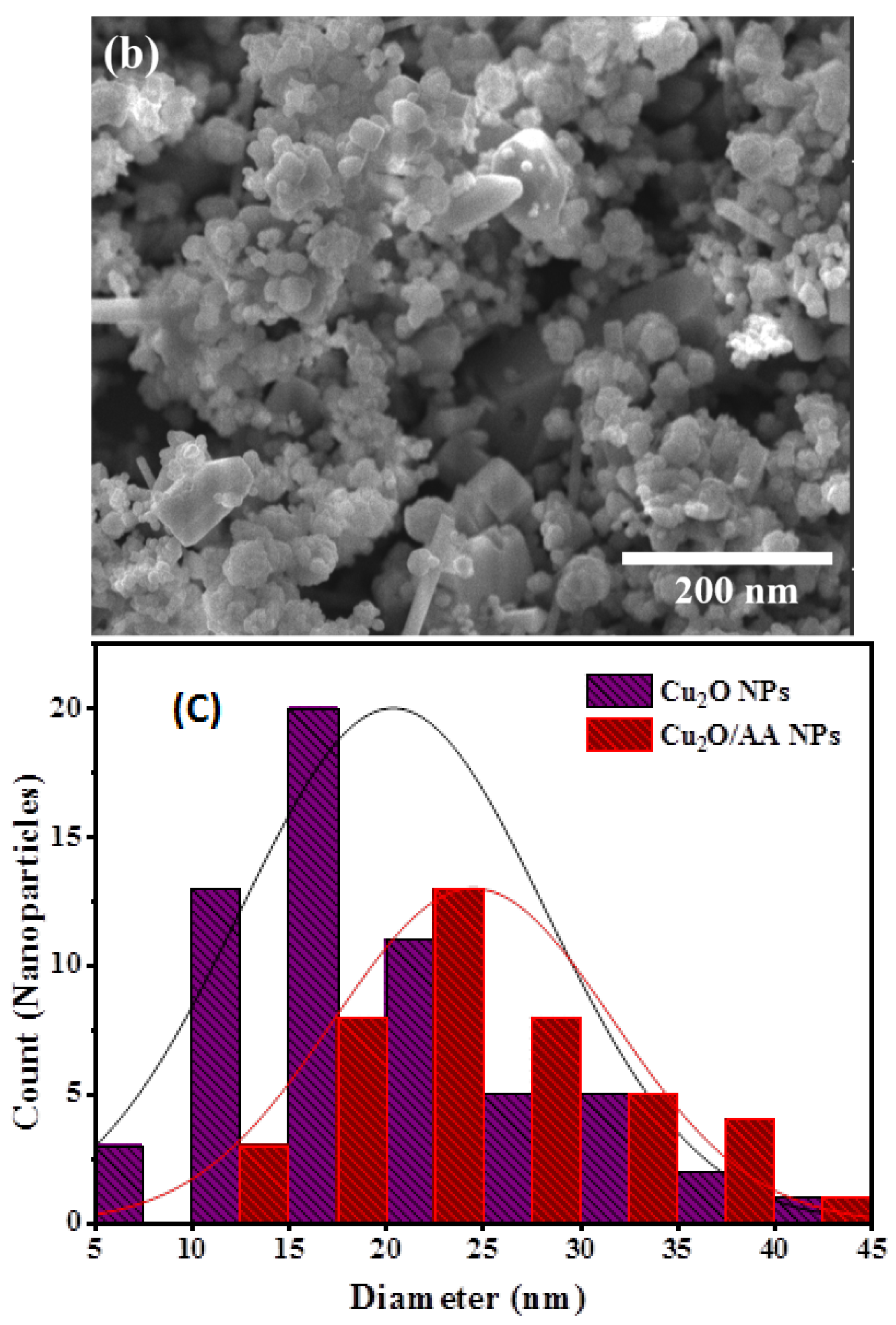
The SEM analysis revealed distinct morphological characteristics of both Cu2O NPs and Cu2O/AA NPs (Figure 4a,b). The SEM images provided a detailed view of the particle morphology, showcasing Cu2O NPs with a uniform spherical shape and an average size distribution of approximately 20 nm (Figure 4c). This uniformity in shape and size suggests a controlled synthesis process, indicative of the stability and reproducibility of the fabrication method. In contrast, Cu2O/AA NPs exhibited a more varied morphology, featuring a combination of spherical and irregular shapes. The average size distribution of these particles was slightly larger, around 25 nm, compared to pure Cu2O NPs. Moreover, the SEM images revealed densely packed particles on the surface of the Cu2O/AA NPs with minimal interparticle space, suggesting a high degree of packing and aggregation. This observation may arise from the interaction between Cu2O NPs and the ascorbic acid modifier, potentially leading to enhanced stability and surface coverage.


Figure 4.
SEM analysis of (a) Cu2O NPs, (b) Cu2O/AA NPs; (c) particle size diameter of Cu2O NPs and Cu2O/AA NPs.
3.5. Zeta Potential Measurements
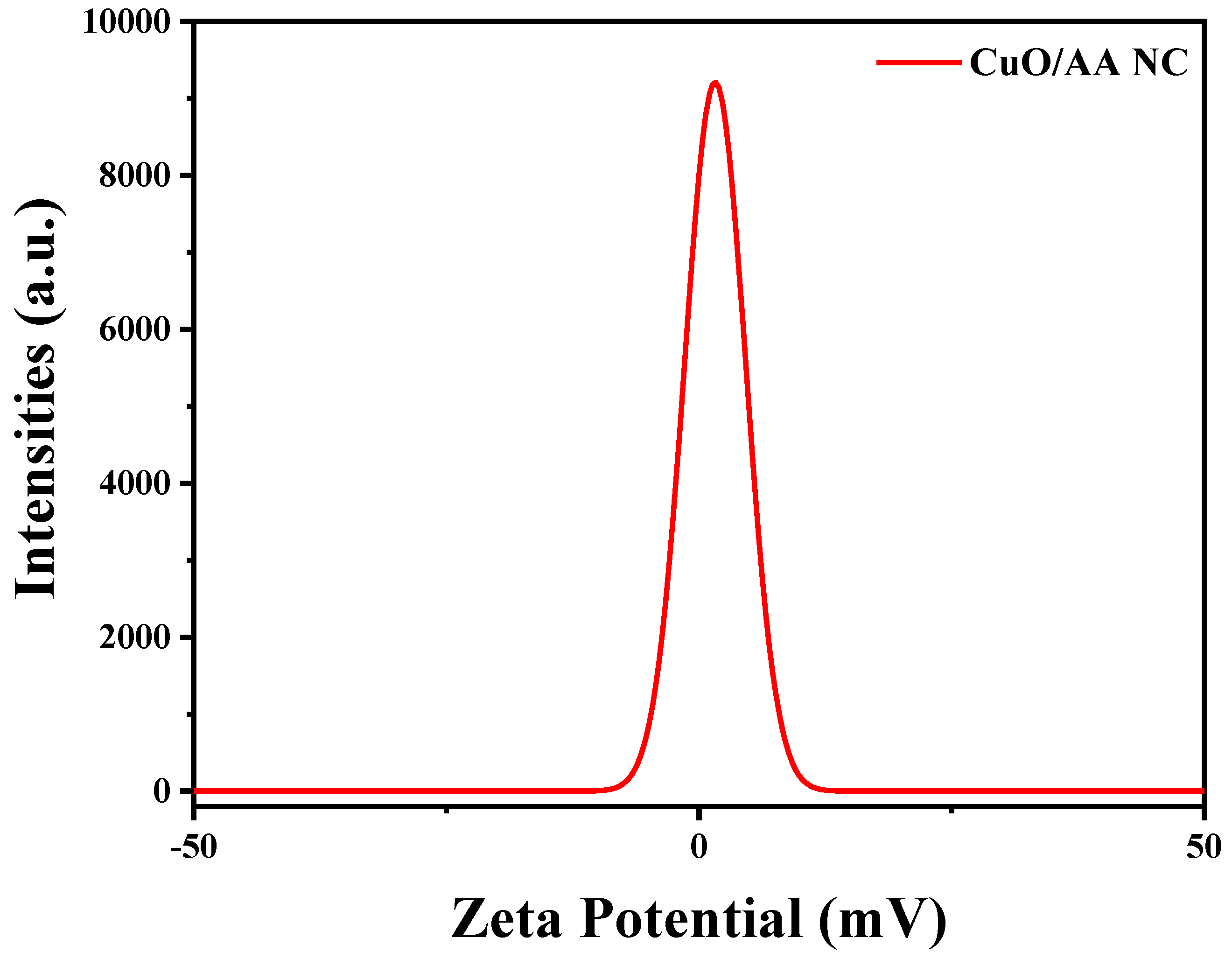
The zeta potential of the Cu2O/AA NPs was determined to be approximately −1.76 mV, indicating that there was a negative surface charge on the modified nanoparticles (Figure 5). This negative value suggests stability in the deionized water dispersion medium used for the measurement. Although the magnitude of −1.76 mV is relatively low compared to highly stable colloidal systems (typically >+30 mV or <−30 mV), it still signifies significant electrostatic repulsion among the particles, playing a crucial role in preventing aggregation or agglomeration and enhancing colloidal stability. Consequently, the obtained zeta potential value implies that the Cu2O/AA NPs could be suitable for applications where surface charge and colloidal stability are critical factors, such as drug delivery systems, where nanoparticles need to remain dispersed and stable in biological fluids for effective delivery and transport [48]. Additionally, the stable dispersion of nanoparticles is essential in catalytic applications, where a high surface area and homogeneous distribution of the catalyst are desired for optimal performance. However, it is important to note that zeta potential is just one factor influencing colloidal stability, and other factors like particle size, shape, and surface chemistry also play important roles [49,50].

Figure 5.
Zeta potential of the Cu2O/AA NPs.
3.6. BET Analysis
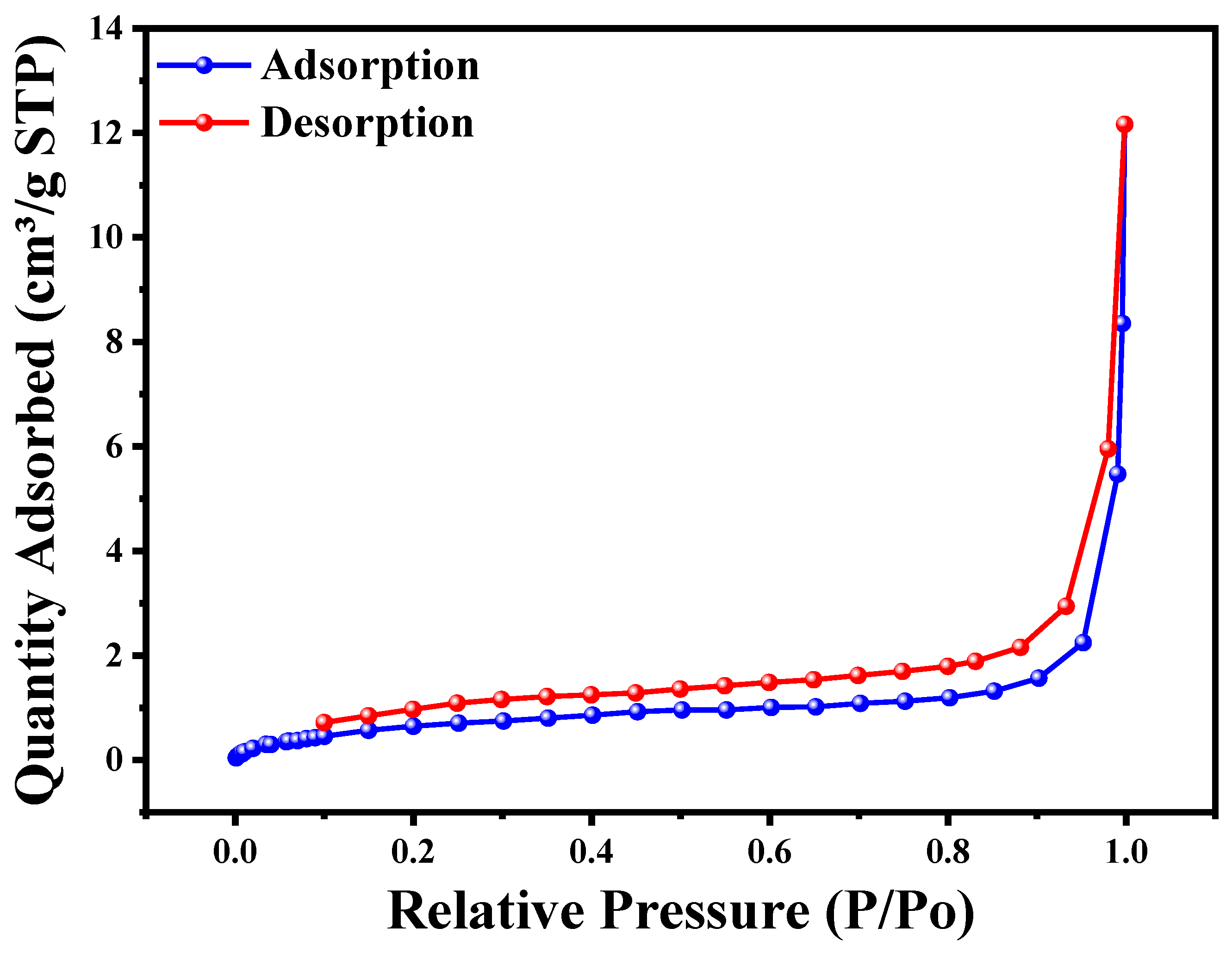
The nitrogen adsorption–desorption isotherms shown in Figure 6 and the data in Table 1 provide insights into the porous structure and surface area of the Cu2O/AA NPs material. The BET surface area of 2.72 m2/g indicates a relatively low surface area, but the material is classified as mesoporous with an average pore diameter of 1.47 nm (BJH adsorption) or 1.07 nm (BJH desorption) in the range of 1–3 nm. Pore volumes of 0.0034 cm3/g (adsorption) or 0.0045 cm3/g (desorption) are relatively low, and the average particle size of 22.02 nm suggests the material consists of nanoparticles. The surface analysis of Cu2O/AA NPs using N2 adsorption–desorption isotherms revealed their mesoporous nature and promising catalytic behavior. The Type IV isotherm with a hysteresis loop confirmed the ordered mesoporous framework texture. While the specific surface area value is not exceptionally high, the presence of mesopores and the nanoparticle size may contribute to the reported “enormous photocatalytic activity” of the Cu2O/AA NPs material. The noticeable improvement in the specific surface area value and the superior N2 adsorption behavior of the Cu2O/AA NPs suggest their potential for high photocatalytic activity. The mesoporous structure, high surface area, and favorable pore characteristics of these nanoparticles are advantageous for catalytic applications. Overall, the results suggest that the Cu2O/AA NPs material possesses a mesoporous structure with a relatively low surface area and pore volume but a favorable nanoparticle size, which may contribute to its reported photocatalytic activity [51].

Figure 6.
BET analysis for Cu2O/AA NC by using N2 adsorption-desorption isotherms.

Table 1.
BET surface area and porosity of Cu2O/AA NC.
3.7. Photocatalytic Degradation of Brilliant Cresyl Blue
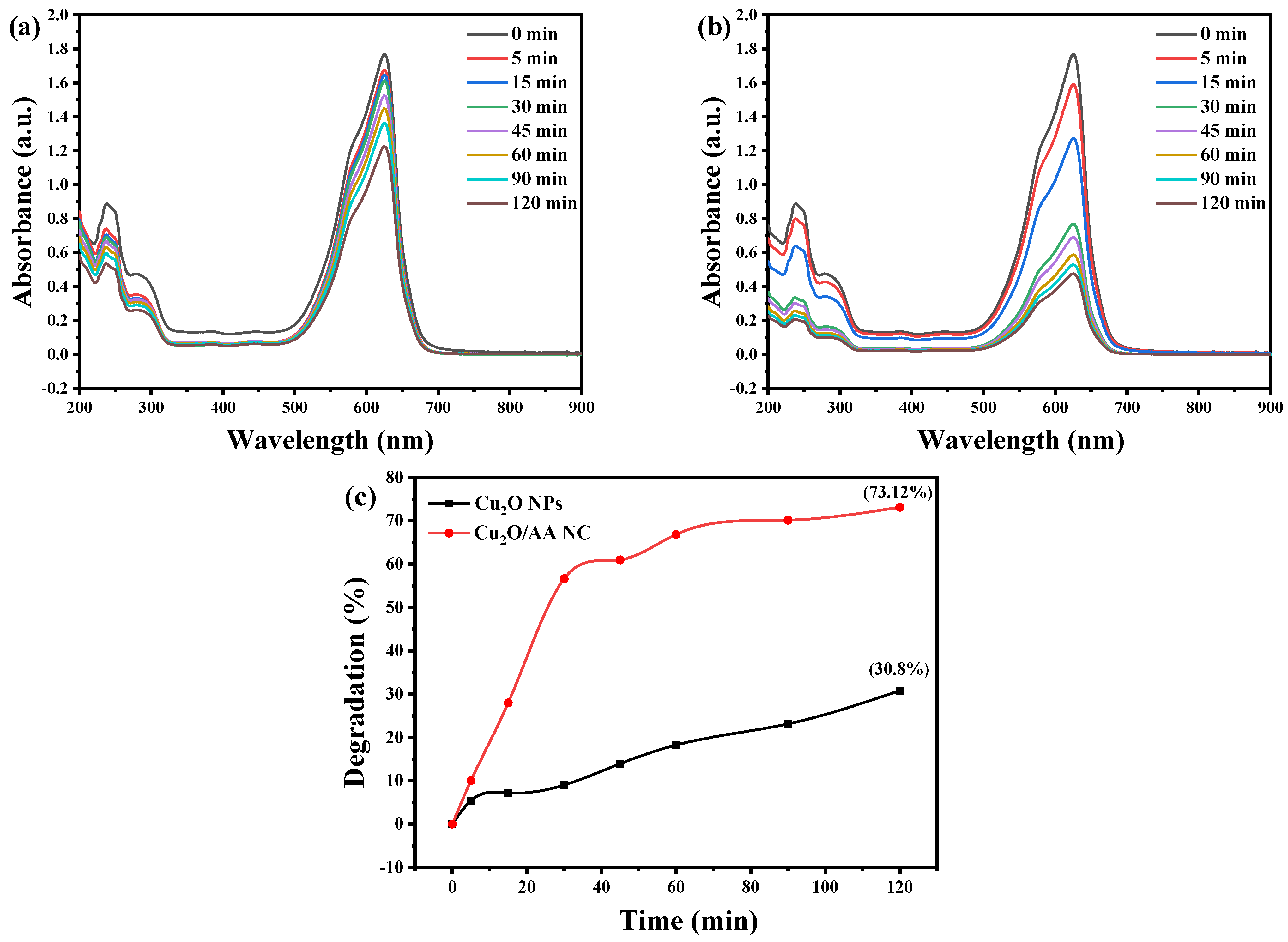
The absorption spectra presented in Figure 7a–c provide a visual representation of the photocatalytic degradation of BCB dye by pure Cu2O NPs and Cu2O/AA NPs under solar light irradiation. The distinct absorption peaks observed at around 625 nm for BCB serve as a spectroscopic fingerprint, enabling the monitoring of the degradation process. As the photocatalytic reaction progresses, these characteristic peaks exhibit a gradual decrease in intensity, indicating the progressive breakdown of the dye molecules over an extended period.

Figure 7.
Photocatalytic behavior of BCB using (a) Cu2O NPs and (b) Cu2O/AA NPs at different irradiation times under UV-Vis irradiation. (c) Rate of degradation under sunlight exposure in the presence of Cu2O NPs and Cu2O/AA NPs.
The absorption spectra reveal a notable reduction in peak intensity for BCB dye, manifesting as a visible fading of its vibrant color over time. Quantitative analysis reveals that the photocatalytic activity towards BCB degradation achieves coefficients of 30.8% and 73.12% for Cu2O NPs and Cu2O/AA NPs, respectively, within a 120 min duration. Significantly, the Cu2O/AA NPs exhibit an improved decomposition efficiency over time, corroborating the findings of previous studies [52,53,54]. This outcome suggests the superior dye removal capability of the Cu2O/AA NPs, likely attributable to the synergistic effects arising from the composite structure and the presence of AA as a hole scavenger.
The gradual decrease in the absorption peak intensities indicates the progressive breakdown of the dye molecules, facilitated by the photocatalytic activity of the Cu2O NPs and Cu2O/AA NPs. The enhanced performance of the Cu2O/AA NPs can be attributed to several factors, including the composite structure, which may facilitate charge separation and prolong the lifetime of charge carriers, thereby improving the overall photocatalytic efficiency. Additionally, the presence of AA as a hole scavenger can potentially mitigate the recombination of photogenerated electron–hole pairs, further enhancing the photocatalytic activity.
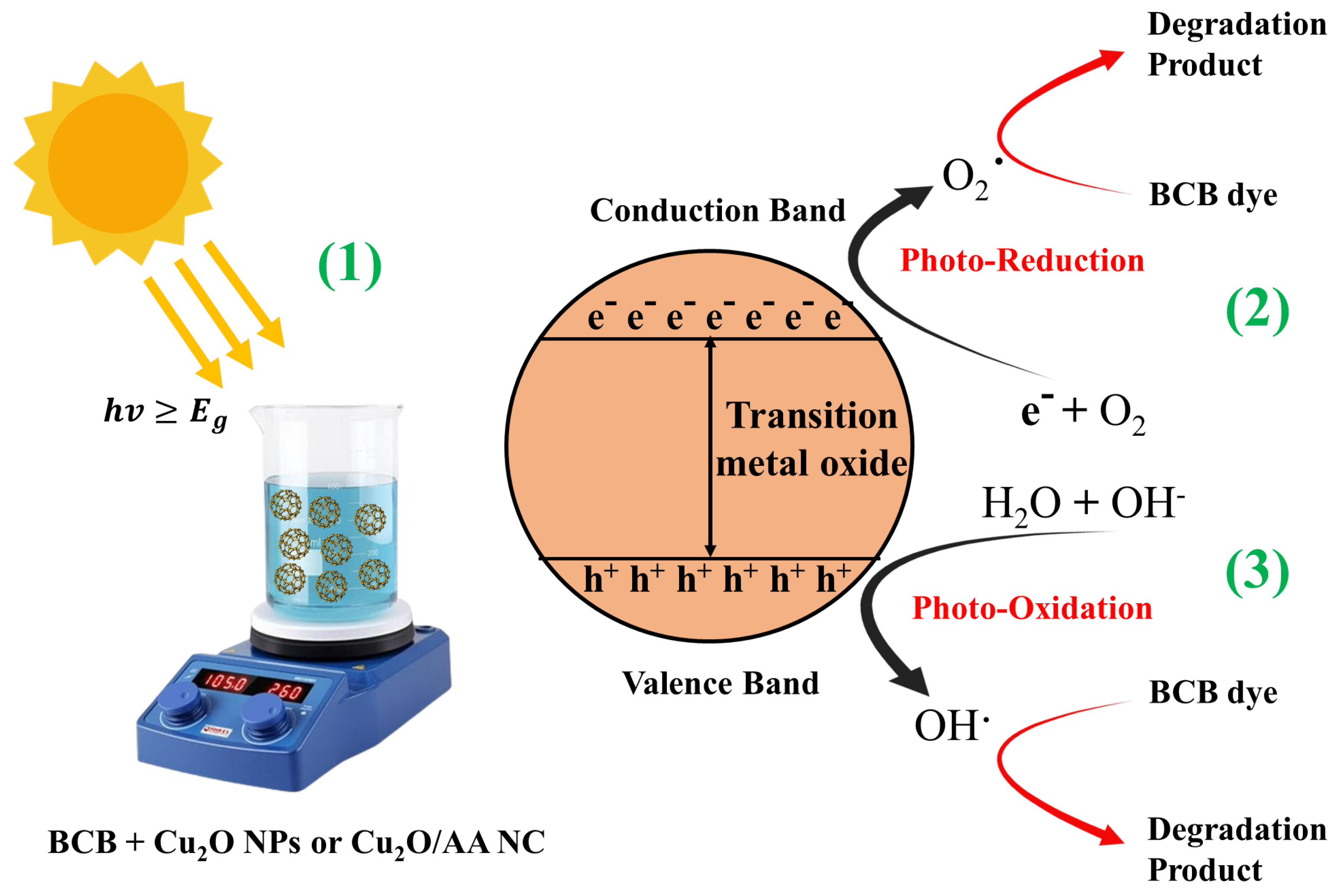
The photocatalytic degradation of BCB dye using pure Cu2O NPs and Cu2O/AA NPs under solar light irradiation involves a series of photochemical reactions that ultimately lead to the breakdown of the organic dye molecules. When exposed to solar radiation, the semiconductor properties of Cu2O NPs and Cu2O/AA NPs initiate a cascade of events that drive the degradation process [51,55].
Upon absorbing solar photons, electron–hole pairs are generated within the semiconductor materials. These photoinduced charge carriers migrate to the surface of the photocatalyst, where they participate in redox reactions with the adsorbed dye molecules. Specifically, electrons in the conduction band (CB) of Cu2O NPs and Cu2O/AA NPs can reduce the dye molecules, while holes in the valence band (VB) facilitate water oxidation, resulting in the formation of reactive oxygen species (ROS) such as hydroxyl radicals () and superoxide radicals () (Figure 8).

Figure 8.
Photodegradation mechanism of BCB using Cu2O NPs and Cu2O/AA NPs.
These highly reactive ROS act as potent oxidizing agents, initiating an attack on the adsorbed BCB molecules and kickstarting the degradation process. This degradation involves breaking chemical bonds and cleaving chromophoric groups, leading to the transformation of complex dye molecules into smaller, less chromatic fragments. The presence of Cu2O NPs and Cu2O/AA NPs can significantly influence the overall efficiency of the photocatalytic procedure [37,56].
The key reactions involved in the photocatalytic degradation process can be summarized as follows:
- Generation of electron–hole pairs: ;
- Water oxidation and hydroxyl radical formation: ;
- Superoxide radical formation: ;
- Additional hydroxyl radical formation: ;
- Degradation of BCB dye by ROS:
- Degradation of BCB dye by ROS:
The photocatalytic degradation process involves a complex interplay between these photochemical reactions, ultimately leading to the breakdown of the organic BCB dye molecules. Here is a breakdown of the steps, going from left to right:
1. Light excitation (hv ≥ Eg): A light particle (hv) with energy greater than or equal to the bandgap (Eg) of the metal oxide excites an electron from the valence band to the conduction band.
2. Photo-oxidation: The positively charged hole (h+) in the valence band can directly oxidize pollutants or generate hydroxyl radicals (OH·) from water.
3. Photo-reduction: The excited electron (e⁻) in the conduction band can reduce molecular oxygen (O2) to superoxide radicals ().
The Cu2O/AA NPs exhibit several distinct advantages over Cu2O NPs in the degradation of BCB dye: Firstly, the presence of the Cu2O component in the Cu2O/AA NPs enables the efficient utilization of visible light, which constitutes a significant portion of the solar spectrum, increasing the overall photocatalytic activity. Secondly, the heterojunction formed between Cu2O and AA facilitates efficient charge separation, reducing recombination and promoting the generation of ROS. Moreover, the incorporation of AA in the Cu2O/AA NPs promotes the formation of highly oxidizing ROS, which are essential for the effective degradation of organic pollutants like BCB dye, with AA not only acting as a capping agent but also contributing to the generation of these reactive species, thereby enhancing the photocatalytic performance. Additionally, the AA capping agent helps to stabilize the Cu2O/AA structure, preventing agglomeration and improving the reusability of the photocatalyst, a crucial factor for practical applications. Overall, the Cu2O/AA NPs represent a promising photocatalytic system for the degradation of BCB dye and other organic pollutants in wastewater treatment applications, offering several advantages over Cu2O NPs alone, including enhanced visible light absorption, improved charge separation, increased ROS generation, and better stability, making it an attractive candidate for efficient and sustainable wastewater remediation strategies.
Table 2 presents a comprehensive comparison of the photocatalytic degradation efficiency of various NPs, as well as Cu2O/AA, in the context of BCB degradation. Each entry in the table denotes the sample name, synthesis method, experimental conditions, catalysis dose, and degradation efficiency (% Deg) achieved within specified timeframes.

Table 2.
Comparative analysis of BCB degradation via photocatalysis using various NPs.
3.8. Recyclability and Stability
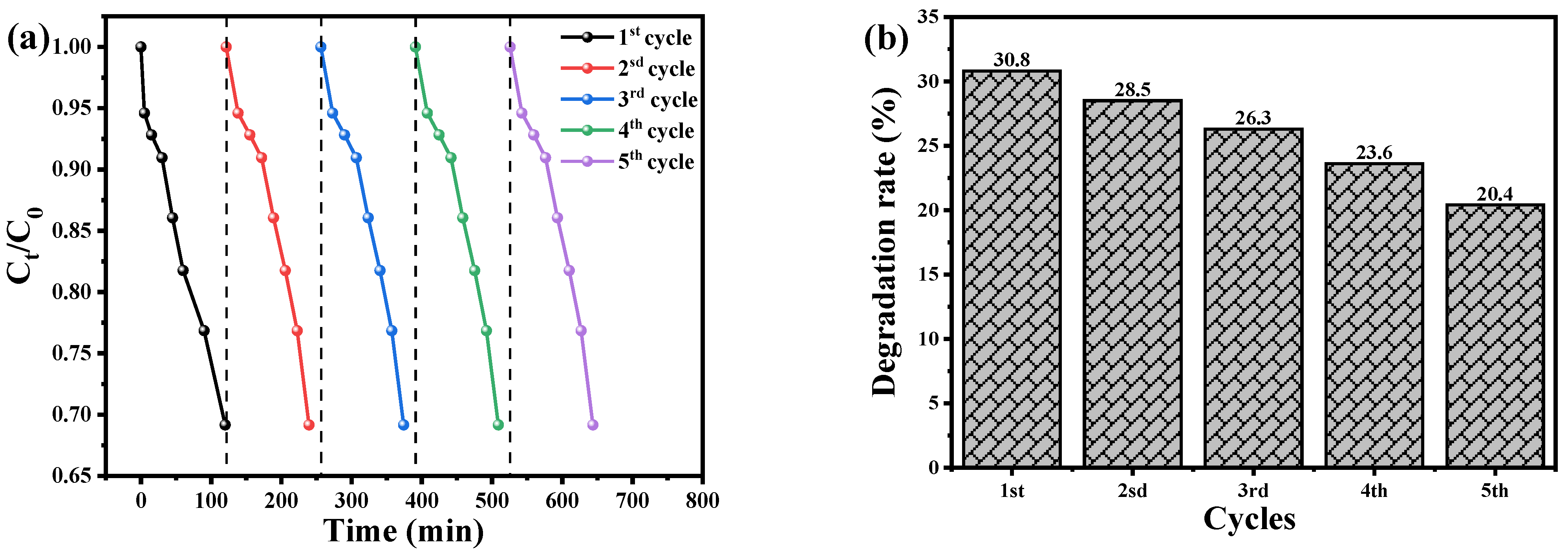
The recyclability and reusability of photocatalysts are crucial factors in assessing their feasibility for practical applications in water remediation. The authors conducted a series of experiments to evaluate the reusability of CuO nanoparticles (NPs) and Cu2O/AA NPs as photocatalysts for the degradation of BCB dye. The experimental procedure involved drying and reusing the photocatalysts for five consecutive cycles under identical conditions as the initial cycle. The results, depicted in Figure 9a,c, demonstrate that both the Cu2O NPs and Cu2O/AA NPs exhibited remarkable initial effectiveness and reusability in photodegrading BCB dye. However, a slight decrease in the decomposition efficiency was observed over the course of the five cycles. Specifically, the efficiency of the Cu2O NPs declined from 30.8% to 20.4%, while that of the Cu2O/AA NPs decreased from 73.12% to 65.3% (Figure 9b,d). This decline can be attributed to the inevitable loss of photocatalyst material during the recycling process, which may occur during washing, centrifugation, or due to the adsorption of intermediate species generated during the photocatalysis process [60,61]. Interestingly, the XRD data presented in Figure 9e revealed that the essential diffraction peaks of Cu2O NPs and Cu2O/AA NPs remained intact both before and after the photodegradation process, throughout the five consecutive photocatalytic cycles. This observation suggests that the catalytic material did not undergo significant structural alterations, highlighting the stability and suitability of these photocatalysts for reuse in water remediation applications.

Figure 9.
The recyclability of Cu2O NPs and Cu2O/AA NPs photocatalysts for degradation of BCB dye. (a) Cu2O NPs, (c) Cu2O/AA NPs, dotted line detain the start point of the Cycle and the next cycle and (b,d) reusability (degradation) efficiency vs. number of cycles in the photodegradation of BCB dye by Cu2O NPs and Cu2O/AA NPs, respectively. (e) XRD analysis of Cu2O NPs and Cu2O/ASA NPs of pure and reused.
The observed decline in decomposition efficiency over successive cycles could be attributed to the depletion of active sites due to catalyst agglomeration or leaching during the recycling process. Optimized techniques for catalyst recovery and regeneration might potentially mitigate these losses, thereby enhancing the overall efficiency. The consistent X-ray diffraction patterns suggest that the Cu2O and Cu2O/AA photocatalysts retain their crystalline integrity even after multiple cycles. This structural stability is a promising indicator of their long-term durability in water treatment applications. Nevertheless, further investigations are warranted to delineate the impact of prolonged usage on the photocatalytic activity and devise strategies to sustain optimal performance. Furthermore, evaluating the photocatalysts under diverse environmental conditions (e.g., pH, light intensity) and with a wide range of pollutant species would provide invaluable insights into their applicability in real-world wastewater remediation scenarios.
4. Conclusions
This study offers a comprehensive investigation into the synthesis, characterization, and application of Cu2O NPs and their ascorbic acid-modified derivatives for the efficient photocatalytic degradation of BCB dye. By employing a range of analytical techniques, including UV-Vis spectroscopy, FTIR, XRD, zeta potential measurements, and BET analysis, we have gained significant insights into the structural, optical, morphological, and surface properties of both Cu2O and Cu2O/AA NPs. Our findings reveal their remarkable efficiency in BCB dye degradation, with Cu2O/AA NPs demonstrating a degradation coefficient of 73.12% within 120 min, surpassing pure Cu2O NPs which achieved 30.8% degradation under similar conditions. This enhanced performance can be attributed to improved charge separation, increased ROS generation, and superior stability conferred by the presence of ascorbic acid. Furthermore, recyclability studies showcased promising reusability of the photocatalysts, albeit with slight efficiency declines over successive cycles. Cu2O/AA NPs exhibited sustained catalytic activity, retaining structural integrity throughout five consecutive cycles of BCB dye degradation, as evidenced by XRD analysis. In light of these findings, Cu2O/AA NPs emerge as promising candidates for effective and sustainable wastewater remediation strategies. Their superior photocatalytic performance, coupled with favorable recyclability and stability characteristics, underscores their potential applicability in addressing water pollution challenges. Future research endeavors could explore optimization strategies to further enhance catalytic efficiency and elucidate the broader applicability of these nanocomposites in diverse environmental remediation scenarios.
Author Contributions
Conceptualization: M.M.S.A., S.Z., S.E.L., H.A.M. and A.B.; Data curation: A.B., M.L.T. and M.M.S.A.; Formal analysis: M.M.S.A., S.E.L. and H.A.M.; Investigation: A.B. and M.L.T.; Methodology: S.Z., S.E.L. and H.A.M.; Project administration: M.M.S.A. and S.Z.; Resources: H.A.M.; Software: A.B.; Supervision: S.E.L.; Validation: T.T. and S.E.L.; Visualization: T.T., A.B. and S.E.L.; Writing—original draft: H.A.M., A.B. and M.L.T.; Writing—review and editing: M.M.S.A., A.B. and S.E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the financial support through Researchers Supporting Project number (RSPD2024R688), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maponya, T.C.; Hato, M.J.; Makhado, E.; Makgopa, K.; Khanuja, M.; Modibane, K.D. Photocatalytic Degradation of Dyes in Wastewater Using Metal Organic Frameworks. In Metal, Metal-Oxides and Metal-Organic Frameworks for Environmental Remediation; Springer International Publishing: Cham, Switzerland, 2021; pp. 261–285. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, S.; Ismail, A.M. Electrical and Optical Properties of Novel Brilliant Cresyl Blue Dye-doped Poly (Methyl Methacrylate) as Selective Cut-off Laser Filters. Polym. Int. 2020, 69, 1308–1318. [Google Scholar] [CrossRef]
- Roy, C.; Dutta, A.; Mahapatra, M.; Karmakar, M.; Roy, J.S.D.; Mitra, M.; Chattopadhyay, P.K.; Singha, N.R. Collagenic Waste and Rubber Based Resin-Cured Biocomposite Adsorbent for High-Performance Removal (s) of Hg (II), Safranine, and Brilliant Cresyl Blue: A Cost-Friendly Waste Management Approach. J. Hazard. Mater. 2019, 369, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Okoye, C.O.; Ezeorba, T.P.C.; Mao, G.; Chen, Y.; Xu, H.; Song, C.; Feng, W.; Wu, X. Emerging bio-dispersant and bioremediation technologies as environmentally friendly management responses toward marine oil spill: A comprehensive review. J. Environ. Manag. 2022, 322, 116123. [Google Scholar] [CrossRef] [PubMed]
- Gamelas, S.R.D.; Tomé, J.P.C.; Tomé, A.C.; Lourenço, L.M.O. Advances in Photocatalytic Degradation of Organic Pollutants in Wastewaters: Harnessing the Power of Phthalocyanines and Phthalocyanine-Containing Materials. RSC Adv. 2023, 13, 33957–33993. [Google Scholar] [CrossRef] [PubMed]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used for Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Franco, J.H.; Castañeda Cárdenas, S.D.; Zea Ramírez, H.R. Photocatalytic Degradation of Organic Dyes from Clinical Laboratory Wastewater. Water 2023, 15, 1238. [Google Scholar] [CrossRef]
- Aroob, S.; Carabineiro, S.A.C.; Taj, M.B.; Bibi, I.; Raheel, A.; Javed, T.; Yahya, R.; Alelwani, W.; Verpoort, F.; Kamwilaisak, K.; et al. Green Synthesis and Photocatalytic Dye Degradation Activity of CuO Nanoparticles. Catalysts 2023, 13, 502. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Stephen, A. Basic Principles, Mechanism, and Challenges of Photocatalysis BT. In Nanocomposites for Visible Light-Induced Photocatalysis; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–40. ISBN 978-3-319-62446-4. [Google Scholar]
- Gurylev, V. Photocatalysis: Basic Principles. In Advancement of Metal Oxide Materials for Photocatalytic Application; Gurylev, V., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–39. ISBN 978-3-031-20553-8. [Google Scholar]
- Ahmadian, A.; Ahmadi, S.; Goharrizi, B.A. Roles of Reactive Species in Photocatalysis: Effect of Scavengers and Inorganic Ions on Dye Removal from Wastewater. Int. J. Environ. Sci. Technol. 2023, 20, 6433–6448. [Google Scholar] [CrossRef]
- Náfrádi, M.; Veréb, G.; Firak, D.S.; Alapi, T. Photocatalysis: Introduction, Mechanism, and Effective Parameters. In Green Photocatalytic Semiconductors: Recent Advances and Applications; Garg, S., Chandra, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–31. ISBN 978-3-030-77371-7. [Google Scholar]
- Maheshwari, K.; Agrawal, M.; Gupta, A.B. Dye Pollution in Water and Wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–25. [Google Scholar]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of Copper Oxide Nanoparticles by Chemical and Biogenic Methods: Photocatalytic Degradation and in Vitro Antioxidant Activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, Selectivity, and Stability of Earth-Abundant CuO/Cu2O/Cu0-Based Photocatalysts toward CO2 Reduction. Chem. Eng. J. 2022, 429, 131579. [Google Scholar] [CrossRef]
- Nwanna, E.C.; Jen, T.-C. CuxOy Nanoparticle Fabrication: Synthesis, Characterization, and Applications. Mater. Sci. Eng. B 2024, 303, 117333. [Google Scholar] [CrossRef]
- Kavitha, S.; Jayamani, N.; Barathi, D. A Study on Preparation of Unique TiO2/Cu2O Nanocomposite with Highly Efficient Photocatalytic Reactivity under Visible-Light Irradiation. Mater. Technol. 2021, 36, 670–683. [Google Scholar] [CrossRef]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Mhamad, S.A.; Aziz, M. Review of Various Strategies to Boost the Photocatalytic Activity of the Cuprous Oxide-Based Photocatalyst. J. Environ. Chem. Eng. 2021, 9, 105138. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-Step Green Synthesis of Gold and Silver Nanoparticles with Ascorbic Acid and Their Versatile Surface Post-Functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Kader, D.A.; Rashid, S.O.; Omer, K.M. Green Nanocomposite: Fabrication, Characterization, and Photocatalytic Application of Vitamin C Adduct-Conjugated ZnO Nanoparticles. RSC Adv. 2023, 13, 9963–9977. [Google Scholar] [CrossRef] [PubMed]
- Bajić, V.; Spremo-Potparević, B.; Živković, L.; Čabarkapa, A.; Kotur-Stevuljević, J.; Isenović, E.; Sredojević, D.; Vukoje, I.; Lazić, V.; Ahrenkiel, S.P. Surface-Modified TiO2 Nanoparticles with Ascorbic Acid: Antioxidant Properties and Efficiency against DNA Damage in Vitro. Colloids Surf. B Biointerfaces 2017, 155, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Khan, A.A.P.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.-H. Engineering Nanostructures of CuO-Based Photocatalysts for Water Treatment: Current Progress and Future Challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-Edge Advances in Tailoring Size, Shape, and Functionality of Nanoparticles and Nanostructures: A Review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Díaz-García, Á.; Law, J.Y.; Romero, A.; Franco, V.; Guerrero, A. Quantifying the Structure and Properties of Nanomagnetic Iron Oxide Particles for Enhanced Functionality through Chemical Synthesis. Nanomaterials 2023, 13, 2242. [Google Scholar] [CrossRef] [PubMed]
- Vivas, L.; Chi-Duran, I.; Enríquez, J.; Barraza, N.; Singh, D.P. Ascorbic Acid Based Controlled Growth of Various Cu and Cu2O Nanostructures. Mater. Res. Express 2019, 6, 65033. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Jiang, L.; Zang, Z. Preparation of Cubic Cu2O Nanoparticles Wrapped by Reduced Graphene Oxide for the Efficient Removal of Rhodamine B. J. Alloys Compd. 2017, 718, 112–115. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Ascorbic Acid-Mediated Synthesis and Characterisation of Iron Oxide/Gold Core–Shell Nanoparticles. J. Exp. Nanosci. 2016, 11, 370–382. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eddine, L.S.; Hasan, G.G.; Meneceur, S.; Salmi, C.; Abdullah, J.A.A.; Abdullah, M.; Menaa, F. Efficient Removal of Heavy Metals, Dyes, and Contaminants from Industrial Wastewater Using Chitosan-Coated Fe3O4 Nanocomposites: Biosynthesis, Characterizations, and Performance Evaluation. Biomass Convers. Biorefinery 2024, 1–16. [Google Scholar] [CrossRef]
- Terea, H.; Selloum, D.; Rebiai, A.; Bouafia, A.; Ben Mya, O. Preparation and Characterization of Cellulose/ZnO Nanoparticles Extracted from Peanut Shells: Effects on Antibacterial and Antifungal Activities. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Allag, N.; Bouafia, A.; Chemsa, B.; Ben Mya, O.; Chala, A.; Siad, C.; Alam, M.W. Effect of Precursors on Structural, Optical and Surface Properties of ZnO Thin Film Prepared by Spray Pyrolysis Method: Efficient Removal of Cu (II) from Wastewater. Transit. Met. Chem. 2023, 49, 39–51. [Google Scholar] [CrossRef]
- Kir, I.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Mohammed, H.A.M. Biosynthesis and Characterization of Novel Nanocomposite ZnO/BaMg2 Efficiency for High-Speed Adsorption of AZO Dye. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Tedjani, M.L.; Khelef, A.; Laouini, S.E.; Bouafia, A.; Albalawi, N. Optimizing the Antibacterial Activity of Iron Oxide Nanoparticles Using Central Composite Design. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3564–3584. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Melo, E.I.; Alves, V.N.; Coelho, N.M.M. Moringa Oleifera Lam. Seeds as a Natural Solid Adsorbent for Removal of AgI in Aqueous Solutions. J. Braz. Chem. Soc. 2010, 21, 1727–1732. [Google Scholar] [CrossRef]
- Sakura, G.B.; Leung, A.Y.T. Experimental Study of Particle Collection Efficiency of Cylindrical Inlet Type Cyclone Separator. Int. J. Environ. Sci. Dev. 2015, 6, 160–164. [Google Scholar] [CrossRef]
- Zohra, R.; Meneceur, S.; Mohammed, H.A.; Hasan, G.G.; Bouafia, A.; Abdullah, J.A.A.; Alharthi, F.; Eddine, L.S. Enhanced Photocatalytic Degradation of Dyes and Antibiotics with Biosynthesized FeMn2O4 Nanocomposite under Sunlight Irradiation: Isotherm and Kinetic Study. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Laid, T.M.; Abdelhamid, K.; Eddine, L.S.; Abderrhmane, B. Optimizing the Biosynthesis Parameters of Iron Oxide Nanoparticles Using Central Composite Design. J. Mol. Struct. 2021, 1229, 129497. [Google Scholar] [CrossRef]
- Anber, A.A.; Essa, M.S.; Kadhim, G.A.; Hashim, S.S. Preparation of Nanoparticles Copper Oxide Using an Atmospheric-Pressure Plasma Jet. J. Phys. Conf. Ser. 2018, 1032, 12009. [Google Scholar] [CrossRef]
- Aloui, M.; Mentar, L.; Beniaiche, A.; Azizi, A. NH4Cl and KCl Effect on the Structural, Morphological, Optical and Electrochemical Properties of Cu2O Nanoparticles and Cu2O Nanostructures, Galvanostatically Electrodeposited. J. Solid State Chem. 2022, 315, 123435. [Google Scholar] [CrossRef]
- Ilie, A.; Ghiţulică, C.; Andronescu, E.; Cucuruz, A.; Ficai, A. New Composite Materials Based on Alginate and Hydroxyapatite as Potential Carriers for Ascorbic Acid. Int. J. Pharm. 2016, 510, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Bestimmung der Größe und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen. Math. Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Meneceur, S.; Bouafia, A.; Laouini, S.E.; Mohammed, H.A.; Daoudi, H.; Chami, S.; Hasan, G.G.; Abdullah, J.A.A.; Salmi, C. Removal Efficiency of Heavy Metals, Oily in Water, Total Suspended Solids, and Chemical Oxygen Demand from Industrial Petroleum Wastewater by Modern Green Nanocomposite Methods. J. Environ. Chem. Eng. 2023, 11, 111209. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Methods in Molecular Biology; Springer International Publishing: Cham, Switzerland, 2011; Volume 697, pp. 63–70. [Google Scholar]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Zidane, Y.; Laouini, S.E.; Bouafia, A.; Meneceur, S.; Tedjani, M.L.; Alshareef, S.A.; Almukhlifi, H.A.; Al-Essa, K.; Al-Essa, E.M.; Rahman, M.M.; et al. Green Synthesis of Multifunctional MgO@AgO/Ag2O Nanocomposite for Photocatalytic Degradation of Methylene Blue and Toluidine Blue. Front. Chem. 2022, 10, 1083596. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, J.L.; Muñoz Villegas, L.F.; Sánchez, V.I.Y.; Perez, F.A.N.; Rodriguez, J.M.Z. Adsorption of Brilliant Cresyl Blue from Aqueous Solution by Silver Nanoparticles on Zinc Oxide. MRS Adv. 2023. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K. Fabrication of Zno/Cuo Hybrid Nanocomposite for Photocatalytic Degradation of Brilliant Cresyl Blue (Bcb) Dye in Aqueous Solutions. J. Water Environ. Nanotechnol. 2021, 6, 196–211. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of Cobalt and Cobalt Oxide Nanoparticles as Nanocatalyst towards Dyes Degradation in Wastewater. Nano Struct. Nano Objects 2020, 21, 100405. [Google Scholar] [CrossRef]
- Meneceur, S.; Hemmami, H.; Bouafia, A.; Laouini, S.E.; Tedjani, M.L.; Berra, D.; Mahboub, M.S. Photocatalytic Activity of Iron Oxide Nanoparticles Synthesized by Different Plant Extracts for the Degradation of Diazo Dyes Evans Blue and Congo Red. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Zohra, R.; Meneceur, S.; Eddine, L.S.; Bouafia, A.; Mohammed, H.A.; Hasan, G.G. Biosynthesis and Characterization of MnO2 and Zn/Mn2O4 NPs Using Ziziphus Spina-Christi Aqueous Leaves Extract: Effect of Decoration on Photodegradation Activity against Various Organic Dyes. Inorg. Chem. Commun. 2023, 156, 111304. [Google Scholar] [CrossRef]
- Asif, S.A.B.; Khan, S.B.; Asiri, A.M. Efficient Solar Photocatalyst Based on Cobalt Oxide/Iron Oxide Composite Nanofibers for the Detoxification of Organic Pollutants. Nanoscale Res. Lett. 2014, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Messai, R.; Ferhat, M.F.; Belmekki, B.; Alam, M.W.; Al-Othoum, M.A.S.; Sadaf, S. GAD Plasma-Assisted Synthesis of ZnO Nanoparticles and Their Photocatalytic Activity. Mater. Res. Express 2024, 11, 015006. [Google Scholar] [CrossRef]
- Singh, P.; MAbdullah, M.; Sagadevan, S.; Kaur, C.; Ikram, S. Highly Sensitive Ethanol Sensor Based on TiO2 Nanoparticles and Its Photocatalyst Activity. Optik 2019, 182, 512–518. [Google Scholar] [CrossRef]
- Baig, A.; Baig, A.; Rathinam, V.; Ramya, V. Facile Fabrication of Zn-Doped SnO2 Nanoparticles for Enhanced Photocatalytic Dye Degradation Performance under Visible Light Exposure. Adv. Compos. Hybrid Mater. 2021, 4, 114–126. [Google Scholar] [CrossRef]
- El-Berry, M.F.; Sadeek, S.A.; Abdalla, A.M.; Nassar, M.Y. Microwave-Assisted Fabrication of Copper Nanoparticles Utilizing Different Counter Ions: An Efficient Photocatalyst for Photocatalytic Degradation of Safranin Dye from Aqueous Media. Mater. Res. Bull. 2021, 133, 111048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).