Preparation of Fe-HMOR with a Preferential Iron Location in the 12-MR Channels for Dimethyl Ether Carbonylation
Abstract
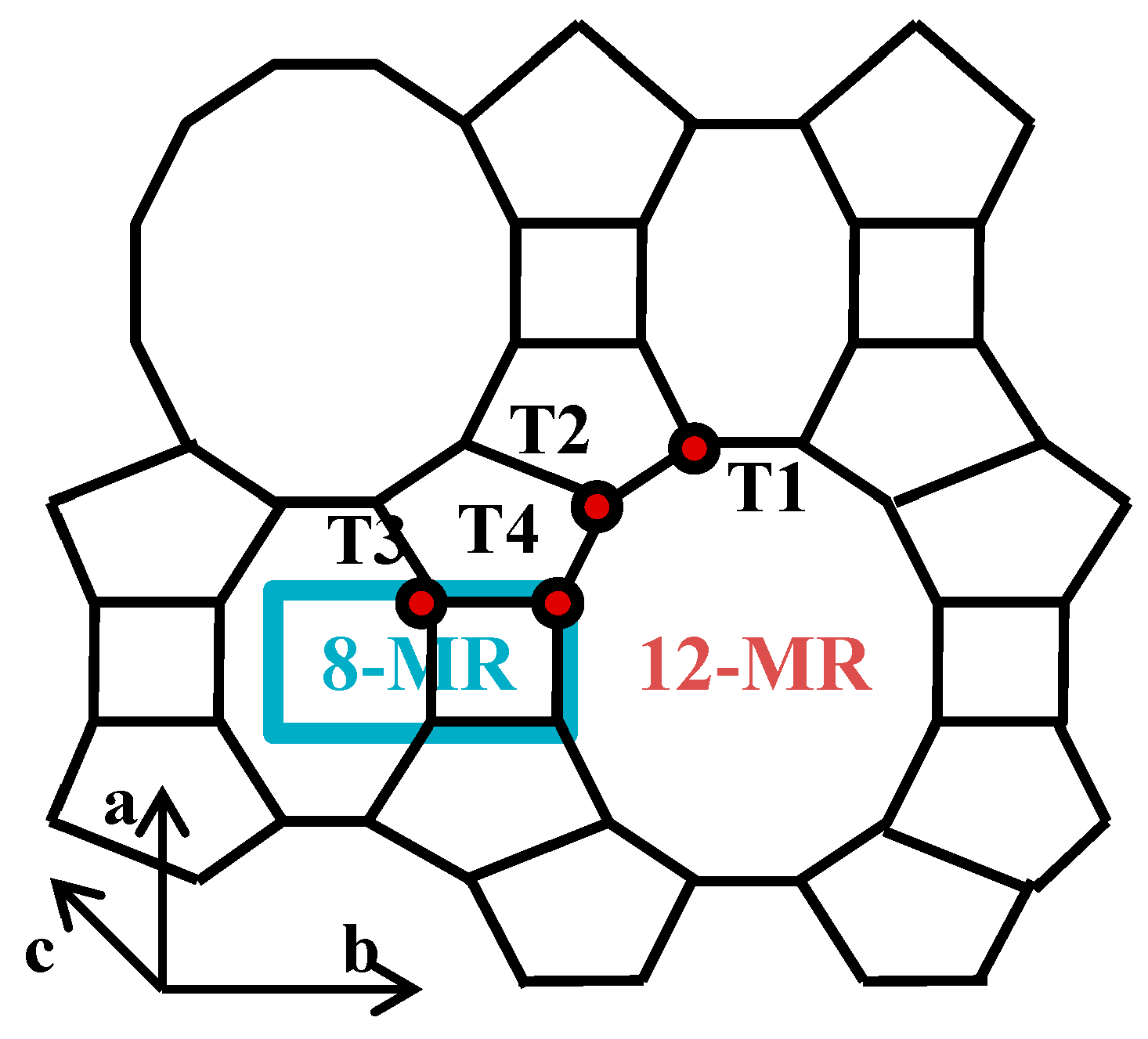
:1. Introduction
2. Materials and Methods
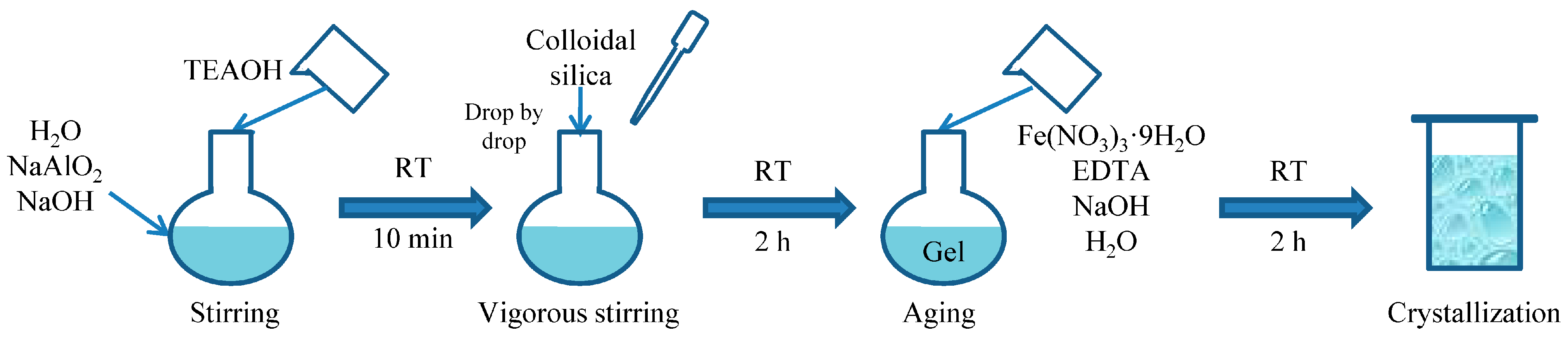
2.1. Synthesis of Fe-Modified Mordenite
2.2. Characterization
2.3. Catalyst Tests
3. Results and Discussion
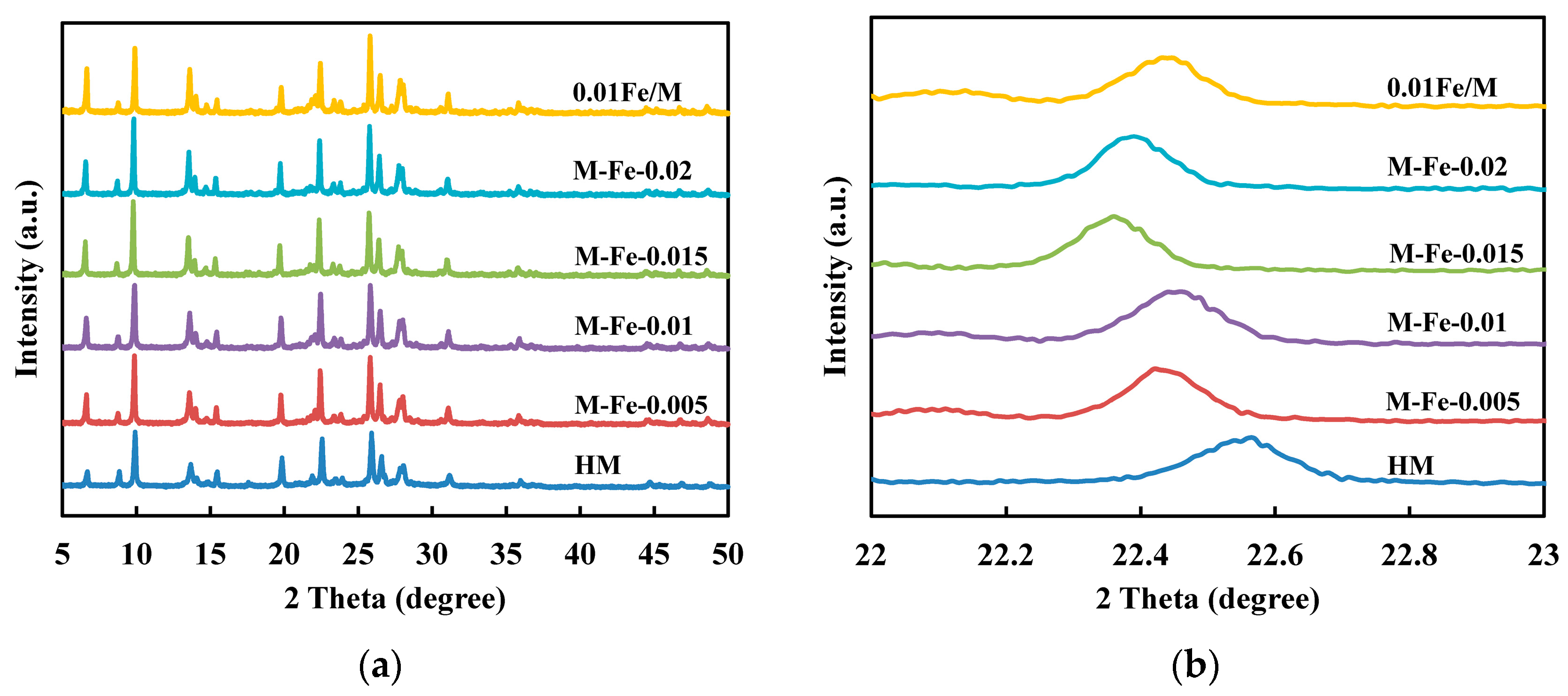
3.1. Fe-Modified Mordenite Zeolites
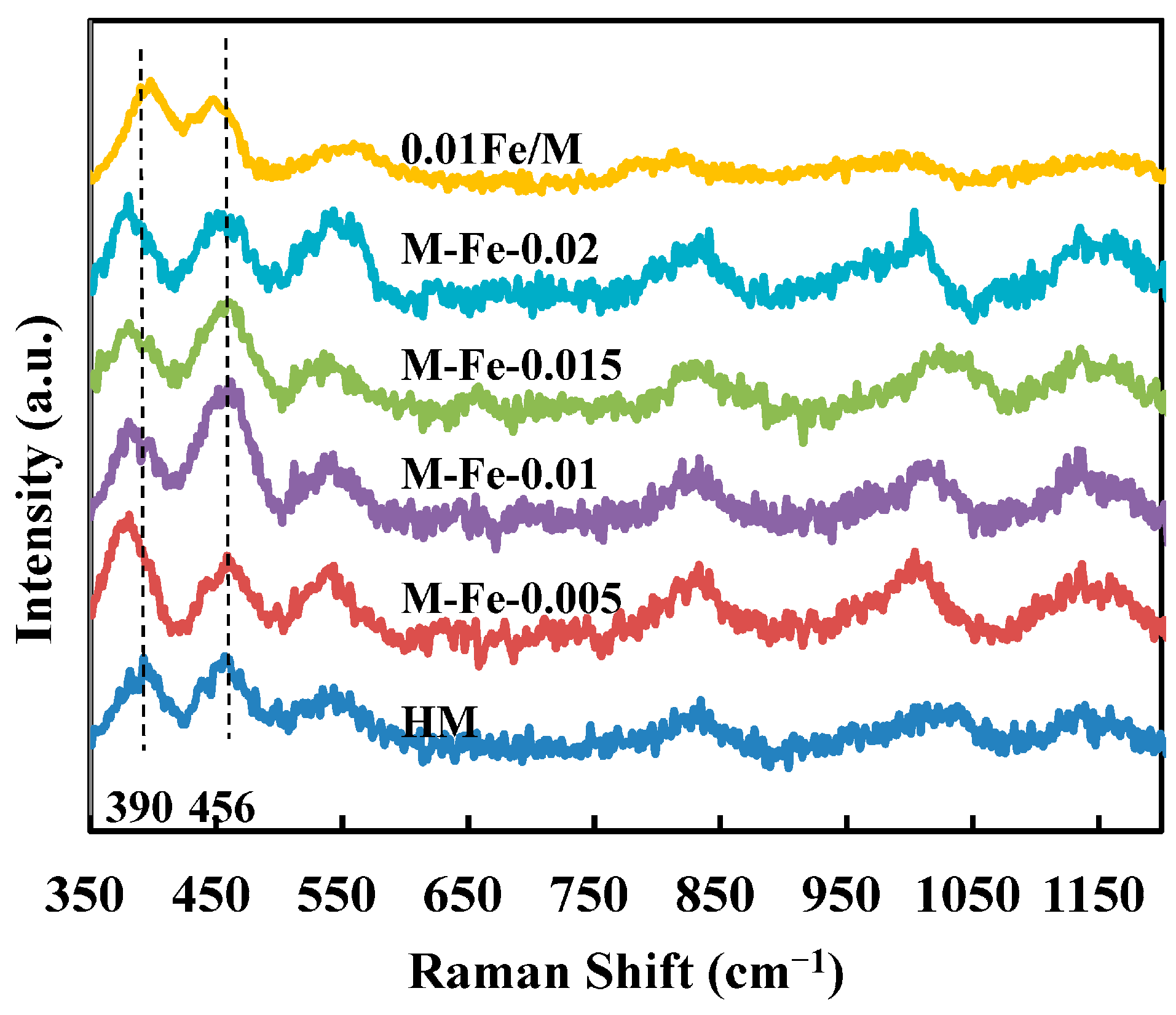
3.2. Framework Incorporation of Fe Atoms
3.3. Acid Properties of the Catalysts
3.4. Catalytic Performance in DME Carbonylation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; He, Z.; Zhang, L.; Zhao, X.; Cao, J. Facile designing a nanosheet HMOR zeolite for enhancing the efficiency of ethanol synthesis from dimethyl ether and syngas. Int. J. Hydrogen Energ. 2022, 47, 9273–9282. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energ. 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Zhan, E.; Xiong, Z.; Shen, W. Dimethyl ether carbonylation over zeolites. J. Energy Chem. 2019, 36, 51–63. [Google Scholar] [CrossRef]
- Chen, F.; Feng, X.; Zhang, L.; Zhao, J.; He, Z.; Yi, F.; Zhao, X.; Cao, J. Selective enrichment of Brønsted acid site in 8-membered ring channels of MOR zeolite to enhance the catalytic reactivity of dimethyl ether carbonylation. Chem. Eng. Sci. 2022, 263, 118110. [Google Scholar] [CrossRef]
- Yao, J.; Wu, Q.; Fan, J.; Komiyama, S.; Yong, X.; Zhang, W.; Zhao, T.; Guo, Z.; Yang, G.; Tsubaki, N. A carbonylation zeolite with specific nanosheet structure for efficient catalysis. ACS Nano 2021, 15, 13568–13578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qian, W.; Ma, H.; Ying, W.; Yuan, P.; Zhang, H. Theoretical study for adsorption-diffusion on H-MOR and pyridine pre-adsorbed H-MOR of dimethyl ether carbonylation. ACS Omega 2023, 8, 22067–22076. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.; Yu, C.; Liu, Y.; Xu, C.; Lu, C. Study on the deactivation process of dimethyl ether carbonylation reaction over mordenite catalyst. Fuel 2021, 286, 119480. [Google Scholar] [CrossRef]
- Chu, Y.; Lo, A.; Wang, C.; Deng, F. Origin of high selectivity of dimethyl ether carbonylation in the 8-membered ring channel of mordenite zeolite. J. Phys. Chem. C 2019, 123, 15503–15512. [Google Scholar] [CrossRef]
- Rasmussen, D.B.; Christensen, J.M.; Temel, B.; Studt, F.; Moses, P.G.; Rossmeisl, J.; Riisager, A.; Jensen, A.D. Ketene as a reaction intermediate in the carbonylation of dimethyl ether to methyl acetate over mordenite. Angew. Chem. Int. Ed. 2015, 54, 7261–7264. [Google Scholar] [CrossRef]
- Chen, W.; Li, G.; Yi, X.; Day, S.J.; Tarach, K.A.; Liu, Z.; Liu, S.; Tsang, S.C.E.; Góra-Marek, K.; Zheng, A. Molecular understanding of the catalytic consequence of ketene intermediates under confinement. J. Am. Chem. Soc. 2021, 143, 15440–15452. [Google Scholar] [CrossRef]
- Cheng, Z.; Huang, S.; Li, Y.; Cai, K.; Wang, Y.; Wang, M.; Lv, J.; Ma, X. Role of Brønsted acid sites within 8-MR of mordenite in the deactivation roadmap for dimethyl ether carbonylation. ACS Catal. 2021, 11, 5647–5657. [Google Scholar] [CrossRef]
- He, T.; Liu, X.; Xu, S.; Han, X.; Pan, X.; Hou, G.; Bao, X. Role of 12-ring channels of mordenite in DME carbonylation investigated by solid-state NMR. J. Phys. Chem. C 2016, 120, 22526–22531. [Google Scholar] [CrossRef]
- Bhan, A.; Allian, A.D.; Sunley, G.J.; Law, D.J.; Iglesia, E. Specificity of sites within eight-membered ring zeolite channels for carbonylation of methyls to acetyls. J. Am. Chem. Soc. 2007, 129, 4919–4924. [Google Scholar] [CrossRef] [PubMed]
- Issa, H.; Chaouati, N.; Toufaily, J.; Hamieh, T.; Sachse, A.; Pinard, L. Toolbox of post-synthetic mordenite modification strategies: Impact on textural, acidic and catalytic properties. ChemCatChem 2019, 18, 4581–4592. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, D.; Li, B.; Lyu, Y.; Wang, X.; Liu, X.; Li, L.; Yu, S.; Liu, X.; Yan, Z. Isomerization of α-pinene with a hierarchical mordenite molecular sieve prepared by the microwave assisted alkaline treatment. Micropor. Mesopor. Mater. 2020, 299, 110117. [Google Scholar] [CrossRef]
- Xu, F.; Lv, J.; Chen, C.; Hong, Z.; Zhao, G.; Miao, L.; Yang, W.; Zhu, Z. Effect of steam treatment on the properties of mordenite and its catalytic performance in a DME carbonylation reaction. Ind. Eng. Chem. Res. 2022, 61, 1258–1266. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Cheng, Z.; Cai, K.; Li, L.; Milan, E.; Lv, J.; Wang, Y.; Sun, Q.; Ma, X. Promoting the activity of Ce-incorporated MOR in dimethyl ether carbonylation through tailoring the distribution of Brønsted acids. Appl. Catal. B Environ. 2019, 256, 117777. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, W.; Shi, L.; Liu, H.; Liu, S.; Xu, S.; Ni, Y.; Liu, Y.; Li, L.; Liu, Z. Promotion effect of Fe in mordenite zeolite on carbonylation of dimethyl ether to methyl acetate. Catal. Sci. Technol. 2015, 5, 1961–1968. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; He, Z.; Chen, F.; Su, C.; Zhao, X.; Cao, J.; He, Y. Enhancing the stability of dimethyl ether carbonylation over Fe-doped MOR zeolites with tunable 8-MR acidity, Chem. Eng. Sci. 2022, 256, 117671. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Bu, L.; Zhi, Y.; Wang, Z.; Yang, M.; Chu, K.; Huang, Y.; Guo, N.; Qu, L.; et al. Wheel-like mordenite nanoassemblies with shortened channel lengths for improved catalytic performance in dimethyl ether carbonylation. ACS Appl. Nano Mater. 2023, 6, 18005–18015. [Google Scholar] [CrossRef]
- Wu, P.; Komatsu, T.; Yashima, T. Isomorphous substitution of Fe3+ in the framework of aluminosilicate mordenite by hydrothermal synthesis. Micropor. Mesopor. Mater. 1998, 20, 139–147. [Google Scholar] [CrossRef]
- Xiong, Z.; Qi, G.; Zhan, E.; Chu, Y.; Xu, J.; Wei, J.; Ta, N.; Hao, A.; Zhou, Y.; Deng, F.; et al. Experimental identification of the active sites over a plate-like mordenite for the carbonylation of dimethyl ether. Chem 2023, 9, 76–92. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, J.; Hou, W.; Liu, Y.; Zhou, Y.; Wang, J. One-pot template-free synthesis of Cu-MOR zeolite toward efficient catalyst support for aerobic oxidation of 5-hydroxymethylfurfural under ambient pressure. ACS Appl. Mater. Inter. 2016, 8, 23122–23132. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Y.; Sun, C.; Zhao, T.; Zhao, J.; Wang, Z.; Liu, W.; Wu, S.; Shi, M.; Bu, L. Novel synthesis and catalytic performance of hierarchical MOR. New J. Chem. 2021, 45, 8629–8638. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Beyer, H.K.; Valyon, J. Properties of the end members in the Pentasil-family of zeolites: Characterization as adsorbents. Zeolites 1981, 1, 161–168. [Google Scholar] [CrossRef]
- Liu, C.; Gu, W.; Kong, D.; Guo, H. The significant effects of the alkali-metal cations on ZSM-5 zeolite synthesis: From mechanism to morphology. Micropor. Mesopor. Mater. 2014, 183, 30–36. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Yi, H.; Yu, Q.; Zhang, Y.; Wei, J.; Yuan, Y. Synthesis, characterization and application of Fe-zeolite: A review. Appl. Catal. A Gen. 2022, 630, 118467. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; He, S.; Liang, T.; Zheng, S.; Lu, L.; Hao, C.; Chen, K.; Li, T.; Yi, L.; et al. Study on the catalytic activity and hydrothermal stability of one-pot synthesized Fe-based FER zeolites for NH3-SCR. Catal. Sci. Technol. 2023, 13, 5435–5448. [Google Scholar] [CrossRef]
- Gomez, S.; Lerici, L.; Saux, C.; Perez, A.L.; Brondino, C.D.; Pierella, L.; Pizzio, L. Fe/ZSM-11 as a novel and efficient photocatalyst to degrade dichlorvos on water solutions. Appl. Catal. B Environ. 2017, 202, 580–586. [Google Scholar] [CrossRef]
- Kotolevich, Y.; Khramov, E.; Sánchez-López, P.; Pestryakov, A.; Zubavichus, Y.; Antúnez-Garcia, J.; Petranovskii, V. Formation of Ag-Fe bimetallic nano-species on mordenite depending on the initial ratio of components. Materials 2023, 16, 3026. [Google Scholar] [CrossRef]
- Yu, D.; Wang, P.; Li, X.; Zhao, H.; Lv, X. Study on the role of Fe species and acid sites in NH3-SCR over the Fe-based zeolites. Fuel 2023, 336, 126759. [Google Scholar] [CrossRef]
- Deng, Y.; Veser, G. Toward accelerated activation of Fe-ZSM-5 in methane dehydroaromatization. Energ. Fuel 2023, 37, 13282–13295. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, G.; Li, C.; Xiao, F. Characterization of aluminosilicate zeolites by UV Raman spectroscopy. Micropor. Mesopor. Mat. 2001, 46, 23–34. [Google Scholar] [CrossRef]
- Twu, J.; Dutta, P.K.; Kresge, C.T. Vibrational spectroscopic examination of the formation of mordenite crystals. J. Phys. Chem. 1991, 13, 5267–5271. [Google Scholar] [CrossRef]
- Boronat, M.; Martínez-Sánchez, C.; Law, D.; Corma, A. Enzyme-like specificity in zeolites: A unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO. J. Am. Chem. Soc. 2008, 130, 16316–16323. [Google Scholar] [CrossRef] [PubMed]
- Reule, A.A.C.; Sawada, J.A.; Semagina, N. Effect of selective 4-membered ring dealumination on mordenite-catalyzed dimethyl ether carbonylation. J. Catal. 2017, 349, 98–109. [Google Scholar] [CrossRef]
- Yakimov, A.V.; Ravi, M.; Verel, R.; Sushkevich, V.L.; van Bokhoven, J.A.; Copéret, C. Structure and framework association of Lewis acid sites in MOR zeolite. J. Am. Chem. Soc. 2022, 144, 10377–10385. [Google Scholar] [CrossRef]
- Lukyanov, D.B.; Vazhnova, T.; Cherkasov, N.; Casci, J.L.; Birtill, J.J. Insights into Brønsted acid sites in the zeolite mordenite. J. Phys. Chem. C 2014, 118, 23918–23929. [Google Scholar] [CrossRef]
- Datka, J.; Gil, B.; Kubacka, A. Acid properties of NaH-mordenites: Infrared spectroscopic studies of ammonia sorption. Zeolites 1995, 15, 501–506. [Google Scholar] [CrossRef]
- Sheng, H.; Ma, H.; Qian, W.; Fei, N.; Zhang, H.; Ying, W. Platinum-copper bimetallic-modified nanoprism mordenite for carbonylation of dimethyl ether. Energ. Fuel. 2019, 33, 10159–10166. [Google Scholar] [CrossRef]
- Lónyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite. Micropor. Mesopor. Mat. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Zhao, W.; Li, X. Selective catalytic reduction of NO by NH3 over MoO3 promoted Fe2O3 catalyst. Kinet. Catal. 2018, 59, 628–634. [Google Scholar] [CrossRef]
- Wang, M.; Huang, S.; Lü, J.; Cheng, Z.; Li, Y.; Wang, S.; Ma, X. Modifying the acidity of H-MOR and its catalytic carbonylation of dimethyl ether. Chin. J. Catal. 2016, 9, 1530–1537. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, N.; Xian, H.; Cheng, Q.; Tan, Y.; Tsubaki, N.; Li, X. Facilely synthesized H-mordenite nanosheet assembly for carbonylation of dimethyl ether. ACS Appl. Mater. Inter. 2015, 7, 8398–8403. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.; Ihm, S. Acid strength control in MFI zeolite for the methanol-to-hydrocarbons (MTH) reaction. Ind. Eng. Chem. Res. 2014, 53, 10072–10079. [Google Scholar] [CrossRef]
- Lew, C.M.; Chen, C.; Long, G.J.; Grandjean, F.; Ichimura, A.S.; Xie, D.; Grosso-Giordano, N.A.; Chakarawet, K.; Lacheen, H.S.; Jensen, K.O.; et al. Synthesis, physicochemical characterization, and catalytic evaluation of Fe3+-containing SSZ-70 zeolite. ACS Catal. 2022, 12, 6464–6477. [Google Scholar] [CrossRef]
- Tsubota, S.; Kokuryo, S.; Tamura, K.; Miyake, K.; Uchida, Y.; Mizusawa, A.; Kubo, T.; Nishiyama, N. Exploring the Effect of Brønsted Acidity of MFI-type Zeolites on Catalytic Cracking Temperature of Low Density Polyethylene. Catal. Sci. Technol. 2024, 14, 1369–1374. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401. [Google Scholar] [CrossRef]
- Palčić, A.; Valtchev, V. Analysis and control of acid sites in zeolites. Appl. Catal. A Gen. 2020, 606, 117795. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared adsorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Chen, F.; Feng, X.; Zhao, J.; He, Z.; Zhang, L.; Wang, Y.; Deng, P.; Gao, X.; Zhao, X.; Cao, J. Designing Mordenite Zeolites with Tunable Distribution of Acid Sites in Channels and Nano-Morphology to Boost the Catalytic Behavior Performance of Dimethyl Ether Carbonylation. Chem. Eng. Sci. 2023, 282, 119250. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, J.; Gu, Y.; Zhang, W.; Cao, K.; Cui, W.; Xu, S.; Fan, D.; Tian, P.; Liu, Z. Designed Synthesis of MOR Zeolites Using Gemini-Type Bis(Methylpyrrolidinium) Dications as Structure Directing Agents and their DME Carbonylation Performance. J. Mater. Chem. A 2022, 10, 8334–8343. [Google Scholar] [CrossRef]
- Lu, P.; Yang, G.; Tanaka, Y.; Tsubaki, N. Ethanol direct synthesis from dimethyl ether and syngas on the combination of noble metal impregnated zeolite with Cu/ZnO catalyst. Catal. Today 2014, 232, 22–26. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Ma, X.; Liu, Y.; Zhu, W.; Liu, Z. Identifying and controlling the acid site distributions in mordenite zeolite for dimethyl ether carbonylation reaction by means of selective ion-exchange. Catal. Sci. Technol. 2020, 1, 4663–4672. [Google Scholar] [CrossRef]
- Liu, S.; Fang, X.; Liu, Y.; Liu, H.; Ma, X.; Zhu, W.; Liu, Z. Dimethyl ether carbonylation over mordenite zeolite modified by alkyimidazolium ions. Catal. Commun. 2020, 147, 106161. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Mezari, B.; Pestman, R.; Wannapakdee, W.; Hensen, E.J.M. On the role of acidity in bulk and nanosheet [T]MFI (T=Al3+, Ga3+, Fe3+, B3+) zeolites in the methanol-to-hydrocarbons reaction. ChemCatChem 2017, 9, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.; Jung, H.S.; Kim, H.S.; Kim, J.; Cho, S.J.; Lee, W.B.; Park, M.; Bae, J.W. Gas-Phase Carbonylation of Dimethyl Ether on the Stable Seed-Derived Ferrierite. ACS Catal. 2020, 10, 5135–5146. [Google Scholar] [CrossRef]
- Liu, J.; Xue, H.; Huang, X.; Wu, P.; Huang, S.; Liu, S.; Shen, W. Stability Enhancement of H-Mordenite in Dimethyl Ether Carbonylation to Methyl Acetate by Pre-Adsorption of Pyridine. Chin. J. Catal. 2010, 31, 729–738. [Google Scholar] [CrossRef]
- Wang, X.; Li, R.; Yu, C.; Zhang, L.; Xu, C.; Zhou, H. Dimethyl ether carbonylation over nanosheet-assembled hierarchical mordenite. Micropor. Mesopor. Mater. 2019, 274, 227–235. [Google Scholar] [CrossRef]
- Vinila, V.S.; Isac, J. Synthesis and structural studies of superconducting perovskite GdBa2Ca3Cu4O10.5þd nanosystems. In Design, Fabrication, and Characterization of Multifunctional Nanomaterials, 1st ed.; Thomas, S., Kalarikkal, N., Abraham, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 319–338. [Google Scholar]



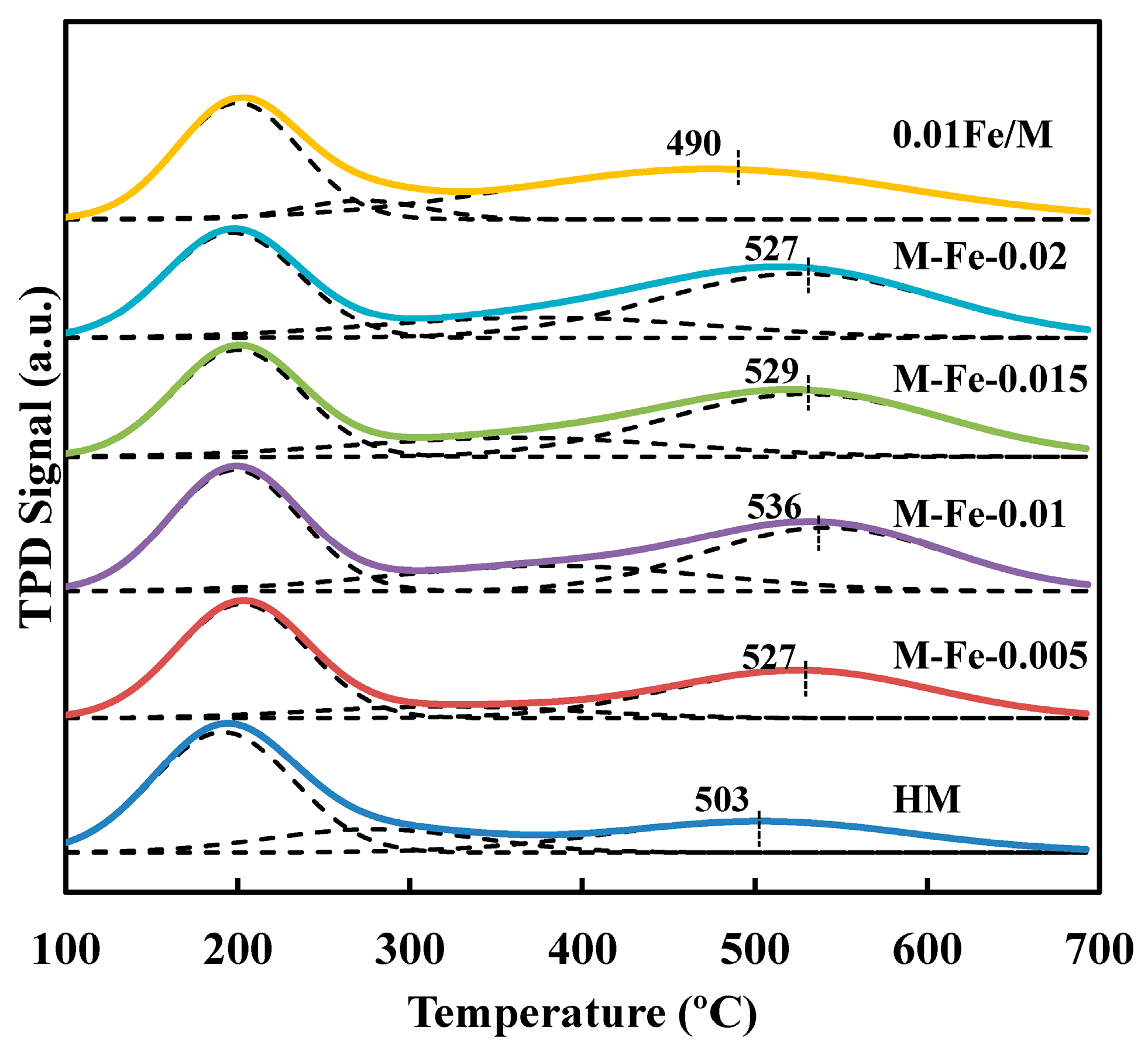


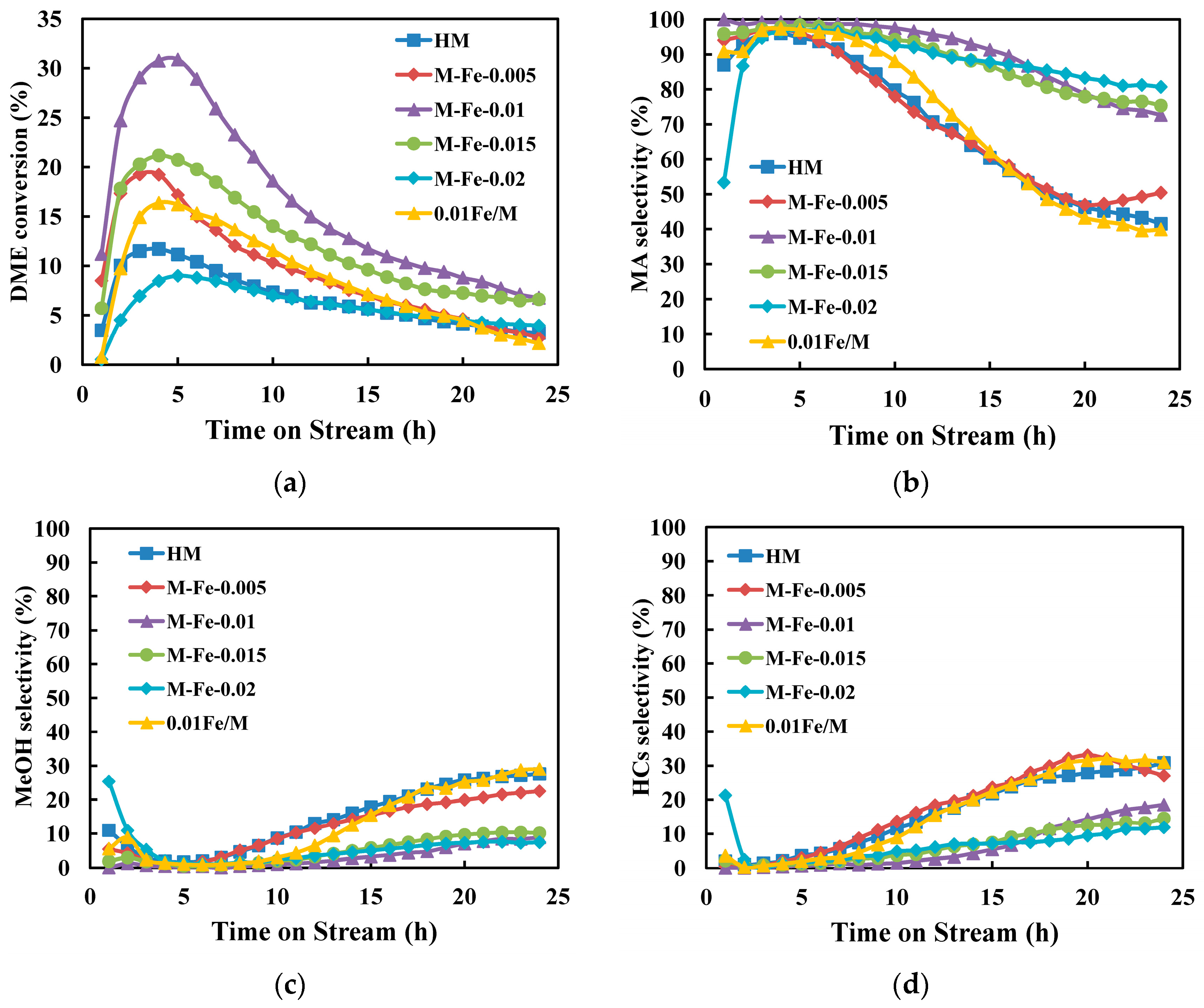

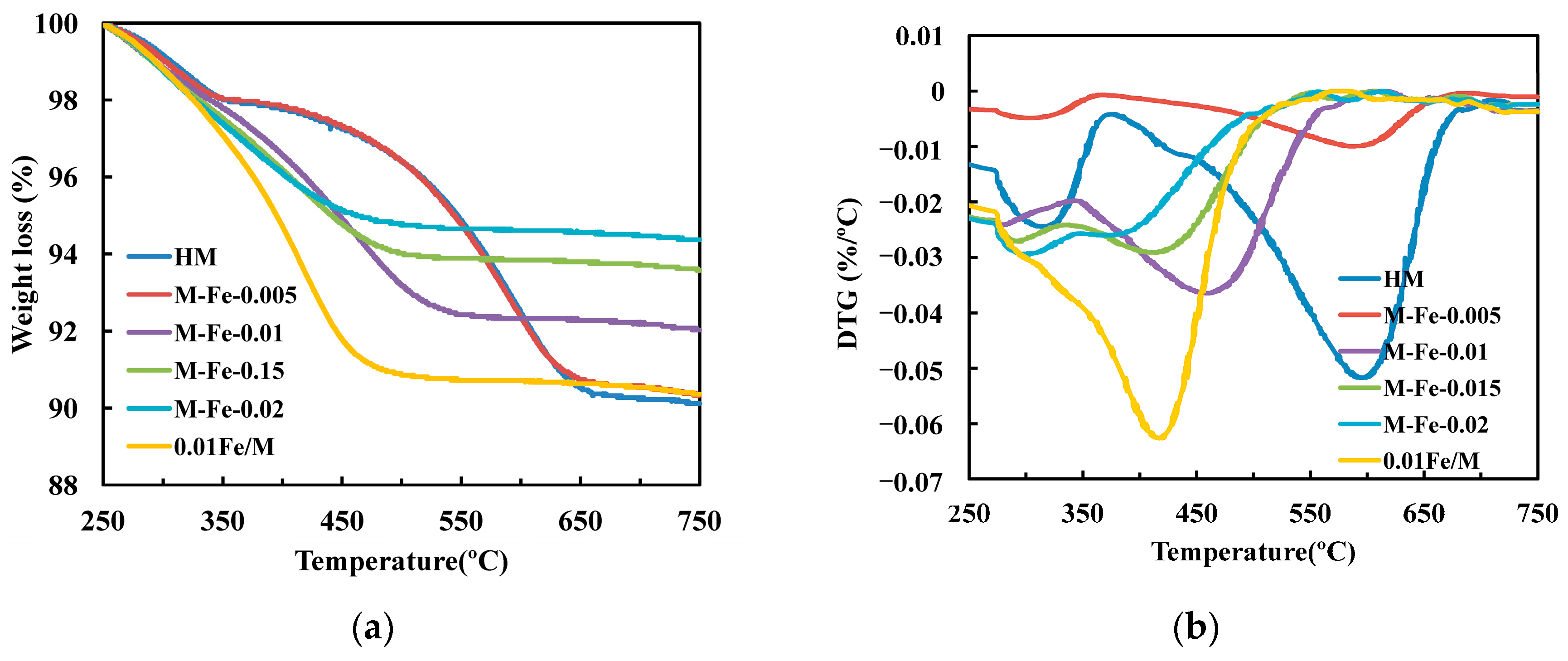
| Sample | Relative Crystallinity (%) | Iron Content a (wt%) | Si/Al a | Si/(Al+Fe) a | 27Al NMR (%) b | |
|---|---|---|---|---|---|---|
| AlF | AlEF | |||||
| HM | 100 | - | 8.6 | - | 78.6 | 21.4 |
| M-Fe-0.005 | 129 | 1.03 | 10.1 | 8.9 | 80.9 | 19.1 |
| M-Fe-0.01 | 125 | 2.06 | 11.3 | 8.8 | 80.5 | 19.5 |
| M-Fe-0.015 | 131 | 2.69 | 11.8 | 8.6 | 89.3 | 10.7 |
| M-Fe-0.02 | 134 | 3.55 | 12.4 | 8.1 | 87.1 | 12.9 |
| 0.01Fe/M | 142 | 2.38 | 10.3 | 7.9 | 81.8 | 18.2 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | ||||
|---|---|---|---|---|---|---|
| SBET | Smicro | Sext | Vtotal | Vmicro | Vmeso | |
| HM | 449 | 412 | 37 | 0.194 | 0.163 | 0.031 |
| M-Fe-0.005 | 489 | 449 | 40 | 0.214 | 0.179 | 0.035 |
| M-Fe-0.01 | 488 | 450 | 38 | 0.211 | 0.179 | 0.032 |
| M-Fe-0.015 | 459 | 424 | 35 | 0.207 | 0.169 | 0.038 |
| M-Fe-0.02 | 364 | 340 | 24 | 0.159 | 0.136 | 0.023 |
| 0.01Fe/M | 445 | 426 | 19 | 0.180 | 0.169 | 0.011 |
| Sample | NH3-TPD (mmol/g) a | Py-IR (mmol/g) | NH3-IR (mmol/g) c | ||||
|---|---|---|---|---|---|---|---|
| Weak | Moderate | Strong | B8-MR | B12-MR b | B | L | |
| HM | 0.550 | 0.164 | 0.307 | 0.157 | 0.150 | 0.304 | 0.157 |
| M-Fe-0.005 | 0.492 | 0.101 | 0.407 | 0.246 | 0.161 | 0.399 | 0.127 |
| M-Fe-0.01 | 0.519 | 0.223 | 0.555 | 0.395 | 0.160 | 0.559 | 0.214 |
| M-Fe-0.015 | 0.451 | 0.207 | 0.568 | 0.336 | 0.232 | 0.566 | 0.208 |
| M-Fe-0.02 | 0.464 | 0.227 | 0.586 | 0.290 | 0.296 | 0.604 | 0.228 |
| 0.01Fe/M | 0.479 | 0.155 | 0.573 | 0.293 | 0.280 | 0.552 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, Y.; Bu, L.; Chu, K.; Huang, Y.; Guo, N.; Qu, L.; Sang, J.; Su, X.; Zhang, X.; et al. Preparation of Fe-HMOR with a Preferential Iron Location in the 12-MR Channels for Dimethyl Ether Carbonylation. Materials 2024, 17, 2417. https://doi.org/10.3390/ma17102417
Liu W, Wang Y, Bu L, Chu K, Huang Y, Guo N, Qu L, Sang J, Su X, Zhang X, et al. Preparation of Fe-HMOR with a Preferential Iron Location in the 12-MR Channels for Dimethyl Ether Carbonylation. Materials. 2024; 17(10):2417. https://doi.org/10.3390/ma17102417
Chicago/Turabian StyleLiu, Wenrong, Yaquan Wang, Lingzhen Bu, Kailiang Chu, Yitong Huang, Niandong Guo, Liping Qu, Juncai Sang, Xuemei Su, Xian Zhang, and et al. 2024. "Preparation of Fe-HMOR with a Preferential Iron Location in the 12-MR Channels for Dimethyl Ether Carbonylation" Materials 17, no. 10: 2417. https://doi.org/10.3390/ma17102417
APA StyleLiu, W., Wang, Y., Bu, L., Chu, K., Huang, Y., Guo, N., Qu, L., Sang, J., Su, X., Zhang, X., & Li, Y. (2024). Preparation of Fe-HMOR with a Preferential Iron Location in the 12-MR Channels for Dimethyl Ether Carbonylation. Materials, 17(10), 2417. https://doi.org/10.3390/ma17102417







