Progress Made in Non-Metallic-Doped Materials for Electrocatalytic Reduction in Ammonia Production
Abstract
1. Introduction
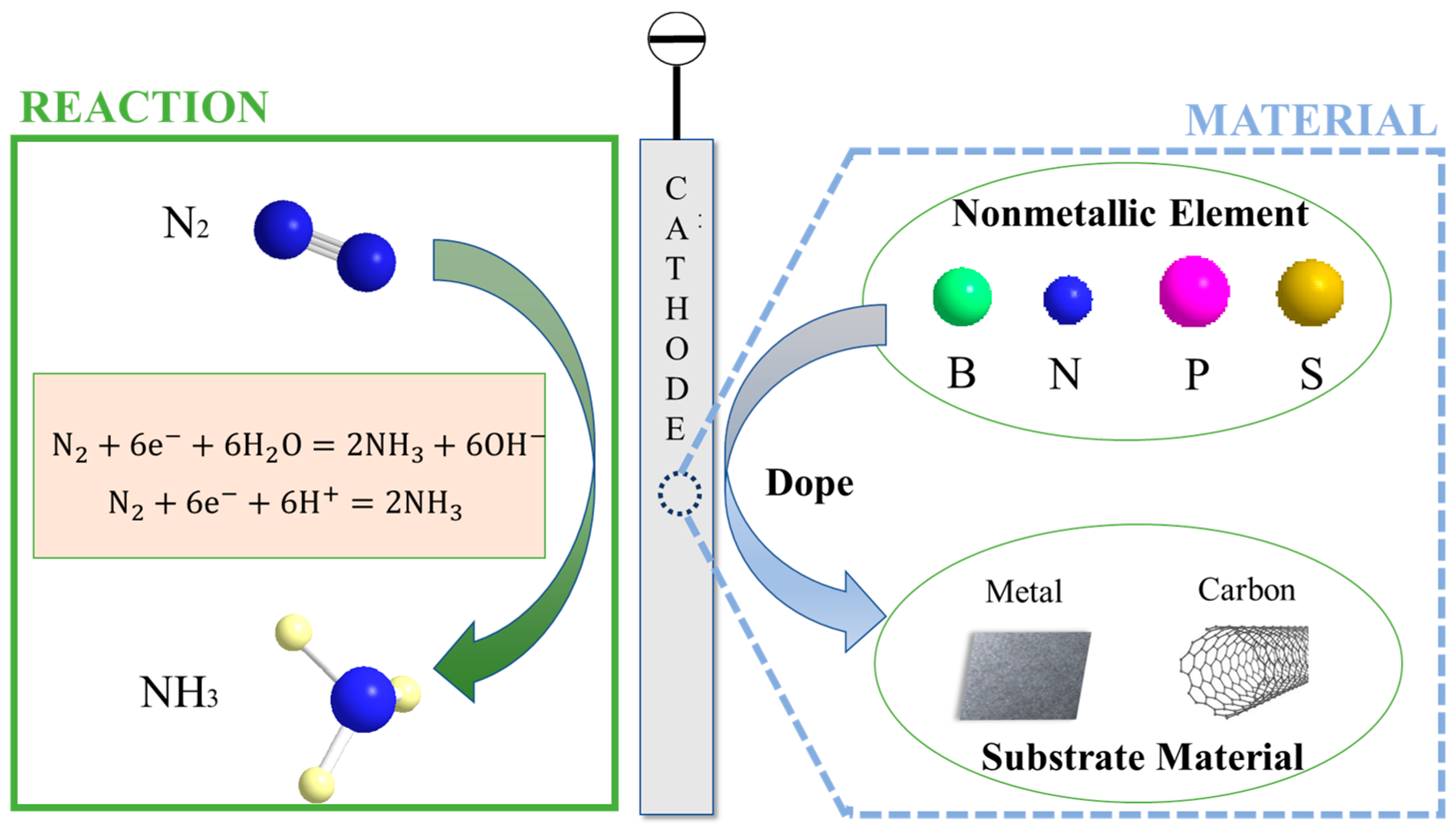
2. Construction Principle of Non-Metallic-Element-Doped Catalyst
2.1. Defect Engineering
2.2. Post-Synthesis Doping Strategies
2.3. In Situ Doping Methods
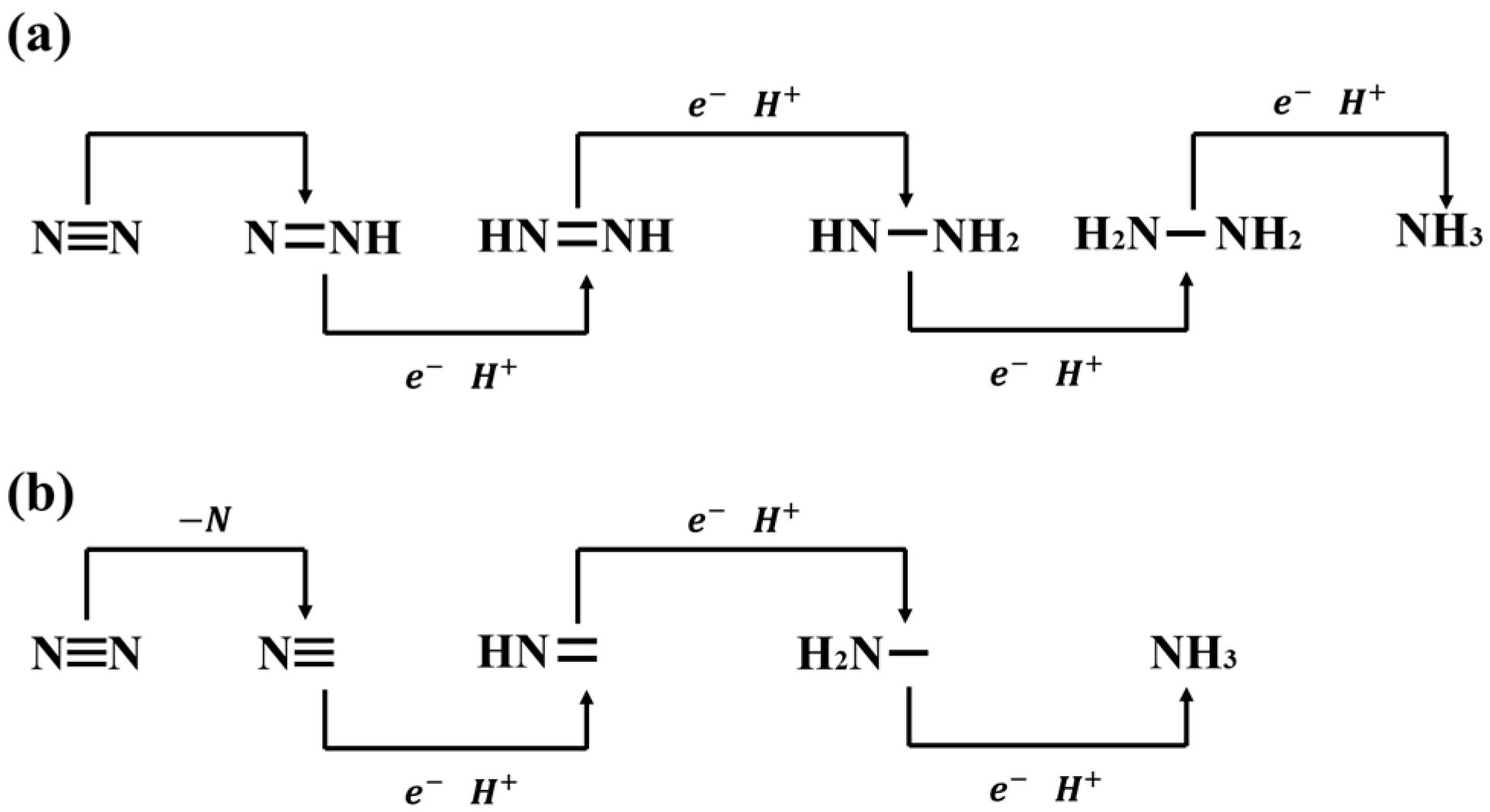
3. Regulation of Nitrogen Reduction by Non-Metallic-Doped Catalysts
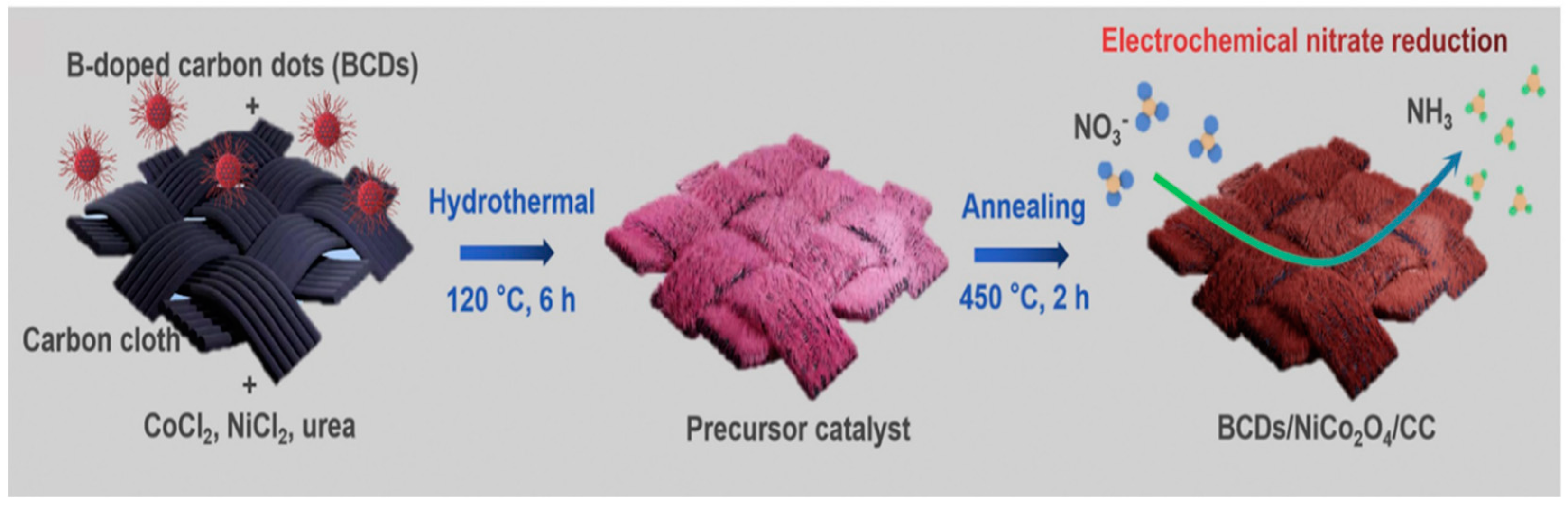
3.1. Boron-Doped (B-Doped) Catalysts
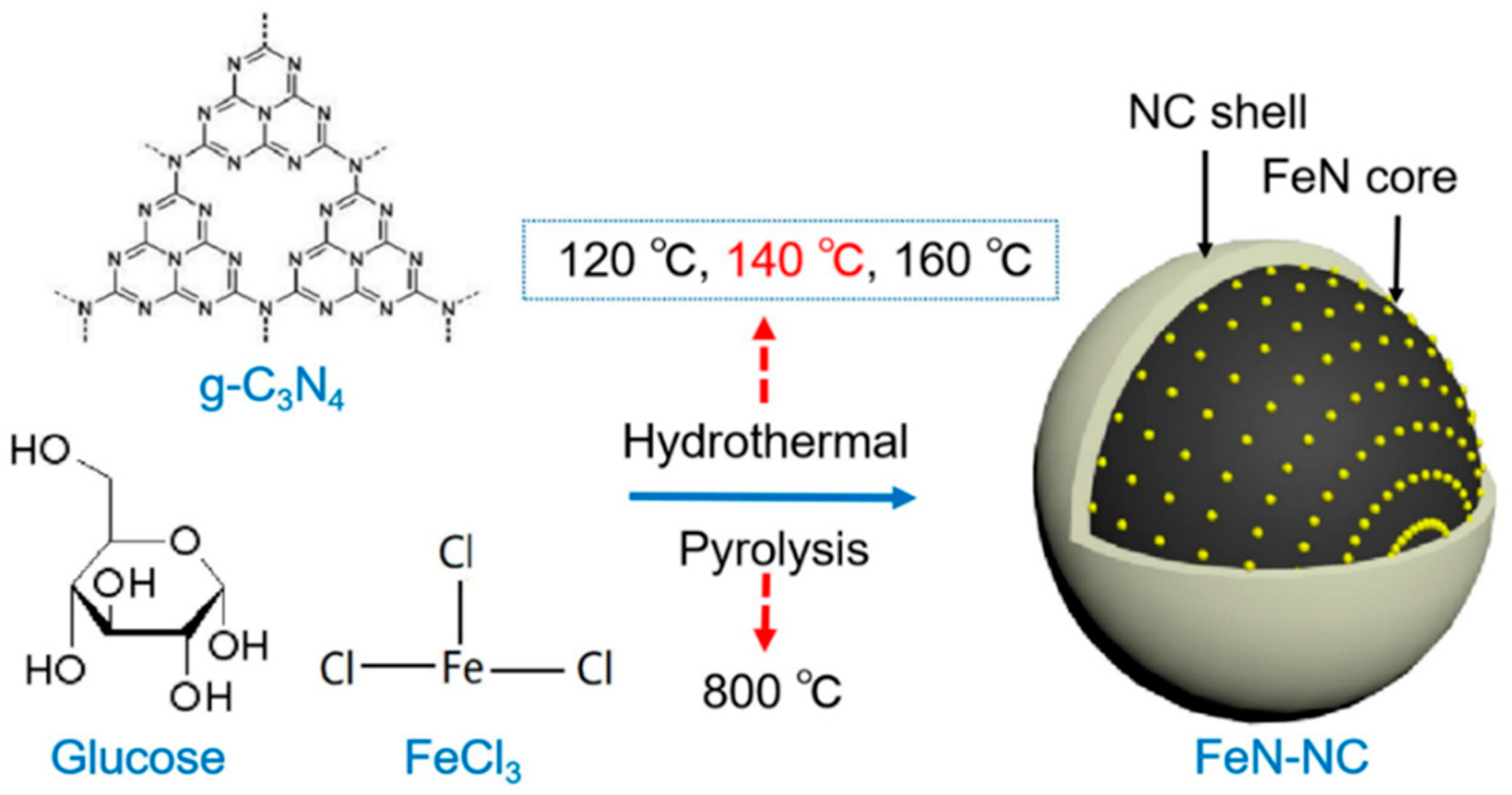
3.2. Nitrogen-Doped Catalysts
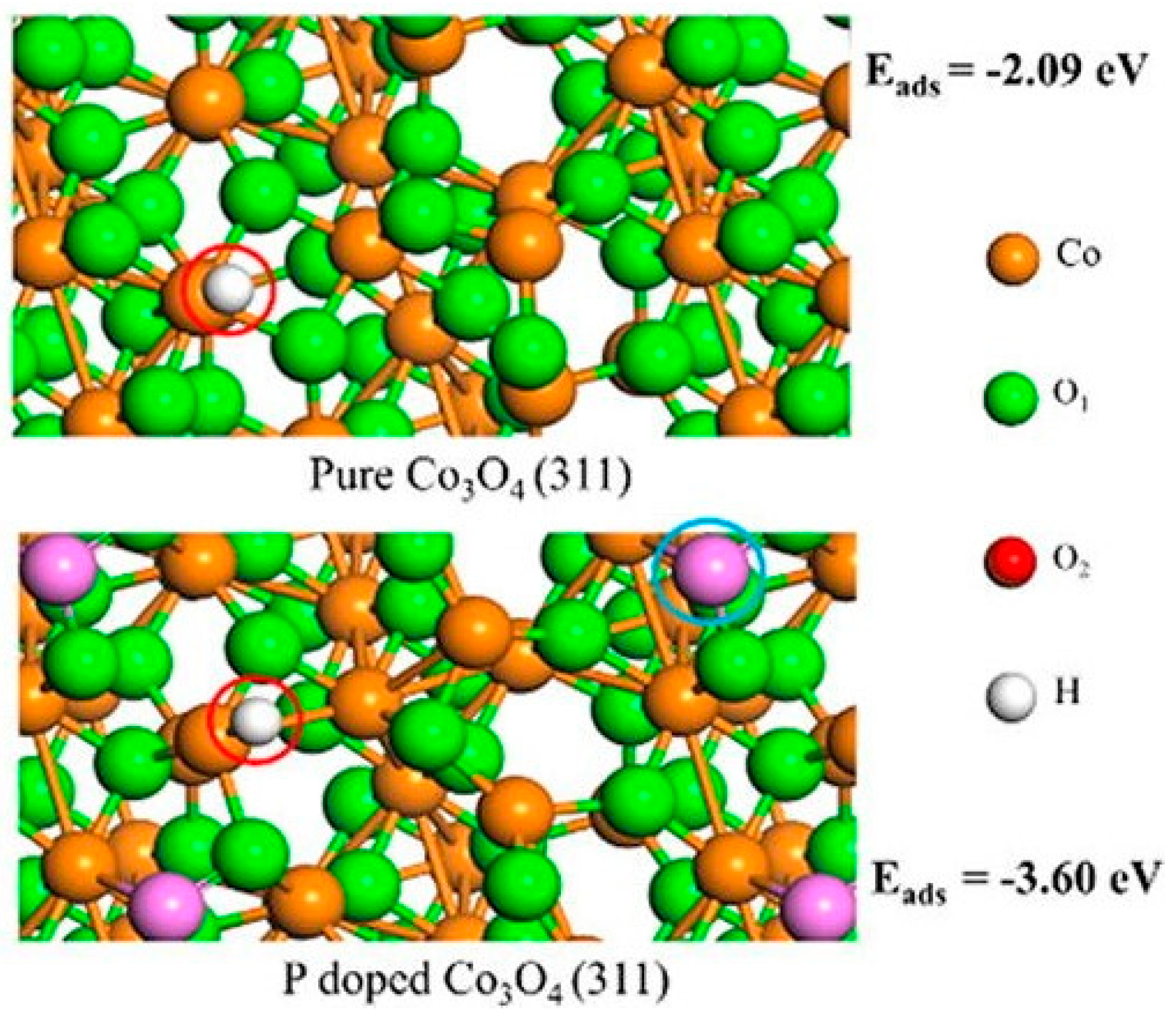
3.3. Phosphorous-Doped Catalysts
3.4. Sulfur-Doped Catalysts
4. Nitrogen Synthesis of Ammonia Using Non-Metal-Doped Catalysts
5. Synthesizing and Activating Biomass-Derived Carbon Materials for NH3 Production
6. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| NV | Nitrogen vacancies |
| B | Boron |
| P | Phosphorus |
| S | Sulfur |
| NH3 | Ammonia |
| NO3− | Nitrate |
| MOFs | Metal–organic frameworks |
| HER | Hydrogen evolution reaction |
| CVD | Chemical vapor deposition |
| HTC | Hydrothermal carbonization |
| ORR | Oxygen reduction reaction |
| GO | Graphene oxide |
| NG | Graphene sheets |
| N-RGO | N-doped reduced graphene oxide |
| CNTs | Carbon nanotubes |
| N-CNFs | N-doped carbon nanofibers |
| NRRs | Nitrogen reduction reactions |
| HER | Hydrogen evolution reaction |
| FE | Faradaic efficiency |
| O-KFCNTs | Hollow carbon microtubes |
| NMP | N-methyl-2-pyrrolidone |
| g | C3N4 porous polymeric carbon nitride |
| C2H4N4 | Dicyandiamide |
| C3H7NO | Dimethylformamide |
| C3H6N6 | Melamine |
| BCMs | Biomass-derived carbon materials |
References
- Tao, W.; Wang, P.; Hu, B.; Wang, X.; Zhou, G. Accelerating the reaction kinetics from nitrate to ammonia by anion substitution in NiCo-based catalysts. J. Environ. Chem. Eng. 2023, 11, 109117. [Google Scholar] [CrossRef]
- Zhai, G.-Y.; Li, Q.-Y.; Zhang, S.-N.; Xu, D.; Xia, S.-Y.; Gao, P.; Lin, X.; Lin, Y.-X.; Cheng, J.-H.; Hu, W.-Y.; et al. Accelerating the Activation of NOx−on Ru Nanoparticles for Ammonia Production by Tuning Their Electron Deficiency. CCS Chem. 2022, 4, 3455–3462. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, W.; Lu, X.; Zhong, B.; Guo, Y.; Lu, X.; Zhao, Y.; He, W.; Wang, S.; Zhang, X.; et al. Exploring global changes in agricultural ammonia emissions and their contribution to nitrogen deposition since 1980. Proc. Natl. Acad. Sci. USA 2022, 119, e2121998119. [Google Scholar] [CrossRef] [PubMed]
- Mehr, H.G.; Torabiardekani, N.; Rahimpour, M.R. Chapter eleven—Ammonia application in fabric, textile, and leather products. In Progresses in Ammonia: Science, Technology and Membranes; Basile, A., Rahimpour, M.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 223–239. [Google Scholar]
- Fan, K.; Xie, W.; Li, J.; Sun, Y.; Xu, P.; Tang, Y.; Li, Z.; Shao, M. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 2022, 13, 7958. [Google Scholar] [CrossRef] [PubMed]
- Yüzbaşıoğlu, A.E.; Avşar, C.; Gezerman, A.O. The current situation in the use of ammonia as a sustainable energy source and its industrial potential. Curr. Res. Green Sustain. Chem. 2022, 5, 100307. [Google Scholar] [CrossRef]
- Shahed Gharahshiran, V.; Zheng, Y. Sustainable ammonia synthesis: An in-depth review of non-thermal plasma technologies. J. Energy Chem. 2024, 96, 1–38. [Google Scholar] [CrossRef]
- Wu, T.; Fan, W.; Zhang, Y.; Zhang, F. Electrochemical synthesis of ammonia: Progress and challenges. Mater. Today Phys. 2021, 16, 100310. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, J.; Peng, Y.; Lin, X.; Lv, X.; Ye, Q.; Liu, S.; Wu, A.; Li, X. Ar-plasma enhanced copper-nickel alloy catalysis for ammonia synthesis. Waste Dispos. Sustain. Energy 2022, 4, 149–155. [Google Scholar]
- Shan, Y.; Huang, Q.; Guan, D.; Hubacek, K. China CO2 emission accounts 2016–2017. Sci. Data 2020, 7, 54. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, C.; Yu, Y.; Lu, S.; Zhang, B. Recent advances in non-noble metal electrocatalysts for nitrate reduction. Chem. Eng. J. 2021, 403, 126269. [Google Scholar] [CrossRef]
- Yuan, S.; Xue, Y.; Ma, R.; Ma, Q.; Chen, Y.; Fan, J. Advances in iron-based electrocatalysts for nitrate reduction. Sci. Total Environ. 2023, 866, 161444. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rafiq, M.; Woldu, A.R.; Tong, Q.-X.; Astruc, D.; Hu, L. Recent progress in electrocatalytic nitrogen reduction to ammonia (NRR). Coord. Chem. Rev. 2023, 478, 214981. [Google Scholar] [CrossRef]
- Davy, H.S.I. The Bakerian Lecture, on some chemical agencies of electricity. Philos. Trans. R. Soc. Lond. 1807, 31, 1–56. [Google Scholar]
- Martín, A.J.; Shinagawa, T.; Pérez-Ramírez, J. Electrocatalytic Reduction of Nitrogen: From Haber-Bosch to Ammonia Artificial Leaf. Chem 2019, 5, 263–283. [Google Scholar] [CrossRef]
- Fichter, F.; Suter, R. Zur Frage der kathodischen Reduktion des elementaren Stickstoffs. Helv. Chim. Acta 2004, 5, 246–255. [Google Scholar] [CrossRef]
- Tamelen, E.E.v.; Åkermark, B. Electrolytic reduction of molecular nitrogen. J. Am. Chem. Soc. 1968, 90, 4492–4493. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, C.; Tan, X.; Huang, H.; Wei, Q.; Qiu, J. Strategies to suppress hydrogen evolution for highly selective electrocatalytic nitrogen reduction: Challenges and perspectives. Energy Environ. Sci. 2021, 14, 1176–1193. [Google Scholar] [CrossRef]
- Tsuneto, A.; Kudo, A.; Sakata, T. Lithium-mediated electrochemical reduction of high pressure N2 to NH3. J. Electroanal. Chem. 1994, 367, 183–188. [Google Scholar] [CrossRef]
- Rod, T.H.; Logadottir, A.; Nørskov, J.K. Ammonia synthesis at low temperatures. J. Chem. Phys. 2000, 112, 5343–5347. [Google Scholar] [CrossRef]
- Kordali, V.; Kyriacou, G.; Lambrou, C. Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell. Chem. Commun. 2000, 6, 1673–1674. [Google Scholar] [CrossRef]
- Köleli, F.; Röpke, T. Electrochemical hydrogenation of dinitrogen to ammonia on a polyaniline electrode. Appl. Catal. B Environ. 2006, 62, 306–310. [Google Scholar] [CrossRef]
- Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef]
- Azofra, L.M.; Li, N.; MacFarlane, D.R.; Sun, C. Promising prospects for 2D d2–d4 M3C2 transition metal carbides (MXenes) in N2 capture and conversion into ammonia. Energy Environ. Sci. 2016, 9, 2545–2549. [Google Scholar] [CrossRef]
- Abghoui, Y.; Skúlason, E. Onset potentials for different reaction mechanisms of nitrogen activation to ammonia on transition metal nitride electro-catalysts. Catal. Today 2017, 286, 69–77. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Xie, X.-Y.; Li, X.; Ma, Y.; Liu, Q.; Fang, W.-H.; Shi, X.; Cui, G.; Sun, X. Ti3C2Tx (T = F, OH) MXene nanosheets: Conductive 2D catalysts for ambient electrohydrogenation of N2 to NH3. J. Mater. Chem. A 2018, 6, 24031–24035. [Google Scholar] [CrossRef]
- Du, H.L.; Chatti, M.; Hodgetts, R.Y.; Cherepanov, P.V.; Nguyen, C.K.; Matuszek, K.; MacFarlane, D.R.; Simonov, A.N. Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency. Nature 2022, 609, 722–727. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, D.; Deng, Z.; Zhang, L.; Li, J.; Li, Z.; Sun, S.; Luo, Y.; Zheng, D.; Wang, Y.; et al. Constructing Co@TiO2 Nanoarray Heterostructure with Schottky Contact for Selective Electrocatalytic Nitrate Reduction to Ammonia. Small 2023, 19, e2208036. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Chen, S.; Peng, F.; Gao, F. Boosting Electrochemical Reduction of Nitrate to Ammonia by Constructing Nitrate-Favored Active Cu–B Sites on SnS2. Small 2024, 20, 2308182. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, G.; Tian, Y.; Tang, Y.; Chu, K. FeS2 nanoparticles on reduced graphene oxide: An efficient electrocatalyst for nitrate electroreduction to ammonia. Dalton Trans. 2022, 51, 16805–16810. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, G.-k.; Shen, P.; Zhang, H.; Ma, D.; Chu, K. Lewis Acid Fe-V Pairs Promote Nitrate Electroreduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2211537. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Li, J.; Song, Q.; Liu, X. N-doped carbon–iron heterointerfaces for boosted electrocatalytic active and selective ammonia production. Proc. Natl. Acad. Sci. USA 2023, 120, e2207080119. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xie, K.; Xie, J.; Wang, X.; Zhang, H.; Chen, S.-P.; Wang, H.; Li, Z.; Li, C. Alloying of Cu with Ru Enabling the Relay Catalysis for Reduction of Nitrate to Ammonia. Adv. Mater. 2023, 35, e2202952. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, M.; Yang, Y.; Luo, R.; Liu, W.; Zhang, F.; Tang, C.; Yang, G.; Zhou, Y. Self-Supported Pd Nanorod Arrays for High-Efficient Nitrate Electroreduction to Ammonia. Small 2023, 19, e2207743. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, Z.; Li, X.; Kang, J.; Ma, D.; Chu, K. Single-Atom Bi Alloyed Pd Metallene for Nitrate Electroreduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2209890. [Google Scholar] [CrossRef]
- Pan, F.; Zhou, J.; Wang, T.; Zhu, Y.; Ma, H.; Niu, J.; Wang, C. Revealing the activity origin of ultrathin nickel metal–organic framework nanosheet catalysts for selective electrochemical nitrate reduction to ammonia: Experimental and density functional theory investigations. J. Colloid Interface Sci. 2023, 638, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Zagalskaya, A.; Ng, J.L.; Hong, J.; Alexandrov, V.; Pham, T.A.; Su, X. Coupling nitrate capture with ammonia production through bifunctional redox-electrodes. Nat. Commun. 2023, 14, 823. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Z.; Zhang, G.; Wang, Y. On-Demand Atomic Hydrogen Provision by Exposing Electron-Rich Cobalt Sites in an Open-Framework Structure toward Superior Electrocatalytic Nitrate Conversion to Dinitrogen. Environ. Sci. Technol. 2022, 56, 614–623. [Google Scholar] [CrossRef]
- Xiong, R.; Xu, B.; Chen, Z.; Cheng, C.; Wang, Y. Self-supporting electrode incorporating active Co sites for ultrafast ammonia production from nitrate reduction. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133557. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J.; Wang, Y.; Yu, C.; Zhou, X.; Xu, B.; László, K.; Li, F.; Zhang, W. Selective electrocatalytic reduction of nitrate to dinitrogen by Cu2O nanowires with mixed oxidation-state. Chem. Eng. J. 2022, 433, 133495. [Google Scholar] [CrossRef]
- Hu, Q.; Qin, Y.J.; Wang, X.D.; Zheng, H.J.; Gao, K.R.; Yang, H.P.; Zhang, P.X.; Shao, M.H.; He, C.X. Grain Boundaries Engineering of Hollow Copper Nanoparticles Enables Highly Efficient Ammonia Electrosynthesis from Nitrate. Ccs Chem. 2022, 4, 2053–2064. [Google Scholar] [CrossRef]
- Chanda, D.; Xing, R.M.; Xu, T.; Liu, Q.; Luo, Y.L.; Liu, S.H.; Tufa, R.A.; Dolla, T.H.; Montini, T.; Sun, X.P. Electrochemical nitrogen reduction: Recent progress and prospects. Chem. Commun. 2021, 57, 7335–7349. [Google Scholar] [CrossRef]
- Lin, J.; Xu, T.; Qi, J. Chapter 6—Defect engineering for modifying transition metal oxides. In Metal Oxides and Related Solids for Electrocatalytic Water Splitting; Qi, J., Korotcenkov, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–190. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, Y.; Xie, M.; Jin, Z.; Li, P.; Yu, G. Structural engineering of catalysts for ammonia electrosynthesis from nitrate: Recent advances and challenges. EES Catal. 2024, 2, 202–219. [Google Scholar] [CrossRef]
- Yu, S.; Xiang, T.; Alharbi, N.S.; Al-aidaroos, B.A.; Chen, C. Recent development of catalytic strategies for sustainable ammonia production. Chin. J. Chem. Eng. 2023, 62, 65–113. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, H.; Yang, X.; Xue, S.; Liang, Z.; Ren, X.; Liu, A.; Wu, G. Recent Advances in Designing Efficient Electrocatalysts for Electrochemical Nitrate Reduction to Ammonia. Small Struct. 2022, 4, 2200202. [Google Scholar] [CrossRef]
- Huang, Y.; Long, J.; Wang, Y.; Meng, N.; Yu, Y.; Lu, S.; Xiao, J.; Zhang, B. Engineering Nitrogen Vacancy in Polymeric Carbon Nitride for Nitrate Electroreduction to Ammonia. ACS Appl. Mater. Interfaces 2021, 13, 54967–54973. [Google Scholar] [CrossRef]
- Zhang, M.; Choi, C.; Huo, R.; Gu, G.H.; Hong, S.; Yan, C.; Xu, S.; Robertson, A.W.; Qiu, J.; Jung, Y.; et al. Reduced graphene oxides with engineered defects enable efficient electrochemical reduction of dinitrogen to ammonia in wide pH range. Nano Energy 2020, 68, 104323. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-doped carbon materials as a promising platform toward the efficient catalysis for hydrogen generation. Appl. Catal. A Gen. 2019, 571, 25–41. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.-S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, S.; Han, M.; Zhang, X.; Liu, Y.; Li, W.; Chen, C.; Wang, G.; Zhang, H.; Zhao, H. Ambient Electrosynthesis of Ammonia on a Biomass-Derived Nitrogen-Doped Porous Carbon Electrocatalyst: Contribution of Pyridinic Nitrogen. ACS Energy Lett. 2019, 4, 377–383. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Zhao, S.; Wu, J.; Li, F.; Tian, M.; Long, X.; Jin, J.; Ma, J. Nitrogen-doped mesoporous carbon nanosheet/carbon nanotube hybrids as metal-free bi-functional electrocatalysts for water oxidation and oxygen reduction. J. Mater. Chem. A 2016, 4, 13133–13141. [Google Scholar] [CrossRef]
- Mandal, B.; Saha, S.; Das, D.; Panda, J.; Das, S.; Sarkar, R.; Tudu, B. Supercapacitor performance of nitrogen doped graphene synthesized via DMF assisted single-step solvothermal method. FlatChem 2022, 34, 100400. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Chen, X.; Fang, Y.; Chen, H. Biomass pyrolysis for nitrogen-containing liquid chemicals and nitrogen-doped carbon materials. J. Anal. Appl. Pyrolysis 2016, 120, 186–193. [Google Scholar] [CrossRef]
- Chen, H.; Ding, L.; Zhang, K.; Chen, Z.; Lei, Y.; Zhou, Z.; Hou, R. Preparation of chemically reduced graphene using hydrazine hydrate as the reduction agent and its NO2 sensitivity at room temperature. Int. J. Electrochem. Sci. 2020, 15, 10231–10242. [Google Scholar] [CrossRef]
- Moorthy, M.; Kumar, V.B.; Porat, Z.e.; Gedanken, A. Novel polymerization of aniline and pyrrole by carbon dots. New J. Chem. 2018, 42, 535–540. [Google Scholar] [CrossRef]
- Guo, H.-L.; Su, P.; Kang, X.; Ning, S.-K. Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J. Mater. Chem. A 2013, 1, 2248–2255. [Google Scholar] [CrossRef]
- González-Gaitán, C.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Functionalization of carbon nanotubes using aminobenzene acids and electrochemical methods. Electroactivity for the oxygen reduction reaction. Int. J. Hydrogen Energy 2015, 40, 11242–11253. [Google Scholar] [CrossRef]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Recent progress in nitrogen-doped carbon and its composites as electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 9370–9386. [Google Scholar] [CrossRef]
- Mohan, T.V.R.; Nallagangula, M.; Kala, K.; Hernandez-Tamargo, C.E.; De Leeuw, N.H.; Namitharan, K.; Bhat, V.T.; Sasidharan, M.; Selvam, P. Pyridinic-nitrogen on ordered mesoporous carbon: A versatile NAD(P)H mimic for borrowing-hydrogen reactions. J. Catal. 2023, 419, 80–98. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Lu, J.; Bo, X.; Wang, H.; Guo, L. Nitrogen-doped ordered mesoporous carbons synthesized from honey as metal-free catalyst for oxygen reduction reaction. Electrochim. Acta 2013, 108, 10–16. [Google Scholar] [CrossRef]
- Wei, X.; Wang, M.-S.; Bando, Y.; Golberg, D. Post-Synthesis Carbon Doping of Individual Multiwalled Boron Nitride Nanotubes via Electron-Beam Irradiation. J. Am. Chem. Soc. 2010, 132, 13592–13593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Kato, Y.; Al-zubaidi, A.; Yamamoto, K.; Kawasaki, S. Effect of post-synthesis nitrogen doping in nanocarbons on cathode reaction of metal-air cells. Mater. Express 2014, 4, 337–342. [Google Scholar] [CrossRef]
- Milliken, S.; Cheong, T.; Cui, K.; Veinot, J.G.C. Post-Synthesis Boron Doping of Silicon Quantum Dots via Hydrosilsesquioxane-Capped Thermal Diffusion. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Kim, Y.; Ryu, J.; Park, M.; Kim, E.S.; Yoo, J.M.; Park, J.; Kang, J.H.; Hong, B.H. Vapor-phase molecular doping of graphene for high-performance transparent electrodes. ACS Nano 2014, 8, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lu, Z.; Shan, G.; Chen, Y. Solvothermal Fabrication of Nitrogen-Doped Carbon Nanoparticles as Efficient Catalyst for Oxygen Reduction in KOH Electrolyte. ChemistrySelect 2017, 2, 5390–5393. [Google Scholar] [CrossRef]
- Dlamini, M.W.; Phaahlamohlaka, T.N.; Kumi, D.O.; Forbes, R.; Jewell, L.L.; Coville, N.J. Post doped nitrogen-decorated hollow carbon spheres as a support for Co Fischer-Tropsch catalysts. Catal. Today 2020, 342, 99–110. [Google Scholar] [CrossRef]
- Han, Q.; Wu, C.; Jiao, H.; Xu, R.; Wang, Y.; Xie, J.; Guo, Q.; Tang, J. Rational Design of High-Concentration Ti3+ in Porous Carbon-Doped TiO2 Nanosheets for Efficient Photocatalytic Ammonia Synthesis. Adv. Mater. 2021, 33, 2008180. [Google Scholar] [CrossRef] [PubMed]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Mainali, K.; Mood, S.H.; Pelaez-Samaniego, M.R.; Sierra-Jimenez, V.; Garcia-Perez, M. Production and applications of N-doped carbons from bioresources: A review. Catal. Today 2023, 423, 114248. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pašti, I.A.; Mitrić, M.; Travas-Sejdić, J.; Ćirić-Marjanović, G.; Mentus, S.V. Electrocatalysis of oxygen reduction reaction on polyaniline-derived nitrogen-doped carbon nanoparticle surfaces in alkaline media. J. Power Sources 2012, 220, 306–316. [Google Scholar] [CrossRef]
- Saha, D.; Kienbaum, M.J. Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous Mesoporous Mater. 2019, 287, 29–55. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Wang, H.; Yuan, J. Advanced Heteroatom-Doped Porous Carbon Membranes Assisted by Poly(ionic liquid) Design and Engineering. Acc. Mater. Res. 2020, 1, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Payattikul, L.; Chen, C.-Y.; Chen, Y.-S.; Raja Pugalenthi, M.; Punyawudho, K. Recent Advances and Synergistic Effects of Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts: A Review. Molecules 2023, 28, 7751. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cao, P.; Chen, W.; Ezeh, C.I.; Chen, Z.; Luo, Y.; Liu, Q.; Zhao, H.; Rui, Z.; Gao, S.; et al. Electrocatalysis enabled transformation of earth-abundant water, nitrogen and carbon dioxide for a sustainable future. Mater. Adv. 2022, 3, 1359–1400. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, K.; Cheng, B.-H.; Jiang, H. Preparation of N-Doped Supercapacitor Materials by Integrated Salt Templating and Silicon Hard Templating by Pyrolysis of Biomass Wastes. ACS Sustain. Chem. Eng. 2017, 5, 6682–6691. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Wang, K.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. In-situ synthesis of graphene/nitrogen-doped ordered mesoporous carbon nanosheet for supercapacitor application. Carbon 2016, 96, 955–964. [Google Scholar] [CrossRef]
- Suboch, A.N.; Podyacheva, O.Y. Pd Catalysts Supported on Bamboo-Like Nitrogen-Doped Carbon Nanotubes for Hydrogen Production. Energies 2021, 14, 1501. [Google Scholar] [CrossRef]
- Wang, B.; Liu, B.; Dai, L. Non-N-Doped Carbons as Metal-Free Electrocatalysts. Adv. Sustain. Syst. 2020, 5, 2000134. [Google Scholar] [CrossRef]
- Lu, L.; Cao, X.; Shen, Z.; Li, L.; Huo, J.; Chen, W.; Liu, C.; Liu, H. Electrospun nitrogen-doped carbon nanofibers for electrocatalysis. Sustain. Mater. Technol. 2020, 26, e00221. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, K.; Pan, Z.; Huang, Z.; Xu, Y.; Hu, G.; Wu, S.; Chen, C.; Lin, L.; Lin, Y. Research on a novel Ni-doped TiN modified N-doped CNTs supported Pt catalysts and their synergistic effect for methanol electrooxidation. Int. J. Hydrogen Energy 2018, 43, 22519–22528. [Google Scholar] [CrossRef]
- Tong, X.; Chen, Z.; Zhuo, H.; Hu, Y.; Jing, S.; Liu, J.; Zhong, L. Tailoring the physicochemical properties of chitosan-derived N-doped carbon by controlling hydrothermal carbonization time for high-performance supercapacitor application. Carbohydr. Polym. 2019, 207, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Hamsa, A.P.; Arulprakasam, M.; Unni, S.M. Electrochemical nitrogen fixation on single metal atom catalysts. Chem. Commun. 2023, 59, 10689–10710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, Q.; Chen, Z.; Jose, V.; Jiang, X.; Fu, G.; Lee, J.-M.; Huang, S. B, N-doped ultrathin carbon nanosheet superstructure for high-performance oxygen reduction reaction in rechargeable zinc-air battery. Carbon 2020, 164, 398–406. [Google Scholar] [CrossRef]
- Wang, J.; Shi, M.; Yi, G.; Meng, J.; Li, Q. Computational identification of B substitutional doped C9N4 monolayer for electrocatalytic N2 reduction. Mol. Catal. 2021, 511, 111726. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; He, C.; Ren, X.; Zhang, P.; Mi, H. Recent Progress in 2D Catalysts for Photocatalytic and Electrocatalytic Artificial Nitrogen Reduction to Ammonia. Adv. Energy Mater. 2021, 11, 2003294. [Google Scholar] [CrossRef]
- Lu, X.; Yu, J.; Cai, J.; Zhang, Q.; Yang, S.; Gu, L.; Waterhouse, G.I.N.; Zang, S.-Q.; Yang, B.; Lu, S. Exclusive nitrate to ammonia conversion via boron-doped carbon dots induced surface Lewis acid sites. Cell Rep. Phys. Sci. 2022, 3, 100961. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, Z.-N.; Fu, M.-L. Boron-doped activated carbon catalyzed reduction of dilute nitrite acid in oxidative esterification reaction in the coal to ethylene glycol process. J. Environ. Chem. Eng. 2022, 10, 107932. [Google Scholar] [CrossRef]
- Gou, F.; Wang, H.; Fu, M.; Jiang, Y.; Shen, W.; He, R.; Li, M. Boron-induced electron localization in Cu nanowires promotes efficient nitrate reduction to ammonia in neutral media. Appl. Surf. Sci. 2023, 612, 155872. [Google Scholar] [CrossRef]
- Gou, Q.; Mao, Y.; Lv, S.; Gou, F.; Jiang, Y.; Shen, W.; Li, M.; Wang, Y.; He, R. Enhancing nitrate reduction to ammonia by synergistic and interface coupling effects of binary metal sites. Appl. Catal. B Environ. Energy 2024, 348, 123810. [Google Scholar] [CrossRef]
- Kuang, P.; Natsui, K.; Feng, C.; Einaga, Y. Electrochemical reduction of nitrate on boron-doped diamond electrodes: Effects of surface termination and boron-doping level. Chemosphere 2020, 251, 126364. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Y.; Li, Y.; Zhang, Y.; Huang, S.; Lin, W.; Ding, K. Whether Corrugated or Planar Vacancy Graphene-like Carbon Nitride (g-C3N4) Is More Effective for Nitrogen Reduction Reaction? J. Phys. Chem. C 2019, 123, 17296–17305. [Google Scholar] [CrossRef]
- Majumder, M.; Saini, H.; Dědek, I.; Schneemann, A.; Chodankar, N.R.; Ramarao, V.; Santosh, M.S.; Nanjundan, A.K.; Kment, Š.; Dubal, D.; et al. Rational Design of Graphene Derivatives for Electrochemical Reduction of Nitrogen to Ammonia. ACS Nano 2021, 15, 17275–17298. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Zhi, Q.; Gong, L.; Sun, H.; Liu, M.; Zhang, J.; Han, X.; Xia, Z.; Zhang, L. Rational design of boron-containing co-doped graphene as highly efficient electro-catalysts for the nitrogen reduction reaction. J. Mater. Chem. A 2021, 9, 24590–24599. [Google Scholar] [CrossRef]
- Singh, S.; Nguyen, T.D.; Siang, T.J.; Phuong, P.T.T.; Huy Phuc, N.H.; Truong, Q.D.; Lam, S.S.; Vo, D.-V.N. Boron-doped Ni/SBA-15 catalysts with enhanced coke resistance and catalytic performance for dry reforming of methane. J. Energy Inst. 2020, 93, 31–42. [Google Scholar] [CrossRef]
- Wang, F.; Xia, L.; Li, X.; Yang, W.; Zhao, Y.; Mao, J. Nano-Ferric Oxide Embedded in Graphene Oxide: High-performance Electrocatalyst for Nitrogen Reduction at Ambient Condition. Energy Environ. Mater. 2020, 4, 88–94. [Google Scholar] [CrossRef]
- Yan, X.; Liu, D.; Cao, H.; Hou, F.; Liang, J.; Dou, S.X. Nitrogen Reduction to Ammonia on Atomic-Scale Active Sites under Mild Conditions. Small Methods 2019, 3, 1800501. [Google Scholar] [CrossRef]
- Peng, X.; Wang, C.; Tan, Z.; Ni, J.; Lin, B.; Lin, J.; Wang, X.; Zheng, L.; Au, C.-t.; Jiang, L. N-Induced Electron Transfer Effect on Low-Temperature Activation of Nitrogen for Ammonia Synthesis over Co-Based Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 1529–1539. [Google Scholar] [CrossRef]
- Nichols, F.; Liu, Q.; Sandhu, J.; Azhar, Z.; Cazares, R.; Mercado, R.; Bridges, F.; Chen, S. Platinum-complexed phosphorous-doped carbon nitride for electrocatalytic hydrogen evolution. J. Mater. Chem. A 2022, 10, 5962–5970. [Google Scholar] [CrossRef]
- Rafeeq, H.; Hussain, A.; Ambreen, A.; Zill e, H.; Waqas, M.; Bilal, M.; Iqbal, H.M.N. Functionalized nanoparticles and their environmental remediation potential: A review. J. Nanostructure Chem. 2022, 12, 1007–1031. [Google Scholar] [CrossRef]
- Sun, J.; Lu, J.; Huang, C.; Wu, Q.; Xia, L.; Xu, Q.; Yao, W. Modification of Ni3N with a Cobalt-Doped Carbon Shell for High-Performance Hydrogen Evolution in Alkaline Media. ACS Sustain. Chem. Eng. 2021, 9, 1994–2002. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Garcia, M.A.S.; Gothe, M.L.; Galvan, D.; Troise, P.C.; Conte-Junior, C.A.; Vidinha, P.; Camargo, P.H.C.; Rossi, L.M. Recent advances in the use of nitrogen-doped carbon materials for the design of noble metal catalysts. Coord. Chem. Rev. 2023, 481, 215053. [Google Scholar] [CrossRef]
- Rossi, L.M.; Fiorio, J.L.; Garcia, M.A.S.; Ferraz, C.P. The role and fate of capping ligands in colloidally prepared metal nanoparticle catalysts. Dalton Trans. 2018, 47, 5889–5915. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, H.; Dai, S. ChemInform Abstract: Porous Carbon Supports: Recent Advances with Various Morphologies and Compositions. ChemCatChem 2015, 7, 2788–2805. [Google Scholar] [CrossRef]
- Yang, N.; Li, L.; Li, J.; Wei, Z. Modifying the sensibility of nonmetal-doped phosphorene by local or global properties. Phys. Chem. Chem. Phys. 2019, 21, 4899–4906. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shen, Z.; Jiao, L.; Yang, L.; Wang, X.; Wu, Q.; Hu, Z. Tuning metal catalysts via nitrogen-doped nanocarbons for energy chemistry: From metal nanoparticles to single metal sites. EnergyChem 2021, 3, 100066. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Xu, G.; Li, H.; Bati, A.S.R.; Bat-Erdene, M.; Nine, M.J.; Losic, D.; Chen, Y.; Shapter, J.G.; Batmunkh, M.; Ma, T. Nitrogen-doped phosphorene for electrocatalytic ammonia synthesis. J. Mater. Chem. A 2020, 8, 15875–15883. [Google Scholar] [CrossRef]
- Batmunkh, M.; Myekhlai, M.; Bati, A.S.R.; Sahlos, S.; Slattery, A.D.; Benedetti, T.M.; Gonçales, V.R.; Gibson, C.T.; Gooding, J.J.; Tilley, R.D.; et al. Microwave-assisted synthesis of black phosphorus quantum dots: Efficient electrocatalyst for oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 12974–12978. [Google Scholar] [CrossRef]
- Gayen, P.; Spataro, J.; Avasarala, S.; Ali, A.-M.; Cerrato, J.M.; Chaplin, B.P. Electrocatalytic Reduction of Nitrate Using Magnéli Phase TiO2 Reactive Electrochemical Membranes Doped with Pd-Based Catalysts. Environ. Sci. Technol. 2018, 52, 9370–9379. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, B.P.; Shapley, J.R.; Werth, C.J. The Selectivity and Sustainability of a Pd–In/γ-Al2O3 Catalyst in a Packed-Bed Reactor: The Effect of Solution Composition. Catal. Lett. 2009, 130, 56–62. [Google Scholar] [CrossRef]
- Machida, M.; Sato, K.; Ishibashi, I.; Hasnat, M.A.; Ikeue, K. Electrocatalytic nitrate hydrogenation over an H+-conducting solid polymer electrolyte membrane–modified cathode assembly. Chem. Commun. 2006, 7, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Karim, M.R.; Machida, M. Electrocatalytic ammonia synthesis: Role of cathode materials and reactor configuration. Catal. Commun. 2009, 10, 1975–1979. [Google Scholar] [CrossRef]
- Gao, Z.; Lai, Y.; Tao, Y.; Xiao, L.; Zhang, L.; Luo, F. Constructing Well-Defined and Robust Th-MOF-Supported Single-Site Copper for Production and Storage of Ammonia from Electroreduction of Nitrate. ACS Cent. Sci. 2021, 7, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.-Y.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Jia, R.; Zhang, C.; Zhang, B. Electrochemical synthesis of nitric acid from air and ammonia through waste utilization. Natl. Sci. Rev. 2019, 6, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, X.; Shen, S.; Wang, G.; Zhang, J. Computational prediction and experimental evaluation of nitrate reduction to ammonia on rhodium. J. Catal. 2021, 402, 1–9. [Google Scholar] [CrossRef]
- Sun, J.; Gao, W.; Fei, H.; Zhao, G. Efficient and selective electrochemical reduction of nitrate to N2 by relay catalytic effects of Fe-Ni bimetallic sites on MOF-derived structure. Appl. Catal. B Environ. 2022, 301, 120829. [Google Scholar] [CrossRef]
- Qin, J.; Wu, K.; Chen, L.; Wang, X.; Zhao, Q.; Liu, B.; Ye, Z. Achieving high selectivity for nitrate electrochemical reduction to ammonia over MOF-supported RuxOy clusters. J. Mater. Chem. A 2022, 10, 3963–3969. [Google Scholar] [CrossRef]
- Jiang, M.; Su, J.; Song, X.; Zhang, P.; Zhu, M.; Qin, L.; Tie, Z.; Zuo, J.-L.; Jin, Z. Interfacial Reduction Nucleation of Noble Metal Nanodots on Redox-Active Metal–Organic Frameworks for High-Efficiency Electrocatalytic Conversion of Nitrate to Ammonia. Nano Lett. 2022, 22, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Liu, C.-Y.; Park, J.; Liu, Y.-H.; Senftle, T.P.; Lee, S.W.; Hatzell, M.C. Structure Sensitivity of Pd Facets for Enhanced Electrochemical Nitrate Reduction to Ammonia. ACS Catal. 2021, 11, 7568–7577. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, H.; Ding, L.-X.; Chen, S.; Zou, Y.; Chen, G.-F.; Wang, H. Selective Synthesis of Either Nitric Acid or Ammonia from Air by Electrolyte Regulation in a Plasma Electrolytic System. ACS Sustain. Chem. Eng. 2023, 11, 11737–11744. [Google Scholar] [CrossRef]
- Kong, J.; Kim, H.; Park, H.S. Electrochemical NH3 production: In-situ evaluation of the activity and durability of nitrogen-reduction catalysis using scanning electrochemical microscopy (SECM). Appl. Catal. B Environ. 2023, 338, 123019. [Google Scholar] [CrossRef]
- Wang, J.; Ling, L.; Deng, Z.; Zhang, W.-x. Nitrogen-doped iron for selective catalytic reduction of nitrate to dinitrogen. Sci. Bull. 2020, 65, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhao, F.; Wang, X.; Wang, Q.; Zhao, Q.; Li, J.; Liu, G. A phosphorus-doped potassium peroxyniobate electrocatalyst with enriched oxygen vacancies boosts electrocatalytic nitrogen reduction to ammonia. Dalton Trans. 2022, 51, 11163–11168. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Jiang, B.; Ni, C.; Qi, Y.; Bi, X. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: Mechanism exploration from both experimental and DFT studies. Chem. Eng. J. 2020, 382, 123034. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, G.; Tian, Y.; Guo, Y.; Chu, K. Boron phosphide as an efficient metal-free catalyst for nitrate electroreduction to ammonia. Dalton Trans. 2023, 52, 4290–4295. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Wang, S.; Sun, H. Rational Catalyst Design for N2 Reduction under Ambient Conditions: Strategies toward Enhanced Conversion Efficiency. ACS Catal. 2020, 10, 6870–6899. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Arif, M.; Yasin, G.; Tabish, M.; Kumar, A.; Ibraheem, S.; Ye, W.; Ajmal, S.; Zhao, J.; Li, P.; et al. Recent developments in heterogeneous electrocatalysts for ambient nitrogen reduction to ammonia: Activity, challenges, and future perspectives. Renew. Sustain. Energy Rev. 2023, 176, 113197. [Google Scholar] [CrossRef]
- Rehman, F.; Delowar Hossain, M.; Tyagi, A.; Lu, D.; Yuan, B.; Luo, Z. Engineering electrocatalyst for low-temperature N2 reduction to ammonia. Mater. Today 2021, 44, 136–167. [Google Scholar] [CrossRef]
- Xia, L.; Yang, J.; Wang, H.; Zhao, R.; Chen, H.; Fang, W.; Asiri, A.M.; Xie, F.; Cui, G.; Sun, X. Sulfur-doped graphene for efficient electrocatalytic N2-to-NH3 fixation. Chem. Commun. 2019, 55, 3371–3374. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, S.; Gao, G.; Ding, S.; Su, Y. A theoretical study on molybdenum and sulfur co-doped graphene for electrocatalytic nitrogen reduction. Mol. Catal. 2022, 517, 112048. [Google Scholar] [CrossRef]
- Ruan, Y.; He, Z.-H.; Liu, Z.-T.; Wang, W.; Hao, L.; Xu, L.; Robertson, A.W.; Sun, Z. Emerging two-dimensional materials for the electrocatalytic nitrogen reduction reaction to yield ammonia. J. Mater. Chem. A 2023, 11, 22590–22607. [Google Scholar] [CrossRef]
- Braun, A.; Bora, D.K.; Lauterbach, L.; Lettau, E.; Wang, H.; Cramer, S.P.; Yang, F.; Guo, J. From inert gas to fertilizer, fuel and fine chemicals: N2 reduction and fixation. Catal. Today 2022, 387, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, G.; Chen, G.F.; Zhang, H.; Zhang, S.; Wang, H. Comprehensive Understanding of the Thriving Ambient Electrochemical Nitrogen Reduction Reaction. Adv. Mater. 2021, 33, 2007650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, G.F.; Ding, L.X.; Wang, H. Advanced Non-metallic Catalysts for Electrochemical Nitrogen Reduction under Ambient Conditions. Chem.–A Eur. J. 2019, 25, 12464–12485. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Zhang, J.G.; Zhao, H.Q.; Fang, Y.S.; Ming, K.; Huang, H.; Chen, J.M.; Wang, X.C. Fluorine-doped graphene with an outstanding electrocatalytic performance for efficient oxygen reduction reaction in alkaline solution. R. Soc. Open Sci. 2018, 5, 180925. [Google Scholar] [CrossRef]
- Gu, J.; Guo, X.; Xia, W.; Peng, P.; Du, F.; Li, L. Facile synthesis of fluorinated graphene for surface self-assembly of aluminum hydride. FirePhysChem 2023, 3, 201–207. [Google Scholar] [CrossRef]
- Insausti, M.; Timmis, R.; Kinnersley, R.; Rufino, M.C. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 2020, 706, 135124. [Google Scholar] [CrossRef] [PubMed]
- Sanetuntikul, J.; Hyun, S.; Ganesan, P.; Shanmugam, S. Cobalt and nitrogen co-doped hierarchically porous carbon nanostructure: A bifunctional electrocatalyst for oxygen reduction and evolution reactions. J. Mater. Chem. A 2018, 6, 24078–24085. [Google Scholar] [CrossRef]
- Singh, K.; Razmjooei, F.; Yu, J.-S. Active sites and factors influencing them for efficient oxygen reduction reaction in metal-N coordinated pyrolyzed and non-pyrolyzed catalysts: A review. J. Mater. Chem. A 2017, 5, 20095–20119. [Google Scholar] [CrossRef]
- Palma, V.; Cortese, M.; Renda, S.; Ruocco, C.; Martino, M.; Meloni, E. A Review about the Recent Advances in Selected NonThermal Plasma Assisted Solid–Gas Phase Chemical Processes. Nanomaterials 2020, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Ngorot Kembo, J.P.; Wang, J.; Luo, N.; Gao, F.; Yi, H.; Zhao, S.; Zhou, Y.; Tang, X. A review of catalytic oxidation of carbon monoxide over different catalysts emphasis on Hopcalite Catalysts. New J. Chem. 2023, 47, 20222–20247. [Google Scholar] [CrossRef]
- Shetty, A.; Molahalli, V.; Sharma, A.; Hegde, G. Biomass-Derived Carbon Materials in Heterogeneous Catalysis: A Step towards Sustainable Future. Catalysts 2022, 13, 20. [Google Scholar] [CrossRef]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Ong, H.-C.; Lee, K.-T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Shen, X.; Wang, H.; Wang, H.; Xia, K.; Yin, Z.; Zhang, Y. Biomass-Derived Carbon Materials: Controllable Preparation and Versatile Applications. Small 2021, 17, 2008079. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Verma, G.; Sekhon, S.S. Biomass derived hierarchical porous carbon materials as oxygen reduction reaction electrocatalysts in fuel cells. Prog. Mater. Sci. 2019, 102, 1–71. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, H.; Zhu, S.; Hou, L.; Yuan, C. Hierarchical micro-/mesoporous N- and O-enriched carbon derived from disposable cashmere: A competitive cost-effective material for high-performance electrochemical capacitors. Green Chem. 2015, 17, 2373–2382. [Google Scholar] [CrossRef]
- Wu, T.; Li, P.; Wang, H.; Zhao, R.; Zhou, Q.; Kong, W.; Liu, M.; Zhang, Y.; Sun, X.; Gong, F. Biomass-derived oxygen-doped hollow carbon microtubes for electrocatalytic N2-to-NH3 fixation under ambient conditions. Chem. Commun. 2019, 55, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, R.; Zhang, P.; Wang, P.; Chen, N.; Qian, B.; Zhang, L.; Yu, J.; Dai, B. Functional Carbon from Nature: Biomass-Derived Carbon Materials and the Recent Progress of Their Applications. Adv. Sci. 2023, 10, 2205557. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yan, M.; Jiang, J.; Huang, A.; Cai, S.; Lan, L.; Ye, K.; Chen, D.; Tang, K.; Zuo, Q.; et al. A state-of-the-art review on biomass-derived carbon materials for supercapacitor applications: From precursor selection to design optimization. Sci. Total Environ. 2024, 912, 169141. [Google Scholar] [CrossRef]
- Lesbayev, B.; Auyelkhankyzy, M.; Ustayeva, G.; Yeleuov, M.; Rakhymzhan, N.; Maltay, A.; Maral, Y. Recent advances: Biomass-derived porous carbon materials. S. Afr. J. Chem. Eng. 2023, 43, 327–336. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Hao, R.; Wang, Y. Synthesis and applications of biomass-derived porous carbon materials in energy utilization and environmental remediation. Chemosphere 2023, 339, 139635. [Google Scholar] [CrossRef] [PubMed]
- Priya, D.S.; Kennedy, L.J.; Anand, G.T. Emerging trends in biomass-derived porous carbon materials for energy storage application: A critical review. Mater. Today Sustain. 2023, 21, 100320. [Google Scholar] [CrossRef]
- Paramasivam, N.; Sambandam, A.; Nastesan, B. Metalloids (B, Si) and non-metal (N, P, S) doped graphene nanosheet as a supercapacitor electrode: A density functional theory study. Mater. Today Commun. 2023, 35, 105905. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, Z.; Yang, W.; Zhou, H.; Fu, C.; Gong, Y.; Chen, L.; Kuang, Y. Different types of nitrogen species in nitrogen-doped carbon material: The formation mechanism and catalytic role on oxygen reduction reaction. Electrochim. Acta 2017, 245, 957–966. [Google Scholar] [CrossRef]
- Ma, G.; Ning, G.; Wei, Q. S-doped carbon materials: Synthesis, properties and applications. Carbon 2022, 195, 328–340. [Google Scholar] [CrossRef]
- Wang, M.J.; Wang, L.; Li, Q.; Bai, Y.; Wang, D.; Feng, Y. Controllable Synthesis of N-/S-Doped and N,S-doped hollow carbon spheres for the oxygen reduction Reaction: A universal Mono-Micelle Self-Assembly strategy. Mater. Lett. 2022, 309, 131315. [Google Scholar] [CrossRef]
- Sudhanshu, R. X-ray Photoelectron Spectroscopy (XPS) Technology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.; Yang, Y. Biomass-Derived Nitrogen-Doped Porous Carbon for Highly Efficient Ambient Electro-Synthesis of NH3. Catalysts 2020, 10, 353. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, J.; Yao, X. Defect electrocatalytic mechanism: Concept, topological structure and perspective. Mater. Chem. Front. 2018, 2, 1250–1268. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward Design of Synergistically Active Carbon-Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dai, L. Carbon-Based Metal-Free Catalysts for Electrocatalysis beyond the ORR. Angew. Chem. Int. Ed. 2016, 55, 11736–11758. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat Commun 2014, 5, 3783. [Google Scholar] [CrossRef]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Quan, X.; Fan, X.; Chen, S.; Yu, H.; Zhao, H.; Zhang, Y.; Zhao, J. Facile Ammonia Synthesis from Electrocatalytic N2 Reduction under Ambient Conditions on N-Doped Porous Carbon. ACS Catal. 2018, 8, 1186–1191. [Google Scholar] [CrossRef]
- Huang, H.; Xia, L.; Cao, R.; Niu, Z.; Chen, H.; Liu, Q.; Li, T.; Shi, X.; Asiri, A.M.; Sun, X. A Biomass-Derived Carbon-Based Electrocatalyst for Efficient N(2) Fixation to NH(3) under Ambient Conditions. Chemistry 2019, 25, 1914–1917. [Google Scholar] [CrossRef]
| Post-Synthesis Doping Method | Source of Non-Metal | Material Doped | Temperature Range (°C) | Time | Result | Ref. |
|---|---|---|---|---|---|---|
| In situ electron-beam irradiation | Paraffin wax | Boron nitride nanotubes | - | - | A well-controlled, little-damaging, simple strategy | [64] |
| Direct nitrogen reaction | Nitrogen and cyanamide | Single-walled carbon nanotubes/graphene sample | 500–800 °C | 1 h | Improved catalytic properties | [65] |
| Thermal-diffusion-based doping | Boron from boric acid | Silicon quantum dots | 400–600 °C | 1 h | Network-interconnected Si particles. | [66] |
| Ethylene amine vapor doping | Ethylene amine | Graphene | 300 °C | 1 h | High-performance transparent electrodes | [67] |
| Solvothermal method | Nitrogen from melamine | Carbon nanoparticles from CCl4 | - | - | Low-cost alternative to commercial Pt/C catalysts | [68] |
| Solid template approach | Nitrogen from melamine | core–shell SiO2@RF composites | 600 and 900 °C | 1 h | High specific surface areas and thermal stabilities | [69] |
| Synthesis Technique | Description | Source/Product | Ref. |
|---|---|---|---|
| CVD | Simultaneous synthesis of carbon material and nitrogen doping. | N-doped carbons | [79] |
| Infiltration and subsequent CVD | A novel approach involving Y zeolite infiltration with an ionic liquid and subsequent CVD. Co-doped microporous carbon exhibited pyridinic, pyrrolic, and graphitic nitrogen. | Co-doped microporous carbons | [70] |
| CVD | Utilization of C2H4/NH3 combinations in CVD for creating N-doped carbon nanofibers (N-CNFs) | N-CNFs | [80] |
| Carbonization | N-doped carbon material preparation from polyaniline (PANI) through carbonization at 800 °C using various salt precursors | Synthesis from PANI | [73] |
| Carbonization | Employing imidazolium/pyridinium ionic liquids (ILs) for N-doped carbon synthesis | N-doped carbons | [81] |
| Electrospinning, carbonization | Creation of hollow-particle-based N-doped carbon nanofibers (N-CNFs) using PAN and ZIF-8, followed by carbonization. | Hollow-particle-based N-CNFs | [82] |
| Hydrothermal carbonization (HTC) | Hydrothermal carbonization (HTC) of biomass for creating N-doped carbon compounds. | Biomass-derived N-doped carbon | [83] |
| Heat treatment, chemical activation | Closed porosity and low surface area in resulting hydrochars necessitating additional treatment. | Biomass-derived N-doped carbon | [71] |
| Pyrolysis | Conventional pyrolysis of biomass for producing N-doped carbon nanosheets without chemical activation. | Advanced pyrolysis for N-doped carbon Nanosheets | [84] |
| Methodology | Description | Reference |
|---|---|---|
| Capping-agent-protected NPs | Uses capping agents for NP growth regulation; concerns about the negative impact on catalytic activity. | [101,102] |
| N-doped carbon supports | Achieves small, evenly distributed NPs. Nitrogen functionalities enhance catalytic performance. | [103,104] |
| Synthetic techniques for NPs on N-doped carbon | Three categories: loading on pre-synthesized N-doped carbon, simultaneous incorporation, and in situ synthesis. | [83,105] |
| Conventional techniques for loading NPs | Includes impregnation, deposition–precipitation, and sol immobilization with subsequent reduction. | [106,107] |
| In situ synthesis of NPs on N-doped carbon | The pyrolysis step allows the simultaneous incorporation of metal and nitrogen. | [107,108] |
| Use of MOF as a sacrificial template | MOFs are used as templates for intriguing metal/N–carbon compositions. | [109,110] |
| Catalysts | NH3 Yield | FE (%) | Electrolyte | Year Published | Reference |
|---|---|---|---|---|---|
| Pd-Cu/TinO2n−1 | - | 22 | 1 mM NaNO3 | 2018 | [113] |
| Pd–In/γ-Al2O3 | - | 71.5 | 1.4 mM NO3− | 2009 | [114] |
| Pt | - | 49 | 3000 mg·L−1 NO3− | 2006 | [115] |
| 30%Cu-70%Pd | - | 58 | 0.05 M KNO3 | 2009 | [116] |
| Fe3Ni-N-C | Nitrate-to-N2 | 99.3 | 100 ppm NO3− | 2021 | [117] |
| Single Fe atom | 0.46 mmol·h−1·cm−2 | 75 | 0.50 M KNO3 | 2021 | [118] |
| Co3O4 nanorod arrays | 0.854 mmol·h−1·cm2 | 33.6 | 100 g L−1 NO3− | 2019 | [119] |
| Rh/C | 34.4 μg h−1cm−2 | 20.8 | 0.1 M KNO3 | 2021 | [120] |
| Cu@Th-BPYDC | 225.3 μmolh−1cm−2 | 92.5 | 100 mM KNO3 | 2022 | [121] |
| RuNi-MOF | 274 mgh−1mgcat−1 | 73 | 50 mg L−1 NO3− | 2022 | [122] |
| Pd-NDs/Zr-MOF | 287.31 mmol·h−1·gcat−1. | 58.1 | 500 ppm NO3− | 2022 | [123] |
| Pd cuboctahedrons | 306.8 μgh−1mgcat−1 | 35 | 20 mM NO3− | 2021 | [124] |
| PTCDA/O-Cu | 436 μg h−1cm−2 | 85.9 | 500 ppm NO3− | 2020 | [125] |
| Catalyst Type/Process | Characteristics | Challenges and Limitations | Ref. |
|---|---|---|---|
| Metallic catalysts (Ru, Rh) | Ru electroplated on titanium felt enhances NH3 production; Ru on porous graphite improves NRR | Catalytic structure sensitivity; cost and scarcity of precious metals | [139,140] |
| Monatomic catalysts | Strong chemical interaction with nitrogen; outstanding activity | High cost and scarcity for large-scale application | [143,144] |
| Non-precious metal catalysts | Low Faraday efficiency; activation via sp hybrid orbitals | Limited Faraday efficiency despite low cost | [145] |
| Metal-free catalysts (N, P) | Carbon-based compounds doped with N and P; PCN-NVs increase ammonia yield and Faraday efficiency | Cost and scarcity challenges for large-scale use | [78] |
| Perovskite catalysts | LaFeO3 effective in oxidizing N2; NTP enhances current density; Cu/CuO catalyst reduces NOx− | Gibbs free energy spectrum investigation; challenges in N2 activation | [146,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quoie Jr, G.D.S.; Jiao, M.; Lászlód, K.; Wang, Y. Progress Made in Non-Metallic-Doped Materials for Electrocatalytic Reduction in Ammonia Production. Materials 2024, 17, 2419. https://doi.org/10.3390/ma17102419
Quoie Jr GDS, Jiao M, Lászlód K, Wang Y. Progress Made in Non-Metallic-Doped Materials for Electrocatalytic Reduction in Ammonia Production. Materials. 2024; 17(10):2419. https://doi.org/10.3390/ma17102419
Chicago/Turabian StyleQuoie Jr, Gerald D. S., Mingshuo Jiao, Krisztina Lászlód, and Ying Wang. 2024. "Progress Made in Non-Metallic-Doped Materials for Electrocatalytic Reduction in Ammonia Production" Materials 17, no. 10: 2419. https://doi.org/10.3390/ma17102419
APA StyleQuoie Jr, G. D. S., Jiao, M., Lászlód, K., & Wang, Y. (2024). Progress Made in Non-Metallic-Doped Materials for Electrocatalytic Reduction in Ammonia Production. Materials, 17(10), 2419. https://doi.org/10.3390/ma17102419









