Influence of Carboxymethyl Cellulose as a Thickening Agent for Glauber’s Salt-Based Low Temperature PCM
Abstract
1. Introduction
2. Materials
3. Characterization Techniques
3.1. Differential Scanning Calorimetry (DSC)
3.2. Temperature History Method (T-History)
3.3. Rheology
4. PCM Preparation Method
5. Results and Discussion
5.1. Preparation Method Comparisons of PCM Thickened with CMC-700
5.2. Preparation of PCM with CMC-250 and CMC-90
5.3. T-History Analysis for Supercooling
5.4. Rheology Analysis
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Junaid, M.F.; ur Rehman, Z.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic phase change materials in thermal energy storage: A review on perspectives and technological advances in building applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Alzuwaid, F.; Ge, Y.T.; Tassou, S.A.; Raeisi, A.; Gowreesunker, L. The novel use of phase change materials in a refrigerated display cabinet: An experimental investigation. Appl. Therm. Eng. 2015, 75, 770–778. [Google Scholar] [CrossRef]
- Tassou, S.A.; De-Lille, G.; Ge, Y.T. Food transport refrigeration—Approaches to reduce energy consumption and environmental impacts of road transport. Appl. Therm. Eng. 2009, 29, 1467–1477. [Google Scholar] [CrossRef]
- Kumar, N.; Von Ness, R.; Chavez, R., Jr.; Banerjee, D.; Muley, A.; Stoia, M. Experimental Analysis of Salt Hydrate Latent Heat Thermal Energy Storage System with Porous Aluminum Fabric and Salt Hydrate as Phase Change Material with Enhanced Stability and Supercooling. J. Energy Resour. Technol. 2021, 143, 042001. [Google Scholar] [CrossRef]
- Bose, P.; Amirtham, V.A. A review on thermal conductivity enhancement of paraffinwax as latent heat energy storage material. Renew. Sustain. Energy Rev. 2016, 65, 81–100. [Google Scholar] [CrossRef]
- Khodadadi, J.; Fan, L.; Babaei, H. Thermal conductivity enhancement of nanostructure-based colloidal suspensions utilized as phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2013, 24, 418–444. [Google Scholar] [CrossRef]
- Selvnes, H.; Allouche, Y.; Manescu, R.I.; Hafner, A. Review on cold thermal energy storage applied to refrigeration systems using phase change materials. Therm. Sci. Eng. Prog. 2021, 22, 100807. [Google Scholar] [CrossRef]
- Cong, L.; Zou, B.; Palacios, A.; Navarro, M.E.; Qiao, G.; Ding, Y. Thickening and gelling agents for formulation of thermal energy storage materials—A critical review. Renew. Sustain. Energy Rev. 2022, 155, 111906. [Google Scholar] [CrossRef]
- Shamseddine, I.; Pennec, F.; Biwole, P.; Fardoun, F. Supercooling of phase change materials: A review. Renew. Sustain. Energy Rev. 2022, 158, 112172. [Google Scholar] [CrossRef]
- Shin, B.C.; Kim, S.D.; Park, W.-H. Phase Separation and Supercooling of a Latent Heat-Storage Material. Energy 1989, 14, 921–930. [Google Scholar] [CrossRef]
- Goswami, M.; Kumar, N.; Li, Y.; Hirschey, J.; LaClair, T.J.; Akamo, D.O.; Sultan, S.; Rios, O.; Gluesenkamp, K.R.; Graham, S. Understanding supercooling mechanism in sodium sulfate decahydrate phase-change material. J. Appl. Phys. 2021, 129, 245109. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xu, X.; Zhang, S. Research progress in nucleation and supercooling induced by phase change materials. J. Energy Storage 2020, 27, 101156. [Google Scholar] [CrossRef]
- Marliacy, P.; Solimando, R.; Bouroukba, M.; Schuffenecker, L. Thermodynamics of crystallization of sodium sulfate decahydrate in H2O–NaCl–Na2SO4: Application to Na2SO4·10H2O-based latent heat storage materials. Thermochim. Acta 2000, 344, 85–94. [Google Scholar] [CrossRef]
- Ayyagari, V.; Cajamarca AP, S.; Shooshtari, A.; Ohadi, M. Experimental study of cyclically stable Glauber’s salt-based PCM for cold thermal energy storage. In Proceedings of the 2023 22nd InterSociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Orlando, FL, USA, 30 May–2 June 2023. [Google Scholar]
- Olson, L.; Lothian, C.; Ådén, U.; Lagercrantz, H.; Robertson, N.J.; Setterwall, F. Phase-changing glauber salt solution for medical applications in the 28–32 °C interval. Materials 2021, 14, 7106. [Google Scholar] [CrossRef] [PubMed]
- De Paola, M.G.; Lopresto, C.G.; Arcuri, N.; Calabrò, V. Crossed analysis by T-history and optical light scattering method for the performance evaluation of Glauber’s salt-based phase change materials. J. Dispers. Sci. Technol. 2022, 43, 760–768. [Google Scholar] [CrossRef]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Marks, S. An investigation of the thermal energy storage capacity of glauber’s salt with respect to thermal cycling. Sol. Energy 1980, 25, 255–258. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Akamo, D.O.; Kumar, N.; Li, Y.; Pekol, C.; Li, K.; Goswami, M.; Hirschey, J.; LaClair, T.J.; Keffer, D.J.; Rios, O.; et al. Stabilization of low-cost phase change materials for thermal energy storage applications. iScience 2023, 26, 107175. [Google Scholar] [CrossRef]
- Redaelli, F.; Sorbona, M.; Rossi, F. Synthesis and processing of hydrogels for medical applications. In Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 205–228. [Google Scholar] [CrossRef]
- Tako, M.; Tamaki, Y.; Teruya, T.; Takeda, Y. The Principles of Starch Gelatinization and Retrogradation. Food Nutr. Sci. 2014, 5, 280–291. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Click Chemistry-Based Injectable Hydrogels and Bioprinting Inks for Tissue Engineering Applications. Tissue Eng. Regen. Med. 2018, 15, 531–546. [Google Scholar] [CrossRef]
- Philp, K. Polysaccharide Ingredients. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Sun, L.; Han, J.; Wu, J.; Huang, W.; Li, Y.; Mao, Y.; Wang, L.; Wang, Y. Cellulose pretreatment with inorganic salt hydrate: Dissolution, regeneration, structure and morphology. Ind. Crops Prod. 2022, 180, 114722. [Google Scholar] [CrossRef]
- Jiang, Z.; Tie, S. Preparation and thermal properties of Glauber’s salt-based phase-change materials for Qinghai-Tibet Plateau solar greenhouses. Int. J. Mod. Phys. B 2017, 31, 1744085. [Google Scholar] [CrossRef]
- Telkes, M. Nucleation of Supersaturated Inorganic Salt Solutions. Ind. Eng. Chem. 1952, 44, 1308–1310. [Google Scholar] [CrossRef]
- Peck, J.H.; Kim, J.J.; Kang, C.; Hong, H. A study of accurate latent heat measurement for a PCM with a low melting temperature using T-history method. Int. J. Refrig. 2006, 29, 1225–1232. [Google Scholar] [CrossRef]
- Thakkar, J.; Annavajjala, S.B.; Kosny, J.; Sobkowicz, M.J. Stabilizing a low temperature phase change material based on Glaubers salt. J. Energy Storage 2024, 91, 111936. [Google Scholar] [CrossRef]
- Fabuel Bartual, C.; Máñez Pitarch, M.J.; García Martínez, E.; Gómez-Tena, M.P. Influence of the molecular weight of carboxymethylcellulose on properties of Binder Jetting manufactured parts. Open Ceram. 2022, 11, 100285. [Google Scholar] [CrossRef]
- Yinping, Z.; Yi, J. A simple method, the T-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201–205. [Google Scholar] [CrossRef]

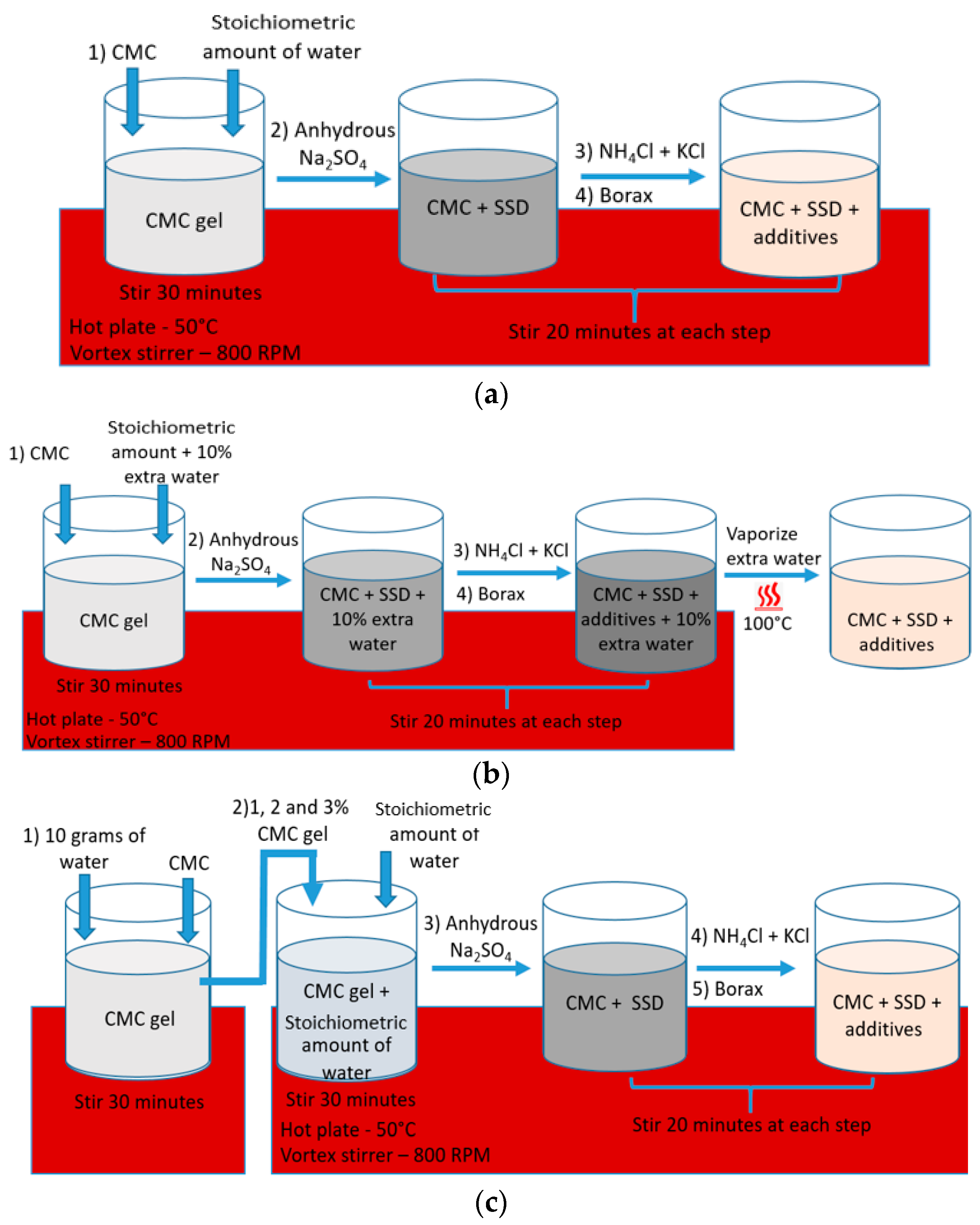




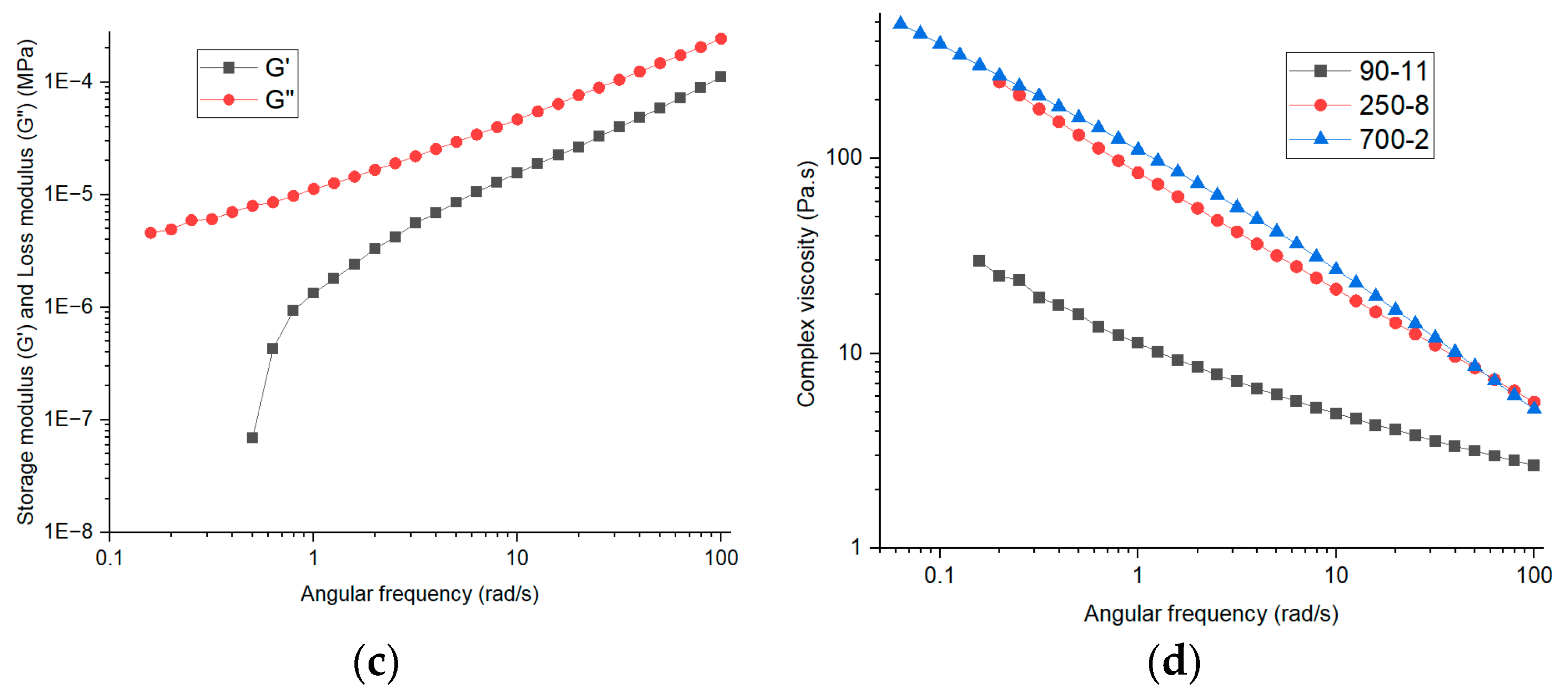
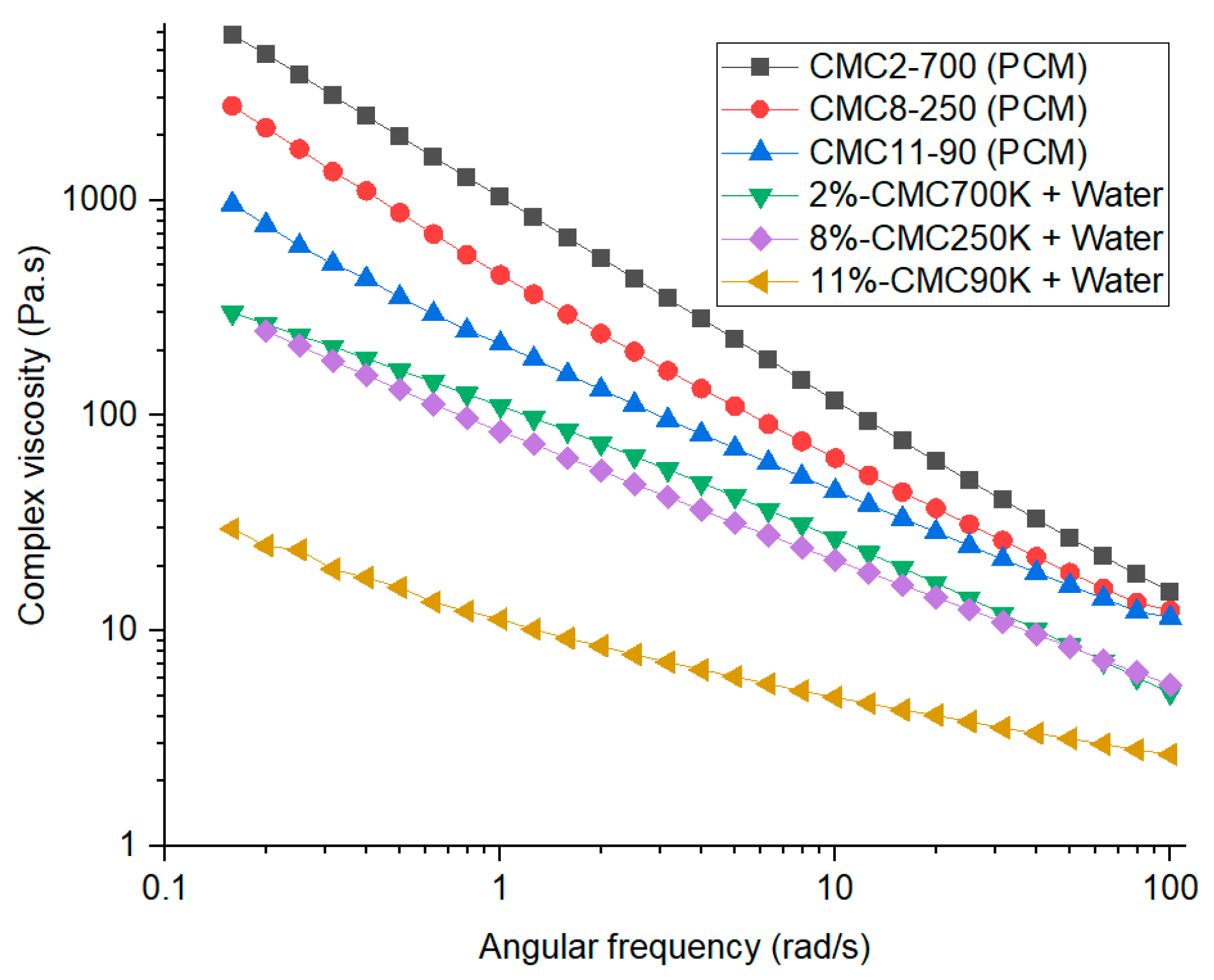
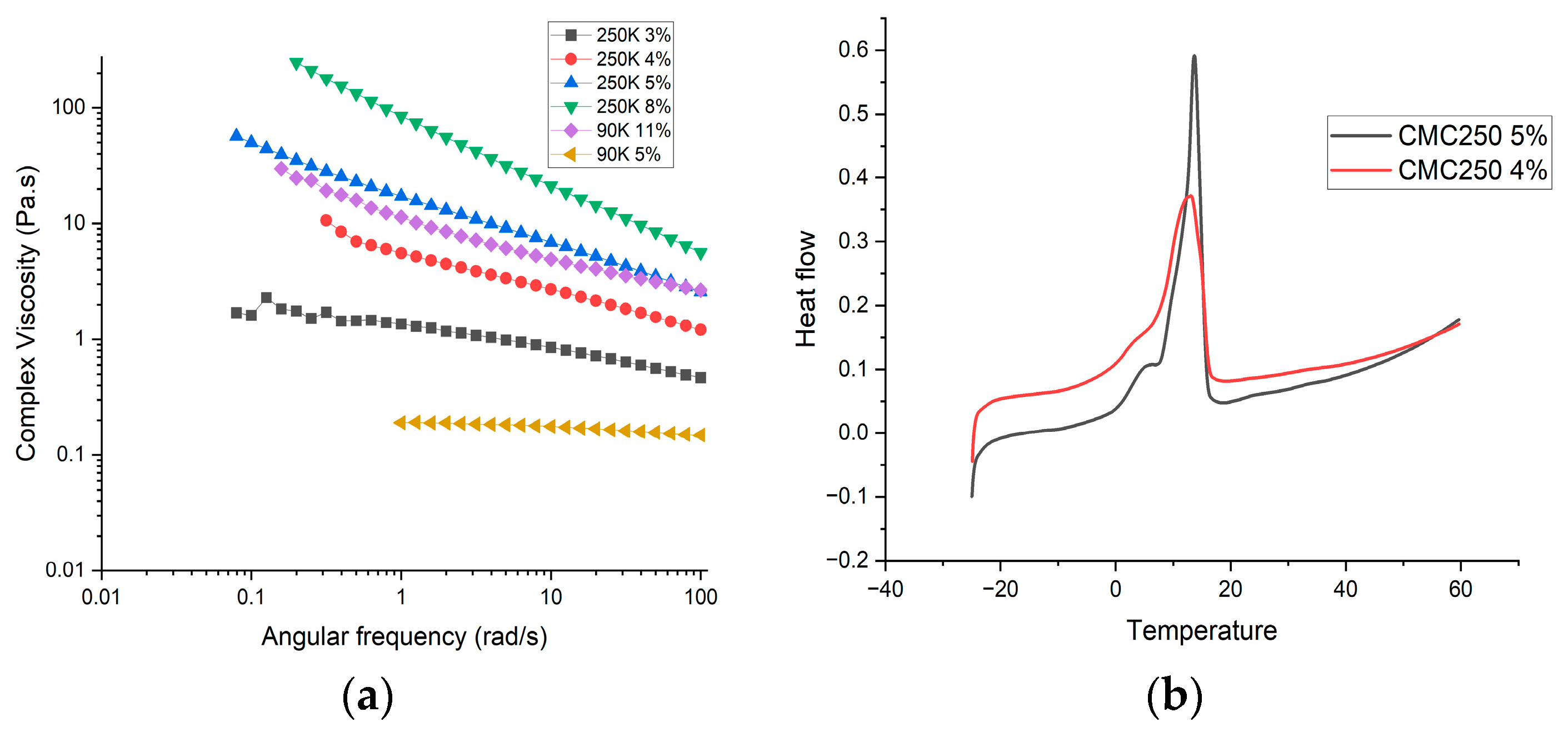
| Method | Sample No. | SSD + KCl + NH4Cl + Borax (%) | CMC Powder (%) | CMC Gel (2 wt.%) |
|---|---|---|---|---|
| 1 | CMC2-700 | 98 | 2 | - |
| 2 | CMC2-700E | 98 | 2 | - |
| 3 | CMC1-700 | 99 | - | 1 |
| 3 | CMC2-700 | 98 | - | 2 |
| 3 | CMC3-700 | 97 | - | 3 |
| 1 | CMC2-250 | 98 | 2 | - |
| 1 | CMC2-90 | 98 | 2 | - |
| 1 | CMC8-250 | 96.32 | 3.68 | - |
| 1 | CMC11-90 | 95.07 | 4.93 | - |
| 1 | CMC4-250 | 98.16 | 1.84 | - |
| 1 | CMC5-250 | 97.70 | 2.30 | - |
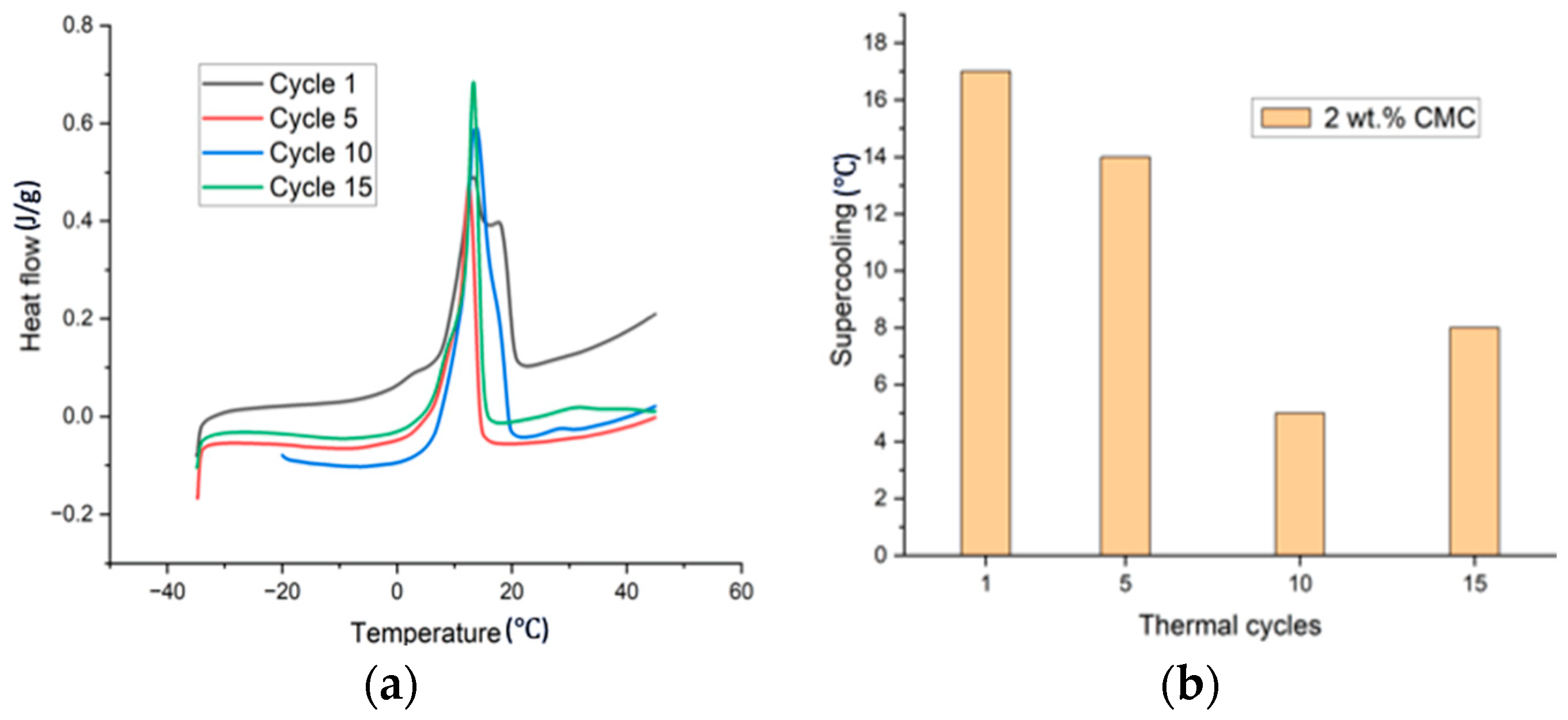
| Cycle No. | Melt Temp. (°C) | Peak Temp. (°C) | Enthalpy (J/g) |
|---|---|---|---|
| 1 | 9.8 | 12.6 | 80 |
| 5 | 9.5 | 13.2 | 90 |
| 10 | 10.5 | 13.8 | 105 |
| 15 | 11.3 | 13.2 | 77 |
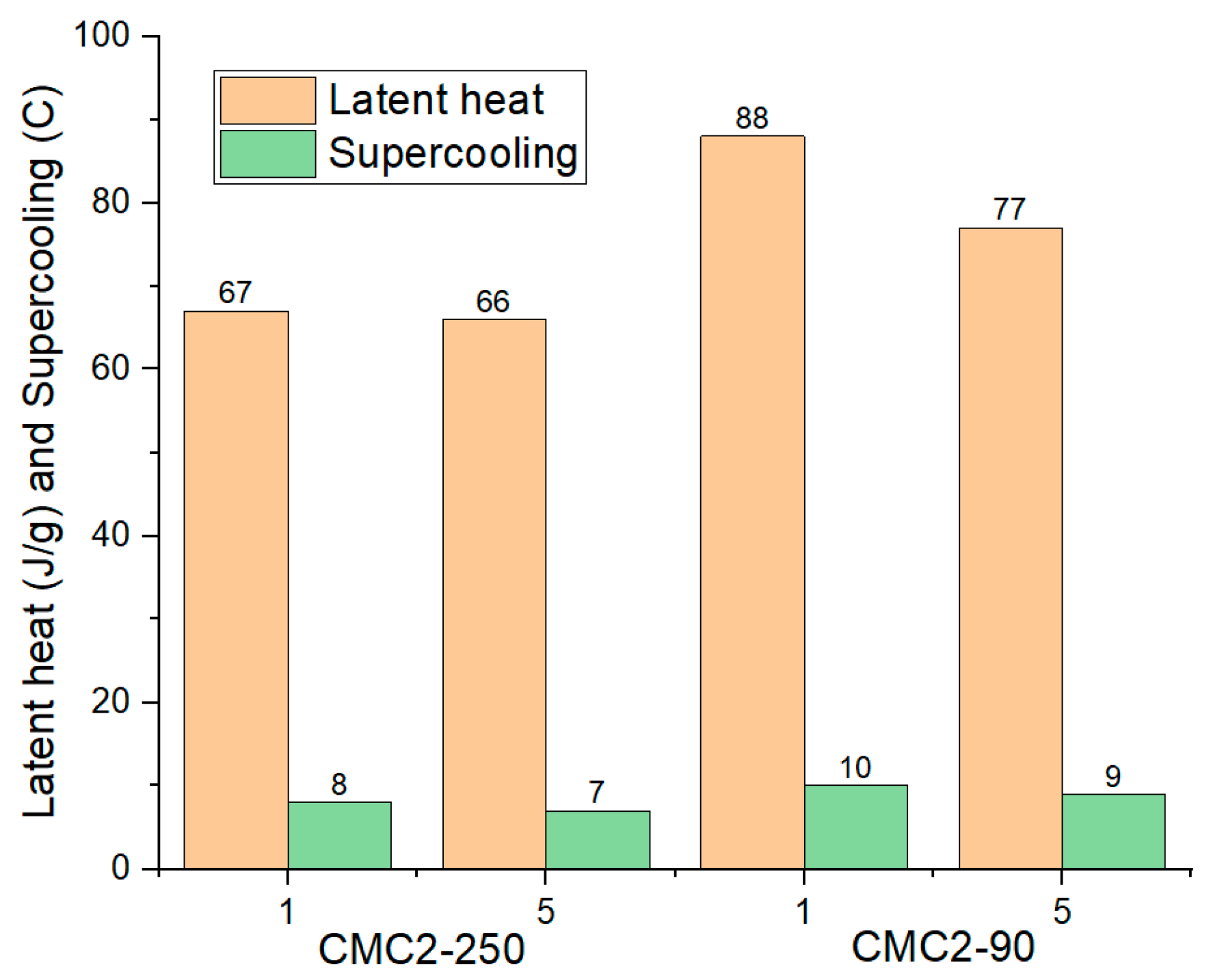
| Sample No. | Cycle No. | Onset Temp. | Peak Temp. | Latent Heat |
|---|---|---|---|---|
| CMC11-90 | 1 | 10.75 | 14.19 | 125 |
| 5 | 10.32 | 13.32 | 124 | |
| 10 | 10.28 | 12.90 | 128 | |
| 15 | 10.15 | 14.12 | 130 | |
| CMC8-250 | 1 | 11.6 | 13.4 | 102 |
| 5 | 10.3 | 13.7 | 107 | |
| 10 | 9.1 | 13.9 | 115 | |
| 15 | 10.2 | 13.8 | 108 |
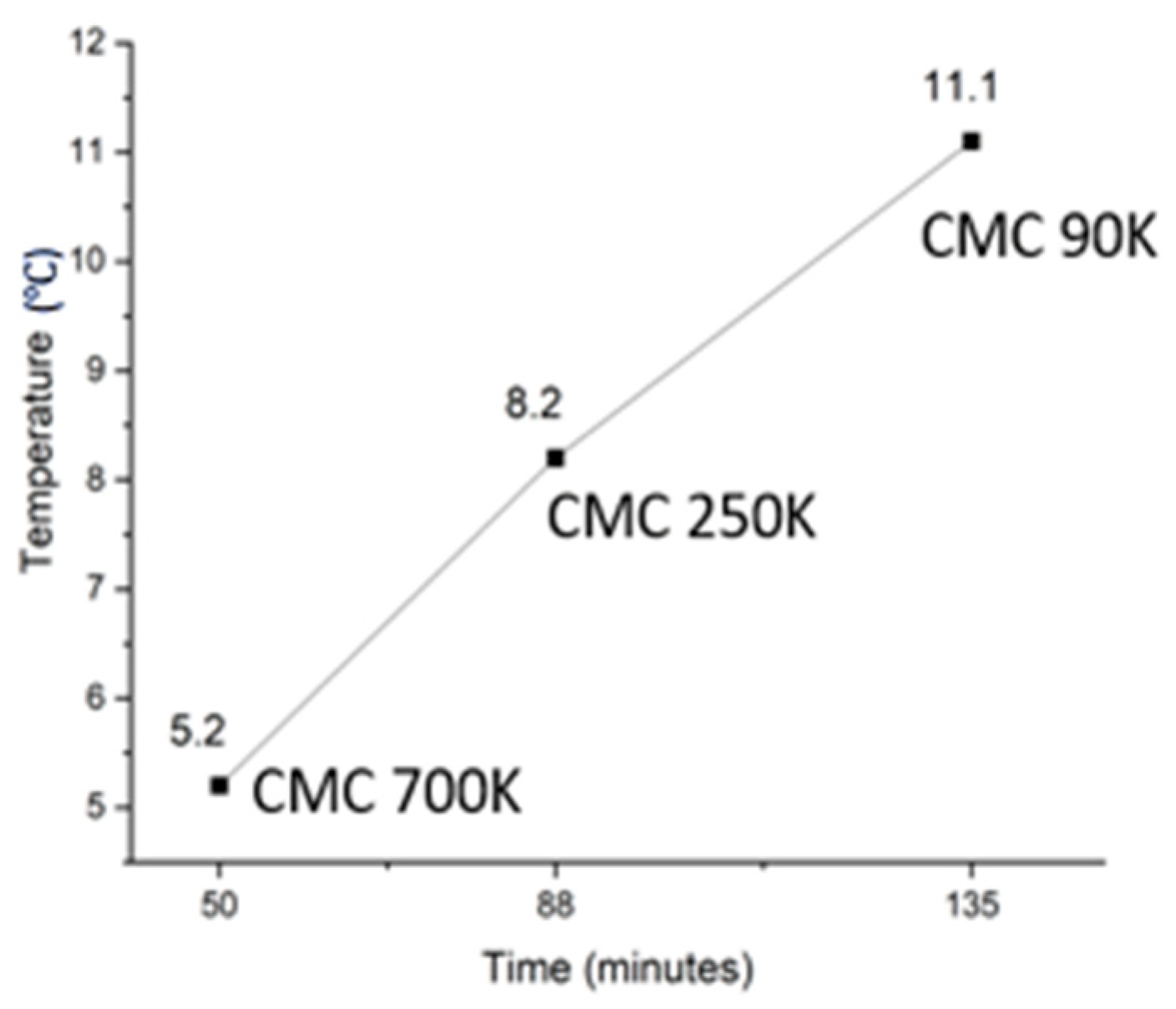
| Sample | Onset Temp. (°C) | Peak Temp. (°C) | Freezing Temp. (°C) | Supercooling (°C) | Time to Reach 0 °C (min) |
|---|---|---|---|---|---|
| CMC2-700 | 10.2 | 13.8 | 5.2 | 5 | 50 |
| CMC8-250 | 11.6 | 13.4 | 8.2 | 3.4 | 88 |
| CMC11-90 | 10.1 | 14.2 | 11.2 | 0 | 135 |
| Na-CMC mw (g/mol) | DS | Na-CMC Mass in Samples (g) | Moles of Sodium (mol) | Mass of Sodium (g) |
|---|---|---|---|---|
| 90,000 | 0.60 0.95 | 2.2 | 1.44 × 10−5 2.28 × 10−5 | 3.30 × 10−4 5.23 × 10−4 |
| 250,000 | 0.89 | 1.6 | 5.70 × 10−6 | 1.31 × 10−4 |
| 700,000 | 0.80 | 0.4 | 4.56 × 10−7 | 1.05 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakkar, J.; Annavajjala, S.B.; Sobkowicz, M.J.; Kosny, J. Influence of Carboxymethyl Cellulose as a Thickening Agent for Glauber’s Salt-Based Low Temperature PCM. Materials 2024, 17, 2442. https://doi.org/10.3390/ma17102442
Thakkar J, Annavajjala SB, Sobkowicz MJ, Kosny J. Influence of Carboxymethyl Cellulose as a Thickening Agent for Glauber’s Salt-Based Low Temperature PCM. Materials. 2024; 17(10):2442. https://doi.org/10.3390/ma17102442
Chicago/Turabian StyleThakkar, Jay, Sai Bhargav Annavajjala, Margaret J. Sobkowicz, and Jan Kosny. 2024. "Influence of Carboxymethyl Cellulose as a Thickening Agent for Glauber’s Salt-Based Low Temperature PCM" Materials 17, no. 10: 2442. https://doi.org/10.3390/ma17102442
APA StyleThakkar, J., Annavajjala, S. B., Sobkowicz, M. J., & Kosny, J. (2024). Influence of Carboxymethyl Cellulose as a Thickening Agent for Glauber’s Salt-Based Low Temperature PCM. Materials, 17(10), 2442. https://doi.org/10.3390/ma17102442







