Recent Advances in Corrosion Inhibition of Bonded NdFeB Magnets
Abstract
:1. Introduction
2. Scientific Literature
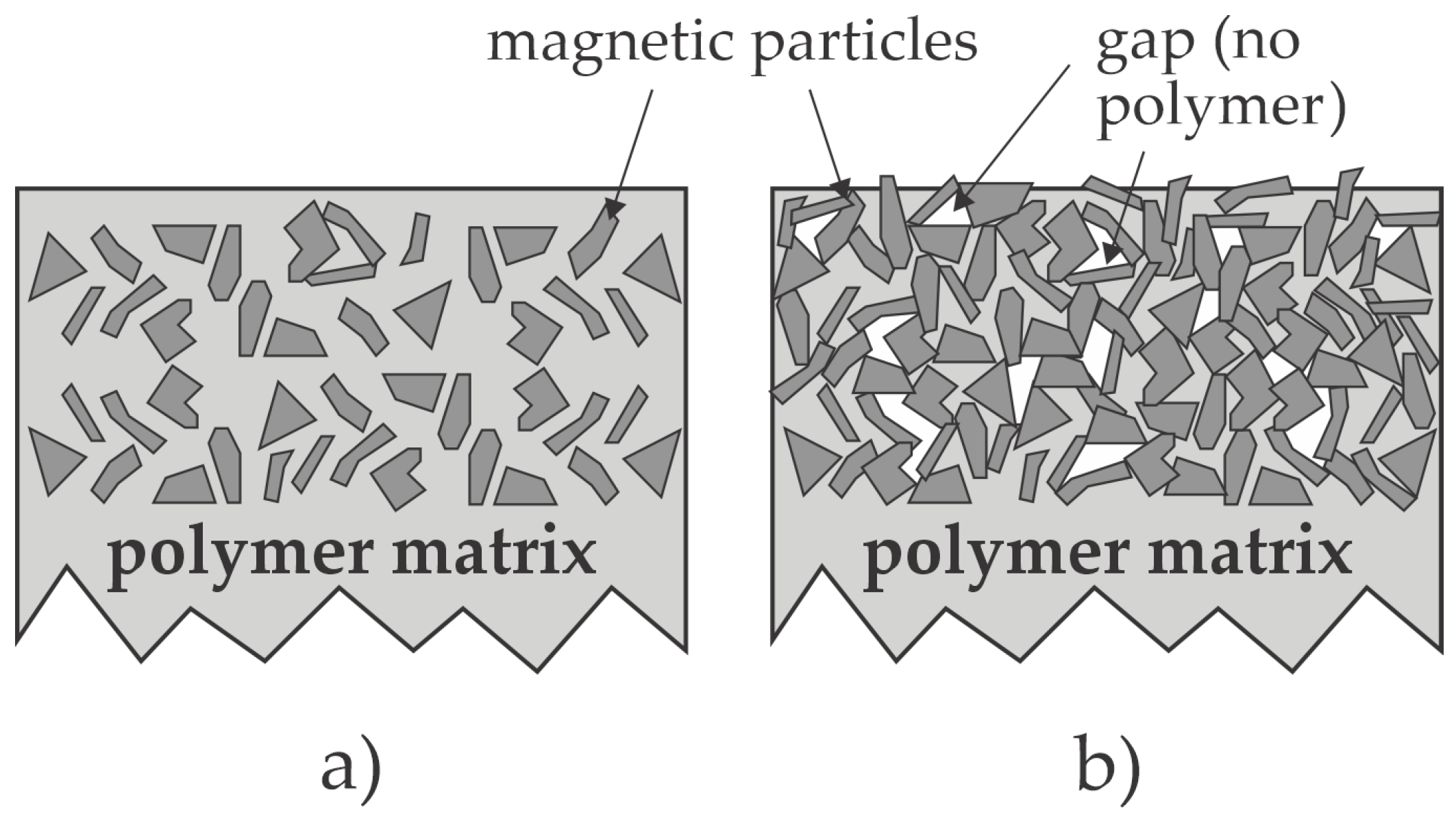
2.1. Modification of the Magnetic Powder
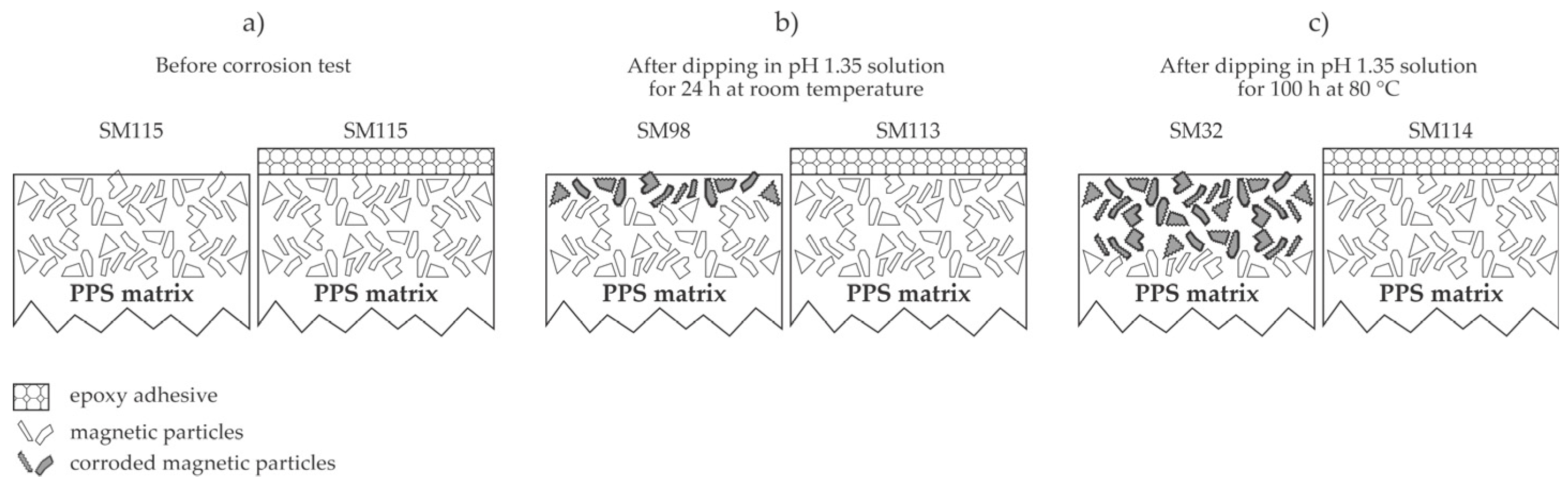
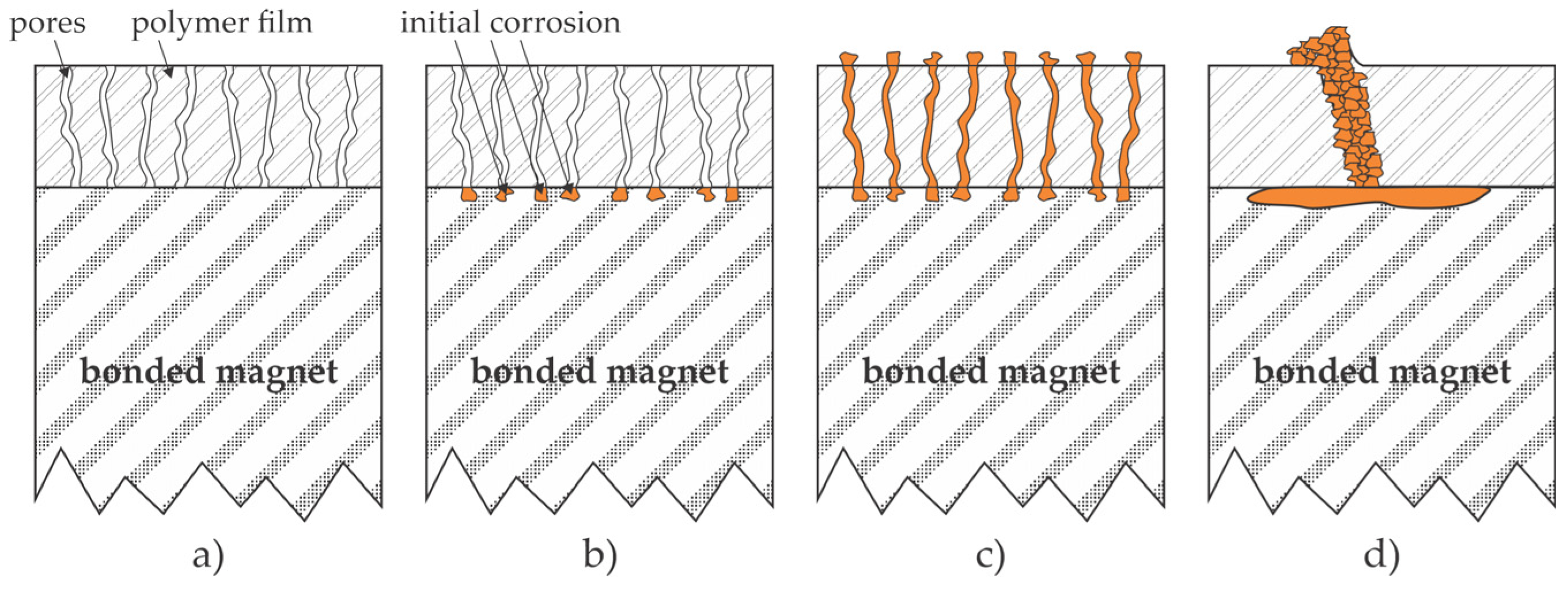
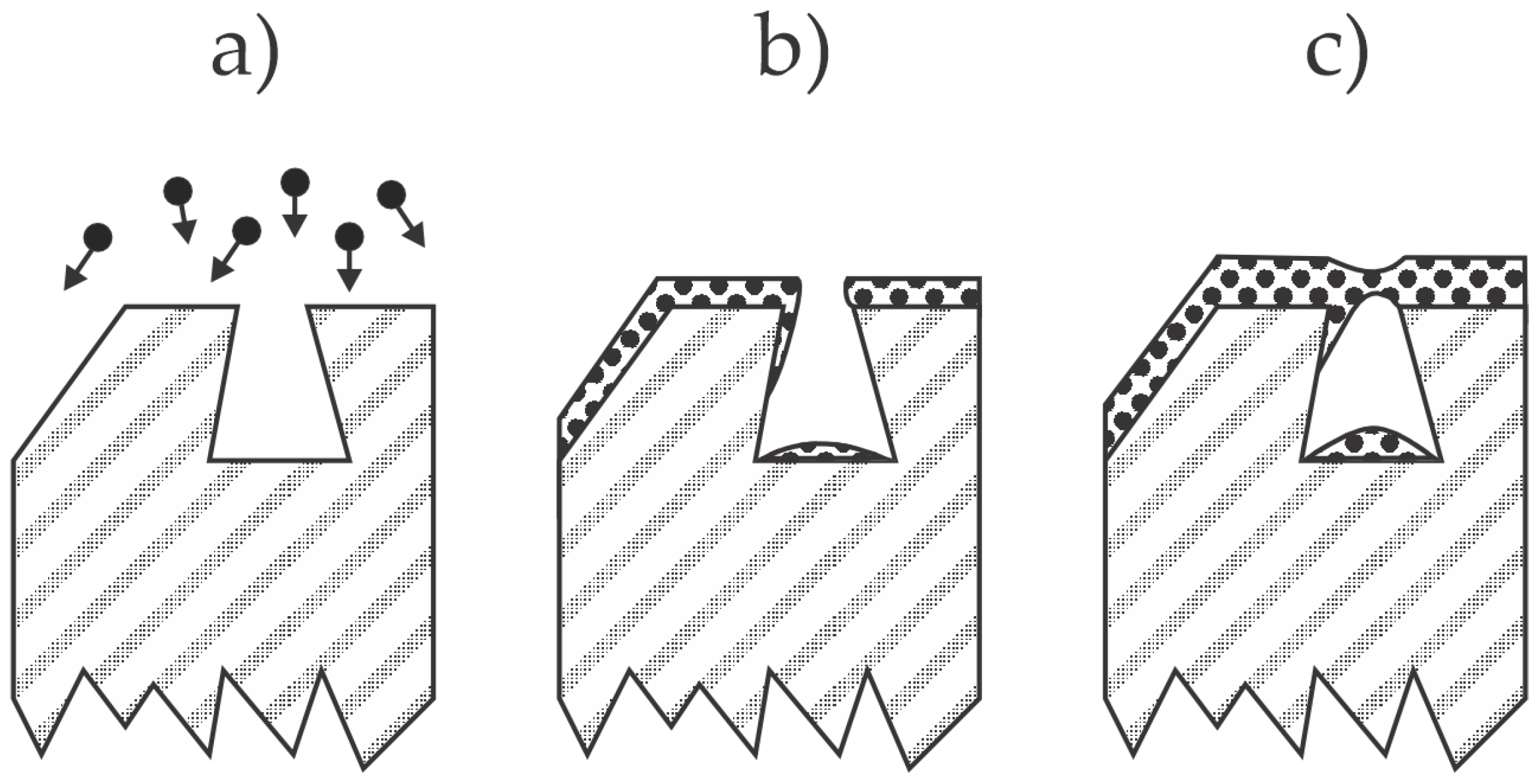
2.2. Adhesive Coatings
2.3. Deposition of Adhesive by Electrophoresis
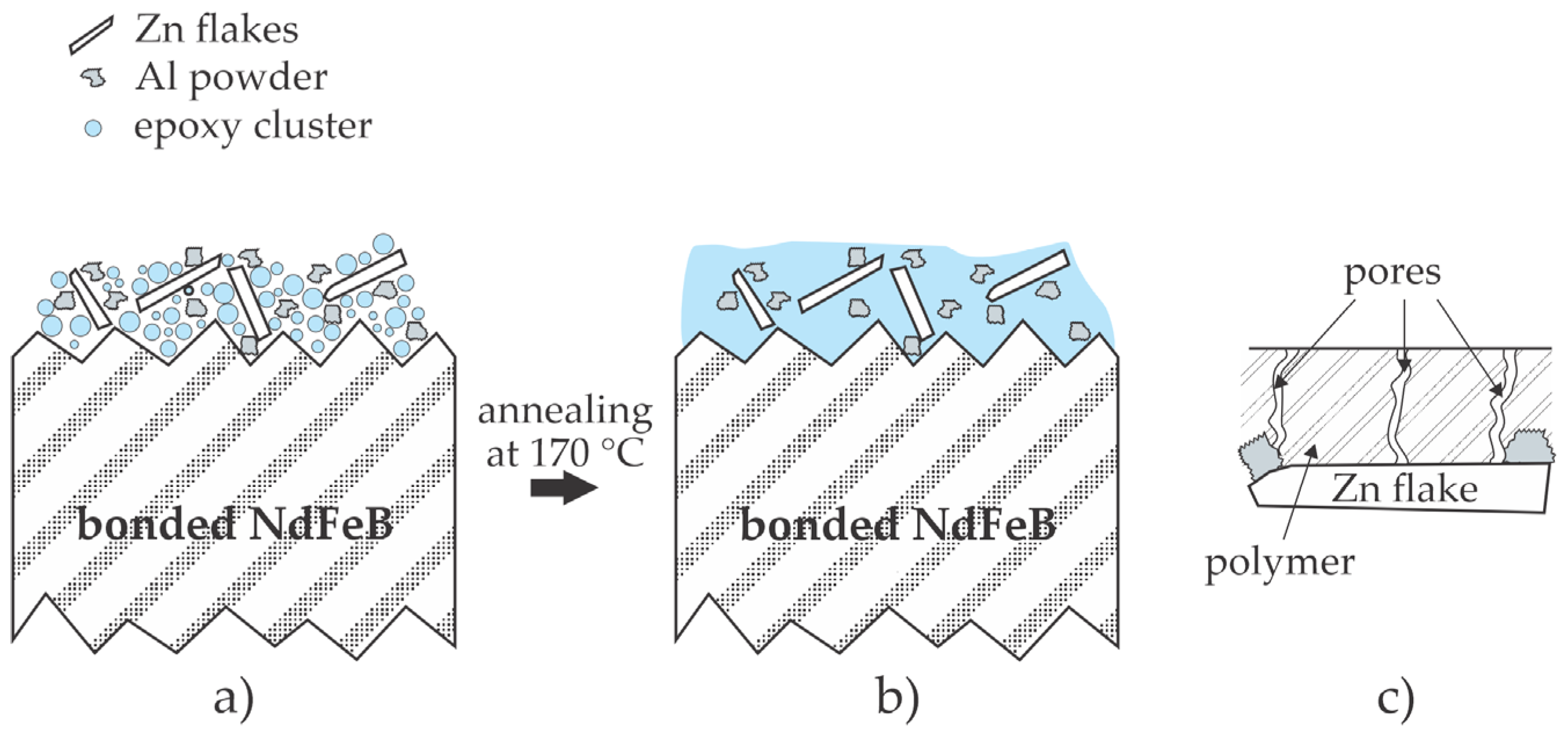
2.4. Deposition of a Composite Coating
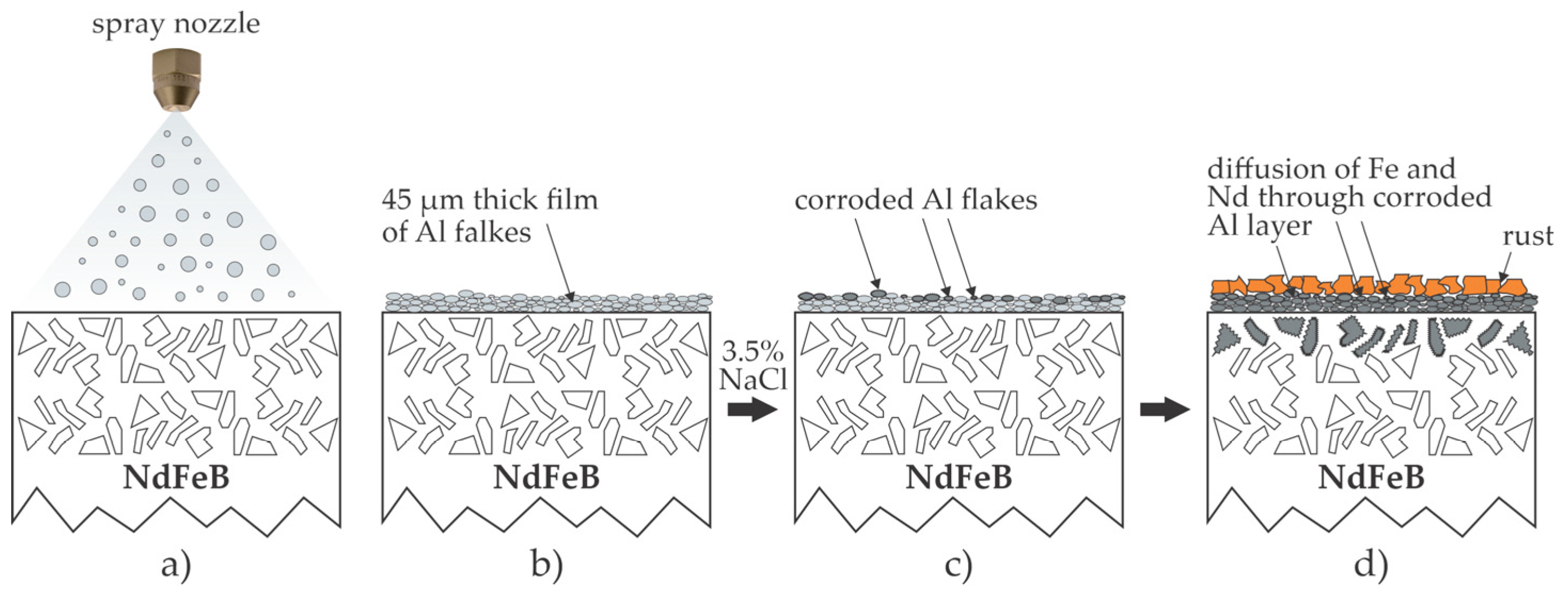
2.5. Deposition of Metallic Coatings
3. Patent Literature
3.1. Deposition of a Metallic Film
3.2. Deposition of a Composite Film
3.3. Surface Oxidation or Nitriding
3.4. More Complex Strucures
4. Discussion and Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brown, D.N. Fabrication, Processing Technologies, and New Advances for RE-Fe-B Magnets. IEEE Trans. Magn. 2016, 52, 2101209. [Google Scholar] [CrossRef]
- Wang, H.; Lamichhane, T.N.; Paranthaman, M.P. Review of additive manufacturing of permanent magnets for electrical machines: A prospective on wind turbine. Mater. Today Phys. 2022, 24, 100675. [Google Scholar] [CrossRef]
- Li, L.; Jones, K.; Sales, B.; Pries, J.L.; Nlebedim, I.C.; Jin, K.; Bei, H.; Post, B.K.; Kesler, M.S.; Rios, O.; et al. Fabrication of highly dense isotropic Nd-Fe-B nylon bonded magnets via extrusion-based additive manufacturing. Addit. Manuf. 2018, 21, 495–500. [Google Scholar] [CrossRef]
- Hemrick, J.; Lara-Curzio, E.; Liu, K.; Ma, B.-M. Mechanical properties of thermally cycled nylon bonded Nd-Fe-B permanent magnets. J. Mater. Sci. 2004, 39, 6509–6522. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, J.; Cheng, X.; Cai, W.; Qiao, L.; Che, S. Surface Modification and Refinement of Nd–Fe–B Magnetic Powder Using ITDT and Phosphoric Acid. JOM 2021, 73, 3941–3949. [Google Scholar] [CrossRef]
- ISO 9227:2017(E); Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. ISO: Geneva, Switzerland, 2017.
- Sagawa, M.; Fujimura, S.; Togawa, N.; Yamamoto, H.; Matsuura, Y. New material for permanent magnets on a base of Nd and Fe (invited). J. Appl. Phys. 1984, 55, 2083–2087. [Google Scholar] [CrossRef]
- Croat, J.J.; Herbst, J.F.; Lee, R.W.; Pinkerton, F.E. Pr-Fe and Nd-Fe-based materials: A new class of high-performance permanent magnets (invited). J. Appl. Phys. 1984, 55, 2078–2082. [Google Scholar] [CrossRef]
- Shimba, K.; Yamazaki, M.; Horikawa, T.; Sugimoto, S.; Mitarai, H. Effect of Phosphate Treatment on the Corrosion Resistance of Nd–Fe–B Anisotropic Magnetic Powder. IEEE Trans. Magn. 2023, 59, 9201104. [Google Scholar] [CrossRef]
- Paranthaman, M.P.; Yildirim, V.; Lamichhane, T.N.; Begley, B.A.; Post, B.K.; Hassen, A.A.; Sales, B.C.; Gandha, K.; Nlebedim, I.C. Additive Manufacturing of Isotropic NdFeB PPS Bonded Permanent Magnets. Materials 2020, 13, 3319. [Google Scholar] [CrossRef] [PubMed]
- Webster, H.F.; Wightman, J.P. Effects of oxygen and ammonia plasma treatment on polyphenylene sulfide thin films and their interaction with epoxy adhesive. J. Adhes. Sci. Technol. 1991, 5, 93–106. [Google Scholar] [CrossRef]
- Gandha, K.; Paranthaman, M.P.; Wang, H.; Liu, X.; Nlebedim, I.C. Thermal stability of anisotropic bonded magnets prepared by additive manufacturing. J. Am. Ceram. Soc. 2022, 106, 166–171. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Sun, Y.; Jiang, N.; Guan, C.; Fang, X.; Liu, J. Preparation and corrosion resistance of epoxy resin coating for bonded NdFeB magnet. Prog. Org. Coat. 2022, 173, 107180. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Yang, L.; Su, L.; Jia, M.; Chen, Y.; Fang, X.; Liu, J. Preparation and Anticorrosion Performance of Double-Layer Epoxy Resin Coatings on Bonded NdFeB Magnets. J. Mater. Eng. Perform. 2023. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, L.; Ren, R.; Chen, Y.; Dong, B.; Liu, J.; Fang, X.; Gao, Q. The Preparation and Properties of ZnAl Coating for Ring-Shaped Bonded NdFeB Magnet with High Corrosion Resistance. J. Mater. Eng. Perform. 2021, 31, 1003–1008. [Google Scholar] [CrossRef]
- Gao, Y.; Bai, Y.; Zhu, H.; Liang, W.; Liu, Q.; Dong, H.; Jia, R.; Ma, W. Corrosion Resistance, Mechanical and Magnetic Properties of Cold-Sprayed Al Coating on Sintered NdFeB Magnet. J. Therm. Spray Technol. 2021, 30, 2117–2127. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, N.; Sun, Y.; Yang, L.; Guan, C.; Zhang, E.; Fang, X.; Liu, J. Structure and Corrosion Resistance Characteristics of ZnAl/EP Coating on Bonded NdFeB Magnet. J. Mater. Eng. Perform. 2022, 32, 5475–5482. [Google Scholar] [CrossRef]
- Fujimura, S.; Sagawa, M.; Matsuura, Y. Isotropic Permanet Magnet and Manufacture Thereof. JPH0467322B2, 19 November 1984. [Google Scholar]
- Croat, J.J. High Energy Product Rare Earth-Iron Magnet Alloys. U.S. Patent 4802931A, 24 June 1983. [Google Scholar]
- Lee, R.W.; Croat, J.J. Bonded Rare Earth-Iron Magnets. AU571497B2, 26 August 1983. [Google Scholar]
- Inoue, K. Production of Magnet. JPS6314827A, 4 July 1986. [Google Scholar]
- Rozendaal, E. Method and Apparatus for the Manufacture of Rare Earth Transition Metal Alloy Magnets. GB2196479A, 20 October 1986. [Google Scholar]
- Hiroyoshi, H.; Wakechigai, T.; Koyama, Y. Single Magnetic Domain Grain Magnet and Manufacture Thereof, and Manufacture of Metallic Binedr Anisotropic Magnet. JPS6481302A, 24 September 1987. [Google Scholar]
- Shi, C.; Zhai, Y.; Guo, B.; Mi, Z. NdFeB Double-Plating Surface Protection Method. CN117248212A, 6 September 2023. [Google Scholar]
- Brown, D.; Ma, B.-M.; Chen, Z. Developments in the Processing and Properties of NdFeb-Type Permanent Magnets. J. Magn. Magn. Mater. 2002, 248, 432–440. [Google Scholar] [CrossRef]
- Cvelbar, U.; Mozetič, M. Method for Improving the Electrical Connection Properties of the Surface of a Product Made from a Polymer-Matrix Composite. EP1828434B1, 16 January 2008. [Google Scholar]
- Xu, G.; Lee, S.; Lyu, J.; Wu, Y.; Wang, D.; Cui, J.; Shen, W. Preparation Method of Neodymium Iron Boron Surface Modified Hexagonal Boron Nitride Reinforced Epoxy Composite Coating. CN116285578A, 24 March 2023. [Google Scholar]
- Shaochun, T.; Hongbin, L.; Chenglong, L.; Weiwei, T. Preparation Method of High-Wear-Resistance and High-Corrosion-Resistance Protective Coating on Neodymium Iron Boron Surface. CN114381786B, 31 October 2023. [Google Scholar]
- Jinhe, W.; Liyi, S.; Di, Z.; Xiong, Z.; Lin, M. Method for Modifying Boron Nitride Nanosheet Surface with Polydopamine. CN106554514B, 23 October 2018. [Google Scholar]
- Yan, M.; Jin, J.; Chen, W.; Wu, C. Method for Improving Corrosion Resistance of neodymium-Iron-Boron Materials by Low-Temperature Oxidation and/or Nitridation Treatment. U.S. Patent 2023282414A1, 7 September 2023. [Google Scholar]
- Godec, M.; Ruiz-Zepeda, F.; Podgornik, B.; Donik, Č.; Kocijan, A.; Skobir Balantič, D.A. The influence of the plasma-nitriding temperature on the microstructure evolution and surface properties of additive-manufactured 18Ni300 maraging steel. Surf. Coat. Technol. 2022, 433, 128089. [Google Scholar] [CrossRef]
- Fujihara, M.; Yoshimura, K.; Kikugawa, A. Process for Production of Surface-Modified Rare Earth Sintered Magnets and Surface-Modified Rare Earth Sintered Magnets. WO2009041639A1, 2 April 2009. [Google Scholar]
- Amherd-Hidalgo, A.; Boulmay, A.; Vuille, P. Corrosion-Inhibiting Protection for Watch Magnets, in Particular Neodymium-Iron-Boron Magnets. U.S. Patent 2022154327A1, 19 May 2022. [Google Scholar]
- Cheng, F.; Li, Y.; Zheng, Q.; Wei, L.; Zhang, C.; Da, B.; Zeng, Z. Sensitivity of ion implantation to low-energy electronic stopping cross-sections. Radiat. Phys. Chem. 2023, 204, 110681. [Google Scholar] [CrossRef]
- Iskanderova, Z.A.; Radjabov, T.D.; Leiderman, R.J.; Rakhimova, G.R.; Tukfatullin, F.K. The influence of ion implantation conditions on depth distribution and retention of implanted impurities. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1986, 14, 542–554. [Google Scholar] [CrossRef]
- Savaloni, H.; Modiri, F. Surface nano-structural modifications and characteristics in nitrogen ion implanted W as a function of temperature and N+ energy. Appl. Surf. Sci. 2006, 253, 1135–1142. [Google Scholar] [CrossRef]
- Bai, X.; Yu, H.; Pan, G.; Han, X. Neodymium-Iron-Boron Sintered Magnet and Method of Anti-Corrosion Treatment. U.S. Patent 2023178274A1, 8 June 2023. [Google Scholar]
- Jiang, L.; Liang, Y.; Ju, W.; Xu, S.; Tao, Z.; Cheng, F.; Wei, G. Rare Earth-Silane Composite Film on Sintered NdFeB Surface and Preparation Method of Rare Earth-Silane Composite Film. CN116190041A, 30 May 2023. [Google Scholar]
- Li, X.; Guo, C.; Lai, H.; Peng, C. NdFeB Plating Trivalent Black Chromium and Graphene Closed Plating Structure. CN218786671U, 4 April 2023. [Google Scholar]
- Xu, G.; Duan, L.; Su-Nam, L.; Zhang, P.; Lyu, J.; Wang, D.; Wu, Y. Preparation Method for Forming Titanium Salt-Silane Composite Passivation Layer on Surface of Electroplated Zn Coating of Sintered NdFeB Magnet. CN115896768A, 4 April 2023. [Google Scholar]
- Gosar, Ž.; Kovač, J.; Mozetič, M.; Primc, G.; Vesel, A.; Zaplotnik, R. Deposition of SiOxCyHz Protective Coatings on Polymer Substrates in an Industrial-Scale PECVD Reactor. Coatings 2019, 9, 234. [Google Scholar] [CrossRef]
- Pigliaru, L.; Paleari, L.; Bragaglia, M.; Nanni, F.; Ghidini, T.; Rinaldi, M. Poly-ether-ether-ketone—Neodymium-iron-boron bonded permanent magnets via fused filament fabrication. Synth. Met. 2021, 279, 116857. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Surface Modification of Polymers by Plasma Treatment for Appropriate Adhesion of Coatings. Materials 2024, 17, 1494. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.L. Thin film nucleation and growth kinetics. Appl. Surf. Sci. 1988, 33–34, 379–394. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Yi, S.-W.; Yu, I.-K.; Kim, W.-J.; Choi, S.-H. Cold Plasma Deposition of Polymeric Nanoprotrusion, Nanoparticles, and Nanofilm Structures on a Slide Glass Surface. Processes 2021, 9, 99. [Google Scholar] [CrossRef]
- Popok, V.N.; Kylián, O. Formation of Advanced Nanomaterials by Gas-Phase Aggregation. Appl. Nano 2021, 2, 82–84. [Google Scholar] [CrossRef]
- Golroudbary, S.R.; Makarava, I.; Kraslawski, A.; Repo, E. Global environmental cost of using rare earth elements in green energy technologies. Sci Total Environ. 2022, 832, 155022. [Google Scholar] [CrossRef]
- Kumari, A.; Sahu, S.K. A comprehensive review on recycling of critical raw materials from spent neodymium iron boron (NdFeB) magnet. Sep. Purif. Technol. 2023, 317, 123527. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primc, G.; Mozetič, M. Recent Advances in Corrosion Inhibition of Bonded NdFeB Magnets. Materials 2024, 17, 2475. https://doi.org/10.3390/ma17112475
Primc G, Mozetič M. Recent Advances in Corrosion Inhibition of Bonded NdFeB Magnets. Materials. 2024; 17(11):2475. https://doi.org/10.3390/ma17112475
Chicago/Turabian StylePrimc, Gregor, and Miran Mozetič. 2024. "Recent Advances in Corrosion Inhibition of Bonded NdFeB Magnets" Materials 17, no. 11: 2475. https://doi.org/10.3390/ma17112475





