Synthesis of Fe-Modified g-C3N4 Nanorod Bunches for the Efficient Photocatalytic Degradation of Oxytetracycline
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of FCNBs and g-C3N4
2.3. Characterizations
2.4. Photoelectrochemical Measurements
2.5. The Performance Measure of Catalysts
3. Results and Discussion
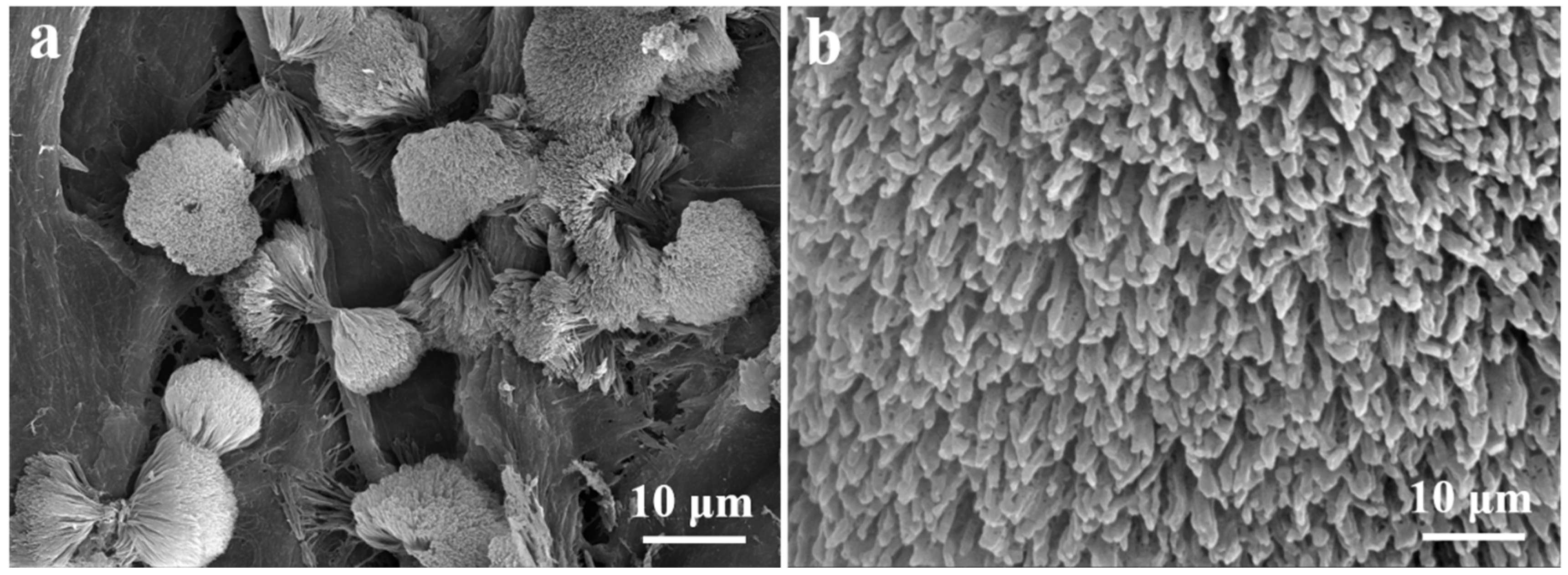
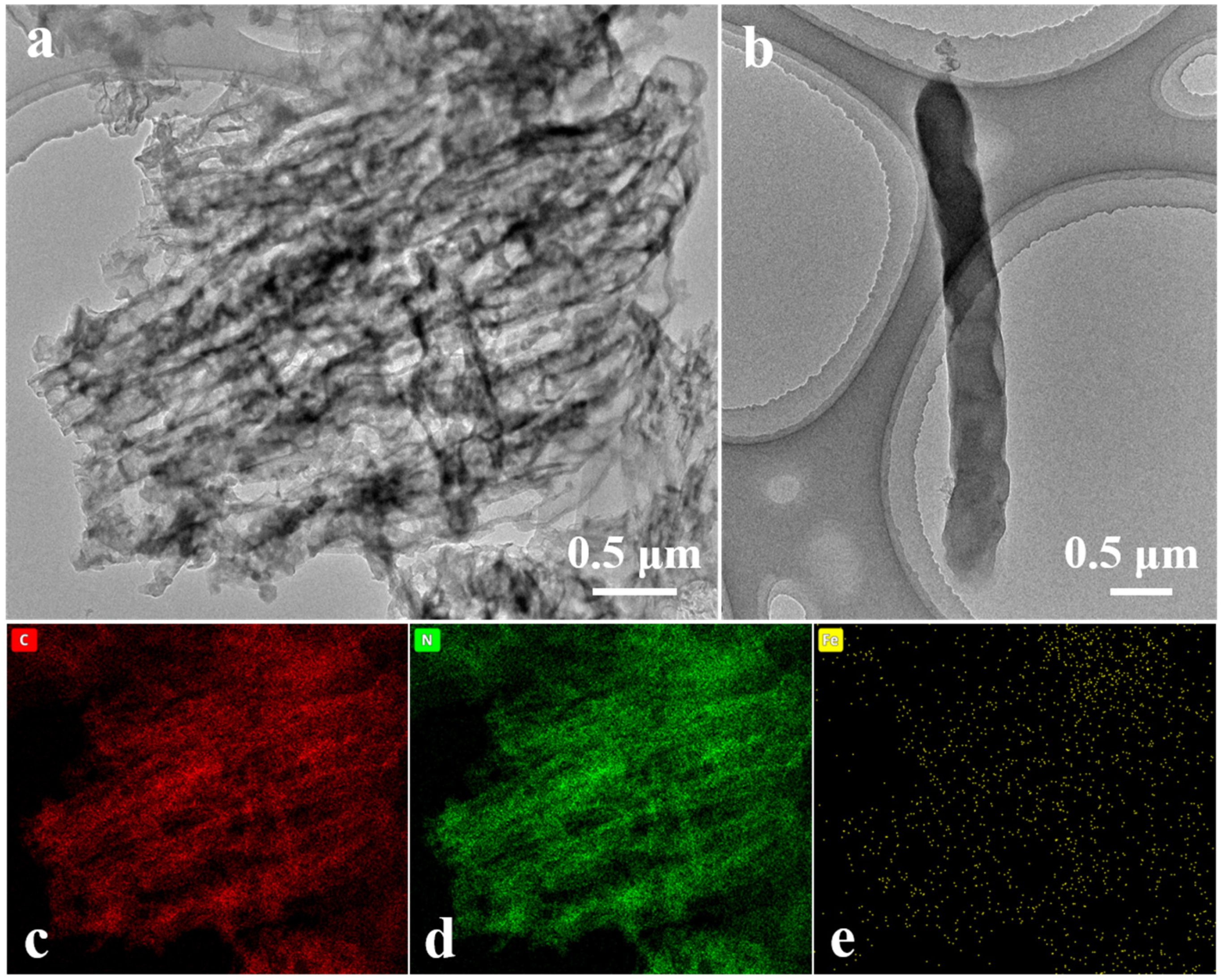
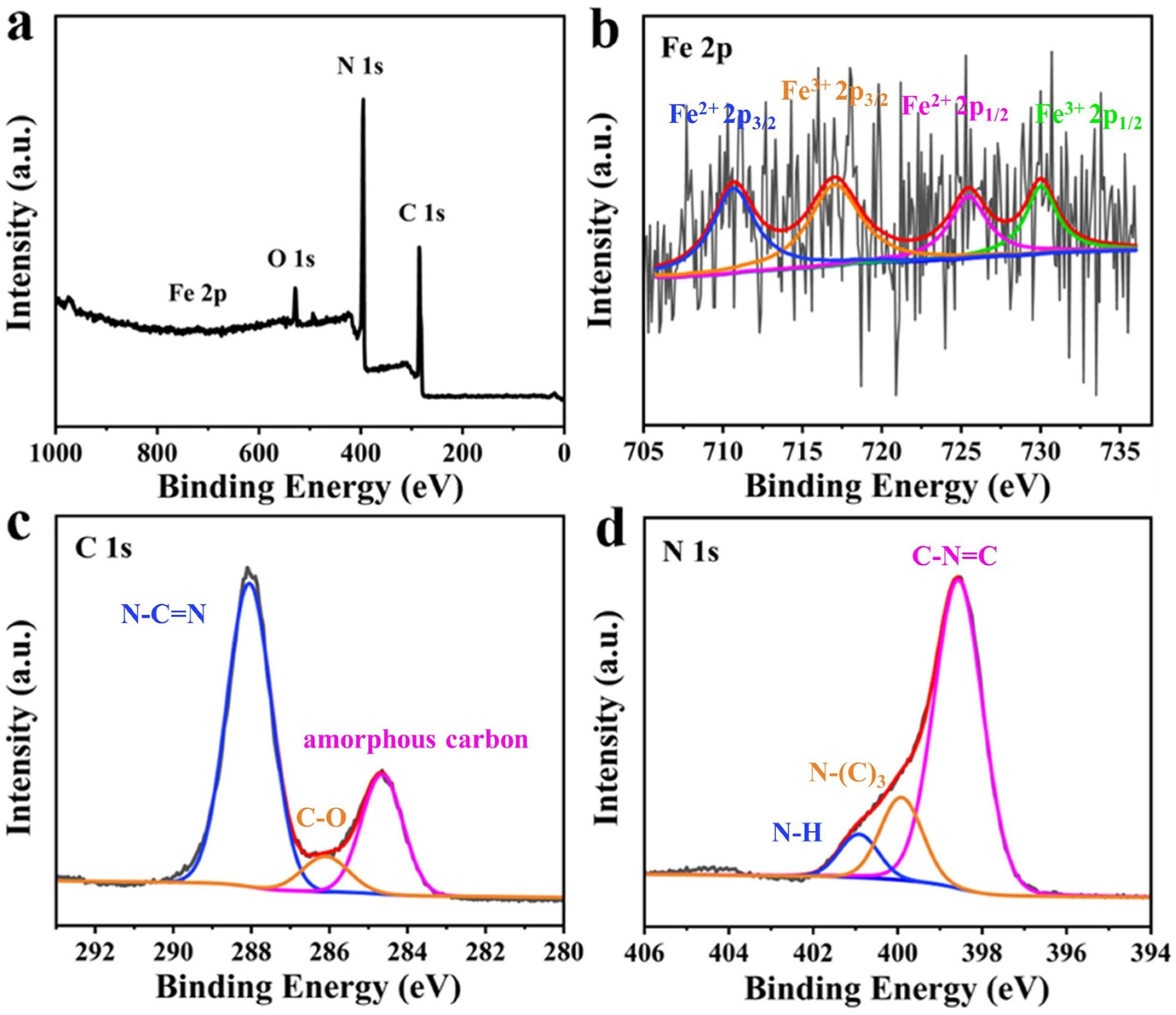
3.1. Characterization of Catalysts
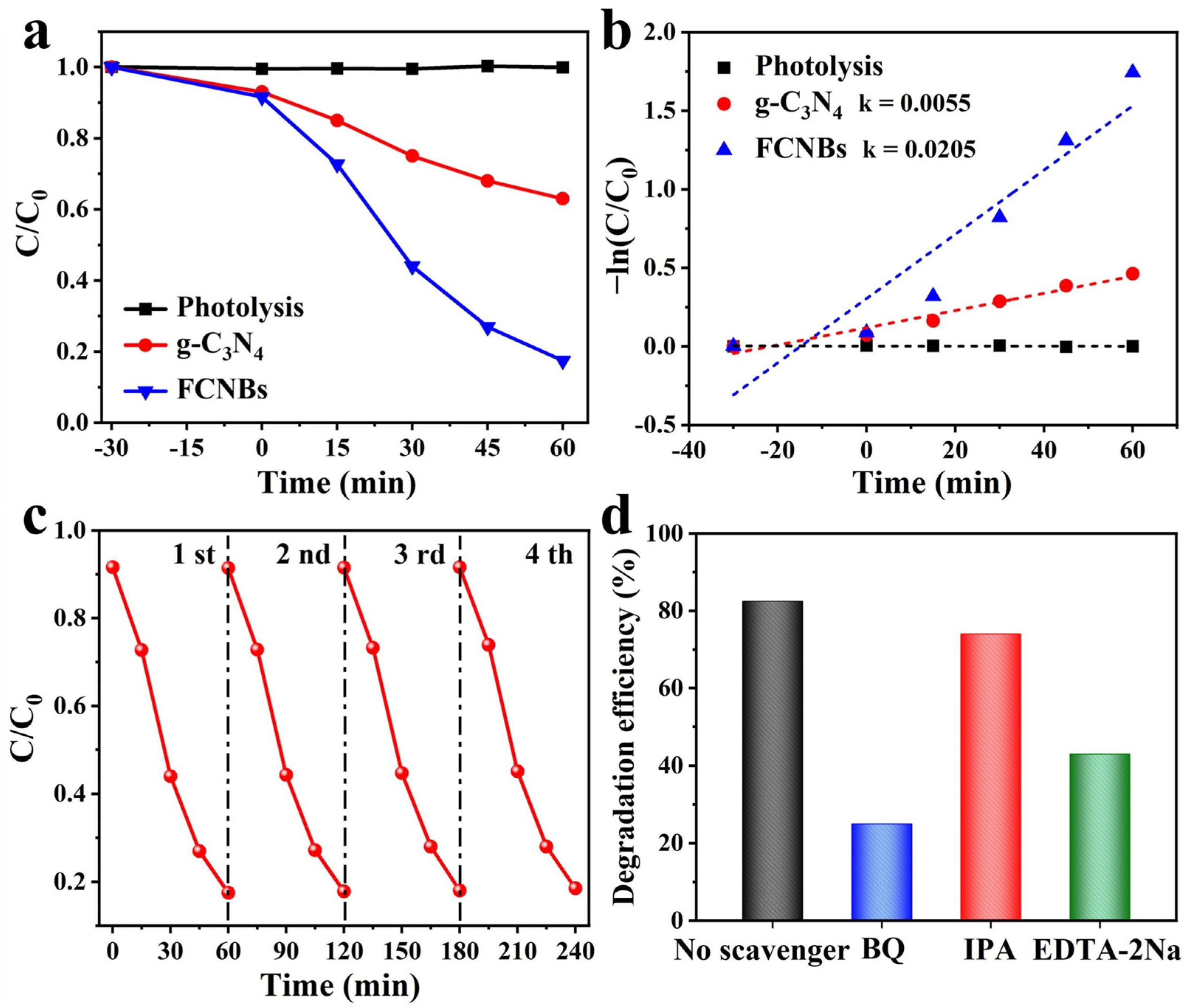
3.2. Photocatalytic Activity Measurement
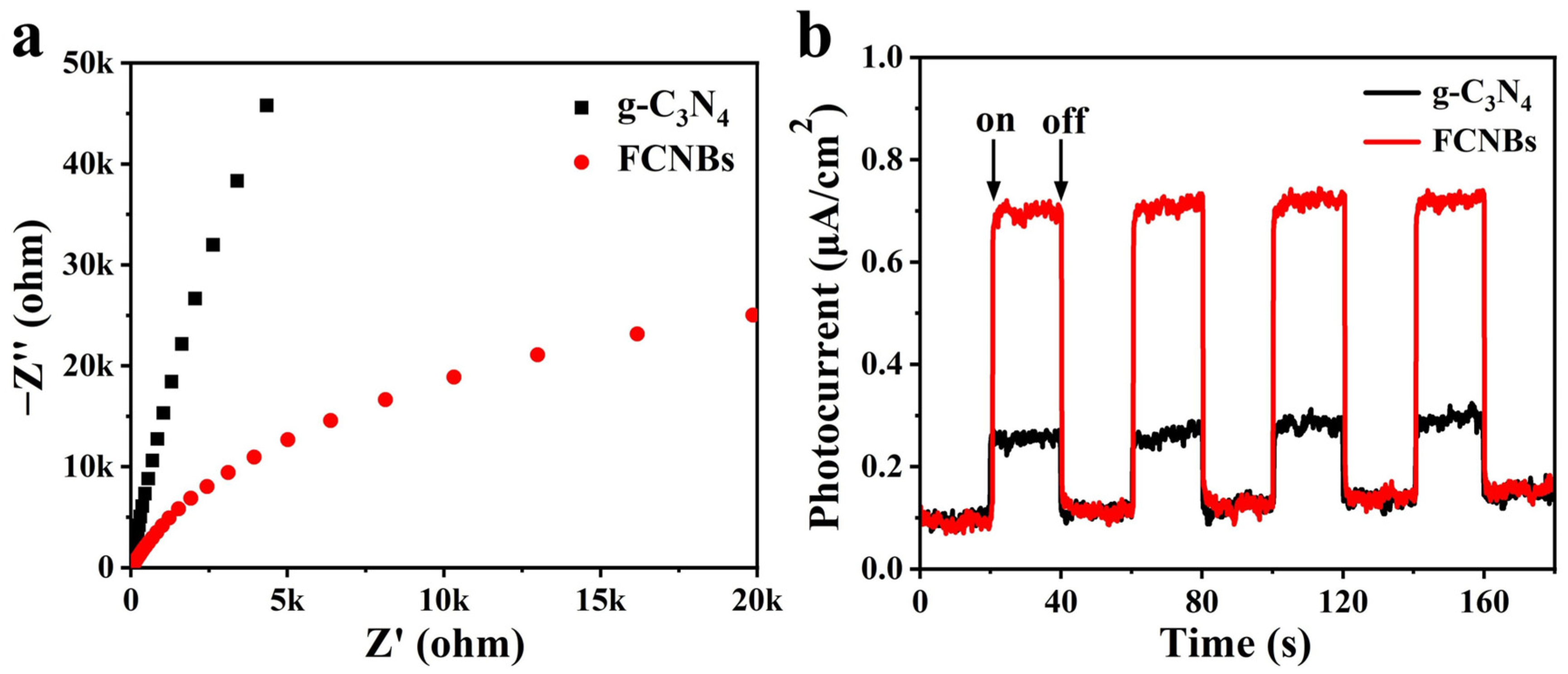
3.3. Photocatalytic Mechanism
3.4. Possible Degradation Pathway of OTC by FCNBs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rej, S.; Hejazi, S.M.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, Š.; Fornasiero, P.; Naldoni, A. Light-Induced Defect Formation and Pt Single Atoms Synergistically Boost Photocatalytic H 2 Production in 2D TiO2-Bronze Nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]
- Ali, H.; Masar, M.; Yasir, M.; Machovsky, M.; Monteiro, O.C.; Kuritka, I. Current Trends in Environmental and Energy Photocatalysis and ISO Standardization. J. Environ. Chem. Eng. 2023, 11, 111541. [Google Scholar] [CrossRef]
- Amen, R.; Elsayed, I.; Schueneman, G.T.; Hassan, E.B. Self-Assembled Aminated and TEMPO Cellulose Nanofibers (Am/TEMPO-CNF) Aerogel for Adsorptive Removal of Oxytetracycline and Chloramphenicol Antibiotics from Water. Gels 2024, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, S.; Kim, D.-G.; Ko, S.-O. Construction of Surface-Rich MoS2 Nanoflowers Decorated on 2D Layered MXene Nanohybrid Heterostructure for Highly Efficient and Rapid Degradation of Oxytetracycline. Chemosphere 2024, 352, 141339. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, Fate, and Risk Assessment of Typical Tetracycline Antibiotics in the Aquatic Environment: A Review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Kong, Y.; Lu, H.; Wang, R.; Yang, Q.; Huang, B.; Zhou, Q.; Hu, W.; Zou, J.; Chen, Q. Adsorption Characteristics of Tetracycline Hydrochloride and Oxytetracycline by a MOF-525(Co) Metal Organic Framework. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132443. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, S. Enhanced Removal of Oxytetracycline from Aquatic Solution Using MnOx@Fe3O4 Bimetallic Nanoparticle Coated Powdered Activated Carbon: Synergism of Adsorption and Chemical Autocatalytic Oxidation Processes. Environ. Sci. Nano 2023, 10, 3171–3183. [Google Scholar] [CrossRef]
- Xia, M.; Niu, Q.; Qu, X.; Zhang, C.; Qu, X.; Li, H.; Yang, C. Simultaneous Adsorption and Biodegradation of Oxytetracycline in Wastewater by Mycolicibacterium Sp. Immobilized on Magnetic Biochar. Environ. Pollut. 2023, 339, 122728. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Chen, D.; Xu, W.; Fang, J.; Liu, Z.; Li, R.; Yao, L.; Qin, J.; Fang, Z. Anatase/Rutile Homojunction Quantum Dots Anchored on g-C3N4 Nanosheets for Antibiotics Degradation in Seawater Matrice via Coupled Adsorption-Photocatalysis: Mechanism Insight and Toxicity Evaluation. Chem. Eng. J. 2022, 432, 134375. [Google Scholar] [CrossRef]
- Hu, C.; Chen, F.; Wang, Y.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Exceptional Cocatalyst-Free Photo-Enhanced Piezocatalytic Hydrogen Evolution of Carbon Nitride Nanosheets from Strong In-Plane Polarization. Adv. Mater. 2021, 33, 2101751. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Sun, C.; Yang, S.; Guan, Y.; He, H. Synthesis and Characterization of g-C3N4/Ag3VO4 Composites with Significantly Enhanced Visible-Light Photocatalytic Activity for Triphenylmethane Dye Degradation. Appl. Catal. B Environ. 2014, 144, 885–892. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Kong, X.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. In Situ Synthesis of a Novel Mn3O4/g-C3N4 p-n Heterostructure Photocatalyst for Water Splitting. J. Colloid Interface Sci. 2021, 586, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X.; Liu, Y.; Jiang, Y.; Ma, Y. Graphitic Carbon Nitride with the Pyridinic N Substituted by Al and Si as Efficient Photocatalysts for CO2 Reduction. J. Mater. Chem. A 2024, 12, 854–863. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, B.; Wu, D.; Tian, C.; Ran, H.; Chen, W.; Huang, Q.; Zhang, W.; Qi, F.; Zhang, N.; et al. Enhanced Photocatalytic Activity of Lead-Free Cs2TeBr6/g-C3N4 Heterojunction Photocatalyst and Its Mechanism. Adv. Funct. Mater. 2024, 34, 2308411. [Google Scholar] [CrossRef]
- Kadam, A.N.; Kim, H.; Lee, S.-W. Low-Temperature in Situ Fabrication of Porous S-Doped g-C3N4 Nanosheets Using Gaseous-Bubble Template for Enhanced Visible-Light Photocatalysis. Ceram. Int. 2020, 46, 28481–28489. [Google Scholar] [CrossRef]
- Tang, J.; Guo, R.; Zhou, W.; Huang, C.; Pan, W. Ball-Flower like NiO/g-C3N4 Heterojunction for Efficient Visible Light Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2018, 237, 802–810. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon Nitride Hollow Spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Dou, T.; Zang, L.; Zhang, Y.; Sun, Z.; Sun, L.; Wang, C. Hybrid G-C3N4 Nanosheet/Carbon Paper Membranes for the Photocatalytic Degradation of Methylene Blue. Mater. Lett. 2019, 244, 151–154. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, X.; Li, Z.; Zhang, J.; Wang, T.; Cao, S. Construction of Local Coordination Environment of Iron Sites over G- C3N4/PCN-222(Fe) Composite with High CO2 Photoreduction Performance. Appl. Catal. B Environ. 2024, 344, 123639. [Google Scholar] [CrossRef]
- Abdelgawad, A.; Salah, B.; Lu, Q.; Abdullah, A.M.; Chitt, M.; Ghanem, A.; Al-Hajri, R.S.; Eid, K. Template-Free Synthesis of M/g-C3N4 (M = Cu, Mn, and Fe) Porous One-Dimensional Nanostructures for Green Hydrogen Production. J. Electroanal. Chem. 2023, 938, 117426. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, F.; Chen, Z.; Huang, S.; Wei, W.; Xie, M.; Xu, H.; Li, H. One-Step Synthesis of Fe-Doped Surface-Alkalinized g- C3N4 and Their Improved Visible-Light Photocatalytic Performance. Appl. Surf. Sci. 2019, 469, 739–746. [Google Scholar] [CrossRef]
- Sudrajat, H. Evidence of Improved Electron–Hole Separation in Fe@g-C3N4 Photocatalysts. Mater. Res. Express 2018, 5, 095501. [Google Scholar] [CrossRef]
- Nguyen Van, M.; Mai, O.; Pham Do, C.; Lam Thi, H.; Pham Manh, C.; Nguyen Manh, H.; Pham Thi, D.; Do Danh, B. Fe-Doped g-C3N4: High-Performance Photocatalysts in Rhodamine B Decomposition. Polymers 2020, 12, 1963. [Google Scholar] [CrossRef] [PubMed]
- Omr, H.A.E.; Putikam, R.; Feng, S.-P.; Lin, M.-C.; Lee, H. Synergistic Role of Cu-C and Cu-N Dual Bonding of Nanostructured g- C3N4/Cu2SnS3 Photocatalysts for Efficient CO2 Conversion to CO. Appl. Catal. B Environ. 2023, 339, 123103. [Google Scholar] [CrossRef]
- Hu, E.; Chen, Q.; Gao, Q.; Fan, X.; Luo, X.; Wei, Y.; Wu, G.; Deng, H.; Xu, S.; Wang, P.; et al. Cyano-Functionalized Graphitic Carbon Nitride with Adsorption and Photoreduction Isosite Achieving Efficient Uranium Extraction from Seawater. Adv. Funct. Mater. 2024, 34, 2312215. [Google Scholar] [CrossRef]
- Ma, T.; Shen, Q.; Zhaoa, B.; Xue, J.; Guan, R.; Liu, X.; Jia, H.; Xu, B. Facile Synthesis of Fe-Doped g-C3N4 for Enhanced Visible-Light Photocatalytic Activity. Inorg. Chem. Commun. 2019, 107, 107451. [Google Scholar] [CrossRef]
- Guo, W.; Fan, K.; Zhang, J.; Xu, C. 2D/2D Z-Scheme Bi2WO6/Porous-g-C3N4 with Synergy of Adsorption and Visible-Light-Driven Photodegradation. Appl. Surf. Sci. 2018, 447, 125–134. [Google Scholar] [CrossRef]
- Xu, Q.; Cheng, B.; Yu, J.; Liu, G. Making Co-Condensed Amorphous Carbon/g-C3N4 Composites with Improved Visible-Light Photocatalytic H2-Production Performance Using Pt as Cocatalyst. Carbon 2017, 118, 241–249. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, C.; Mei, J.; Yang, S.; Wong, P.K. Faster Electron Injection and Higher Interface Reactivity in g-C3N4/Fe2O3 Nanohybrid for Efficient Photo-Fenton-like Activity toward Antibiotics Degradation. Environ. Res. 2021, 195, 110842. [Google Scholar] [CrossRef]
- Khaing, K.K.; Yin, D.; Xiao, S.; Deng, L.; Zhao, F.; Liu, B.; Chen, T.; Li, L.; Guo, X.; Liu, J.; et al. Efficient Solar Light Driven Degradation of Tetracycline by Fe-EDTA Modified g-C3N4 Nanosheets. J. Phys. Chem. C 2020, 124, 11831–11843. [Google Scholar] [CrossRef]
- Wang, J.; Zang, L.; Wang, L.; Tian, Y.; Yang, Z.; Yue, Y.; Sun, L. Magnetic Cobalt Ferrite/Reduced Graphene Oxide (CF/rGO) Porous Balls for Efficient Photocatalytic Degradation of Oxytetracycline. J. Environ. Chem. Eng. 2022, 10, 108259. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Long, M.; Bai, X.; Wen, Q.; Song, F. Solvothermal Synthesis of MIL-53Fe@g-C3N4 for Peroxymonosulfate Activation towards Enhanced Photocatalytic Performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130646. [Google Scholar] [CrossRef]
- Feng, J.; Ran, X.; Wang, L.; Xiao, B.; Lei, L.; Zhu, J.; Liu, Z.; Xi, X.; Feng, G.; Dai, Z.; et al. The Synergistic Effect of Adsorption-Photocatalysis for Removal of Organic Pollutants on Mesoporous Cu2V2O7/Cu3V2O8/g-C3N4 Heterojunction. Int. J. Mol. Sci. 2022, 23, 14264. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, Z.; Zhang, Q.; He, X.; Feng, Q.; Wang, D.; Ma, D. Biomass Porous Carbon as the Active Site to Enhance Photodegradation of Oxytetracycline on Mesoporous G-C3N4. RSC Adv. 2022, 12, 1840–1849. [Google Scholar] [CrossRef]
- Jingyu, H.; Ran, Y.; Zhaohui, L.; Yuanqiang, S.; Lingbo, Q.; Nti Kani, A. In-Situ Growth of ZnO Globular on g-C3N4 to Fabrication Binary Heterojunctions and Their Photocatalytic Degradation Activity on Tetracyclines. Solid State Sci. 2019, 92, 60–67. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Wang, X.; Wang, L.; Wang, J.; Wang, X.; Cheng, W. Synthesis of Fe-Modified g-C3N4 Nanorod Bunches for the Efficient Photocatalytic Degradation of Oxytetracycline. Materials 2024, 17, 2488. https://doi.org/10.3390/ma17112488
Zhao D, Wang X, Wang L, Wang J, Wang X, Cheng W. Synthesis of Fe-Modified g-C3N4 Nanorod Bunches for the Efficient Photocatalytic Degradation of Oxytetracycline. Materials. 2024; 17(11):2488. https://doi.org/10.3390/ma17112488
Chicago/Turabian StyleZhao, Dongmei, Xinyao Wang, Libin Wang, Jingzhen Wang, Xu Wang, and Weipeng Cheng. 2024. "Synthesis of Fe-Modified g-C3N4 Nanorod Bunches for the Efficient Photocatalytic Degradation of Oxytetracycline" Materials 17, no. 11: 2488. https://doi.org/10.3390/ma17112488
APA StyleZhao, D., Wang, X., Wang, L., Wang, J., Wang, X., & Cheng, W. (2024). Synthesis of Fe-Modified g-C3N4 Nanorod Bunches for the Efficient Photocatalytic Degradation of Oxytetracycline. Materials, 17(11), 2488. https://doi.org/10.3390/ma17112488


_Low.png)


