Corrosion Behavior of Nacre-Inspired (TiBw-TiB2)/Al Composites Fabricated by Freeze Casting
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bio-Inspired (TiB2-TiBw)/2024Al Composites
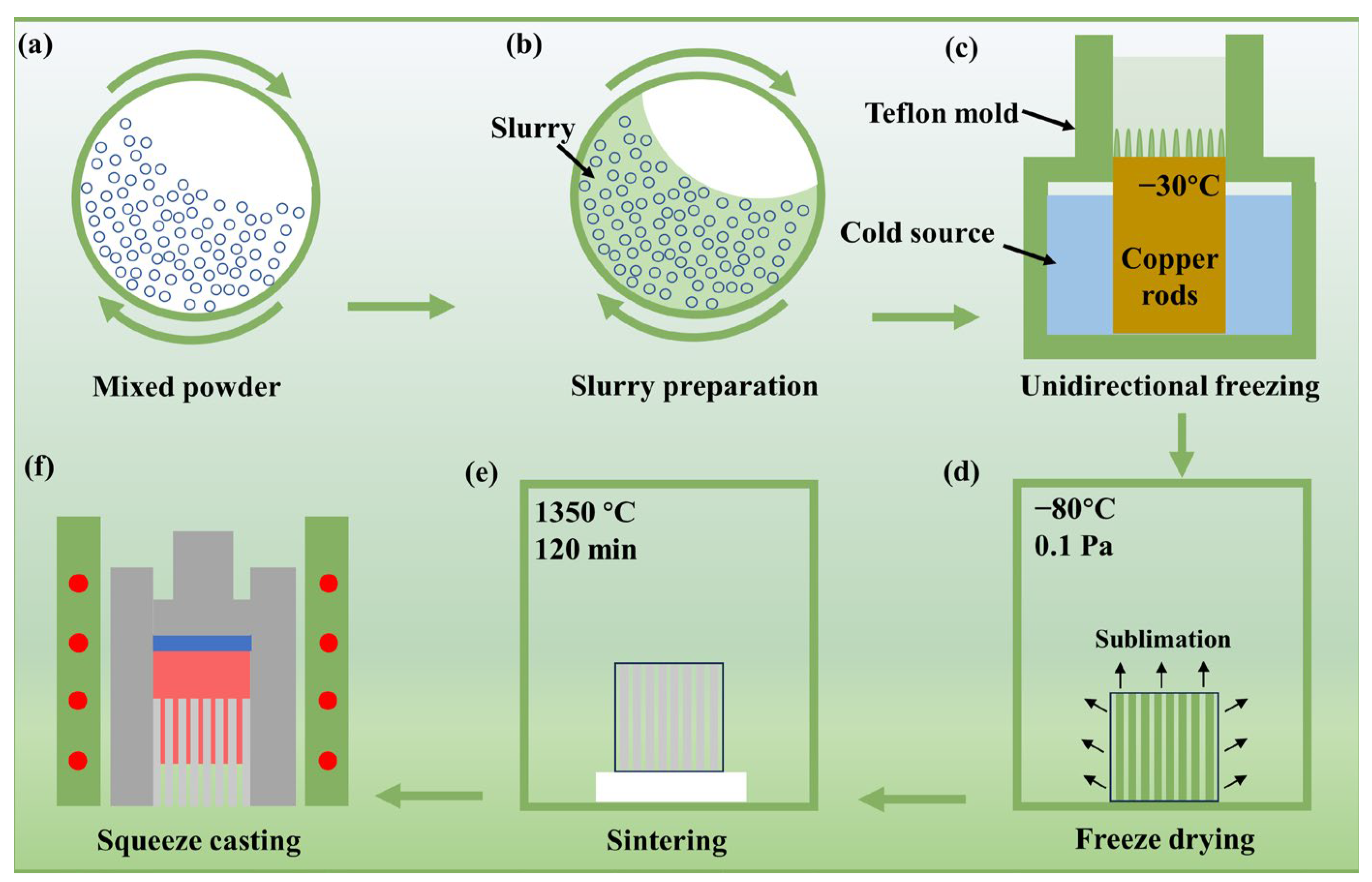
2.2. Corrosion Tests
2.3. Characterization Analysis
3. Results
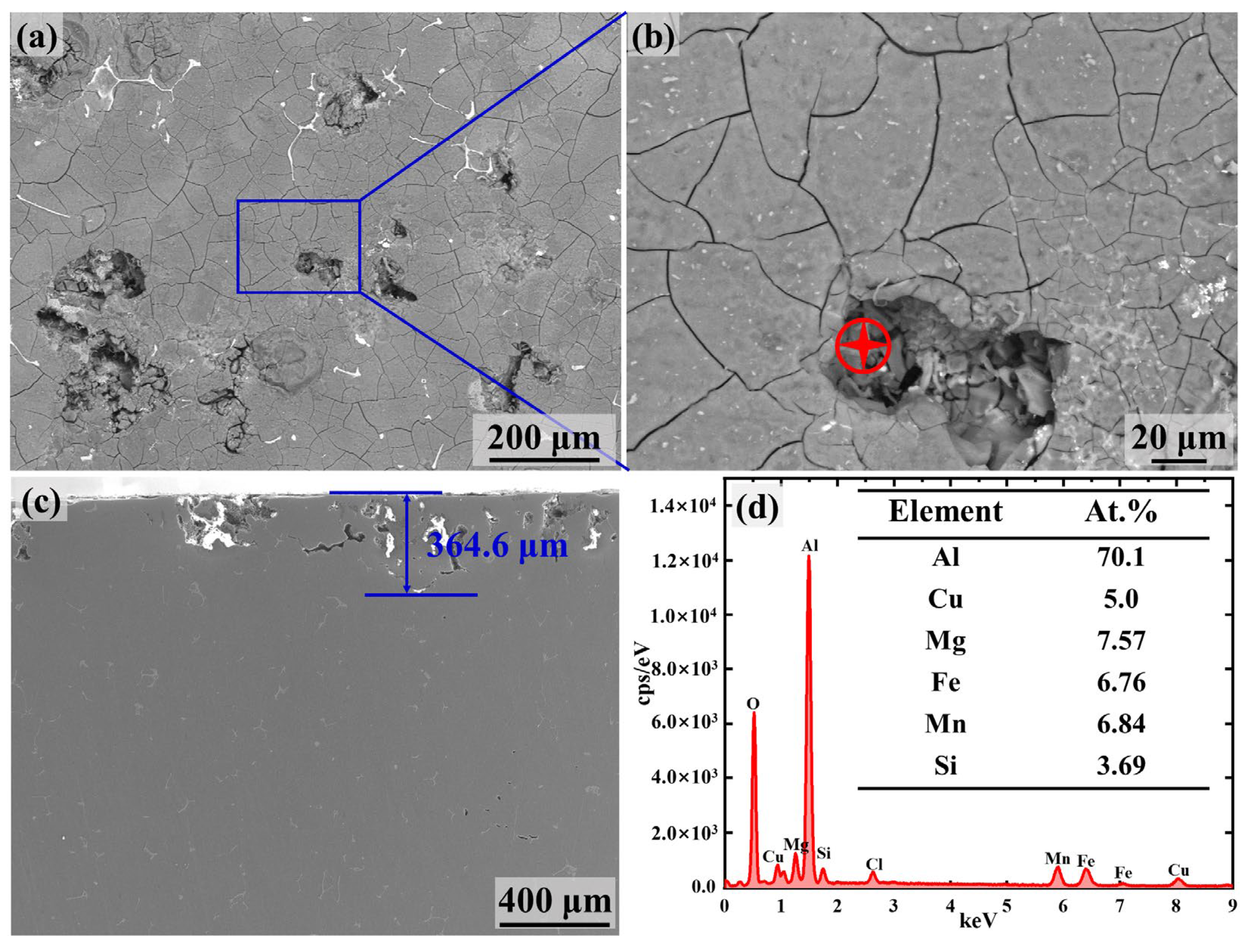
3.1. Microstructure Characterization
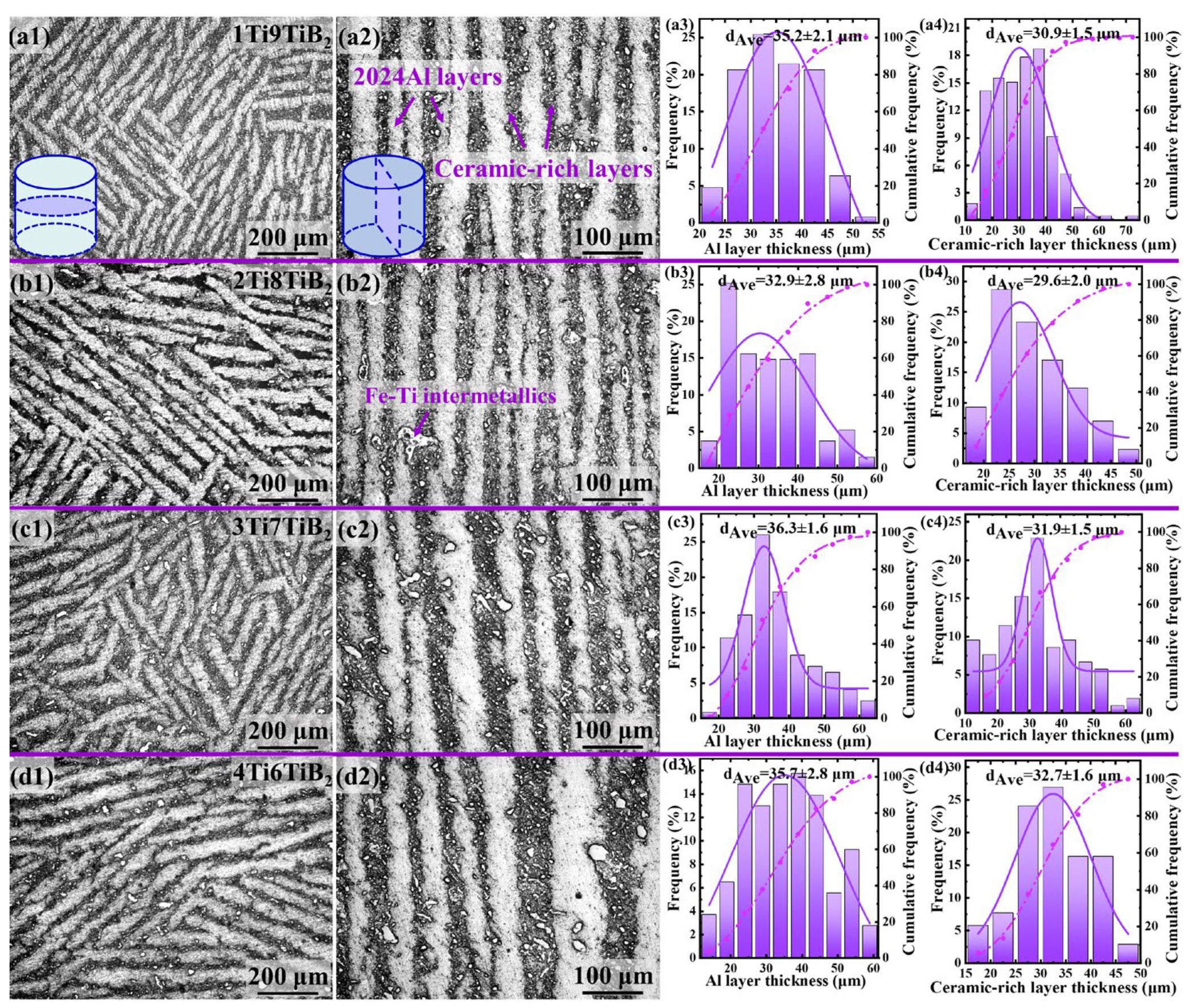
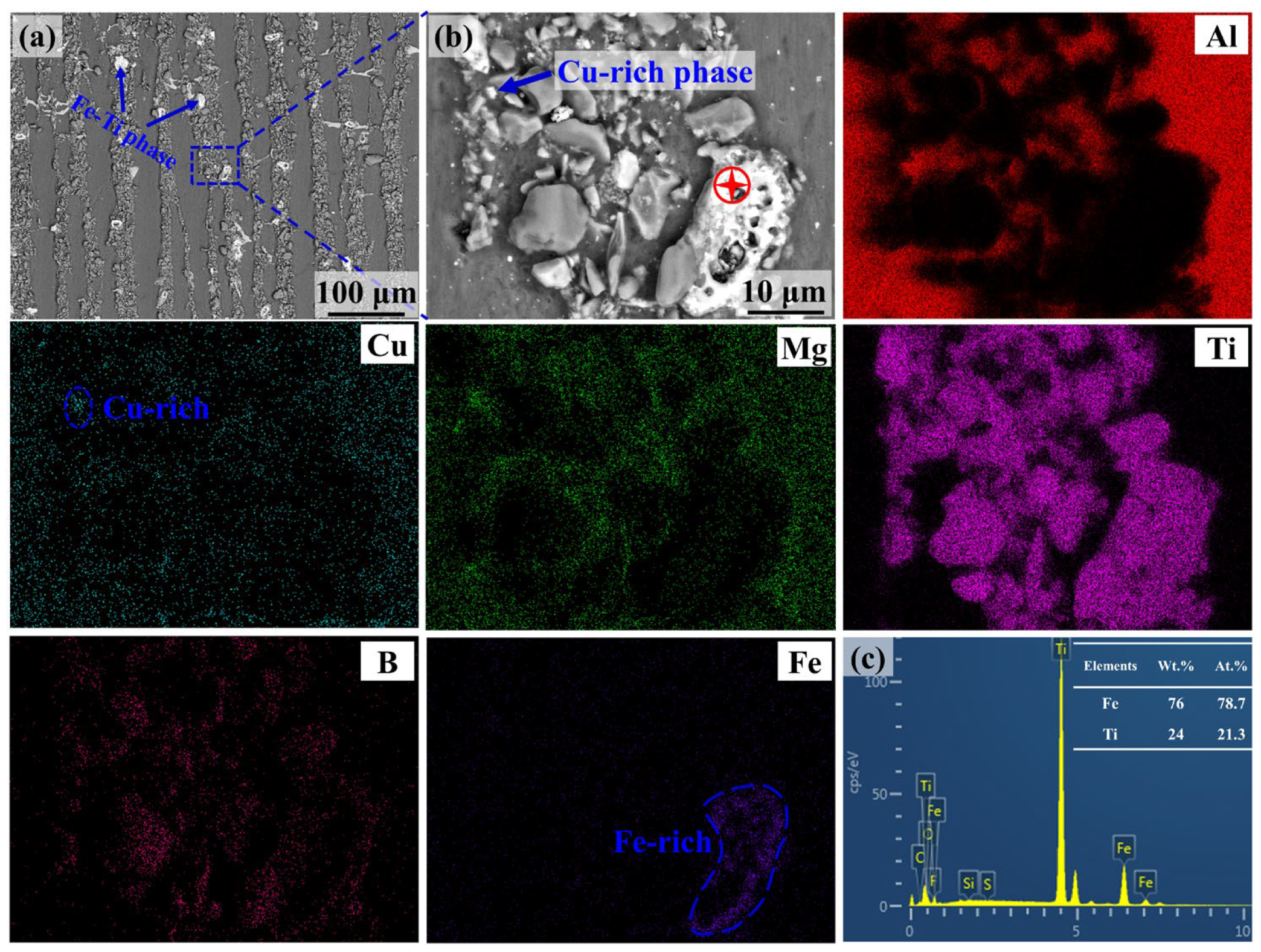
3.2. Electrochemical Corrosion Tests
3.2.1. Potentiodynamic Polarization

| Samples | Ecorr (V) | Icorr (μA/cm2) | Ep (V) | Ip (mA/cm2) | Eb (V) |
|---|---|---|---|---|---|
| 1Ti9TiB2 | −1.019 ± 0.08 | 46.75 ± 2.1 | −0.921 ± 0.05 | 58.0 ± 2.5 | −0.613 ± 0.03 |
| 2Ti8TiB2 | −0.858 ± 0.06 | 33.6 ± 2.7 | −0.815 ± 0.03 | 37.3 ± 2.0 | −0.661 ± 0.05 |
| 3Ti7TiB2 | −1.006 ± 0.06 | 15.9 ± 1.9 | −0.875 ± 0.03 | 29.4 ± 1.3 | −0.620 ± 0.02 |
| 4Ti6TiB2 | −1.017 ± 0.07 | 22.9 ± 1.3 | −0.934 ± 0.01 | 28.6 ± 1.1 | −0.629 ± 0.03 |

3.2.2. Electrochemical Impedance Spectroscopy (EIS)

| 1Ti9TiB2 | 2Ti8TiB2 | 3Ti7TiB2 | 4Ti6TiB2 | |
|---|---|---|---|---|
| Rs (ohm·cm2) | 6.022 ± 0.005 | 5.218 ± 0.004 | 6.95 ± 0.15 | 5.7 ± 0.4 |
| Rct (ohm·cm2) | 63.95 ± 0.03 | 118 ± 3 | 1914 ± 8 | 307.6 ± 6 |
| Q (ohm−1·cm−2·sn) | (5.8 ± 0.1) × 10−3 | (1.26 ± 0.02) × 10−3 | (1.65 ± 0.03) × 10−2 | (4.06 ± 0.05) × 10−3 |
| α | 0.46 ± 0.02 | 0.539 ± 0.006 | 0.819 ± 0.004 | 0.48 ± 0.03 |
| Ws (S·s1/2) | 346 ± 6 | 113.7 ± 3 | – | 104.9 ± 4 |
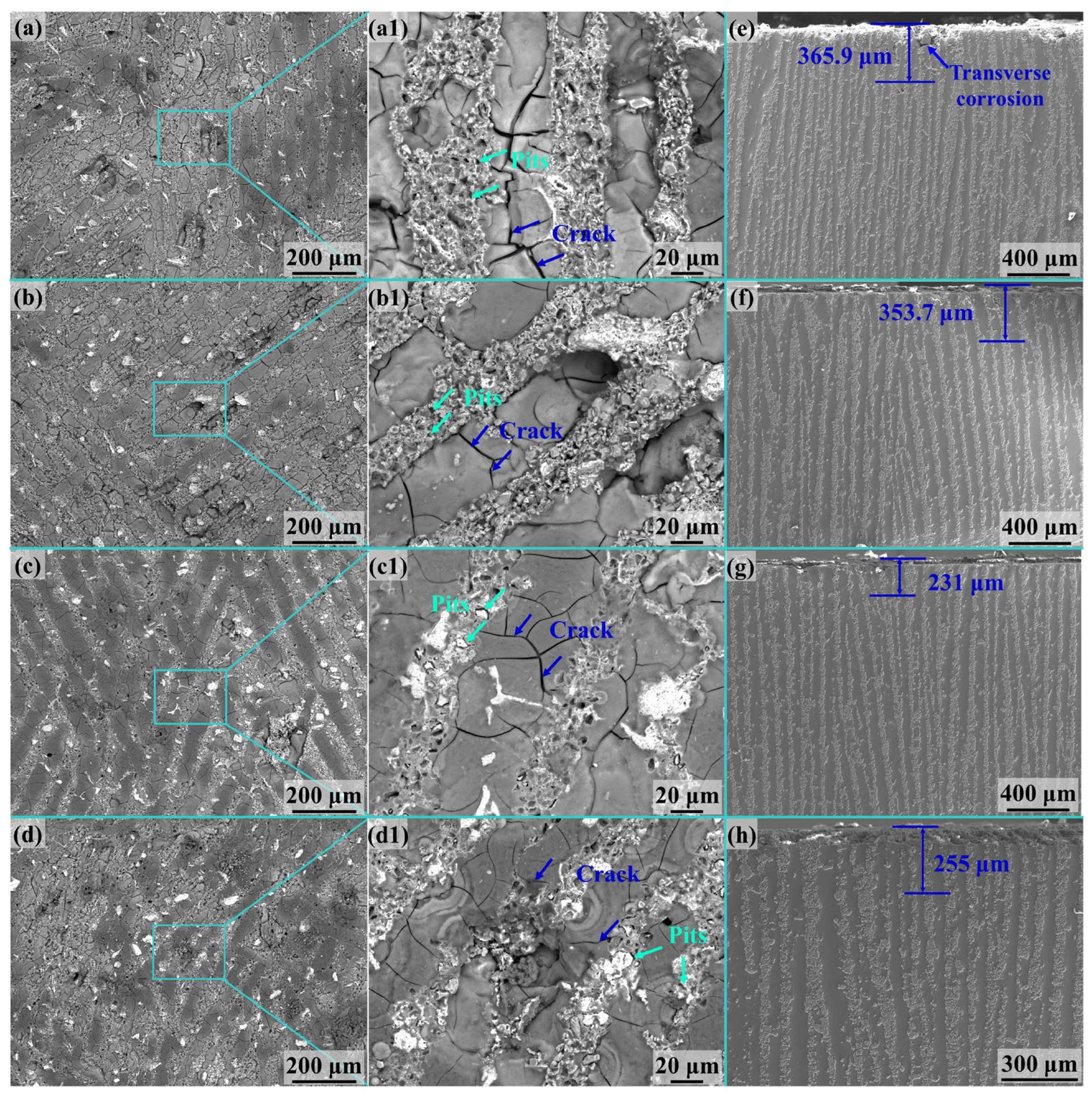
3.3. Intergranular Corrosion Testing
4. Discussion
4.1. Pitting Corrosion
4.2. Intergranular Corrosion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondou, E.; Proietti, A.; Charvillat, C.; Berziou, C.; Feaugas, X.; Sinopoli, D.; Blanc, C. Understanding the mechanisms of intergranular corrosion in 2024 Al alloy at the polycrystal scale. Corros. Sci. 2023, 221, 111338. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Z.; Zhu, Q.; Wang, G.; Zuo, Y.; Li, Q.; Qin, G. Hot-top direct chill casting assisted by a twin-cooling field: Improving the ingot quality of a large-size 2024 Al alloy. J. Mater. Sci. Technol. 2022, 112, 114–122. [Google Scholar] [CrossRef]
- Kim, B.-J.; Kim, S.-H.; Kayani, S.H.; Lee, Y.-H.; Kim, W.-K.; Cheon, H.-S.; Kim, J.; Cho, Y.-H. Melt thermal-rate treatment for uniform solute distribution and improved mechanical properties of an Al-Zn-Mg-Cu alloy prepared by direct-chill casting. J. Alloy. Compd. 2023, 967, 171745. [Google Scholar] [CrossRef]
- Mei, X.; Mei, Q.; Peng, Y.; Chen, Z.; Xu, T.; Wang, Y. Achieving enhanced mechanical properties of SiC/Al–Cu nanocomposites via simultaneous solid-state alloying of Cu and dispersing of SiC nanoparticles. Mater. Sci. Eng. A 2022, 860, 144338. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, G.; Tan, Z.; Zhao, H.; Xu, Y.; Xiong, D.; Li, Z. Towards the strength-ductility synergy of Al2O3/Al composite through the design of roughened interface. Compos. Part B 2021, 224, 109251. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, W.; Kang, P.; Chao, Z.; Han, H.; Zhang, R.; Du, S.; Han, B.; Zhao, Q.; Wu, G. Enhanced ductility of B4C/Al composites by controlling thickness of interfacial oxide layer through high temperature oxidation of B4C particles. J. Alloy. Compd. 2023, 937, 168486. [Google Scholar] [CrossRef]
- Liu, G.F.; Chen, T.J.; Wang, Z.J. Effects of solid solution treatment on microstructure and mechanical properties of SiCp/2024 Al composite: A comparison with 2024 Al alloy. Mater. Sci. Eng. A 2021, 817, 141413. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Chen, H.; Liu, J.; Dan, C.; Ma, S.; Wang, H.; Chen, Z. Enhancing mechanical properties and improving mechanical anisotropy of rolled 2024 Al sheet by TiB2 nanoparticles. Mater. Sci. Eng. A 2023, 874, 145077. [Google Scholar] [CrossRef]
- Zhao, D.-X.; Li, S.-S.; Yang, L.-K.; Zhang, Z.-H.; Shen, P. Near-net shaping of laminated Al/Al2O3 composites by direct ink writing and pressure infiltration. Mater. Sci. Eng. A 2022, 860, 144267. [Google Scholar] [CrossRef]
- Zhong, Z.; Jiang, X.; Wang, X.; Sun, H.; Du, P.; Wu, Z.; Yang, L.; Zhong, Z.; Jiang, X.; Wang, X.; et al. Enhanced strength and ductility in nanocarbon hybrid reinforced B4C/Al laminated composites fabricated by vacuum hot pressing. Vacuum 2023, 218, 112651. [Google Scholar] [CrossRef]
- Han, B.; Jiang, L.; Han, H.; Zhang, R.; Du, S.; Luo, T.; Gong, D.; Xue, W.; Chao, Z.; Chen, G. Mitigating the strength-ductility inversion in B4C/Al composites by constructing heterogeneous structure. Mater. Sci. Eng. A 2023, 888, 145792. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Q.; Li, Z.; Fan, G.; Xiong, D.-B.; Su, Y.; Zhang, J.; Zhang, D. Enhanced Mechanical Properties of Graphene (Reduced Graphene Oxide)/Aluminum Composites with a Bioinspired Nanolaminated Structure. Nano Lett. 2015, 15, 8077–8083. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-K.; Jin, Q.; Guo, R.-F.; Shen, P. Exploiting bio-inspired high energy-absorbent metal/ceramic composites through emulsion-ice-templating and melt infiltration. Materialia 2020, 14, 100884. [Google Scholar] [CrossRef]
- Chao, Z.; Wang, Z.; Jiang, L.; Chen, S.; Pang, B.; Zhang, R.; Du, S.; Chen, G.; Zhang, Q.; Wu, G. Microstructure and mechanical properties of B4C/2024Al functionally gradient composites. Mater. Des. 2022, 215, 110449. [Google Scholar] [CrossRef]
- Ma, K.; Li, X.N.; Liu, K.; Chen, X.G.; Liu, Z.Y.; Xiao, B.L.; Ma, Z.Y. Improving the high-cycle fatigue strength of heterogeneous carbon nanotube/Al-Cu-Mg composites through grain size design in ductile-zones. Compos. Part B 2021, 222, 109094. [Google Scholar] [CrossRef]
- Ma, K.; Liu, Z.; Bi, S.; Zhang, X.; Xiao, B.; Ma, Z. Microstructure evolution and hot deformation behavior of carbon nanotube reinforced 2009Al composite with bimodal grain structure. J. Mater. Sci. Technol. 2020, 70, 73–82. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, N.; He, C. The superior mechanical and physical properties of nanocarbon reinforced bulk composites achieved by architecture design—A review. Prog. Mater. Sci. 2020, 113, 100672. [Google Scholar] [CrossRef]
- Aydin, F. A review of recent developments in the corrosion performance of aluminium matrix composites. J. Alloy. Compd. 2023, 949, 169508. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Zheng, W.; Wang, M.; Liu, B.; Sun, W. Investigation on corrosion behaviors and mechanical properties of TiB2/7075Al composites with various particle contents. J. Mater. Res. Technol. 2023, 23, 2911–2923. [Google Scholar] [CrossRef]
- Zhao, K.; Han, G.; Gao, T.; Yang, H.; Qian, Z.; Hu, K.; Liu, G.; Nie, J.; Liu, X. Interface precipitation and corrosion mechanisms in a model Al-Zn-Mg-Cu alloy strengthened by TiC particles. Corros. Sci. 2022, 206, 110533. [Google Scholar] [CrossRef]
- Pan, S.; Yuan, J.; Linsley, C.; Liu, J.; Li, X. Corrosion behavior of nano-treated AA7075 alloy with TiC and TiB2 nanoparticles. Corros. Sci. 2022, 206, 110533. [Google Scholar] [CrossRef]
- Keshavarz, H.; Moosavi, S.E.; Eslamian, H.; Kokabi, A. Corrosion behavior and tribological-microstructural characterization of Al/ZrO2-Gr hybrid composite fabricated via Friction Stir Processing. J. Alloy. Compd. 2023, 960, 170770. [Google Scholar] [CrossRef]
- Samal, P.; Vundavilli, P.R.; Meher, A.; Mahapatra, M.M. Recent progress in aluminum metal matrix composites: A review on processing, mechanical and wear properties. J. Manuf. Process. 2020, 59, 131–152. [Google Scholar] [CrossRef]
- Wang, P.; Gammer, C.; Brenne, F.; Niendorf, T.; Eckert, J.; Scudino, S. A heat treatable TiB2/Al-3.5Cu-1.5Mg-1Si composite fabricated by selective laser melting: Microstructure, heat treatment and mechanical properties. Compos. Part B 2018, 147, 162–168. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, C.; Liu, J.; Zhang, C.; Chen, H.; Shi, Q.; Dan, C.; Wang, H.; Chen, Z. Improving the ductility of Al matrix composites through bimodal structures: Precise manipulation and mechanical responses to coarse grain fraction. Mater. Sci. Eng. A 2023, 875, 145139. [Google Scholar] [CrossRef]
- GB/T 7998-2005; Test Method for Intergranular Corrosion of Aluminium Alloy. Standard Press of China: Beijing, China, 2005.
- Chaudry, U.M.; Farooq, A.; bin Tayyab, K.; Malik, A.; Kamran, M.; Kim, J.-G.; Li, C.; Hamad, K.; Jun, T.-S. Corrosion behavior of AZ31 magnesium alloy with calcium addition. Corros. Sci. 2022, 199, 110205. [Google Scholar] [CrossRef]
- Xi, K.; Wu, H.; Zhou, C.; Qi, Z.; Yang, K.; Fu, R.K.Y.; Xiao, S.; Wu, G.; Ding, K.; Chen, G.; et al. Improved corrosion and wear resistance of micro-arc oxidation coatings on the 2024 aluminum alloy by incorporation of quasi-two-dimensional sericite microplates. Appl. Surf. Sci. 2022, 585, 152693. [Google Scholar] [CrossRef]
- Ao, M.; Liu, H.; Dong, C.; Feng, S.; Liu, J. Degradation mechanism of 6063 aluminium matrix composite reinforced with TiC and Al2O3 particles. J. Alloy. Compd. 2021, 859, 157838. [Google Scholar] [CrossRef]
- Toptan, F.; Alves, A.; Kerti, I.; Ariza, E.; Rocha, L. Corrosion and tribocorrosion behaviour of Al–Si–Cu–Mg alloy and its composites reinforced with B4C particles in 0.05 M NaCl solution. Wear 2013, 306, 27–35. [Google Scholar] [CrossRef]
- Peng, C.; Cao, G.; Gu, T.; Wang, C.; Wang, Z.; Sun, C. The corrosion behavior of the 6061 Al alloy in simulated Nansha marine atmosphere. J. Mater. Res. Technol. 2022, 19, 709–721. [Google Scholar] [CrossRef]
- Yuan, S.; Gao, Z.; Fu, H.; Cheung, C.F.; Yang, X.-S. Superior corrosion-resistant nanostructured hypoeutectic CrCoNi-based medium-entropy alloy processed by laser surface remelting. J. Alloy. Compd. 2023, 967, 171802. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, Z.; Lu, M. Combined effect of pH and H2S on the structure of passive film formed on type 316L stainless steel. Appl. Surf. Sci. 2018, 458, 686–699. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Gu, X.; Dai, N.; Qin, P.; Zhang, L.-C. Distinction of corrosion resistance of selective laser melted Al-12Si alloy on different planes. J. Alloy. Compd. 2018, 747, 648–658. [Google Scholar] [CrossRef]
- Zhao, C.; Bai, Y.; Zhang, Y.; Wang, X.; Xue, J.M.; Wang, H. Influence of scanning strategy and building direction on microstructure and corrosion behaviour of selective laser melted 316L stainless steel. Mater. Des. 2021, 209, 109999. [Google Scholar] [CrossRef]
- Revilla, R.I.; Wouters, B.; Andreatta, F.; Lanzutti, A.; Fedrizzi, L.; De Graeve, I. EIS comparative study and critical Equivalent Electrical Circuit (EEC) analysis of the native oxide layer of additive manufactured and wrought 316L stainless steel. Corros. Sci. 2020, 167, 108480. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Fan, X.; Dai, N.; Xiao, Y.; Zhang, L.-C. Abnormal corrosion behavior of selective laser melted AlSi10Mg alloy induced by heat treatment at 300 °C. J. Alloy. Compd. 2019, 803, 314–324. [Google Scholar] [CrossRef]
- Deng, P.; Mo, W.; Ouyang, Z.; Tang, C.; Luo, B.; Bai, Z. Mechanical properties and corrosion behaviors of (Sc, Zr) modified Al-Cu-Mg alloy. Mater. Charact. 2023, 196, 112619. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, G.; Chen, M.-S.; Zhang, J.-L.; Chen, Z.-G.; Jiang, Y.-Q.; Li, J. Corrosion resistance of a two-stage stress-aged Al–Cu–Mg alloy: Effects of external stress. J. Alloy. Compd. 2016, 661, 221–230. [Google Scholar] [CrossRef]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Rong, X.; Li, Y.; Chen, X.; Zhang, X.; Zhao, D.; He, C.; Zhao, N. Plain interface strategy toward the high corrosion performance of Al matrix composites. Sci. China Mater. 2023, 66, 4295–4305. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Wu, Z.; Wang, D.; Li, B.; Cui, J. Effects of Ag on the age hardening response and intergranular corrosion resistance of Al-Mg alloys. Mater. Charact. 2019, 147, 84–92. [Google Scholar] [CrossRef]
- Ke, B.; Ye, L.; Zhang, Y.; Tang, J.; Liu, S.; Liu, X.; Dong, Y.; Wang, P. Enhanced mechanical properties and corrosion resistance of an Al-Zn-Mg aluminum alloy through variable-rate non-isothermal aging. J. Alloy. Compd. 2022, 890, 161933. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Qian, M.; Yang, R.; Yu, F.; Zhang, X.; Jia, Z.; Li, A.; Wang, G.; Geng, L. Corrosion Behavior of Nacre-Inspired (TiBw-TiB2)/Al Composites Fabricated by Freeze Casting. Materials 2024, 17, 2534. https://doi.org/10.3390/ma17112534
Zhang J, Qian M, Yang R, Yu F, Zhang X, Jia Z, Li A, Wang G, Geng L. Corrosion Behavior of Nacre-Inspired (TiBw-TiB2)/Al Composites Fabricated by Freeze Casting. Materials. 2024; 17(11):2534. https://doi.org/10.3390/ma17112534
Chicago/Turabian StyleZhang, Jidong, Mingfang Qian, Ruiqing Yang, Feng Yu, Xuexi Zhang, Zhenggang Jia, Aibin Li, Guisong Wang, and Lin Geng. 2024. "Corrosion Behavior of Nacre-Inspired (TiBw-TiB2)/Al Composites Fabricated by Freeze Casting" Materials 17, no. 11: 2534. https://doi.org/10.3390/ma17112534
APA StyleZhang, J., Qian, M., Yang, R., Yu, F., Zhang, X., Jia, Z., Li, A., Wang, G., & Geng, L. (2024). Corrosion Behavior of Nacre-Inspired (TiBw-TiB2)/Al Composites Fabricated by Freeze Casting. Materials, 17(11), 2534. https://doi.org/10.3390/ma17112534








