Optimization of Chitosan Synthesis Process Parameters to Enhance PES/Chitosan Membrane Performance for the Treatment of Acid Mine Drainage (AMD)
Abstract
:1. Introduction
2. Experimental Set-Up
2.1. Materials and Chemicals
2.2. Equipment
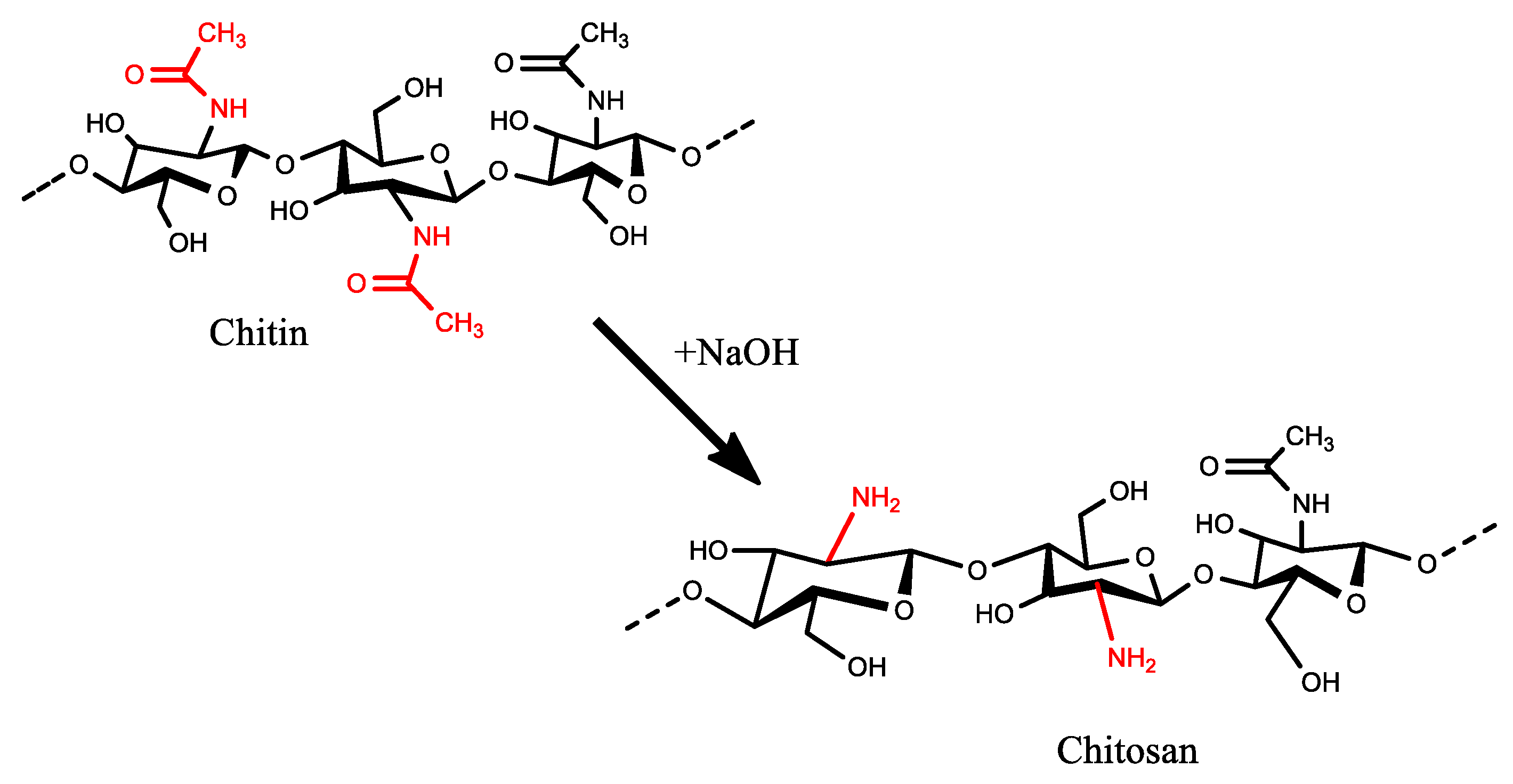
2.3. Production of Chitosan
- Step 1: Pulverization of shells
- Step 2: Chemical Deproteinization and demineralization
- Step 3: Deacetylation
2.4. Optimization Studies
2.5. Fabrication of Membranes
2.6. Performance Evaluation of Membrane
3. Results and Discussion
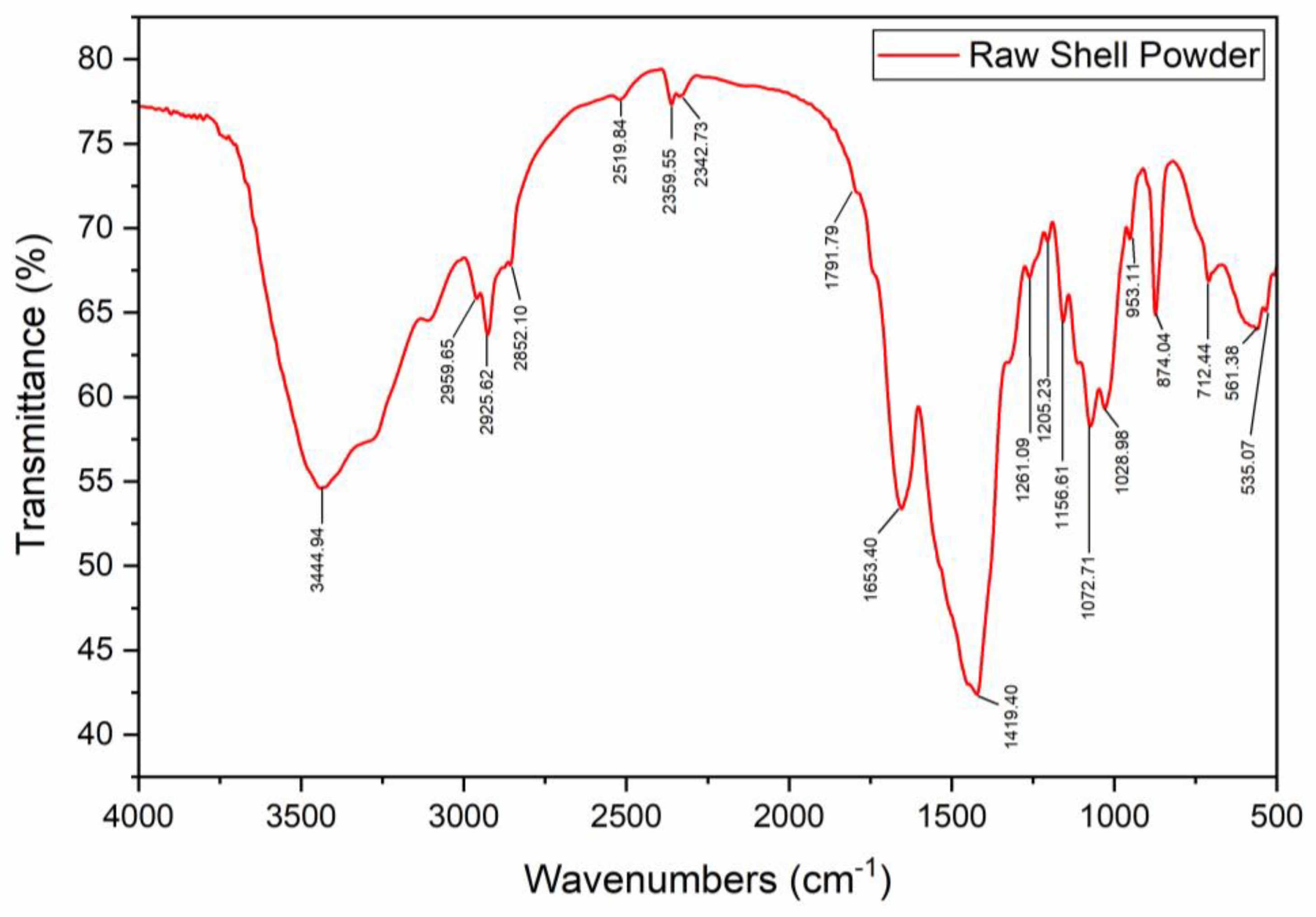
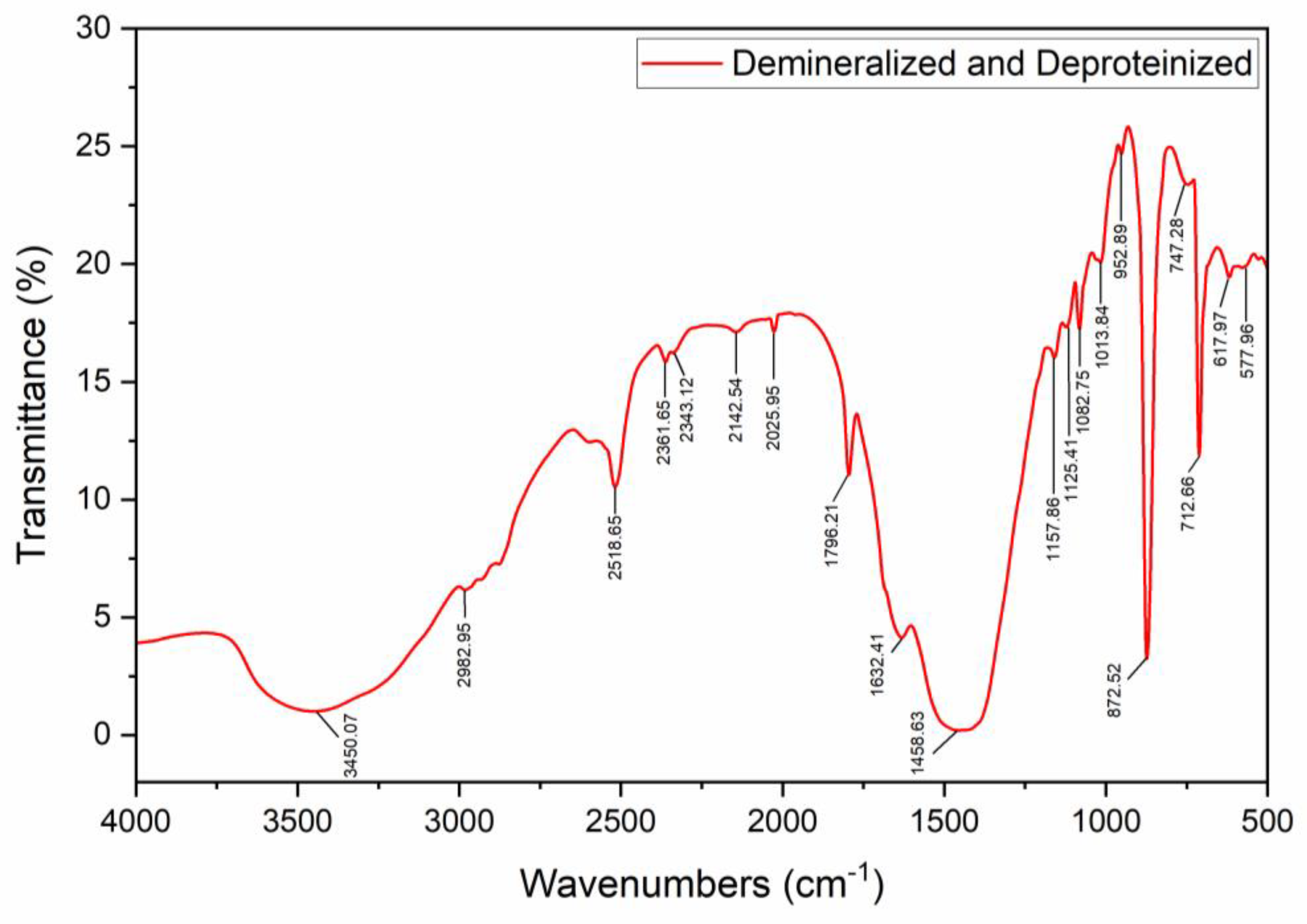
3.1. Shell and Chitin Characterization
3.2. Chitin Yield
3.3. Degree of Deacetylation
3.4. Effect of Time on Chitosan’s Degree of Deacetylation

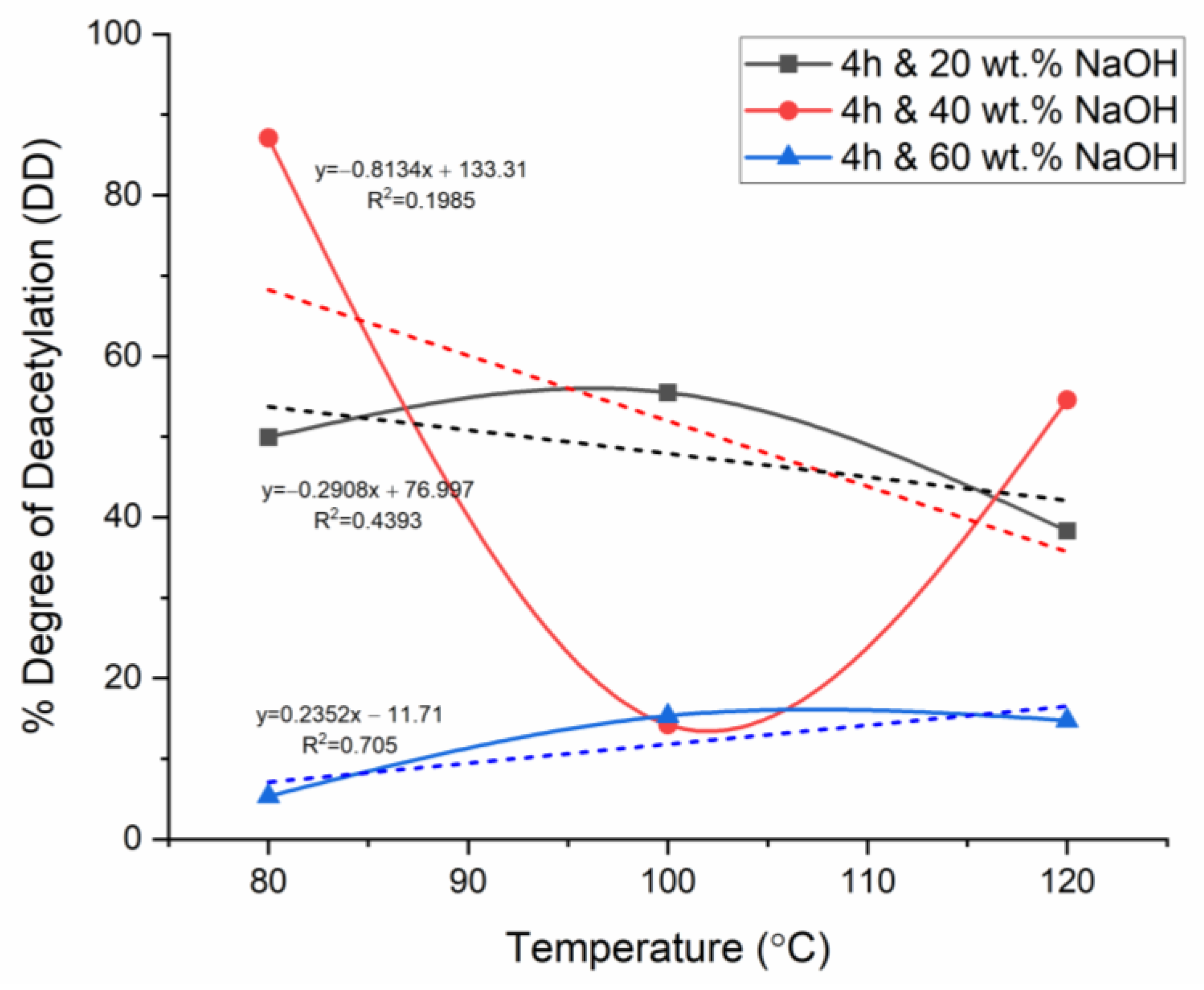
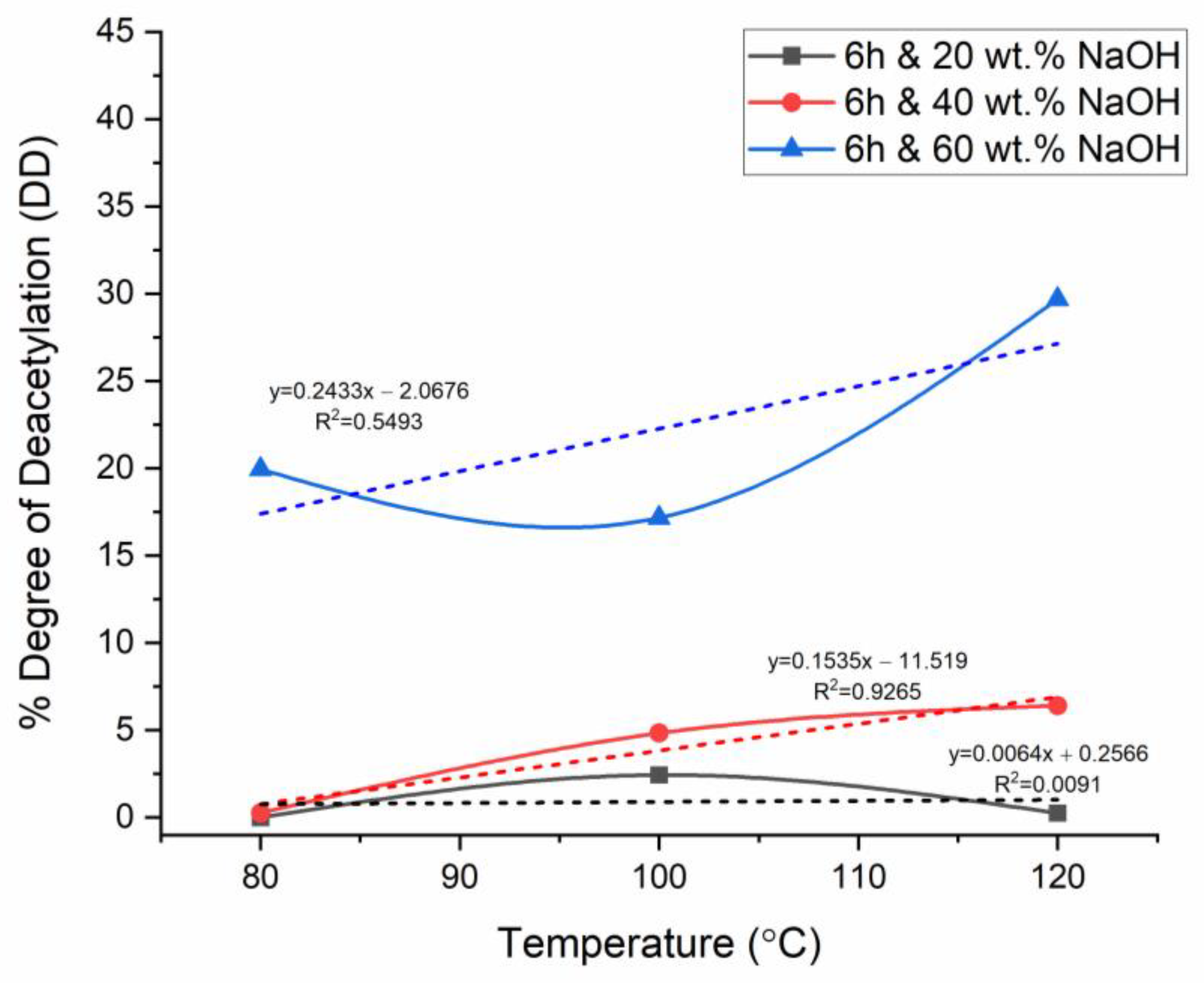
3.5. Effect of Temperature on Chitosan’s Degree of Deacetylation
3.6. Effect of NaOH Concentration on Chitosan’s Degree of Deacetylation
3.7. Quality Evaluation of PES Membranes vs. PES/Chitosan Membrane
3.8. Physiochemical Characterization of Pristine PES and PES/0.75 Chitosan Membranes
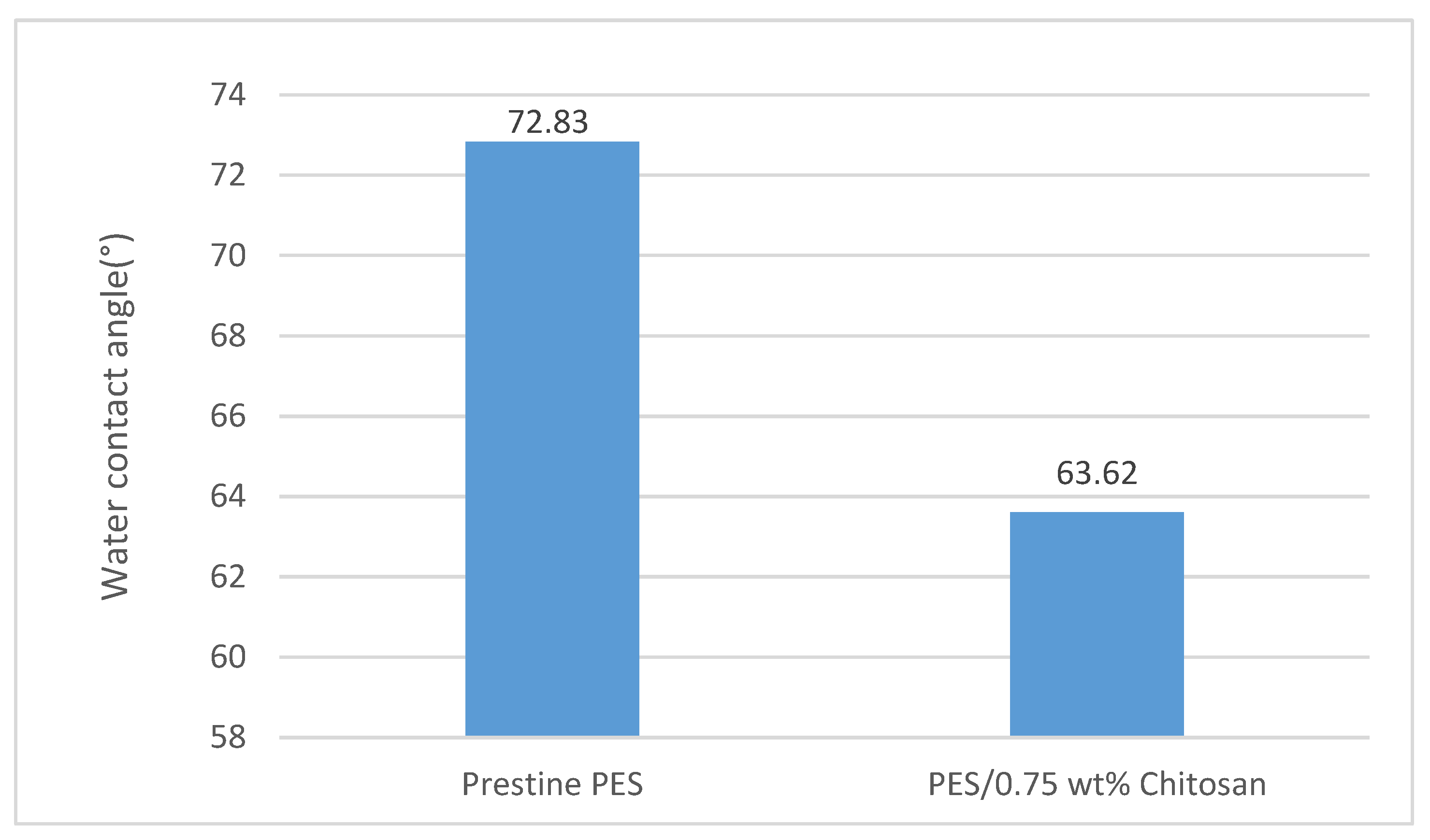
3.9. Membrane Hydrophilicity (Water Contact Angle)
3.10. Techno-Economic Discussion of the Established Optimum Conditions
3.11. Challenges in the Implementation of PES/Chitosan Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iji, O.T.; Njoya, E.M.; Madikizela, B.; Myburgh, J.G.; McGaw, L.J. Evaluation of the genotoxic potential of water impacted by acid mine drainage from a coal mine in Mpumalanga, South Africa, using the Ames test and Comet assay. Water SA 2021, 47, 456–465. [Google Scholar] [CrossRef]
- Singh, P.; Milshina, Y.; Tian, K.; Gusain, D.; Bassin, J.P. Water Conservation and Wastewater Treatment in BRICS Nations: Technologies, Challenges, Strategies and Policies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–374. [Google Scholar] [CrossRef]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Wright, I.A.; Paciuszkiewicz, K.; Belmer, N. Increased Water Pollution after Closure of Australia’s Longest Operating Underground Coal Mine: A 13-Month Study of Mine Drainage, Water Chemistry and River Ecology. Water Air Soil Pollut. 2018, 229, 55. [Google Scholar] [CrossRef]
- BussiÈre, B. Acid Mine Drainage from Abandoned Mine Sites: Problematic and Reclamation Approaches. In Advances in Environmental Geotechnics: Proceedings of the International Symposium on Geoenvironmental Engineering in Hangzhou, China, 8–10 September 2009; Springer: Berlin/Heidelberg, Germany, 2010; pp. 111–125. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Machodi, M.J.; Daramola, M.O. Synthesis and performance evaluation of PES/chitosan membranes coated with polyamide for acid mine drainage treatment. Sci. Rep. 2019, 9, 17657. [Google Scholar] [CrossRef] [PubMed]
- Makhani, T.; Sadare, O.O.; Wagenaar, S.; Moothi, K.; Moutloali, R.M.; Daramola, M.O. Fabrication and performance evaluation of tannin iron complex (TA-FeIII/PES) UF membrane in treatment of BTEX wastewater. Water SA 2022, 48, 457–466. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng. Sci. Technol. Int. J. 2017, 20, 1155–1165. [Google Scholar] [CrossRef]
- Mathaba, M.; Daramola, M.O. Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Agboola, O. The role of membrane technology in acid mine water treatment: A review. Korean J. Chem. Eng. 2019, 36, 1389–1400. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Jeong, S.; Lee, E.J.; Tabatabai, S.A.A.; Leiknes, T.O. High flux and antifouling properties of negatively charged membrane for dyeing wastewater treatment by membrane distillation. Water Res. 2016, 103, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Y.; Wang, X.; Quan, X.; Chen, S. Improvement of Antifouling and Antimicrobial Abilities on Silver-Carbon Nanotube Based Membranes under Electrochemical Assistance. Environ. Sci. Technol. 2019, 53, 5292–5300. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Z.; Chew, J.W.; Krantz, W.B. Effect of humic-acid fouling on membrane distillation. J. Membr. Sci. 2016, 504, 263–273. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Nguyen, L.; Lippich, H.; Wagner, M.; Cornel, P.; Lutze, R.; Engelhart, M. Protein separation from alkaline process water of shrimp waste processing industry using membrane filtration. Vietnam. J. Chem. 2014, 52, 174. [Google Scholar] [PubMed]
- Shi, Z.F.; Zhang, S.M.; Guo, S. Treatment of waste seawater from shrimp farm using a photocatalytic membrane reactor. Appl. Mech. Mater. 2013, 409–410, 199–203. [Google Scholar] [CrossRef]
- Tröstl, J.; Tritscher, T.; Bischof, O.F.; Horn, H.G.; Krinke, T.; Baltensperger, U.; Gysel, M. Fast and precise measurement in the sub-20nm size range using a Scanning Mobility Particle Sizer. J. Aerosol Sci. 2015, 87, 75–87. [Google Scholar] [CrossRef]
- Seghir, B.B.; Benhamza, M.H. Preparation, optimization and characterization of chitosan polymer from shrimp shells. J. Food Meas. Charact. 2017, 11, 1137–1147. [Google Scholar] [CrossRef]
- Hashem, A.M.; Ismail, S.A.; Hosny, A.E.D.M.S.; Awad, G.; Ismail, S.A. Optimization of Dothideomycetes sp. css035 chitosanase productivity and activity using response surface methodology. Egypt. J. Chem. 2018, 61, 973–987. [Google Scholar] [CrossRef]
- Fatima, B. Quantitative Analysis by IR: Determination of Chitin/Chitosan DD, Undefined; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef]
- Chakravarty, J.; Yang, C.-L.; Palmer, J.D.; Brigham, C. Chitin Extraction from Lobster Shell Waste using Microbial Culture-based Methods. Appl. Food Biotechnol. 2018, 5, 141–154. [Google Scholar] [CrossRef]
- Bajaj, M.; Freiberg, A.; Winter, J.; Xu, Y.; Gallert, C. Pilot-scale chitin extraction from shrimp shell waste by deproteination and decalcification with bacterial enrichment cultures. Appl. Microbiol. Biotechnol. 2015, 99, 9835–9846. [Google Scholar] [CrossRef] [PubMed]
- Ahlafi, H.; Moussout, H.; Boukhlifi, F. Kinetics of N-Deacetylation of Chitin Extracted from Shrimp Shells Collected from Coastal Area of Morocco. Mediterr. J. Chem. 2013, 2, 503–513. [Google Scholar] [CrossRef]
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Characterization of Shrimp Shell Deproteinization. Biomacromolecules 2003, 4, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Sapna; Sharma, R.; Kumar, D. Chitosan-Based Membranes for Wastewater Desalination and Heavy Metal Detoxification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 799–814. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Mao, Y. Large-pore-size membranes tuned by chemically vapor deposited nanocoatings for rapid and controlled desalination. RSC Adv. 2020, 10, 40562–40568. [Google Scholar] [CrossRef] [PubMed]
- Satoshi, O.; Yasunori, K. Recent Status and Issues on Techno-economic Analysis. J. Life Cycle Assess. Jpn. 2019, 15, 325–331. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Barrera-Zapata, R.; Ríos-Estepa, R. Comparison of process technologies for chitosan production from shrimp shell waste: A techno-economic approach using Aspen Plus®. Food Bioprod. Process. 2017, 103, 49–57. [Google Scholar] [CrossRef]
- Lee, J.Y.; Foo, D.C.Y. Simultaneous Targeting and Scheduling for Batch Water Networks. Ind. Eng. Chem. Res. 2017, 56, 1559–1569. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Knidri, H.E.L.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Mahardika, R.G.; Jumnahdi, M.; Widyaningrum, Y. Chitin Deacetylation Shells of Portunus pelagicus L. Using Microwave Irradiation. IOP Conf. Ser. Earth Environ. Sci. 2019, 353, 012037. [Google Scholar] [CrossRef]
- Echarri, I.; Casado-Coterillo, C.; Rumayor, M.; Navajas, A.; Gandía, L.M. Environmental Impact Improvement of Chitosan-Based Mixed-Matrix Membranes Manufacture for CO2 Gas Separation by Life Cycle Assessment. Chem. Eng. Technol. 2023, 46, 2184–2191. [Google Scholar] [CrossRef]
- Mulyati, S.; Rosnelly, C.M.; Syamsuddin, Y.; Arahman, N.; Muchtar, S.; Wahyuni; Lauzia, T.; Ambarita, A.C.; Bilad, M.R.; Samsuri, S. Enhancing the Anti-Fouling Property of Polyethersulfone-based Membrane using Chitosan Additive from Golden Snail (Pomacea canaliculata) Shell Waste for Water Purification. ASEAN J. Chem. Eng. 2023, 23, 224–239. [Google Scholar] [CrossRef]
- Hashimah, S.; Hanif, M. Fabrication of Chitosan Membrane: The Effetcs of Different Polyethylene Glycol Compositions on Membrane Performance in Oily Wastewater Treatment. Ph.D. Thesis, UMP, Siyabuswa, South Africa, 2008. [Google Scholar]
- Mohamed, A.; Yousef, S. Green and sustainable membrane fabrication development. Sustain. Technol. Green Econ. 2021, 1, 14–23. [Google Scholar] [CrossRef]
- Kim, J.F. Recent Progress on Improving the Sustainability of Membrane Fabrication. J. Membr. Sci. Res. 2020, 6, 241–250. [Google Scholar] [CrossRef]
- Shi, S.; Liu, X.; Li, W.; Li, Z.; Tu, G.; Deng, B.; Liu, C. Tuning the Biodegradability of Chitosan Membranes: Characterization and Conceptual Design. ACS Sustain. Chem. Eng. 2020, 8, 14484–14492. [Google Scholar] [CrossRef]

| Level | Temperature (°C) | Time (h) | NaOH Concentration (wt.%) |
|---|---|---|---|
| −1 | 80 | 2 | 20 |
| 0 | 100 | 4 | 40 |
| 1 | 120 | 6 | 60 |
| Sample Number | Temperature (°C) | Time (h) | NaOH (wt.%) |
|---|---|---|---|
| 1 | 80 | 2 | 20 |
| 2 | 80 | 2 | 40 |
| 3 | 80 | 2 | 60 |
| 4 | 80 | 4 | 20 |
| 5 | 80 | 4 | 40 |
| 6 | 80 | 4 | 60 |
| 7 | 80 | 6 | 20 |
| 8 | 80 | 6 | 40 |
| 9 | 80 | 6 | 60 |
| 10 | 100 | 2 | 20 |
| 11 | 100 | 2 | 40 |
| 12 | 100 | 2 | 60 |
| 13 | 100 | 4 | 20 |
| 14 | 100 | 4 | 40 |
| 15 | 100 | 4 | 60 |
| 16 | 100 | 6 | 20 |
| 17 | 100 | 6 | 40 |
| 18 | 100 | 6 | 60 |
| 19 | 120 | 2 | 20 |
| 20 | 120 | 2 | 40 |
| 21 | 120 | 2 | 60 |
| 22 | 120 | 4 | 20 |
| 23 | 120 | 4 | 40 |
| 24 | 120 | 4 | 60 |
| 25 | 120 | 6 | 20 |
| 26 | 120 | 6 | 40 |
| 27 | 120 | 6 | 60 |
| Initial Dry Mass (g) | Chitin Mass (g) | %Yield |
|---|---|---|
| 117.86 | 72.864 | 61.4 |
| Sample Number | Temperature (°C) | Time (h) | NaOH (wt.%) | %DD | Standard Deviation, CV |
|---|---|---|---|---|---|
| 1 | 80 | 2 | 20 | 5.30 | 0.04 |
| 2 | 80 | 2 | 40 | 49.20 | 0.17 |
| 3 | 80 | 2 | 60 | 0.16 | 0.01 |
| 4 | 80 | 4 | 20 | 49.94 | 0.44 |
| 5 | 80 | 4 | 40 | 87.11 | 1.30 |
| 6 | 80 | 4 | 60 | 5.35 | 0.03 |
| 7 | 80 | 6 | 20 | 407.97 | 32.05 |
| 8 | 80 | 6 | 40 | 0.26 | 0.02 |
| 9 | 80 | 6 | 60 | 19.94 | 0.12 |
| 10 | 100 | 2 | 20 | 0.75 | 0.07 |
| 11 | 100 | 2 | 40 | 1.83 | 0.08 |
| 12 | 100 | 2 | 60 | 0.71 | 0.00 |
| 13 | 100 | 4 | 20 | 55.50 | 0.36 |
| 14 | 100 | 4 | 40 | 14.22 | 0.04 |
| 15 | 100 | 4 | 60 | 15.33 | 0.04 |
| 16 | 100 | 6 | 20 | 2.43 | 0.06 |
| 17 | 100 | 6 | 40 | 4.83 | 0.04 |
| 18 | 100 | 6 | 60 | 17.17 | 0.05 |
| 19 | 120 | 2 | 20 | 8.94 | 0.08 |
| 20 | 120 | 2 | 40 | 7.43 | 0.05 |
| 21 | 120 | 2 | 60 | 8.92 | 0.04 |
| 22 | 120 | 4 | 20 | 38.30 | 0.16 |
| 23 | 120 | 4 | 40 | 54.58 | 0.55 |
| 24 | 120 | 4 | 60 | 14.76 | 0.06 |
| 25 | 120 | 6 | 20 | 0.26 | 0.04 |
| 26 | 120 | 6 | 40 | 6.41 | 0.06 |
| 27 | 120 | 6 | 60 | 29.67 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasifudi, N.F.; Mekuto, L.; Mathaba, M.J. Optimization of Chitosan Synthesis Process Parameters to Enhance PES/Chitosan Membrane Performance for the Treatment of Acid Mine Drainage (AMD). Materials 2024, 17, 2562. https://doi.org/10.3390/ma17112562
Rasifudi NF, Mekuto L, Mathaba MJ. Optimization of Chitosan Synthesis Process Parameters to Enhance PES/Chitosan Membrane Performance for the Treatment of Acid Mine Drainage (AMD). Materials. 2024; 17(11):2562. https://doi.org/10.3390/ma17112562
Chicago/Turabian StyleRasifudi, Ndiwanga F., Lukhanyo Mekuto, and Machodi J. Mathaba. 2024. "Optimization of Chitosan Synthesis Process Parameters to Enhance PES/Chitosan Membrane Performance for the Treatment of Acid Mine Drainage (AMD)" Materials 17, no. 11: 2562. https://doi.org/10.3390/ma17112562





