Research Progress of Bioinspired Structural Color in Camouflage
Abstract
1. Introduction
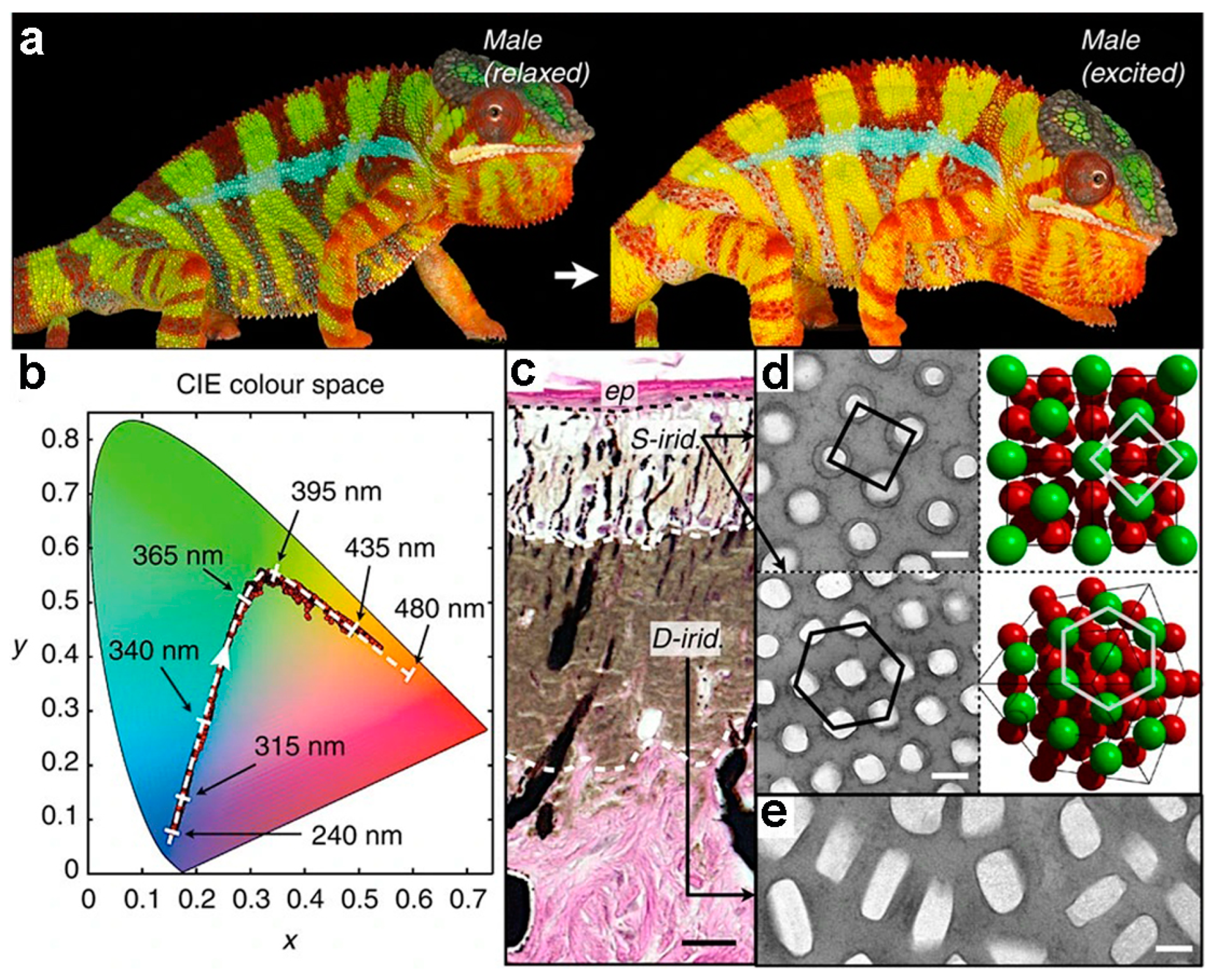
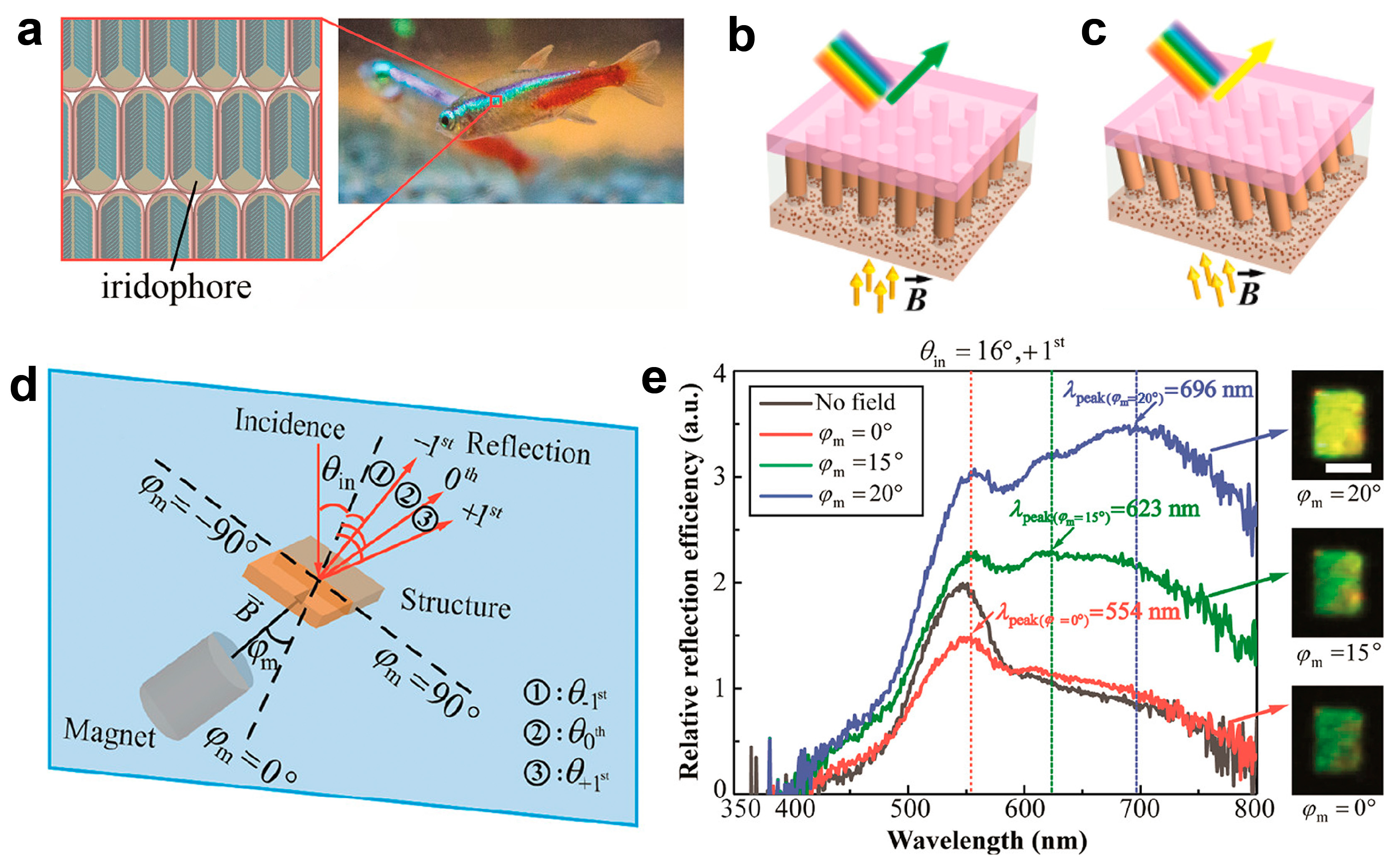
2. Structural Colors in Natural Creatures
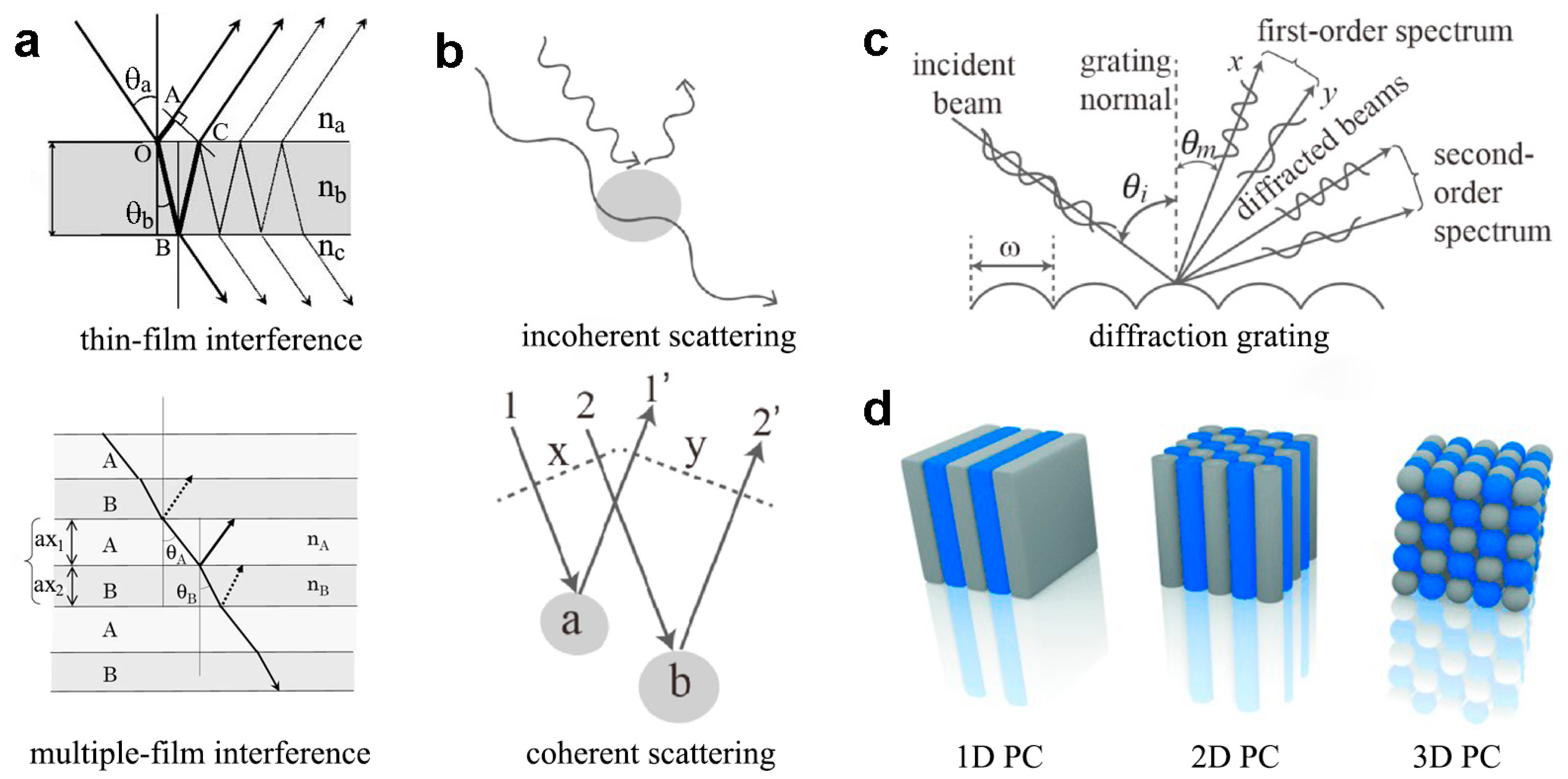
3. Bioinspired Materials with Tunable Structural Colors
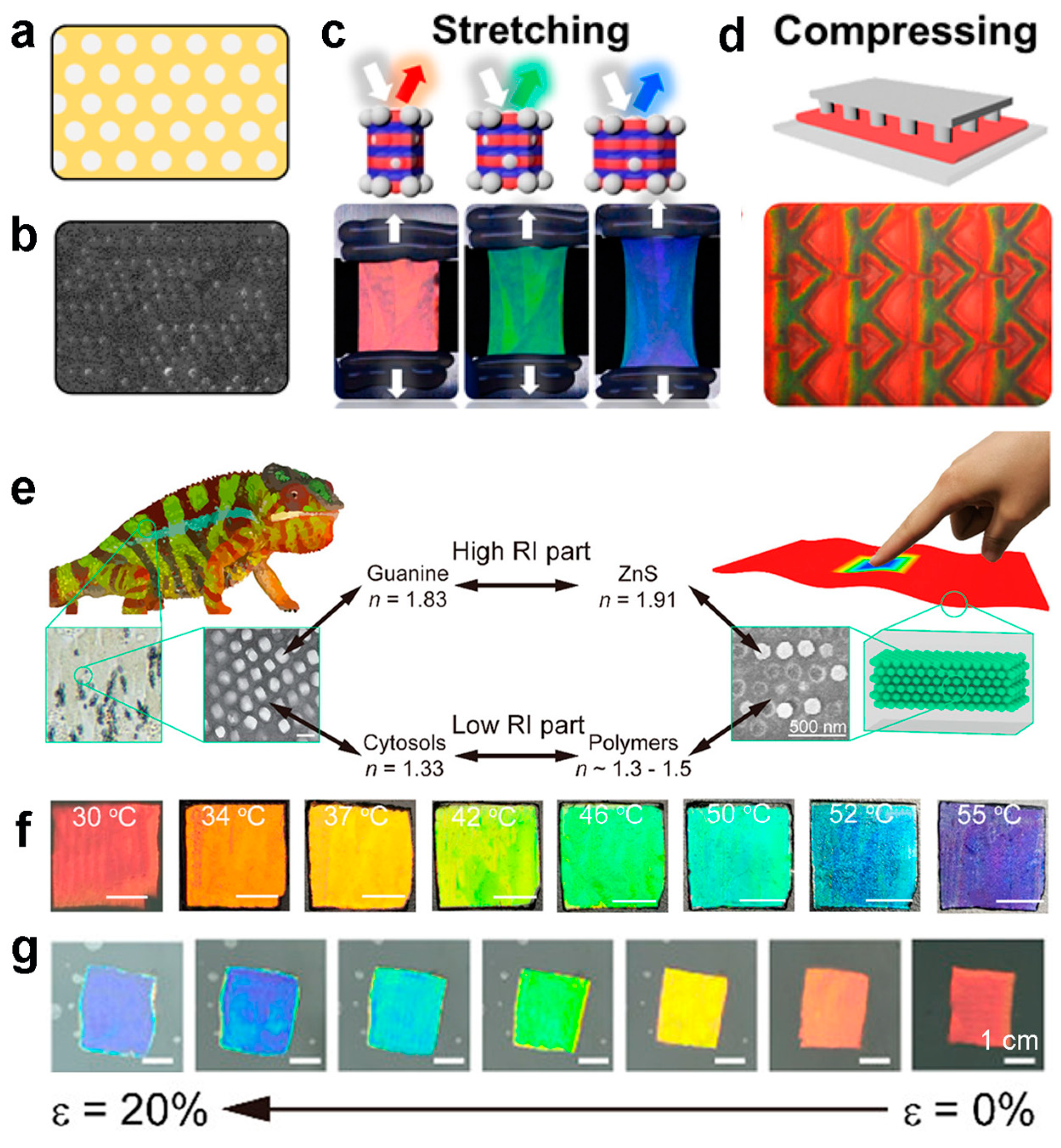
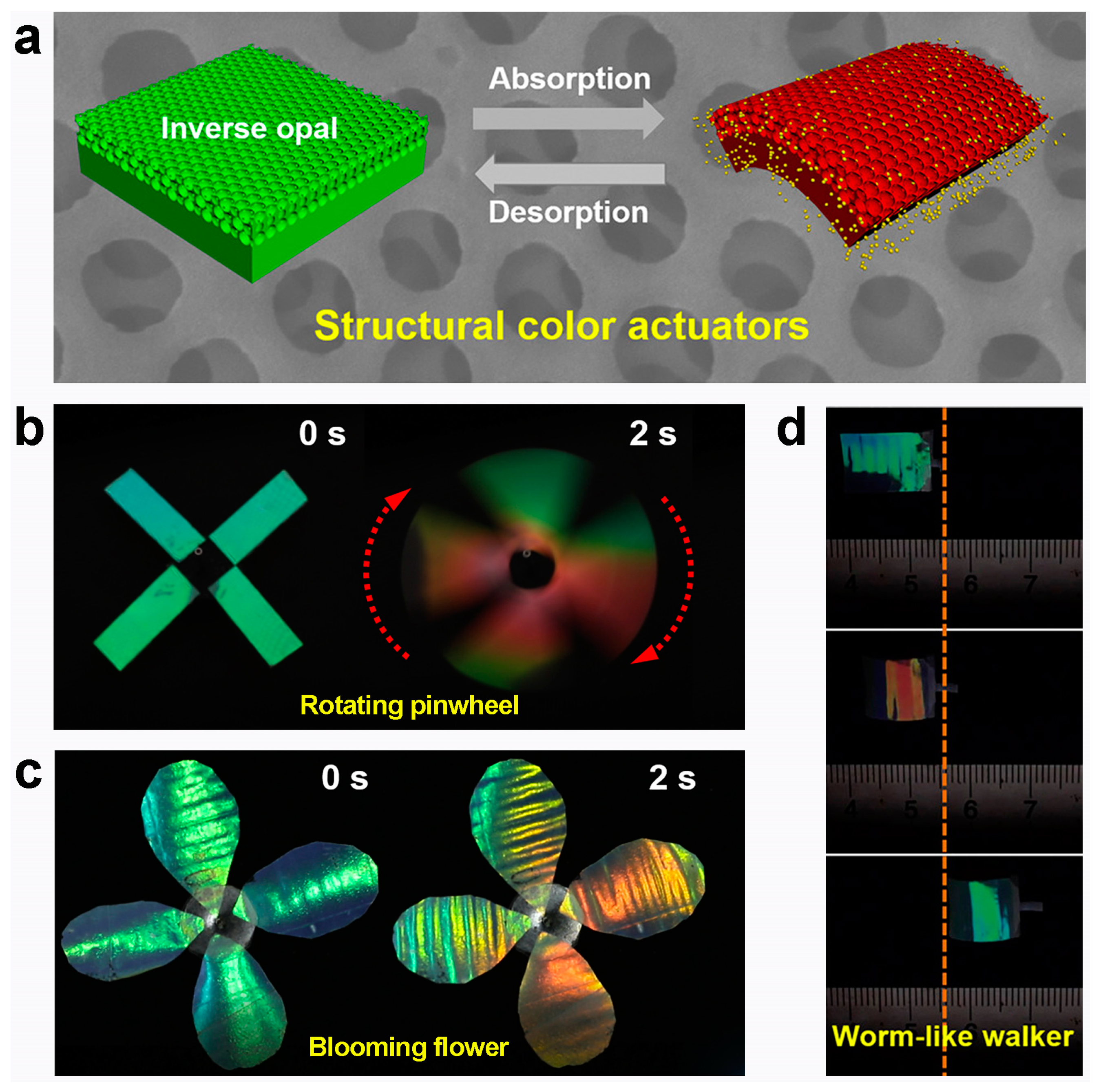
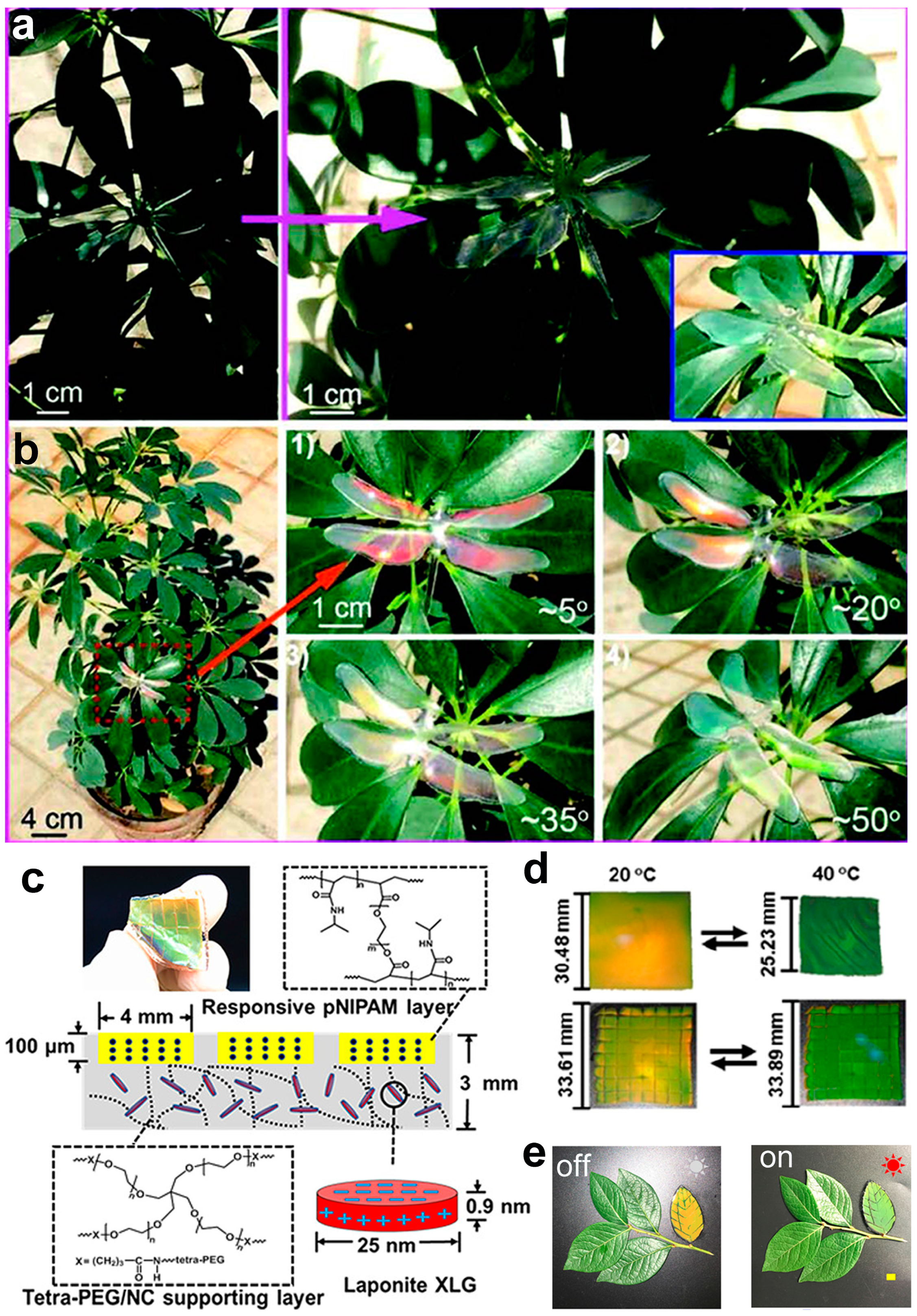
3.1. Photonic Crystal Modulation
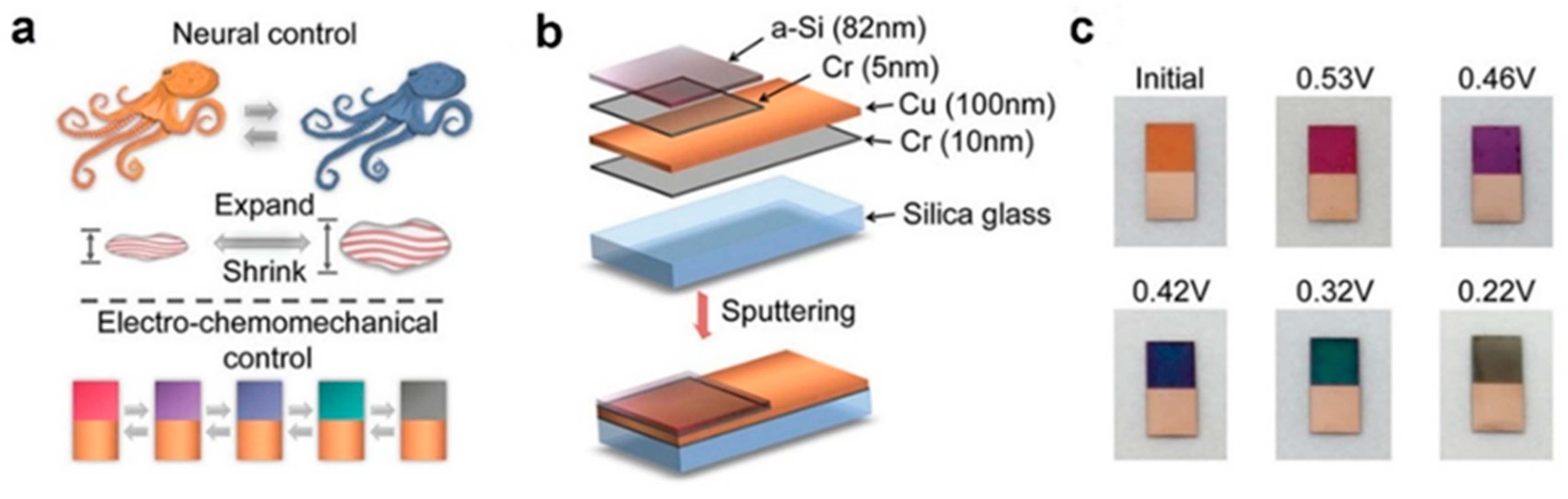
3.2. Plasmonic Modulation
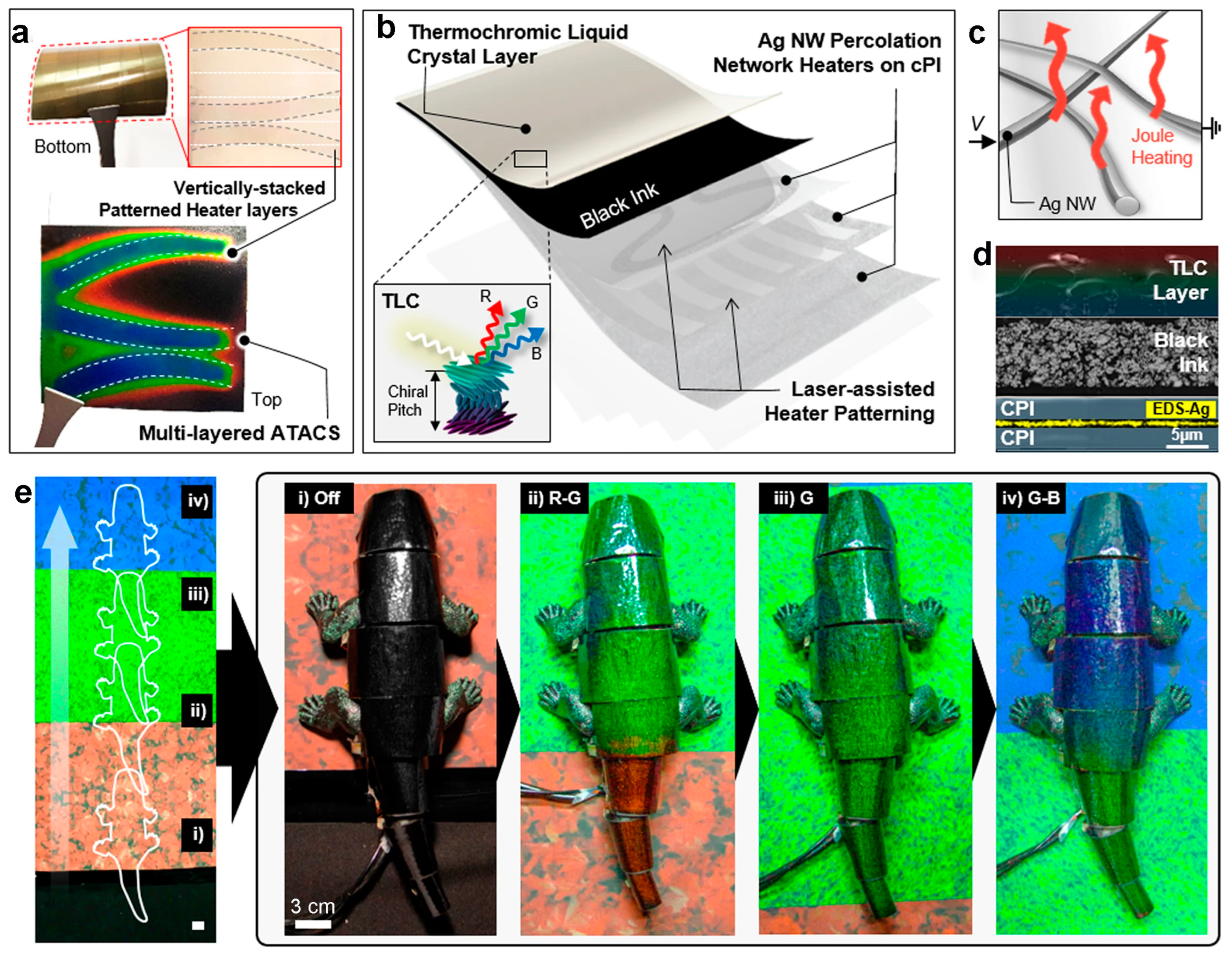
3.3. Film Interference Modulation
4. The Application of Environmental Fusion Modulation
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, Y.; Meng, Z.; Wang, P.; Yan, D. Research Progress of Bionic Adaptive Camouflage Materials. Front. Mater. 2021, 8, 637664. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Cheng, H.; Peng, L.; Zu, M. Manipulating metals for adaptive thermal camouflage. Sci. Adv. 2020, 6, eaba3494. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lim, J.-S.; Chang, I.; Bae, H.M.; Nam, J.; Cho, H.H. Flexible Assembled Metamaterials for Infrared and Microwave Camouflage. Adv. Opt. Mater. 2022, 10, 2200448. [Google Scholar] [CrossRef]
- Xu, C.; Stiubianu, G.T.; Gorodetsky, A.A. Adaptive infrared-reflecting systems inspired by cephalopods. Science 2018, 359, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sul, H.; Jung, Y.; Kim, H.; Han, S.; Choi, J.; Shin, J.; Kim, D.; Jung, J.; Hong, S.; et al. Thermally Controlled, Active Imperceptible Artificial Skin in Visible-to-Infrared Range. Adv. Funct. Mater. 2020, 30, 2003328. [Google Scholar] [CrossRef]
- Xu, C.; Colorado Escobar, M.; Gorodetsky, A.A. Stretchable Cephalopod-Inspired Multimodal Camouflage Systems. Adv. Mater. 2020, 32, 1905717. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Zhang, X.; Wang, L.; Feng, W.; Li, Q. Beyond the Visible: Bioinspired Infrared Adaptive Materials. Adv. Mater. 2021, 33, 2004754. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, Q.; Cai, L.; Pan, M.; Ghosh, P.; Du, K.; Qiu, M. Thermal camouflage based on the phase-changing material GST. Light Sci. Appl. 2018, 7, 26. [Google Scholar] [CrossRef]
- Isapour, G.; Lattuada, M. Bioinspired Stimuli-Responsive Color-Changing Systems. Adv. Mater. 2018, 30, 1707069. [Google Scholar] [CrossRef]
- Deparis, O.; Mouchet, S.; Dellieu, L.; Colomer, J.F.; Sarrazin, M. Nanostructured Surfaces: Bioinspiration for Transparency, Coloration and Wettability. Mater. Today Proc. 2014, 1, 122–129. [Google Scholar] [CrossRef]
- Kinoshita, S. Structural Colors in the Realm of Nature; World Scientific: Hackensack, NJ, USA, 2008. [Google Scholar]
- Deparis, O.; Mouchet, S.R. Natural Photonics and Bioinspiration; Artech House: Boston, MA, USA, 2021. [Google Scholar]
- Chatterjee, A.; Pratakshya, P.; Kwansa, A.L.; Kaimal, N.; Cannon, A.H.; Sartori, B.; Marmiroli, B.; Orins, H.; Feng, Z.; Drake, S.; et al. Squid Skin Cell-Inspired Refractive Index Mapping of Cells, Vesicles, and Nanostructures. ACS Biomater. Sci. Eng. 2023, 9, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Z.; Xu, C.; Chatterjee, A.; Gorodetsky, A.A. Reconfigurable Micro- and Nano-Structured Camouflage Surfaces Inspired by Cephalopods. ACS Nano 2021, 15, 17299–17309. [Google Scholar] [CrossRef] [PubMed]
- Pratakshya, P.; Xu, C.; Dibble, D.J.; Mukazhanova, A.; Liu, P.; Burke, A.M.; Kurakake, R.; Lopez, R.; Dennison, P.R.; Sharifzadeh, S.; et al. Octopus-inspired deception and signaling systems from an exceptionally-stable acene variant. Nat. Commun. 2023, 14, 8528. [Google Scholar] [CrossRef] [PubMed]
- Linn, N.C.; Sun, C.-H.; Jiang, P.; Jiang, B. Self-assembled biomimetic antireflection coatings. Appl. Phys. Lett. 2007, 91, 101108. [Google Scholar] [CrossRef]
- Min, W.-L.; Betancourt, A.P.; Jiang, P.; Jiang, B. Bioinspired broadband antireflection coatings on GaSb. Appl. Phys. Lett. 2008, 92, 141109. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Xie, G.; Liu, Z.; Shao, H. Cicada Wings: A Stamp from Nature for Nanoimprint Lithography. Small 2006, 2, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862–14889. [Google Scholar] [CrossRef]
- Huang, Y.; Bisoyi, H.K.; Huang, S.; Wang, M.; Chen, X.-M.; Liu, Z.; Yang, H.; Li, Q. Bioinspired Synergistic Photochromic Luminescence and Programmable Liquid Crystal Actuators. Angew. Chem. Int. Ed. 2021, 60, 11247–11251. [Google Scholar] [CrossRef] [PubMed]
- Pris, A.D.; Utturkar, Y.; Surman, C.; Morris, W.G.; Vert, A.; Zalyubovskiy, S.; Deng, T.; Ghiradella, H.T.; Potyrailo, R.A. Towards high-speed imaging of infrared photons with bio-inspired nanoarchitectures. Nat. Photonics 2012, 6, 195–200. [Google Scholar] [CrossRef]
- Poncelet, O.; Tallier, G.; Mouchet, S.R.; Crahay, A.; Rasson, J.; Kotipalli, R.; Deparis, O.; Francis, L.A. Vapour sensitivity of an ALD hierarchical photonic structure inspired by Morpho. Bioinspiration Biomim. 2016, 11, 036011. [Google Scholar] [CrossRef]
- Rasson, J.; Poncelet, O.; Mouchet, S.R.; Deparis, O.; Francis, L.A. Vapor sensing using a bio-inspired porous silicon photonic crystal. Mater. Today Proc. 2017, 4, 5006–5012. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Verstraete, C.; Bokic, B.; Mara, D.; Dellieu, L.; Orr, A.G.; Deparis, O.; Van Deun, R.; Verbiest, T.; Vukusic, P.; et al. Revealing natural fluorescence in transparent insect wings by linear and nonlinear optical techniques. J. Lumin. 2023, 254, 119490. [Google Scholar] [CrossRef]
- Young, R.E. Oceanic bioluminescence: An overview of general functions. Bull. Mar. Sci. 1983, 33, 829–845. [Google Scholar]
- Greven, H.; Zwanzig, N. Courtship, Mating, and Organisation of the Pronotum in the Glowspot Cockroach Lucihormetica verrucosa (Brunner von Wattenwyl, 1865) (Blattodea: Blaberidae). Entomol. Heute 2013, 25, 77–97. [Google Scholar]
- Merritt, D.J. Standards of evidence for bioluminescence in cockroaches. Naturwissenschaften 2013, 100, 697–698. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Giraldo, M.A.; Leertouwer, H.L. Butterfly wing colors: Glass scales of Graphium sarpedon cause polarized iridescence and enhance blue/green pigment coloration of the wing membrane. J. Exp. Biol. 2010, 213, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Wilts, B.D.; Trzeciak, T.M.; Vukusic, P.; Stavenga, D.G. Papiliochrome II pigment reduces the angle dependency of structural wing colouration in nireus group papilionids. J. Exp. Biol. 2012, 215, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, N.I.; Vukusic, P.; Rutowski, R. Pterin pigment granules are responsible for both broadband light scattering and wavelength selective absorption in the wing scales of pierid butterflies. Proc. R. Soc. B Biol. Sci. 2006, 274, 359–366. [Google Scholar] [CrossRef]
- Fu, Y.; Tippets, C.A.; Donev, E.U.; Lopez, R. Structural colors: From natural to artificial systems. WIREs. Nanomed. Nanobiotechnol. 2016, 8, 758–775. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Yoshioka, S.; Miyazaki, J. Physics of structural colors. Rep. Prog. Phys. 2008, 71, 076401. [Google Scholar] [CrossRef]
- Li, K.; Li, C.; Li, H.; Li, M.; Song, Y. Designable structural coloration by colloidal particle assembly: From nature to artificial manufacturing. iScience 2021, 24, 102121. [Google Scholar] [CrossRef] [PubMed]
- Schwind, B.; Wu, X.; Tiemann, M.; Fabritius, H.-O. Broadband Mie scattering effects by structural features of setae from the Saharan silver ant Cataglyphis bombycina. J. Opt. Soc. Am. B 2023, 40, B49–B58. [Google Scholar] [CrossRef]
- Ladouce, M.; Barakat, T.; Su, B.-L.; Deparis, O.; Mouchet, S.R. Scattering of ultraviolet light by avian eggshells. Faraday Discuss. 2020, 223, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, S.R.; Lobet, M.; Kolaric, B.; Kaczmarek, A.M.; Van Deun, R.; Vukusic, P.; Deparis, O.; Van Hooijdonk, E. Photonic scales of Hoplia coerulea beetle: Any colour you like. Mater. Today Proc. 2017, 4, 4979–4986. [Google Scholar] [CrossRef]
- Djeghdi, K.; Schumacher, C.; Bauernfeind, V.; Gunkel, I.; Wilts, B.D.; Steiner, U. Anoplophora graafi longhorn beetle coloration is due to disordered diamond-like packed spheres. Soft Matter 2024, 20, 2509–2517. [Google Scholar] [CrossRef]
- Bauernfeind, V.; Ronikier, A.; Ronikier, M.; Kozlowski, G.; Steiner, U.; Wilts, B.D. Thin film structural color is widespread in slime molds (Myxomycetes, Amoebozoa). Opt. Express 2024, 32, 5429–5443. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, J.P.; Pasteels, J.M.; Windsor, D.M.; Vértesy, Z.; Rassart, M.; Seldrum, T.; Dumont, J.; Deparis, O.; Lousse, V.; Biró, L.P.; et al. Switchable reflector in the Panamanian tortoise beetle Charidotella egregia (Chrysomelidae: Cassidinae). Phys. Rev. E 2007, 76, 031907. [Google Scholar] [CrossRef]
- Vukusic, P.; Sambles, J.R.; Lawrence, C.R.; Wootton, R.J. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1999, 266, 1403–1411. [Google Scholar] [CrossRef]
- Ghiradella, H. Structure and development of iridescent butterfly scales: Lattices and laminae. J. Morphol. 1989, 202, 69–88. [Google Scholar] [CrossRef]
- Burresi, M.; Cortese, L.; Pattelli, L.; Kolle, M.; Vukusic, P.; Wiersma, D.S.; Steiner, U.; Vignolini, S. Bright-White Beetle Scales Optimise Multiple Scattering of Light. Sci. Rep. 2014, 4, 6075. [Google Scholar] [CrossRef]
- Lafait, J.; Andraud, C.; Berthier, S.; Boulenguez, J.; Callet, P.; Dumazet, S.; Rassart, M.; Vigneron, J.P. Modeling the vivid white color of the beetle Calothyrza margaritifera. Mater. Sci. Eng. B 2010, 169, 16–22. [Google Scholar] [CrossRef]
- Vukusic, P.; Hallam, B.; Noyes, J. Brilliant Whiteness in Ultrathin Beetle Scales. Science 2007, 315, 348. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.R.; McPhedran, R.C.; McKenzie, D.R.; Botten, L.C.; Nicorovici, N.A.P. Aphrodite’s iridescence. Nature 2001, 409, 36–37. [Google Scholar] [CrossRef]
- Parker, A.R.; Welch, V.L.; Driver, D.; Martini, N. Opal analogue discovered in a weevil. Nature 2003, 426, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Pouya, C.; Stavenga, D.G.; Vukusic, P. Discovery of ordered and quasi-ordered photonic crystal structures in the scales of the beetle Eupholus magnificus. Opt. Express 2011, 19, 11355–11364. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, T.M.; Vukusic, P. Photonic crystal fiber in the polychaete worm Pherusa sp. Phys. Rev. E 2009, 80, 061908. [Google Scholar] [CrossRef]
- Yoshioka, S.; Kinoshita, S. Effect of macroscopic structure in iridescent color of the peacock feathers. Forma 2002, 17, 169–181. [Google Scholar]
- Li, G.; Leng, M.; Wang, S.; Ke, Y.; Luo, W.; Ma, H.; Guan, J.; Long, Y. Printable structural colors and their emerging applications. Mater. Today 2023, 69, 133–159. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Srinivasarao, M. Nano-Optics in the Biological World: Beetles, Butterflies, Birds, and Moths. Chem. Rev. 1999, 99, 1935–1962. [Google Scholar] [CrossRef]
- Vaz, R.; Frasco, M.F.; Sales, M.G.F. Photonics in nature and bioinspired designs: Sustainable approaches for a colourful world. Nanoscale Adv. 2020, 2, 5106–5129. [Google Scholar] [CrossRef]
- Vigneron, J.-P.; Simonis, P. Chapter 5—Structural Colours. In Advances in Insect Physiology; Casas, J., Simpson, S.J., Eds.; Academic Press: New York, NY, USA, 2010; Volume 38, pp. 181–218. [Google Scholar]
- Potyrailo, R.A.; Ghiradella, H.; Vertiatchikh, A.; Dovidenko, K.; Cournoyer, J.R.; Olson, E. Morpho butterfly wing scales demonstrate highly selective vapour response. Nat. Photonics 2007, 1, 123–128. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Tabarrant, T.; Lucas, S.; Su, B.-L.; Vukusic, P.; Deparis, O. Vapor sensing with a natural photonic cell. Opt. Express 2016, 24, 12267–12280. [Google Scholar] [CrossRef]
- Kertész, K.; Piszter, G.; Jakab, E.; Bálint, Z.; Vértesy, Z.; Biró, L.P. Color change of Blue butterfly wing scales in an air—Vapor ambient. Appl. Surf. Sci. 2013, 281, 49–53. [Google Scholar] [CrossRef]
- Teyssier, J.; Saenko, S.V.; van der Marel, D.; Milinkovitch, M.C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 6368. [Google Scholar] [CrossRef]
- Mäthger, L.M.; Denton, E.J.; Marshall, N.J.; Hanlon, R.T. Mechanisms and behavioural functions of structural coloration in cephalopods. J. R. Soc. Interface 2009, 6, S149–S163. [Google Scholar] [CrossRef]
- Goda, M. Rapid integumental color changes due to novel iridophores in the chameleon sand tilefish Hoplolatilus chlupatyi. Pigm. Cell Melanoma Res. 2017, 30, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dong, B.Q.; Liu, X.H.; Zheng, Y.M.; Zi, J. Structural color change in longhorn beetles Tmesisternus isabellae. Opt. Express 2009, 17, 16183–16191. [Google Scholar] [CrossRef] [PubMed]
- Gur, D.; Leshem, B.; Farstey, V.; Oron, D.; Addadi, L.; Weiner, S. Light-Induced Color Change in the Sapphirinid Copepods: Tunable Photonic Crystals. Adv. Funct. Mater. 2016, 26, 1393–1399. [Google Scholar] [CrossRef]
- Saenko, S.V.; Teyssier, J.; van der Marel, D.; Milinkovitch, M.C. Precise colocalization of interacting structural and pigmentary elements generates extensive color pattern variation in Phelsumalizards. BMC Biol. 2013, 11, 105. [Google Scholar] [CrossRef]
- Mäthger, L.M.; Hanlon, R.T. Malleable skin coloration in cephalopods: Selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell Tissue Res. 2007, 329, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kreit, E.; Mäthger, L.M.; Hanlon, R.T.; Dennis, P.B.; Naik, R.R.; Forsythe, E.; Heikenfeld, J. Biological versus electronic adaptive coloration: How can one inform the other? J. R. Soc. Interface 2013, 10, 20120601. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Q.; Yin, Y. Colloidal Self-Assembly Approaches to Smart Nanostructured Materials. Chem. Rev. 2022, 122, 4976–5067. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, Z.; Ravaine, S.; He, M.; Song, Y.; Yin, Y.; Zheng, H.; Teng, J.; Zhang, A. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Chen, G.; Chen, H.; Zhao, Y. Structural Color Ionic Hydrogel Patches for Wound Management. ACS Nano 2023, 17, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, G.; Kong, H.; Yang, G.; Wei, G.; Zhou, X. Recent advances in photonic crystal-based sensors. Coord. Chem. Rev. 2023, 475, 214909. [Google Scholar] [CrossRef]
- Luo, W.; Yan, J.; Tan, Y.; Ma, H.; Guan, J. Rotating 1-D magnetic photonic crystal balls with a tunable lattice constant. Nanoscale 2017, 9, 9548–9555. [Google Scholar] [CrossRef]
- Lee, G.H.; Choi, T.M.; Kim, B.; Han, S.H.; Lee, J.M.; Kim, S.-H. Chameleon-Inspired Mechanochromic Photonic Films Composed of Non-Close-Packed Colloidal Arrays. ACS Nano 2017, 11, 11350–11357. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zhang, S.; Wu, S. Artificial Chameleon Skin with Super-Sensitive Thermal and Mechanochromic Response. ACS Nano 2021, 15, 15720–15729. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhao, Q.; Du, X. Chameleon-Inspired Structural-Color Actuators. Matter 2019, 1, 626–638. [Google Scholar] [CrossRef]
- Moirangthem, M.; Schenning, A.P.H.J. Full Color Camouflage in a Printable Photonic Blue-Colored Polymer. ACS Appl. Mater. Interfaces 2018, 10, 4168–4172. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; He, Q.; Zhang, L. Embedded Physical Intelligence in Liquid Crystalline Polymer Actuators and Robots. Adv. Mater. 2024, 2312313. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tang, Y.; Martella, D.; Guo, J.; Wiersma, D.S.; Li, Q. Stimuli-responsive photonic actuators for integrated biomimetic and intelligent systems. Responsive Mater. 2023, 1, e20230008. [Google Scholar] [CrossRef]
- Kim, S.-U.; Lee, Y.-J.; Liu, J.; Kim, D.S.; Wang, H.; Yang, S. Broadband and pixelated camouflage in inflating chiral nematic liquid crystalline elastomers. Nat. Mater. 2022, 21, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; de Haan, L.T.; Debije, M.G.; Schenning, A.P.H.J. Liquid crystal-based structural color actuators. Light Sci. Appl. 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Li, D.; Huang, S.; Huang, K.; Zhang, X. Bioinspired Multi-Stimuli Responsive Actuators with Synergistic Color- and Morphing-Change Abilities. Adv. Sci. 2021, 8, 2101295. [Google Scholar] [CrossRef]
- Sol, J.A.H.P.; Smits, L.G.; Schenning, A.P.H.J.; Debije, M.G. Direct Ink Writing of 4D Structural Colors. Adv. Funct. Mater. 2022, 32, 2201766. [Google Scholar] [CrossRef]
- Park, J.G.; Rogers, W.B.; Magkiriadou, S.; Kodger, T.; Kim, S.H.; Kim, Y.S.; Manoharan, V.N. Photonic-crystal hydrogels with a rapidly tunable stop band and high reflectivity across the visible. Opt. Mater. Express 2017, 7, 253–263. [Google Scholar] [CrossRef]
- Ye, B.F.; Zhao, Y.J.; Cheng, Y.; Li, T.T.; Xie, Z.Y.; Zhao, X.W.; Gu, Z.Z. Colorimetric photonic hydrogel aptasensor for the screening of heavy metal ions. Nanoscale 2012, 4, 5998–6003. [Google Scholar] [CrossRef]
- Cai, Z.; Luck, L.A.; Punihaole, D.; Madura, J.D.; Asher, S.A. Photonic crystal protein hydrogel sensor materials enabled by conformationally induced volume phase transition. Chem. Sci. 2016, 7, 4557–4562. [Google Scholar] [CrossRef]
- Yoshioka, S.; Matsuhana, B.; Tanaka, S.; Inouye, Y.; Oshima, N.; Kinoshita, S. Mechanism of variable structural colour in the neon tetra: Quantitative evaluation of the Venetian blind model. J. R. Soc. Interface 2011, 8, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Evans, B.A.; Chang, C.-H. Magnetically Actuated Dynamic Iridescence Inspired by the Neon Tetra. ACS Nano 2019, 13, 4657–4666. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, D.; Peana, S.; Choudhury, S.; Nyga, P.; Kudyshev, Z.A.; Yu, H.; Boltasseva, A.; Shalaev, V.M.; Kildishev, A.V. Colors with plasmonic nanostructures: A full-spectrum review. Appl. Phys. Rev. 2019, 6, 041308. [Google Scholar] [CrossRef]
- Kristensen, A.; Yang, J.K.W.; Bozhevolnyi, S.I.; Link, S.; Nordlander, P.; Halas, N.J.; Mortensen, N.A. Plasmonic colour generation. Nat. Rev. Mater. 2016, 2, 16088. [Google Scholar] [CrossRef]
- Højlund-Nielsen, E.; Clausen, J.; Mäkela, T.; Thamdrup, L.H.; Zalkovskij, M.; Nielsen, T.; Li Pira, N.; Ahopelto, J.; Mortensen, N.A.; Kristensen, A. Plasmonic Colors: Toward Mass Production of Metasurfaces. Adv. Mater. Technol. 2016, 1, 1600054. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Liu, S.; Wong, C.; Chu, S. Mechanical Chameleon through Dynamic Real Time-Plasmonic Tuning. ACS Nano 2016, 10, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Han, Y.; Yang, L.; Li, N.; Luo, J.; Qu, W.; Chen, R.; Jen, A.K.Y.; Li, T.; Chen, H.; et al. Bioinspired Controllable Electro-Chemomechanical Coloration Films. Adv. Funct. Mater. 2019, 29, 201806383. [Google Scholar] [CrossRef]
- Shevtsova, E.; Hansson, C.; Janzen, D.H.; Kjærandsen, J. Stable structural color patterns displayed on transparent insect wings. Proc. Natl. Acad. Sci. USA 2011, 108, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Huang, B.; Wu, S.; Li, L.; Zhang, S. Bio-inspired transparent structural color film and its application in biomimetic camouflage. Nanoscale 2019, 11, 13377–13384. [Google Scholar] [CrossRef]
- Dong, Y.; Bazrafshan, A.; Pokutta, A.; Sulejmani, F.; Sun, W.; Combs, J.D.; Clarke, K.C.; Salaita, K. Chameleon-Inspired Strain-Accommodating Smart Skin. ACS Nano 2019, 13, 9918–9926. [Google Scholar] [CrossRef]
- Shang, S.; Zhang, Q.; Wang, H.; Li, Y. Facile fabrication of magnetically responsive PDMS fiber for camouflage. J. Colloid Interface Sci. 2016, 483, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, J.; Kim, K.K.; Won, P.; Hong, S.; Ko, S.H. Biomimetic chameleon soft robot with artificial crypsis and disruptive coloration skin. Nat. Commun. 2021, 12, 4658. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Wang, H.; Luo, J.; Chen, J.; Qu, Z. Research Progress of Bioinspired Structural Color in Camouflage. Materials 2024, 17, 2564. https://doi.org/10.3390/ma17112564
Gong Y, Wang H, Luo J, Chen J, Qu Z. Research Progress of Bioinspired Structural Color in Camouflage. Materials. 2024; 17(11):2564. https://doi.org/10.3390/ma17112564
Chicago/Turabian StyleGong, Yimin, Haibin Wang, Jianxin Luo, Jiwei Chen, and Zhengyao Qu. 2024. "Research Progress of Bioinspired Structural Color in Camouflage" Materials 17, no. 11: 2564. https://doi.org/10.3390/ma17112564
APA StyleGong, Y., Wang, H., Luo, J., Chen, J., & Qu, Z. (2024). Research Progress of Bioinspired Structural Color in Camouflage. Materials, 17(11), 2564. https://doi.org/10.3390/ma17112564





