Metal–Organic-Framework-Derived Nitrogen-Doped Carbon-Matrix-Encapsulating Co0.5Ni0.5 Alloy as a Bifunctional Oxygen Electrocatalyst for Zinc–Air Batteries
Abstract
:1. Introduction
2. Experiment
Preparation of CoxNiyMOF
3. Results and Discussion
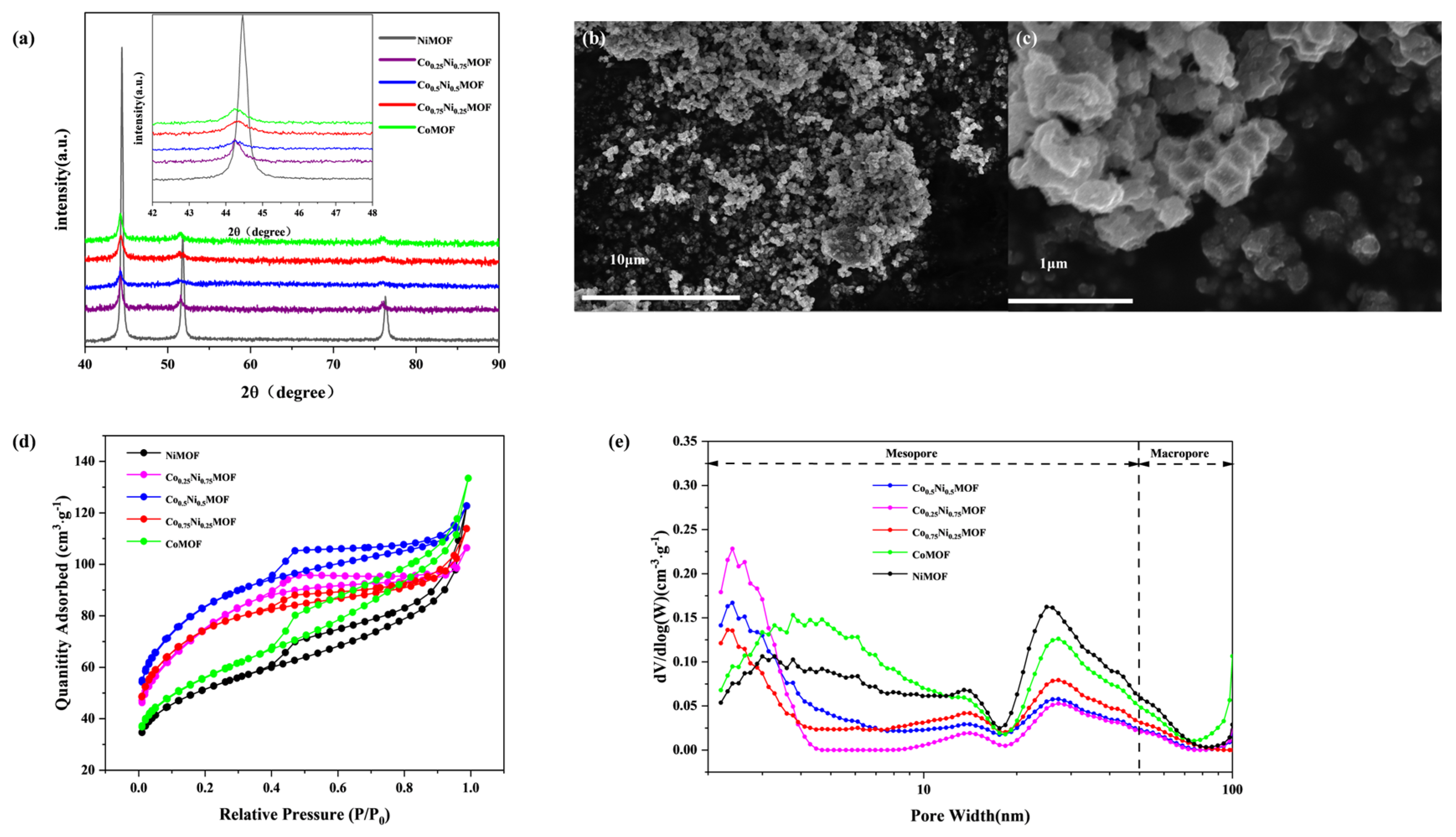
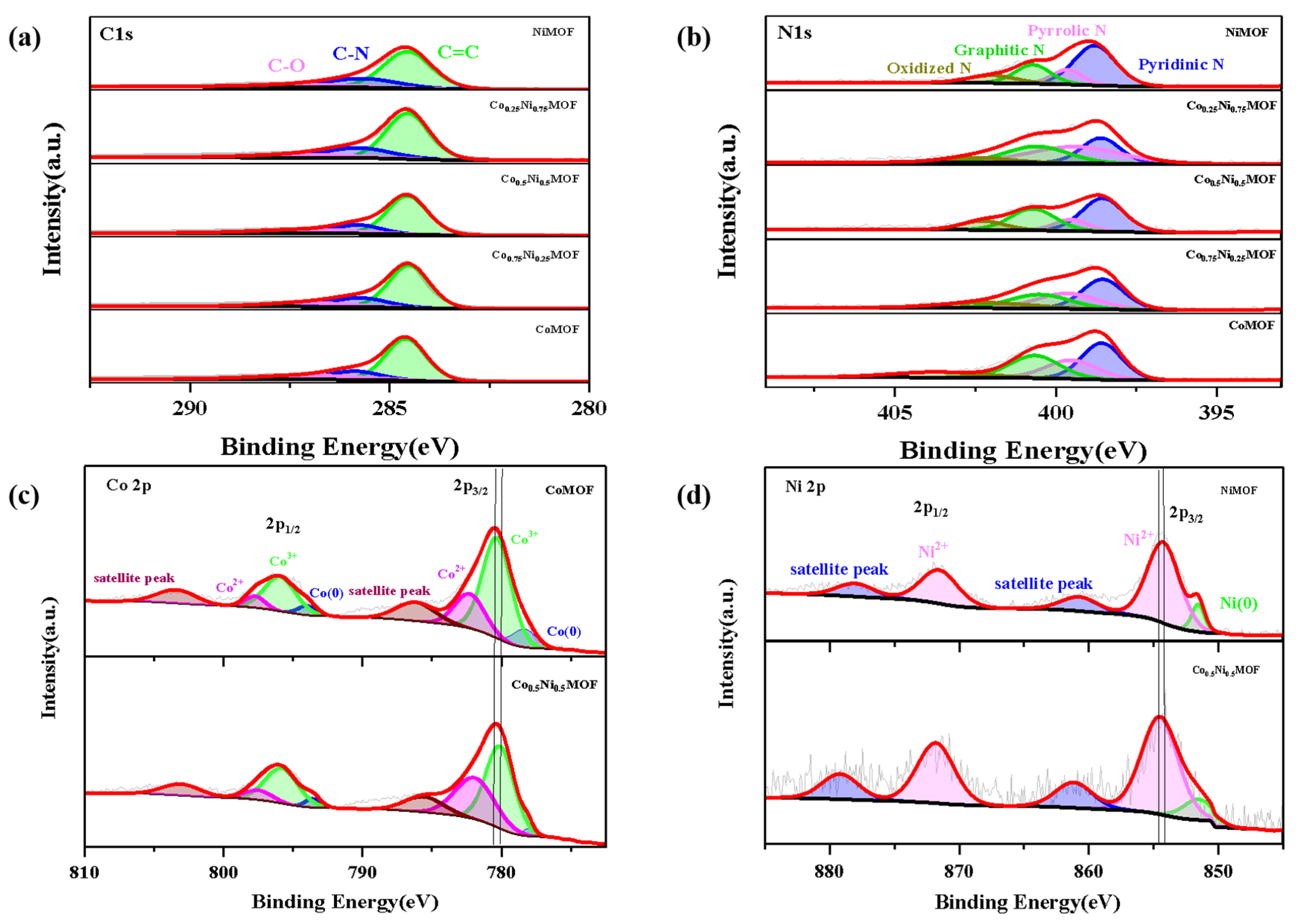
3.1. Preparation and Characterization of CoxNiyMOF
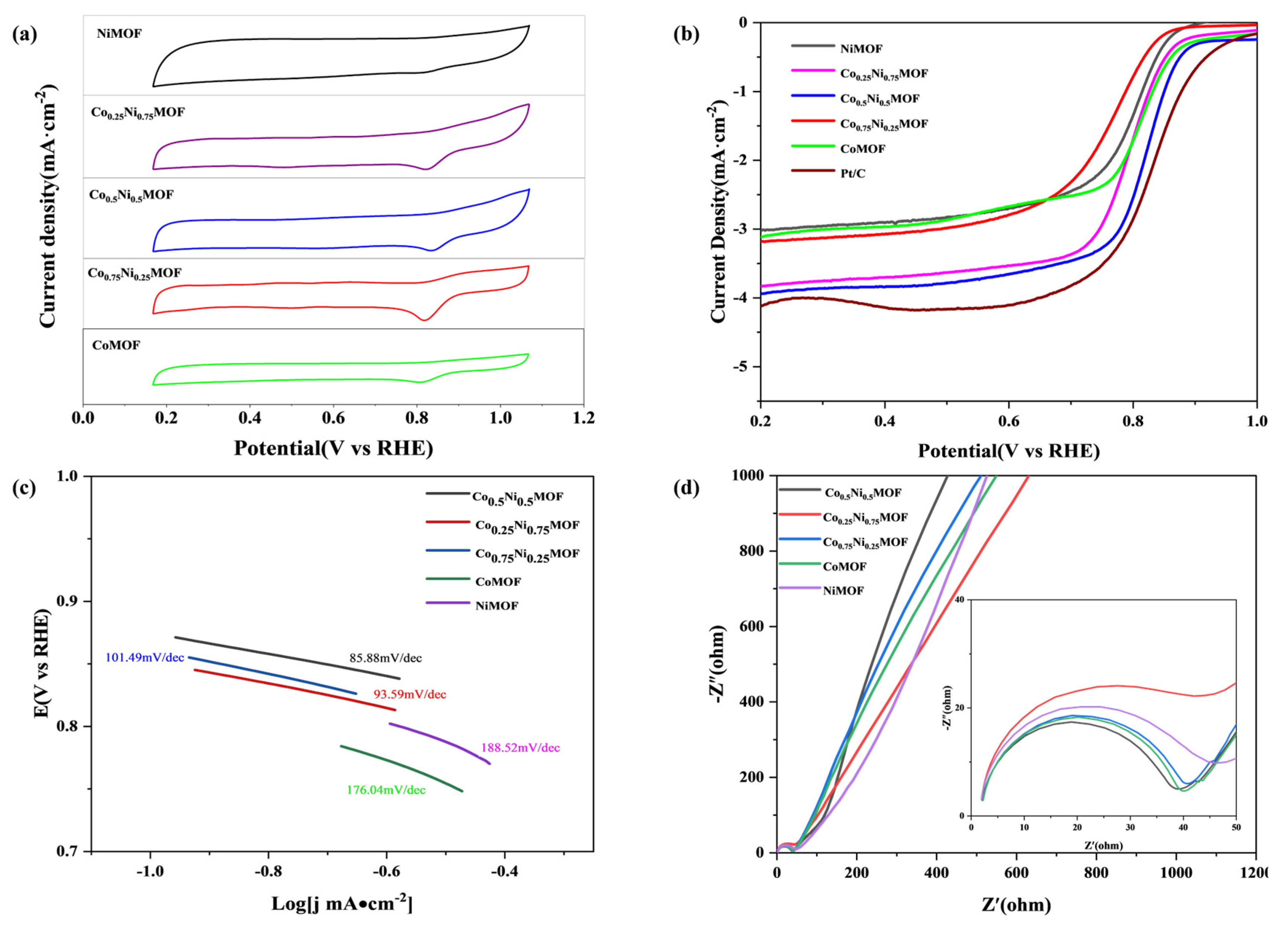
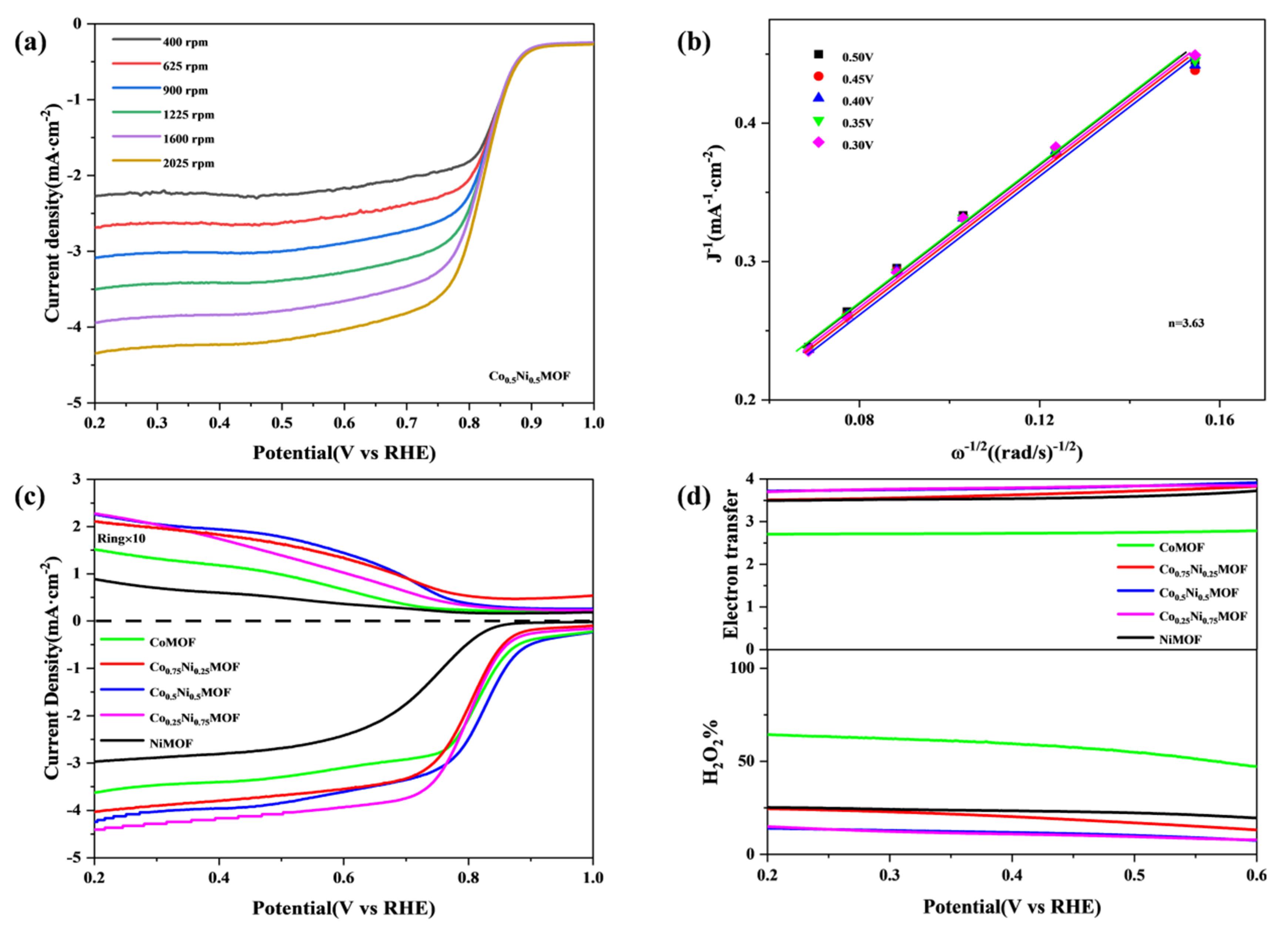
3.2. ORR/OER Activity and Durability
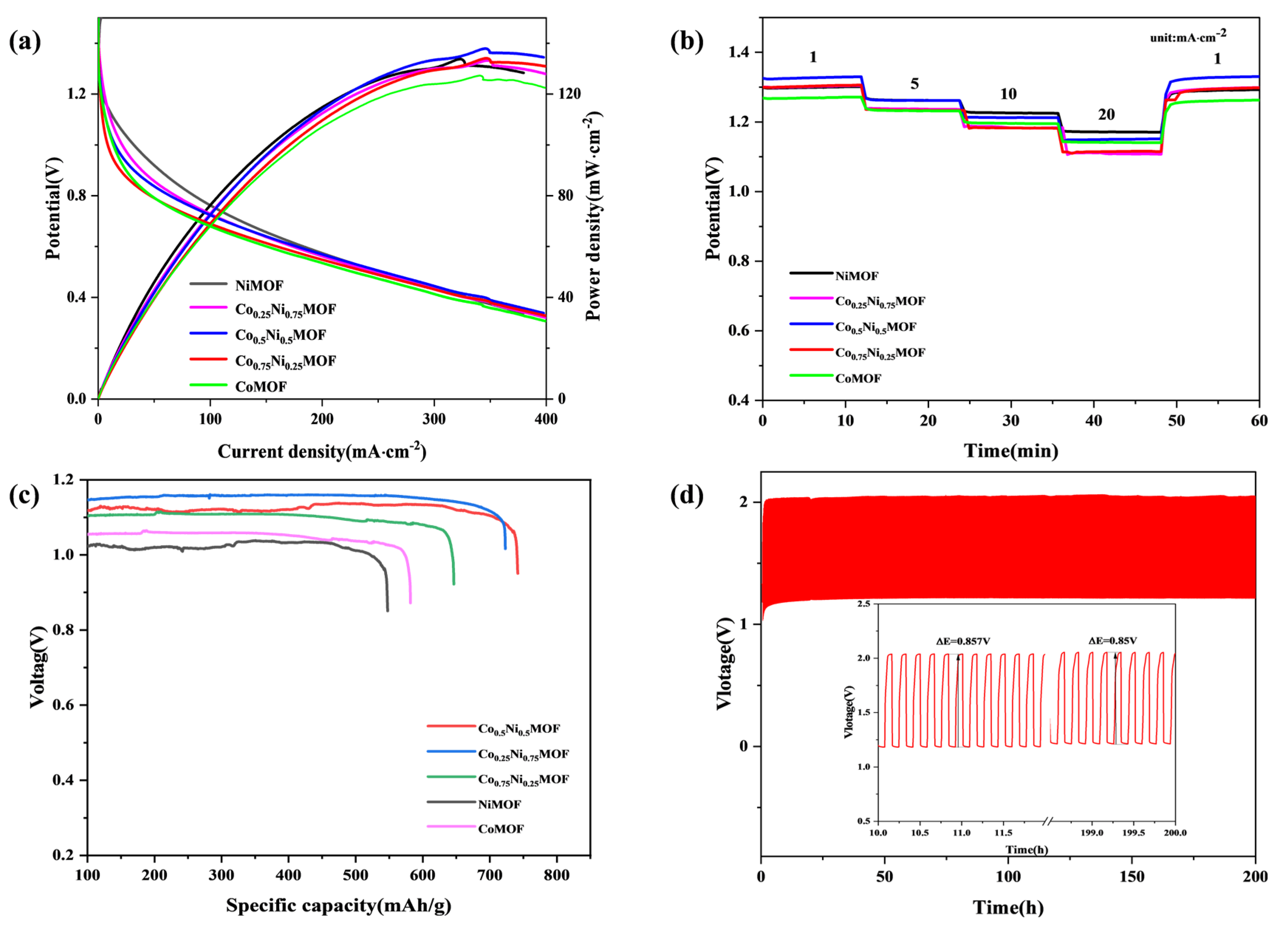
3.3. Zn–Air Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afsahi, F.; Kaliaguine, S. Non-precious electrocatalysts synthesized from metal-organic frameworks. J. Mater. Chem. A 2014, 2, 12270–12279. [Google Scholar] [CrossRef]
- Batool, N.; Shahzad, B.; Iqbal, W.; Han, X.F.; Wang, W.T.; Yan, J.; Shi, R.H.; Tian, J.H.; Yang, R.Z. Metal-Organic Framework-Derived PtNi on N-Doped Carbon Boosting Efficient Oxygen Electrocatalysis. ACS Appl. Energy Mater. 2022, 5, 13134–13141. [Google Scholar] [CrossRef]
- Byeon, A.; Yun, W.C.; Kim, J.M.; Lee, J.W. Non-precious Metal Catalysts for Two-Electron Oxygen Reduction Reaction. ChemElectroChem 2023, 10, 17. [Google Scholar] [CrossRef]
- Das, I.; Noori, M.T.; Shaikh, M.; Ghangrekar, M.M.; Ananthakrishnan, R. Synthesis and Application of Zirconium Metal-Organic Framework in Microbial Fuel Cells as a Cost-Effective Oxygen Reduction Catalyst with Competitive Performance. ACS Appl. Energy Mater. 2020, 3, 3512–3520. [Google Scholar] [CrossRef]
- Kundu, A.; Samanta, A.; Raj, C.R. Hierarchical Hollow MOF-Derived Bamboo-like N-doped Carbon Nanotube-Encapsulated Co0.25Ni0.75 Alloy: An Efficient Bifunctional Oxygen Electrocatalyst for Zinc-Air Battery. ACS Appl. Mater. Interfaces 2021, 13, 30486–30496. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, M.; Etesami, M.; Theerthagiri, J.; Choi, M.Y.; Wannapaiboon, S.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. Tailoring the MOF structure via ligand optimization afforded a dandelion flower like CoS/Co-Nx/CoNi/NiS catalyst to enhance the ORR/OER in zinc-air batteries. Nanoscale 2022, 14, 17908–17920. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.M.; Fujiwara, Y.; Kitagawa, S.; Horike, S. Homogenized Bimetallic Catalysts from Metal-Organic Framework Alloys. Chem. Mat. 2019, 31, 4205–4212. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Kao-Ian, W.; Rittiruam, M.; Praserthdam, S.; Praserthdam, P.; Limphirat, W.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. 3D Hierarchical MOF-Derived Defect-Rich NiFe Spinel Ferrite as a Highly Efficient Electrocatalyst for Oxygen Redox Reactions in Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2024, 16, 11537–11551. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Saher, S.; Xuan, S.; Noman, M.; Durani, M.W.; Ali, A. MOF Derived Catalysts for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell. Key Eng. Mater. 2018, 778, 275–282. [Google Scholar] [CrossRef]
- Lionet, Z.; Nishijima, S.; Kim, T.H.; Horiuchi, Y.; Lee, S.W.; Matsuoka, M. Bimetallic MOF-templated synthesis of alloy nanoparticle-embedded porous carbons for oxygen evolution and reduction reactions. Dalton Trans. 2019, 48, 13953–13959. [Google Scholar] [CrossRef]
- Nadeem, M.; Yasin, G.; Arif, M.; Bhatti, M.H.; Sayin, K.; Mehmood, M.; Yunus, U.; Mehboob, S.; Ahmed, I.; Flörke, U. Pt-Ni@PC900 Hybrid Derived from Layered-Structure Cd-MOF for Fuel Cell ORR Activity. ACS Omega 2020, 5, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Parkash, A. Synthesis of Bimetal Doped Metal-Organic Framework (MOF-5): An Electrocatalyst with Low Noble Metal Content and High Electrochemical Activity. ECS J. Solid State Sci. Technol. 2020, 9, 12. [Google Scholar] [CrossRef]
- Parkash, A.; Islam, M.; Qureshi, K.M.; Arain, A.M. MOF-74 Derived Carbon-Stabilized Pt/Cu-PC-900 Nanoparticles: Ultra-low Pt Content and Improved Electrocatalytic Activity. ECS J. Solid State Sci. Technol. 2022, 11, 8. [Google Scholar] [CrossRef]
- Shao, C.K.; Luo, M.S.; Song, H.Q.; Zhang, S.X.; Wang, F.L.; Liu, X.Y.; Huang, Z.T. Highly Active Porous Carbon-Supported CoNi Bimetallic Catalysts for Four-Electron Reduction of Oxygen. Energy Fuels 2023, 37, 4026–4037. [Google Scholar] [CrossRef]
- Saifi, S.; Dey, G.; Karthikeyan, J.; Kumar, R.; Bhattacharyya, D.; Sinha, A.S.K.; Aijaz, A. Coupling Single-Ni-Atom with Ni-Co Alloy Nanoparticle for Synergistically Enhanced Oxygen Reduction Reaction. Inorg. Chem. 2023, 62, 8200–8209. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cai, W.J.; Yu, B.B.; Qiu, H.; Li, M.L.; Zhu, L.W.; Yan, Z.; Hou, L.; Wang, Y.Y. Performance enhancement of oxygen evolution reaction through incorporating bimetallic electrocatalysts in two-dimensional metal-organic frameworks. Catal. Sci. Technol. 2020, 10, 3897–3903. [Google Scholar] [CrossRef]
- Yu, S.; Wu, Y.; Xue, Q.; Zhu, J.J.; Zhou, Y.Z. A novel multi-walled carbon nanotube-coupled CoNi MOF composite enhances the oxygen evolution reaction through synergistic effects. J. Mater. Chem. A 2022, 10, 4936–4943. [Google Scholar] [CrossRef]
- Sours, T.; Patel, A.; Norskov, J.; Siahrostami, S.; Kulkarni, A. Circumventing Scaling Relations in Oxygen Electrochemistry Using Metal-Organic Frameworks. J. Phys. Chem. Lett. 2020, 11, 10029–10036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Liang, B.L.; Lin, Z.Y.; Zhong, M.; Li, K.X.; Wang, H.; Lv, C.C. Engineering heterostructured Co0.7Fe0.3@Co doped leaf-like carbon nanoplates from dual metal-organic frameworks for high-efficiency oxygen reduction reaction in microbial fuel cell. J. Power Sources 2022, 520, 11. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, C.; Wang, H.F.; Wang, J.L. An ultra-dispersive, nonprecious metal MOF-FeZn catalyst with good oxygen reduction activity and favorable stability in acid. J. Mater. Sci. 2021, 56, 8600–8612. [Google Scholar] [CrossRef]
- Fu, S.F.; Zhu, C.Z.; Shi, Q.R.; Xia, H.B.; Dua, D.; Lin, Y.H. Highly branched PtCu bimetallic alloy nanodendrites with superior electrocatalytic activities for oxygen reduction reactions. Nanoscale 2016, 8, 5076–5081. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Zhu, E.B.; Chen, Y.; Li, Y.J.; Chiu, C.Y.; Xu, Y.X.; Lin, Z.Y.; Duan, X.F.; Huang, Y. A Facile Strategy to Pt3Ni Nanocrystals with Highly Porous Features as an Enhanced Oxygen Reduction Reaction Catalyst. Adv. Mater. 2013, 25, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Xu, Y.; Deng, K.; Yin, S.L.; Wang, Z.Q.; Xue, H.R.; Li, X.N.; Wang, L.; Wang, H.J. Metal-nonmetal nanoarchitectures: Quaternary PtPdNiP mesoporous nanospheres for enhanced oxygen reduction electrocatalysis. J. Mater. Chem. A 2019, 7, 3910–3916. [Google Scholar] [CrossRef]
- Liu, H.M.; Liu, X.Y.; Li, Y.M.; Jia, Y.F.; Tang, Y.W.; Chen, Y. Hollow PtNi alloy nanospheres with enhanced activity and methanol tolerance for the oxygen reduction reaction. Nano Res. 2016, 9, 3494–3503. [Google Scholar] [CrossRef]
- Lu, X.Y.; Tan, X.; Zhang, Q.R.; Daiyan, R.; Pan, J.; Chen, R.; Tahini, H.A.; Wang, D.W.; Smith, S.C.; Amal, R. Versatile electrocatalytic processes realized by Ni, Co and Fe alloyed core coordinated carbon shells. J. Mater. Chem. A 2019, 7, 12154–12165. [Google Scholar] [CrossRef]
- Park, J.; Kabiraz, M.K.; Kwon, H.; Park, S.; Baik, H.; Choi, S.I.; Lee, K. Radially Phase Segregated PtCu@PtCuNi Dendrite@Frame Nanocatalyst for the Oxygen Reduction Reaction. ACS Nano 2017, 11, 10844–10851. [Google Scholar] [CrossRef]
| Cobalt Nitrate (g) | Nickel Nitrate (g) | 2-Methylimidazole (g) | |
|---|---|---|---|
| CoMOF | 1.63 | 0 | 3.99 |
| Co0.75Ni0.25MOF | 1.222 | 0.407 | 3.99 |
| Co0.5Ni0.5MOF | 0.815 | 0.814 | 3.99 |
| Co0.25Ni0.75MOF | 0.407 | 1.221 | 3.99 |
| NiMOF | 0 | 1.628 | 3.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Han, L.; Xiao, S.; Zhu, A.; Zhang, Y.; Zeng, X.; Dong, P. Metal–Organic-Framework-Derived Nitrogen-Doped Carbon-Matrix-Encapsulating Co0.5Ni0.5 Alloy as a Bifunctional Oxygen Electrocatalyst for Zinc–Air Batteries. Materials 2024, 17, 2629. https://doi.org/10.3390/ma17112629
Liu J, Han L, Xiao S, Zhu A, Zhang Y, Zeng X, Dong P. Metal–Organic-Framework-Derived Nitrogen-Doped Carbon-Matrix-Encapsulating Co0.5Ni0.5 Alloy as a Bifunctional Oxygen Electrocatalyst for Zinc–Air Batteries. Materials. 2024; 17(11):2629. https://doi.org/10.3390/ma17112629
Chicago/Turabian StyleLiu, Jinglin, Lina Han, Shicai Xiao, Anqi Zhu, Yingjie Zhang, Xiaoyuan Zeng, and Peng Dong. 2024. "Metal–Organic-Framework-Derived Nitrogen-Doped Carbon-Matrix-Encapsulating Co0.5Ni0.5 Alloy as a Bifunctional Oxygen Electrocatalyst for Zinc–Air Batteries" Materials 17, no. 11: 2629. https://doi.org/10.3390/ma17112629





