Research on Resource Recovery and Disposal of Copper-Containing Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
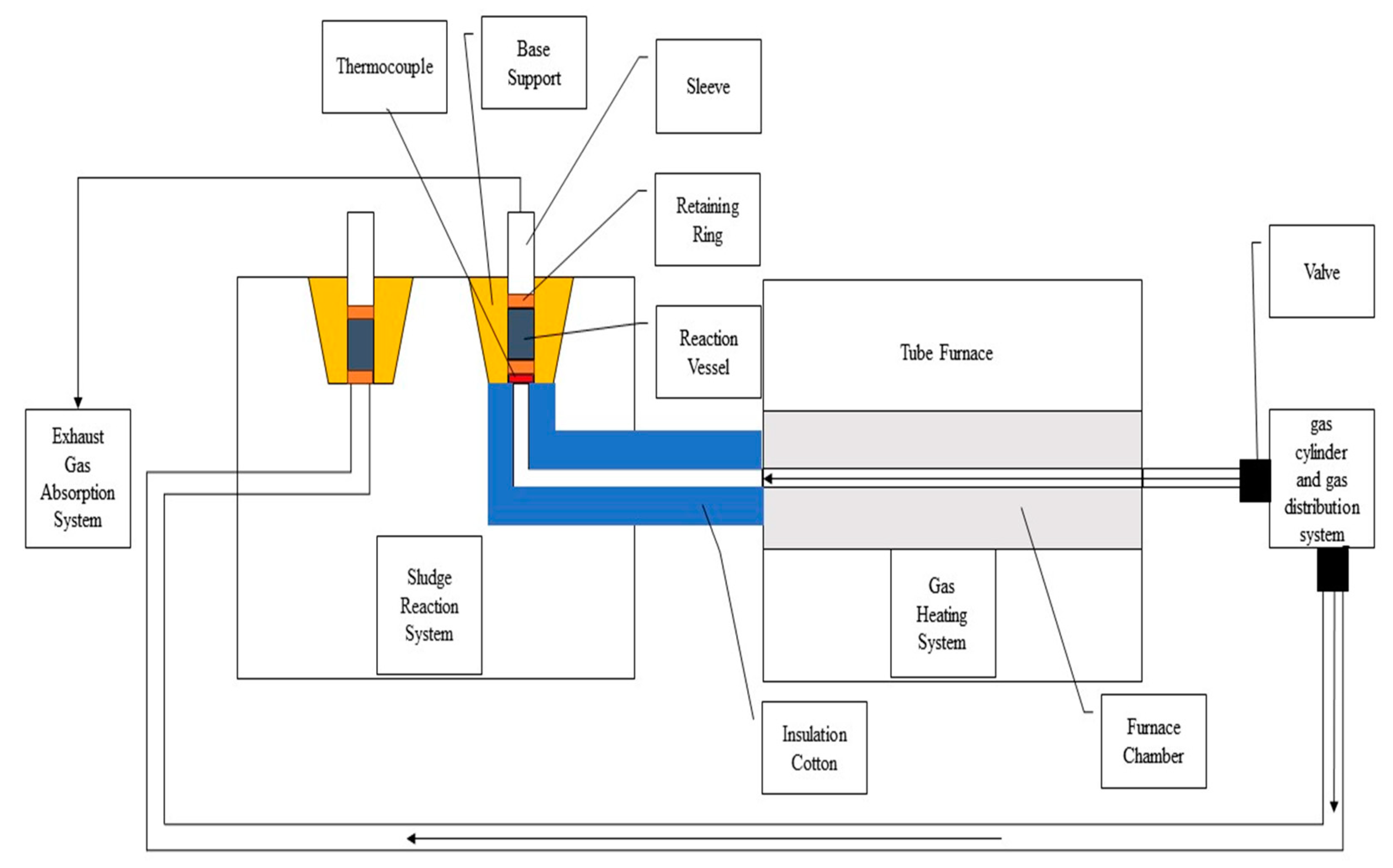
2.2. Experimental Setup
2.3. Experimental Methods
2.3.1. Experimental Method for Decomposition of Sludge under Oxidizing Atmosphere
2.3.2. Experimental Methods for Sludge Reduction
3. Results and Discussion
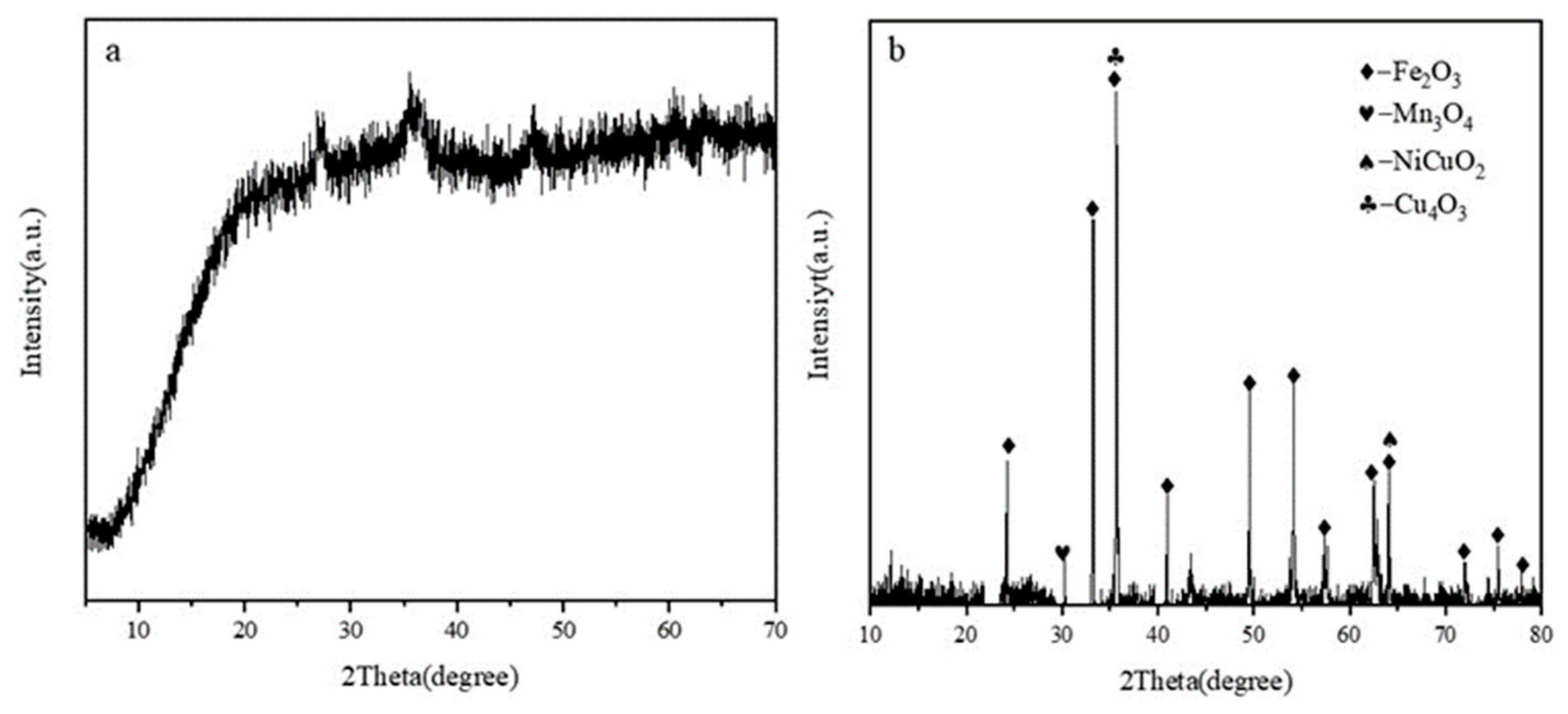
3.1. Copper-Containing Sludge Characteristics
3.2. Decomposition of Copper-Containing Sludge under Oxidising Atmosphere
3.2.1. Orthogonal Experiment
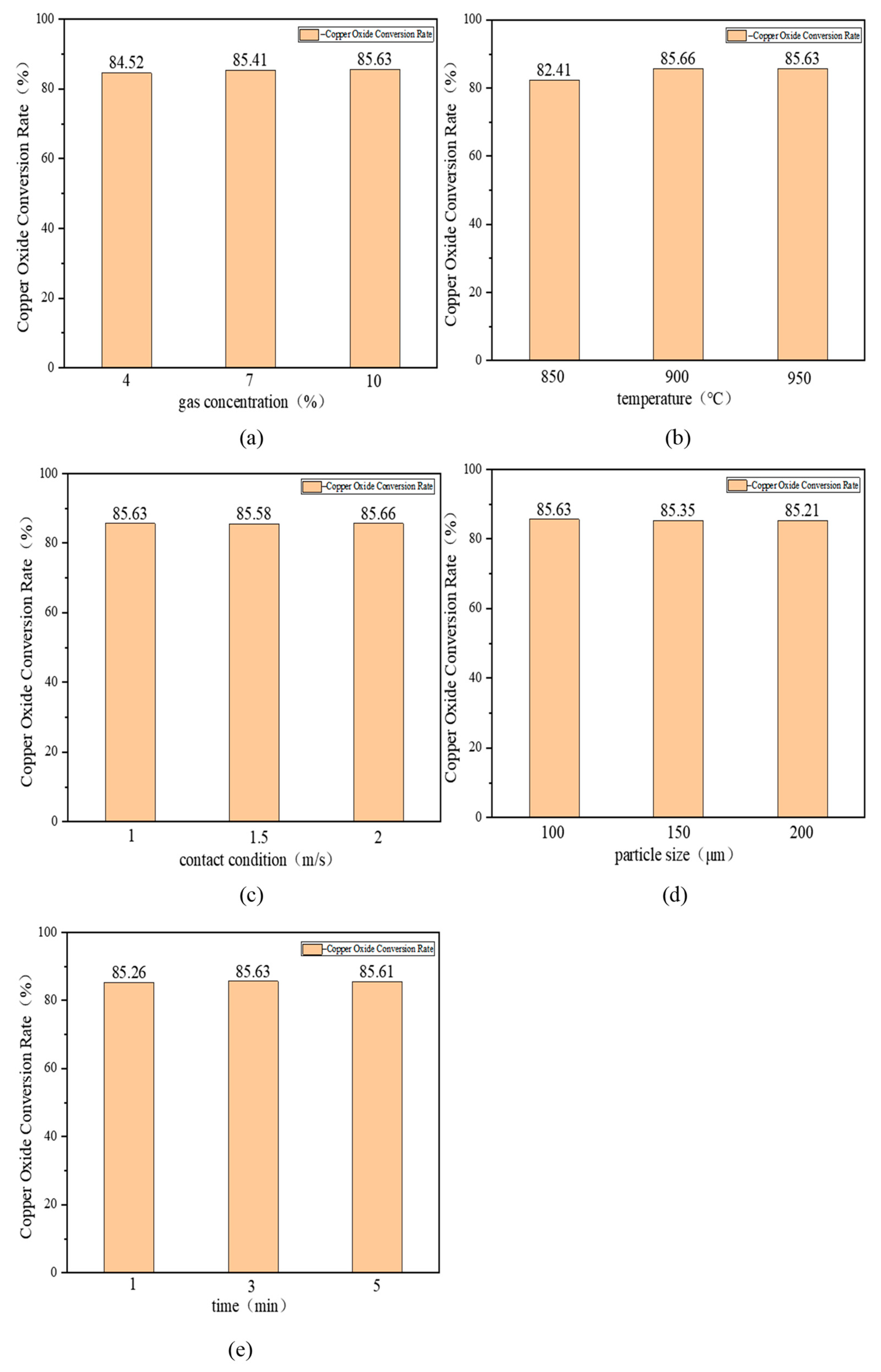
3.2.2. Single-Factor Experiments on the Decomposition of Copper-Containing Sludge under Oxidizing Atmosphere
3.3. Copper-Containing Sludge Reduction Experiment
3.3.1. Orthogonal Experiment
3.3.2. Single-Factor Experiments on the Reduction of Copper-Containing Sludge
4. Conclusions
- (1)
- In the decomposition experiments of the copper-containing sludge under an oxidising atmosphere, the factors influencing the conversion rate of copper oxide were ranked as follows: temperature > gas concentration > particle size > time > contact state. The highest conversion rate of copper oxide in the copper-containing sludge was achieved under the following process parameters: a gas concentration of 10%, temperature of 950 °C, contact state of 1 m/s, particle size of 100 μm, and a duration of 3 min. Based on these findings, single-factor experiments were conducted within the selected ranges of each influencing factor. By analysing the patterns of copper oxide conversion rate under various conditions, the optimal process parameters for the decomposition of the copper-containing sludge in an oxidising atmosphere were determined to be a gas concentration of 10%, temperature of 900 °C, the contact state of 1 m/s, particle size of 100 μm, and a duration of 3 min. The copper oxide conversion rate was 85.66%.
- (2)
- In the reduction experiments of the copper-containing sludge, the factors influencing the conversion rate of copper were ranked in the following order: temperature > gas concentration > time > particle size > contact state. The highest copper conversion rate was achieved under the following process parameters: a gas concentration of 7%, temperature of 850 °C, contact state of 2 m/s, particle size of 200 μm, and a duration of 5 min. Based on these findings, single-factor experiments were conducted within the selected ranges of each influencing factor. The patterns of copper conversion rate under different conditions were studied in order to determine the optimal process parameters for the reduction of copper-containing sludge. These were found to be a gas concentration of 7%, temperature of 800 °C, contact state of 1.5 m/s, particle size of 200 μm, and a duration of 5 min. The copper conversion rate was 83.75%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.; Wang, Y.; Cao, W.; Li, W. Study on the Process Parameters of Sulfuric Acid Leaching of Copper and Nickel Containing Sludge. China Resour. Compr. Util. 2022, 40, 46–49+52. [Google Scholar]
- Li, P.P.; Peng, C.S.; Li, F.M.; Song, S.X.; Juan, A.O. Copper and Nickel Recovery from Electroplating Sludge by the Process of Acid-leaching and Electro-depositing. Int. J. Environ. Res. 2011, 5, 797–804. [Google Scholar]
- Li, C.; Xie, F.; Ma, Y.; Cai, T.; Li, H.; Huang, Z.; Yuan, G. Multiple heavy metals extraction and recovery from hazardous electroplating sludge waste via ultrasonically enhanced two-stage acid leaching. J. Hazard. Mater. 2010, 178, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Deng, C.; Liu, T. Selective recovery of copper from electroplating sludge by integrated EDTA mixed with citric acid leaching and electrodeposition. Sep. Purif. Technol. 2022, 303, 101917. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, T.; Chen, H.; Zhang, Y.; Geng, Z.; Zhu, S.; Xie, X.; Zhang, H.; Gao, Y.; Huo, Y. Stepwise recycling of Fe, Cu, Zn and Ni from real electroplating sludge via coupled acidic leaching and hydrothermal and extraction routes. Environ. Res. 2023, 216, 114462. [Google Scholar]
- Su, R.; Liang, B.; Guan, J. Leaching Effects of Metal from Electroplating Sludge under Phosphate Participation in Hydrochloric Acid Medium. Procedia Environ. Sci. 2016, 31, 361–365. [Google Scholar] [CrossRef]
- Yi, L.; Wu, Q.; Zhao, L.; Li, X.; Liu, M.; Liu, T. Reduction ammonia leaching process and reaction kinetics analysis of electroplating sludge containing copper and nickel. Electroplat. Finish. 2020, 39, 887–892. [Google Scholar]
- Xu, W.; Liu, W.; Zhu, H.; Xu, J.; Li, G.; Fu, D.; Luo, L. Highly selective copper and nickel separation and recovery from electroplating sludge in light industry. Pol. J. Environ. Stud. 2017, 24, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, L.; He, J.; Sun, Y.; Lei, Y. A metallurgical approach for separation and recovery of Cu, Cr, and Ni from electroplating sludge. Sci. Total Environ. 2024, 921, 171130. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Shi, C.; Zuo, X.; Yan, B. Selective recovery of nickel from stainless steel pickling sludge with NH3-(NH4)2CO3 leaching system. Environ. Technol. 2022, 44, 3249–3262. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Gong, J.; Gong, B.; Li, Y.; Jie, F.; Wang, Z.; Xiong, G.; Zhu, X. Removal of copper from electroplating wastewater using adsorption by Bacillus subtilis. Hydrometallurgy 2022, 41, 40–46. [Google Scholar]
- Yang, Y.; Liu, X.; Wang, J.; Huang, Q.; Xin, Y.; Xin, B. Screening Bioleaching Systems and Operational Conditions for Optimal Ni Recovery from Dry Electroplating Sludge and Exploration of the Leaching Mechanisms Involved. Geomicrobiol. J. 2016, 33, 179–184. [Google Scholar] [CrossRef]
- Nikfar, S.; Parsa, A.; Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced bioleaching of Cr and Ni from a chromium-rich electroplating sludge using the filtrated culture of Aspergillus niger. J. Clean. Prod. 2020, 264, 121622. [Google Scholar] [CrossRef]
- Tian, B.; Cui, Y.; Qin, Z.; Wen, L.; Li, Z.; Chu, H.; Xin, B. Indirect bioleaching recovery of valuable metals from electroplating sludge and optimization of various parameters using response surface methodology (RSM). J. Environ. Manag. 2022, 312, 112927. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, X.; Lu, J.; Yin, C. Migration and transformation of heavy metals Cu and Ni during the thermal treatment of electroplating sludge. Environ. Sci. Manag. 2016, 41, 48–50. [Google Scholar]
- Xu, J.; Li, L.; Xu, Z.; Xiao, Y.; Lei, Y.; Liu, Y. Co-treatment of copper electrolytic sludges and copper scraps for the recycled utilization of copper and arsenic. Chemosphere 2023, 341, 140065. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.R.; Lv, J.F.; Zhou, J.S.; Li, Y.; Li, Z.Y. Innovative technology and mechanism for comprehensive recovery of copper, nickel, zinc and iron in electroplating sludge. Sep. Purif. Technol. 2024, 336, 126226. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Y.; Jiao, S.Q.; Chou, K.C.; Zhang, G.H. Recovery of Ni matte from Ni-bearing electroplating sludge. J. Environ. Manag. 2023, 326, 116744. [Google Scholar] [CrossRef]
- Tang, Z.; Ding, X.; Yan, X.; Dong, Y.; Liu, C. Recovery of Iron, Chromium, and Nickel from Pickling Sludge Using Smelting Reduction. Met.-Open Access Metall. J. 2018, 8, 936. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, X.; Gao, P.; Han, Y. A semi-industrial experiment of suspension magnetization roasting technology for separation of iron minerals from red mud. J. Hazard. Mater. 2020, 394, 122579. [Google Scholar] [CrossRef] [PubMed]
- Song, H.W.; Park, K.J.; Han, S.K.; Jung, H.S. Thermal conductivity characteristics of dewatered sewage sludge by thermal hydrolysis reaction. J. Air Waste Manag. Assoc. 2014, 64, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.L.; Chang, C.Y.; Lin, J.P.; Wu, C.H.; Lee, D.J. Resources recovery of oil sludge by pyrolysis: Kinetics study. J. Chem. Technol. Biotechnol. 2000, 75, 443–450. [Google Scholar] [CrossRef]
- Zhang, H.; Jiu, S.; Gao, Q.; Zhao, S.; Chen, Y.; Cheng, F.; Han, D.; Shi, R.; Yuan, K.; Li, J.; et al. Activation of Low-Quality Coal Gangue Using Suspension Calcination for the Preparation of High-Performance Low-Carbon Cementitious Materials: A Pilot Study. Processes 2024, 12, 550. [Google Scholar] [CrossRef]
- Cheng, S.; Jiu, S.; Li, H. Preparation of Calcined Kaolin by Efficient Decarburization of Coal-Series Kaolinite in a Suspended Bed Reactor. Processes 2024, 10, 2048. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Y.; Lu, X.; Ding, C.; Zhao, L. Study on the suspension reduction roasting process of soft manganese ore. China Min. Mag. 2010, 19, 66–70. [Google Scholar]
- Dong, M.; Gui, L.X.; Zhi, H.T. High-temperature reduction of phosphogypsum in a fluidized bed of coal gasification fine slag. Chem. Ind. Eng. Prog. 2023, 8, 1–17. [Google Scholar]
- Zhang, G.; Yang, R.; Jiang, Q.; Liu, Y.; Zhang, P.; Pan, Z. Study on the effect of hydrogen reduction atmosphere under electromagnetic levitation on the dephosphorization of ferrosilicon alloy. Mod. Chem. Ind. 2023, 43, 133–136. [Google Scholar]



| Chemical Composition | Fe | S | Cu | Ni | P | Ti | Mg | Na | Mn |
| Content/% | 38.14 | 2.92 | 2.44 | 1.80 | 0.877 | 0.561 | 0.360 | 0.290 | 0.228 |
| Serial Number | Gas Concentration (%) | Temperature (°C) | Contact Condition (m/s) | Particle Size (μm) | Time (min) |
|---|---|---|---|---|---|
| 1 | 4 | 850 | 1 | 100 | 1 |
| 2 | 7 | 900 | 1.5 | 150 | 3 |
| 3 | 10 | 950 | 2 | 200 | 5 |
| Serial Number | Factors | Copper Oxide Conversion Rate (%) | ||||
|---|---|---|---|---|---|---|
| Gas Concentration (%) | Temperature (°C) | Contact Condition (m/s) | Particle Size (μm) | Time (min) | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 78.14 |
| 2 | 1 | 1 | 2 | 2 | 3 | 79.21 |
| 3 | 1 | 2 | 1 | 3 | 3 | 81.83 |
| 4 | 1 | 2 | 3 | 1 | 2 | 82.94 |
| 5 | 1 | 3 | 2 | 3 | 2 | 84.15 |
| 6 | 1 | 3 | 3 | 2 | 1 | 83.21 |
| 7 | 2 | 1 | 1 | 3 | 2 | 79.41 |
| 8 | 2 | 1 | 3 | 1 | 3 | 81.42 |
| 9 | 2 | 2 | 2 | 2 | 2 | 82.75 |
| 10 | 2 | 2 | 3 | 3 | 1 | 83.12 |
| 11 | 2 | 3 | 1 | 2 | 3 | 84.86 |
| 12 | 2 | 3 | 2 | 1 | 1 | 84.36 |
| 13 | 3 | 1 | 2 | 3 | 1 | 80.23 |
| 14 | 3 | 1 | 3 | 2 | 2 | 81.15 |
| 15 | 3 | 2 | 1 | 2 | 1 | 83.52 |
| 16 | 3 | 2 | 2 | 1 | 3 | 84.32 |
| 17 | 3 | 3 | 1 | 1 | 2 | 85.63 |
| 18 | 3 | 3 | 3 | 3 | 3 | 84.13 |
| Serial Number | Factors | ||||
|---|---|---|---|---|---|
| Gas Concentration (%) | Temperature (°C) | Contact Condition (m/s) | Particle Size (μm) | Time (min) | |
| K1 | 489.48 | 479.56 | 493.39 | 496.81 | 492.58 |
| K2 | 495.92 | 498.48 | 495.02 | 494.70 | 496.03 |
| K3 | 498.98 | 506.34 | 495.97 | 492.87 | 495.77 |
| k1 | 81.58 | 79.93 | 82.23 | 82.80 | 82.10 |
| k2 | 82.65 | 83.08 | 82.50 | 82.45 | 82.67 |
| k3 | 83.16 | 84.39 | 82.66 | 82.15 | 82.63 |
| Range(R) | 1.58 | 4.46 | 0.43 | 0.66 | 0.58 |
| Serial Number | Gas Concentration (%) | Temperature (°C) | Contact Condition (m/s) | Particle Size (μm) | Time (min) |
|---|---|---|---|---|---|
| 1 | 1 | 750 | 1 | 100 | 1 |
| 2 | 4 | 800 | 2 | 150 | 3 |
| 3 | 7 | 850 | 3 | 200 | 5 |
| Serial Number | Factors | Copper Conversion Rate (%) | ||||
|---|---|---|---|---|---|---|
| Gas Concentration (%) | Temperature (°C) | Contact Condition (m/s) | Particle Size (μm) | Time (min) | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 75.58 |
| 2 | 1 | 1 | 2 | 2 | 3 | 76.73 |
| 3 | 1 | 2 | 1 | 3 | 3 | 78.53 |
| 4 | 1 | 2 | 3 | 1 | 2 | 79.42 |
| 5 | 1 | 3 | 2 | 3 | 2 | 80.84 |
| 6 | 1 | 3 | 3 | 2 | 1 | 80.81 |
| 7 | 2 | 1 | 1 | 3 | 2 | 76.85 |
| 8 | 2 | 1 | 3 | 1 | 3 | 77.84 |
| 9 | 2 | 2 | 2 | 2 | 2 | 79.46 |
| 10 | 2 | 2 | 3 | 3 | 1 | 80.24 |
| 11 | 2 | 3 | 1 | 2 | 3 | 82.79 |
| 12 | 2 | 3 | 2 | 1 | 1 | 81.21 |
| 13 | 3 | 1 | 2 | 3 | 1 | 77.54 |
| 14 | 3 | 1 | 3 | 2 | 2 | 78.72 |
| 15 | 3 | 2 | 1 | 2 | 1 | 81.25 |
| 16 | 3 | 2 | 2 | 1 | 3 | 82.41 |
| 17 | 3 | 3 | 1 | 1 | 2 | 83.64 |
| 18 | 3 | 3 | 3 | 3 | 3 | 83.75 |
| Serial Number | Factors | ||||
|---|---|---|---|---|---|
| Gas Concentration (%) | Temperature (°C) | Contact Condition (m/s) | Particle Size (μm) | Time (min) | |
| K1 | 471.91 | 463.26 | 478.64 | 480.10 | 476.63 |
| K2 | 478.39 | 481.31 | 478.19 | 479.76 | 478.93 |
| K3 | 487.31 | 493.04 | 480.78 | 477.75 | 482.05 |
| k1 | 78.65 | 77.21 | 79.77 | 80.02 | 79.44 |
| k2 | 79.73 | 80.22 | 79.70 | 79.96 | 79.82 |
| k3 | 81.22 | 82.17 | 80.13 | 79.63 | 80.34 |
| Range(R) | 2.57 | 4.96 | 0.36 | 0.39 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhou, Y. Research on Resource Recovery and Disposal of Copper-Containing Sludge. Materials 2024, 17, 2636. https://doi.org/10.3390/ma17112636
Yu J, Zhou Y. Research on Resource Recovery and Disposal of Copper-Containing Sludge. Materials. 2024; 17(11):2636. https://doi.org/10.3390/ma17112636
Chicago/Turabian StyleYu, Jinao, and Yongmin Zhou. 2024. "Research on Resource Recovery and Disposal of Copper-Containing Sludge" Materials 17, no. 11: 2636. https://doi.org/10.3390/ma17112636





