Effect of Negative Pulse on the Stability of Black Electrolytes for Magnesium Alloy Microarc Oxidation
Abstract
:1. Introduction
2. Details of Experiment
2.1. Experiment Preparation
2.2. Characterization of Coatings
3. Results and Discussion
3.1. Growth Characteristics of Magnesium Alloy Microarc Oxidation Coatings
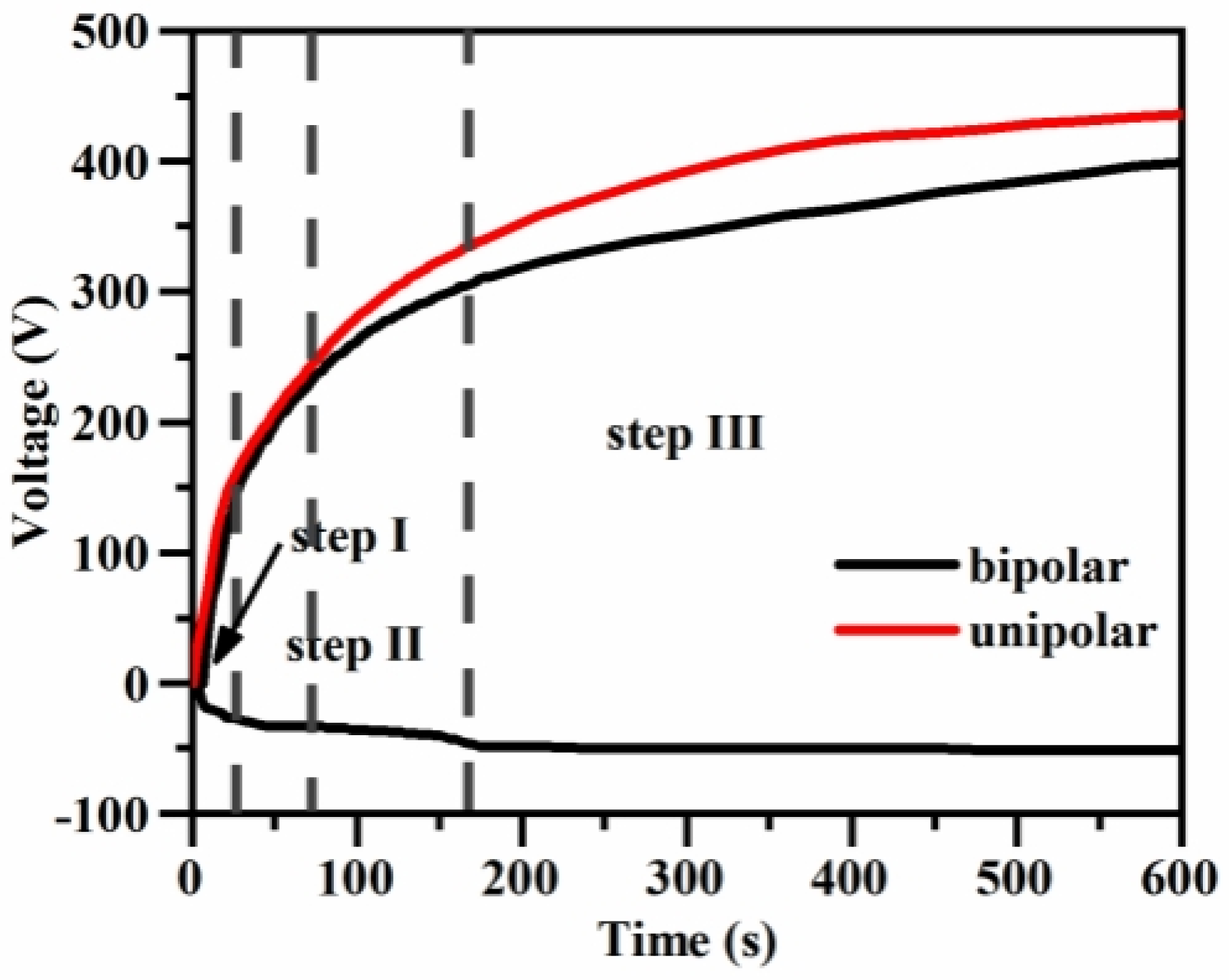
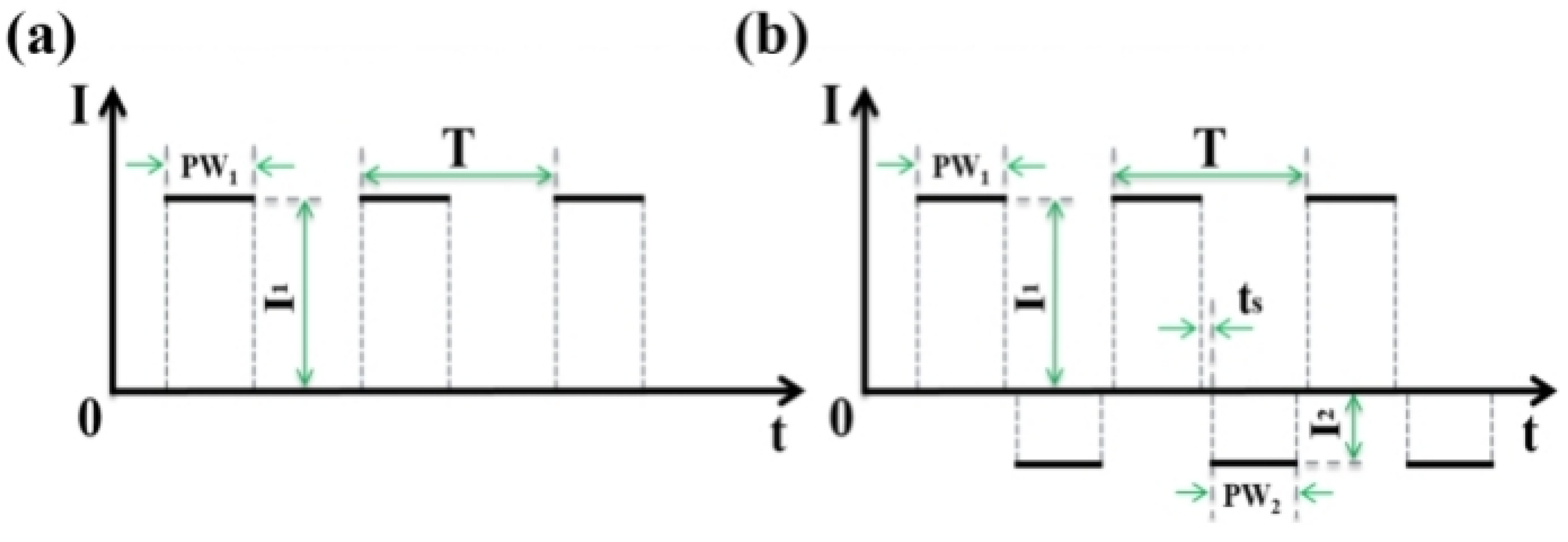
3.1.1. Effects of Different Power Supply Modes on the Growth Characteristics of Magnesium Alloy Microarc Oxidized Coatings
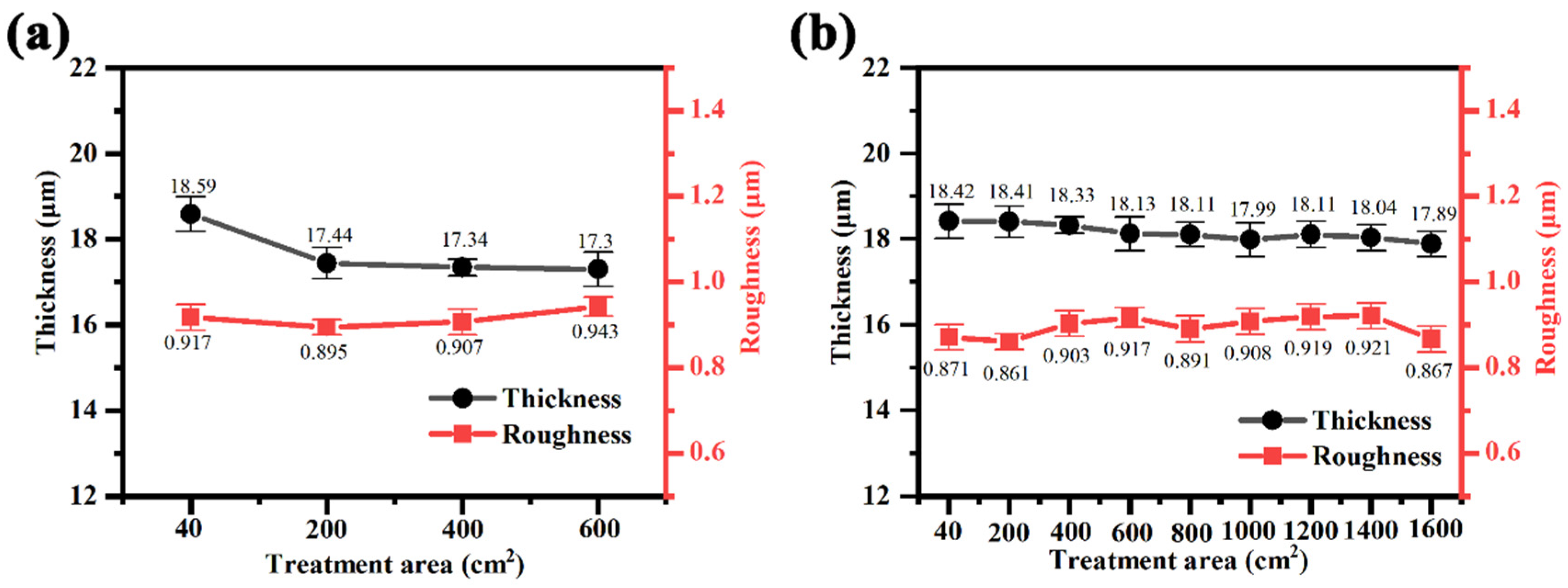
3.1.2. Effect of Treatment Area on the Growth Characteristics of Magnesium Alloy Microarc Oxidized Coatings in Different Power Modes
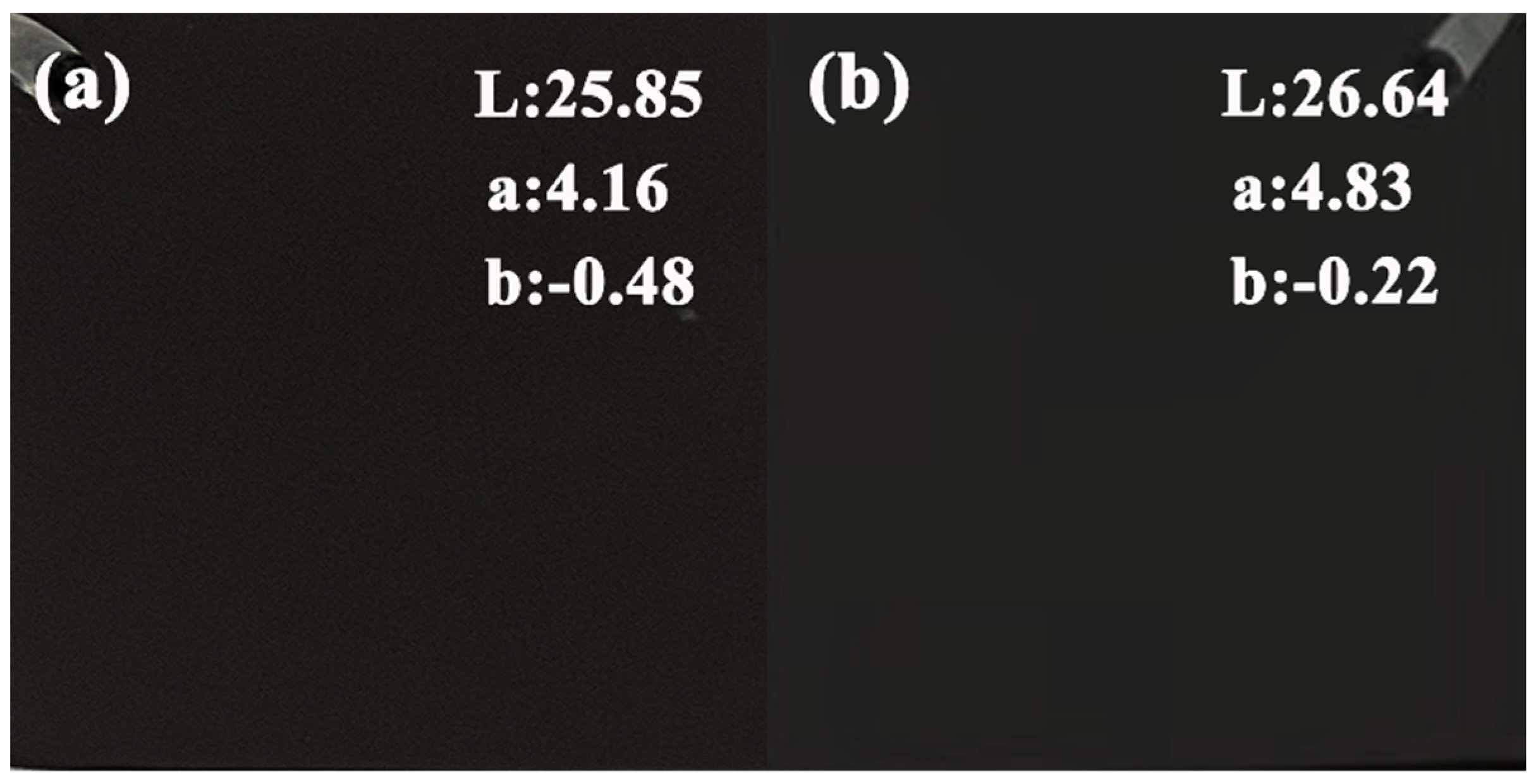
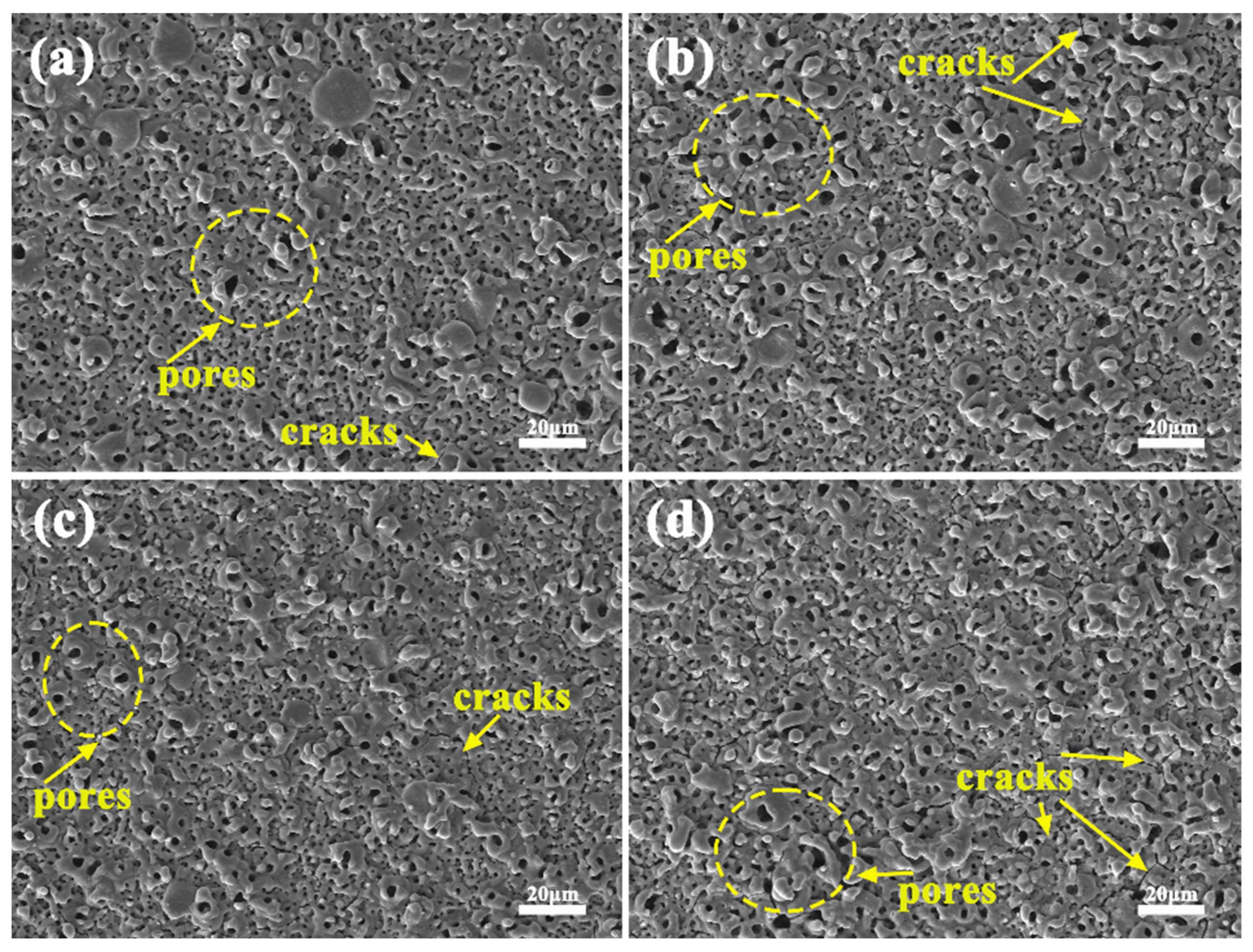
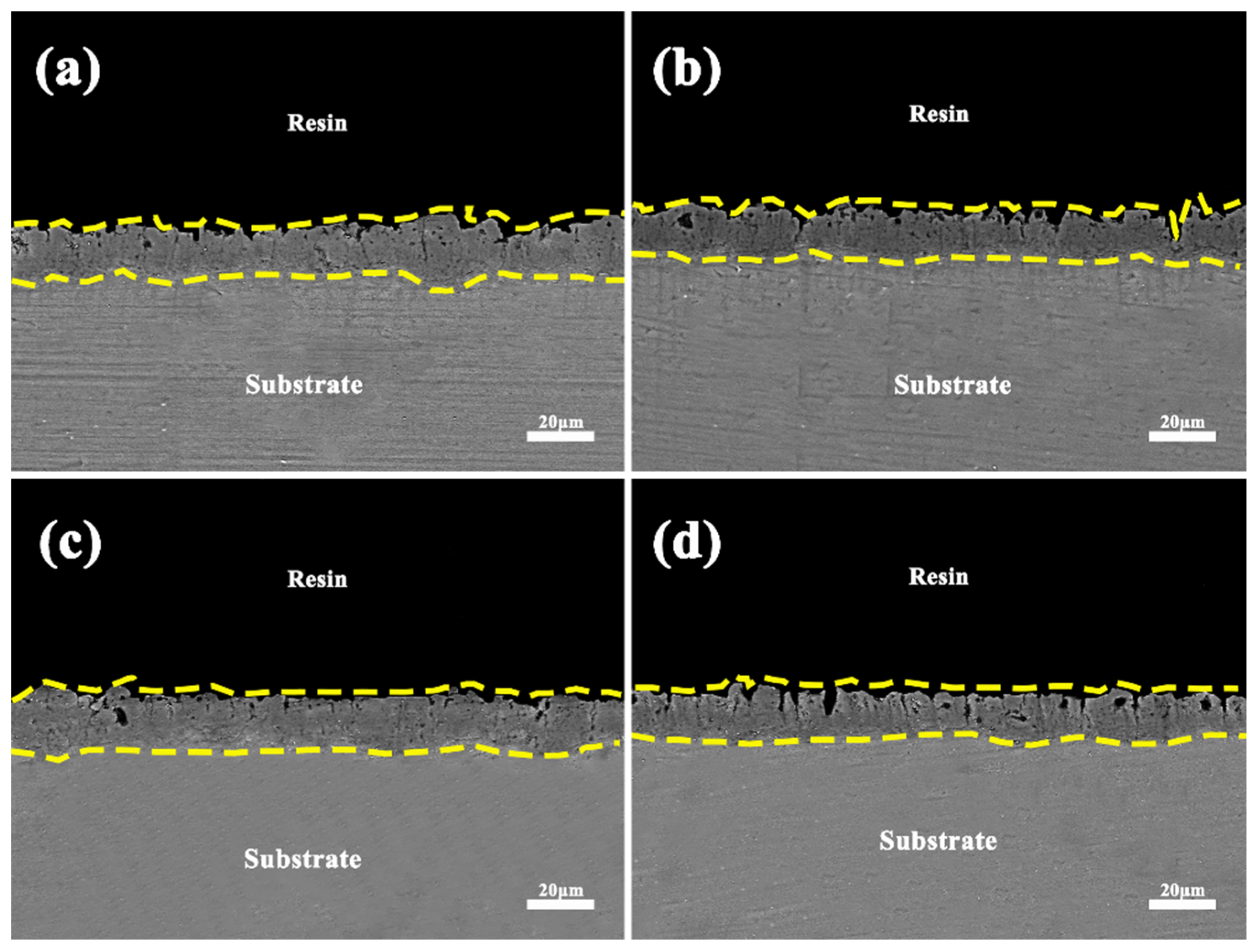
3.2. Influence of Phase Composition and Microstructure of Microarc Oxidized Coatings before and after Electrolyte Failure under Different Power Modes
3.3. Effect of Treatment Area on Electrolyte Base Properties in Different Power Modes
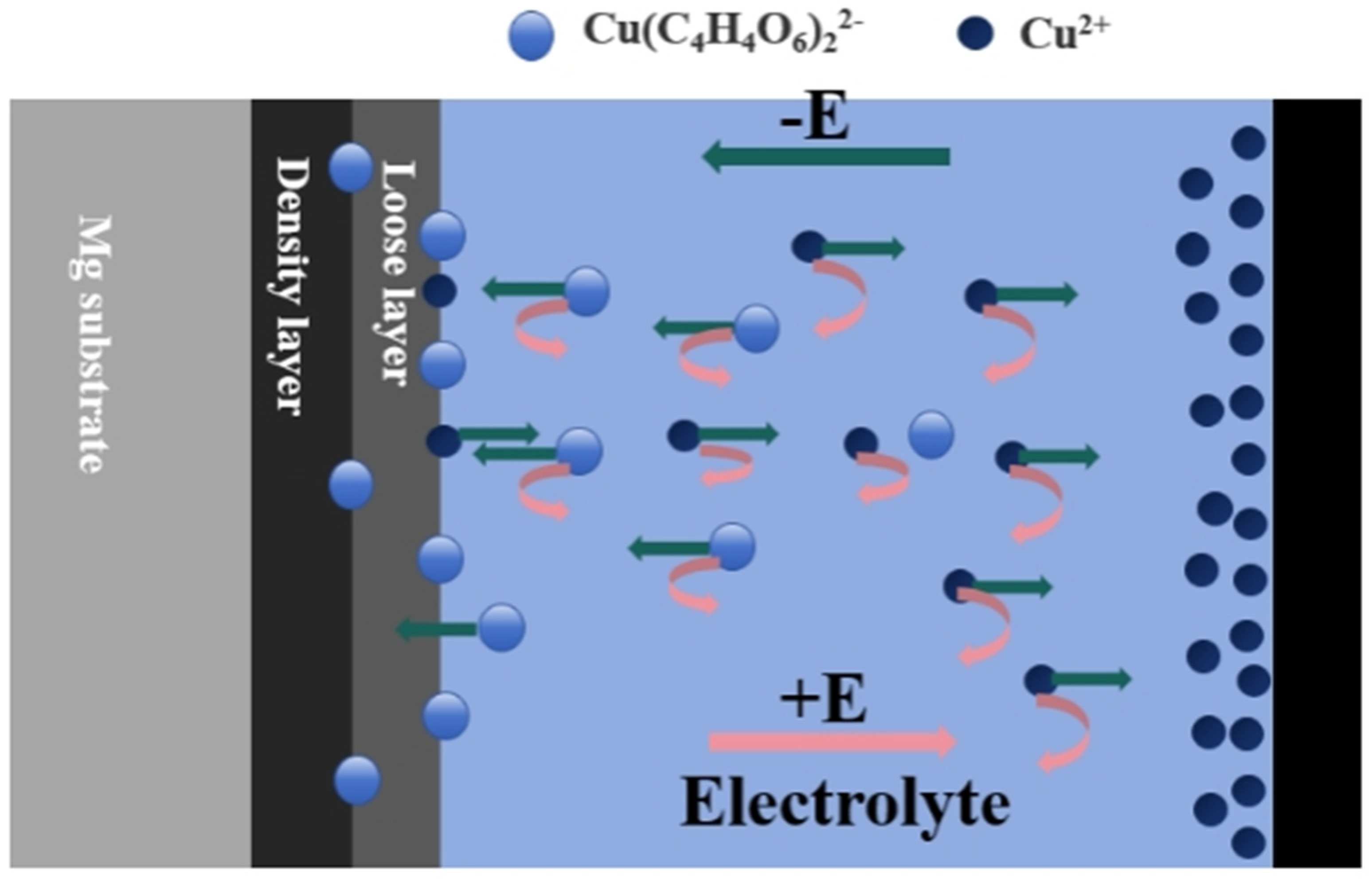
3.4. Failure Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Zahedi Asl, V.; Chini, S.F.; Zhao, J.; Palizdar, Y.; Shaker, M.; Sadeghi, A. Corrosion properties and surface free energy of the Zn Al LDH/rGO coating on MAO pretreated AZ31 magnesium alloy. Surf. Coat. Technol. 2021, 426, 127764. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloys 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Enhanced corrosion resistance and in-vitro biodegradation of plasma electrolytic oxidation coatings prepared on AZ91 Mg alloy using ZnO nanoparticles-incorporated electrolyte. Surf. Coat. Technol. 2019, 360, 153–171. [Google Scholar] [CrossRef]
- Daavari, M.; Atapour, M.; Mohedano, M.; Arrabal, R.; Matykina, E.; Taherizadeh, A. Biotribology and biocorrosion of MWCNTs-reinforced PEO coating on AZ31B Mg alloy. Surf. Interfaces 2021, 22, 100850. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Hang, R.; Shi, H.; Wang, L.; Ma, J.; Liu, X.; Yao, X. Enhanced anticorrosive and antibacterial performances of silver nanoparticles/polyethyleneimine/MAO composite coating on magnesium alloys. J. Mater. Res. Technol. 2021, 11, 2354–2364. [Google Scholar] [CrossRef]
- Yang, C.; Chen, P.; Wu, W.; Sheng, L.; Zheng, Y.; Chu, P.K. A Review of Corrosion-Resistant PEO Coating on Mg Alloy. Coatings 2024, 14, 451. [Google Scholar] [CrossRef]
- Paramsothy, M.; Hassan, S.F.; Srikanth, N.; Gupta, M. Adding carbon nanotubes and integrating with AA5052 aluminium alloy core to simultaneously enhance stiffness, strength and failure strain of AZ31 magnesium alloy. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1490–1500. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-Alhosseini, A.; Esfahani, H. Increasing the in-vitro corrosion resistance of AZ31B-Mg alloy via coating with hydroxyapatite using plasma electrolytic oxidation. J. Asian Ceram. Soc. 2019, 8, 39–49. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of combined organic and inorganic corrosion inhibitors on the nanostructure cerium based conversion coating performance on AZ31 magnesium alloy: Morphological and corrosion studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Liu, S.; Qi, Y.; Peng, Z.; Liang, J. A chemical-free sealing method for Micro-arc oxidation coatings on AZ31 Mg alloy. Surf. Coat. Technol. 2021, 406, 126655. [Google Scholar] [CrossRef]
- Hao, J.; Liu, H. Study on the decay law of magnesium alloy microarc oxidation electrolyte in silicate system. In Proceedings of the Aluminum Profile Surface Treatment and Thermal Insulation Aluminum Profile Technology Exchange Meeting, Suzhou, China, 14 July 2007. [Google Scholar]
- Tai, H. One-Step Coloring and Electrolyte Life Study of Magnesium Alloy Microarc Oxidation; Changchun University of Science and Technology: Changchun, China, 2010. [Google Scholar]
- Liu, H.; Song, H. Effect of solution concentration on the failure of magnesium alloy microarc oxidation electrolytes. Mater. Dev. Appl. 2007, 4, 15–17. [Google Scholar] [CrossRef]
- Hou, Z. Study on the Effect of Additives on the Micro-Arc Oxidation Behavior of Magnesium Alloy and Electrolyte Failure; Xi’an University of Science and Technology: Xi’an, China, 2016. [Google Scholar]
- Bai, L.; Dong, B.; Chen, G.; Xin, T.; Wu, J.; Sun, X. Effect of positive pulse voltage on color value and corrosion property of magnesium alloy black micro-arc oxidation ceramic coating. Surf. Coat. Technol. 2019, 374, 402–408. [Google Scholar] [CrossRef]
- Madhan Kumar, A.; Kwon, S.H.; Jung, H.C.; Shin, K.S. Corrosion protection performance of single and dual Plasma Electrolytic Oxidation (PEO) coating for aerospace applications. Mater. Chem. Phys. 2015, 149–150, 480–486. [Google Scholar] [CrossRef]
- Yu, J.-M.; Kim, H.-J.; Ahn, S.-G.; Choe, H.-C. Plasma electrolytic oxidation of Ti-6Al-4V alloy in electrolytes containing bone formation ions. Appl. Surf. Sci. 2020, 513, 145776. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Su, J.F.; Nie, X. A study of the interactive effects of hybrid current modes on the tribological properties of a PEO (plasma electrolytic oxidation) coated AM60B Mg-alloy. Surf. Coat. Technol. 2013, 215, 421–430. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution. Electrochim. Acta 2007, 52, 5002–5009. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Effect of Pulse Current Mode on Microstructure, Composition and Corrosion Performance of the Coatings Produced by Plasma Electrolytic Oxidation on AZ31 Mg Alloy. Coatings 2019, 9, 688. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Corrosion and wear resistance of coatings produced on AZ31 Mg alloy by plasma electrolytic oxidation in silicate-based K2TiF6 containing solution: Effect of waveform. J. Magnes. Alloys 2022, 10, 2574–2587. [Google Scholar] [CrossRef]
- Blawert, C.; Bala Srinivasan, P. Characterisation of tribological and corrosion behaviour of plasma electrolytic oxidation coated AM50 magnesium alloy. Surf. Eng. 2010, 26, 340–346. [Google Scholar] [CrossRef]






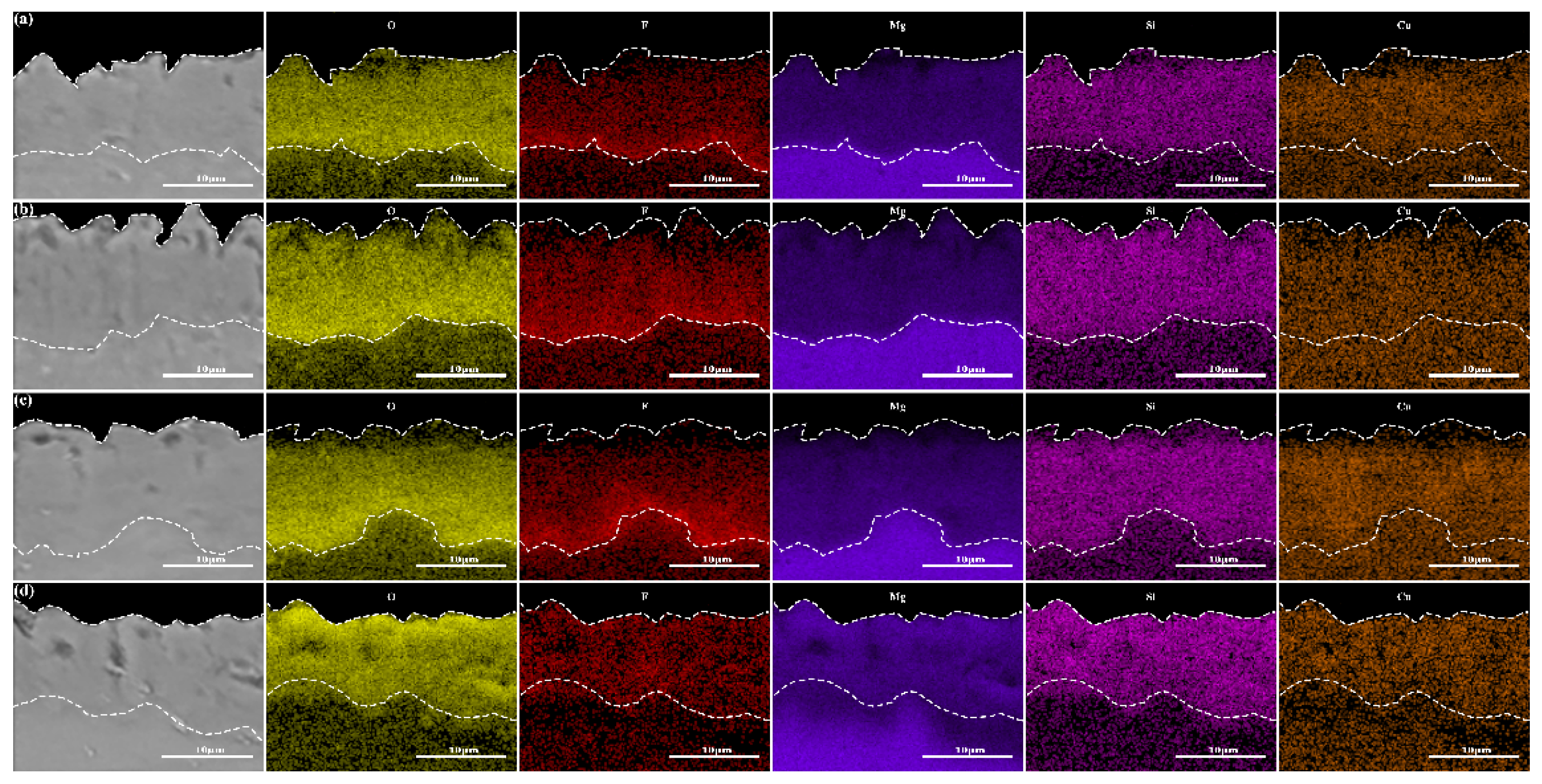

| Concentration (g/L) | Percentage of Element Content (wt. %) | ||||
|---|---|---|---|---|---|
| Mg | O | Si | Cu | F | |
| D1 | 52.91 | 33.33 | 7.89 | 3.39 | 2.48 |
| D2 | 55.36 | 35.08 | 7.29 | 0.01 | 2.27 |
| D3 | 53.49 | 33.33 | 7.59 | 3.70 | 1.88 |
| D4 | 56.14 | 35.48 | 6.31 | 0.44 | 1.62 |
| Power Mode | Pre-Invalidation (Cu mg/L) | Post-Invalidation (Cu mg/L) |
|---|---|---|
| unipolar | 1495 | 448 |
| bipolar | 1495 | 770 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Tong, R.; Li, H.; Wang, W.; Chen, X.; Wang, H.; Yang, Y.; Zhou, S. Effect of Negative Pulse on the Stability of Black Electrolytes for Magnesium Alloy Microarc Oxidation. Materials 2024, 17, 2654. https://doi.org/10.3390/ma17112654
Chen B, Tong R, Li H, Wang W, Chen X, Wang H, Yang Y, Zhou S. Effect of Negative Pulse on the Stability of Black Electrolytes for Magnesium Alloy Microarc Oxidation. Materials. 2024; 17(11):2654. https://doi.org/10.3390/ma17112654
Chicago/Turabian StyleChen, Bo, Rui Tong, Hongtao Li, Wenqiang Wang, Xuanyu Chen, Hao Wang, Yifeng Yang, and Shiquan Zhou. 2024. "Effect of Negative Pulse on the Stability of Black Electrolytes for Magnesium Alloy Microarc Oxidation" Materials 17, no. 11: 2654. https://doi.org/10.3390/ma17112654





