Mo-Doped Na4Fe3(PO4)2P2O7/C Composites for High-Rate and Long-Life Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
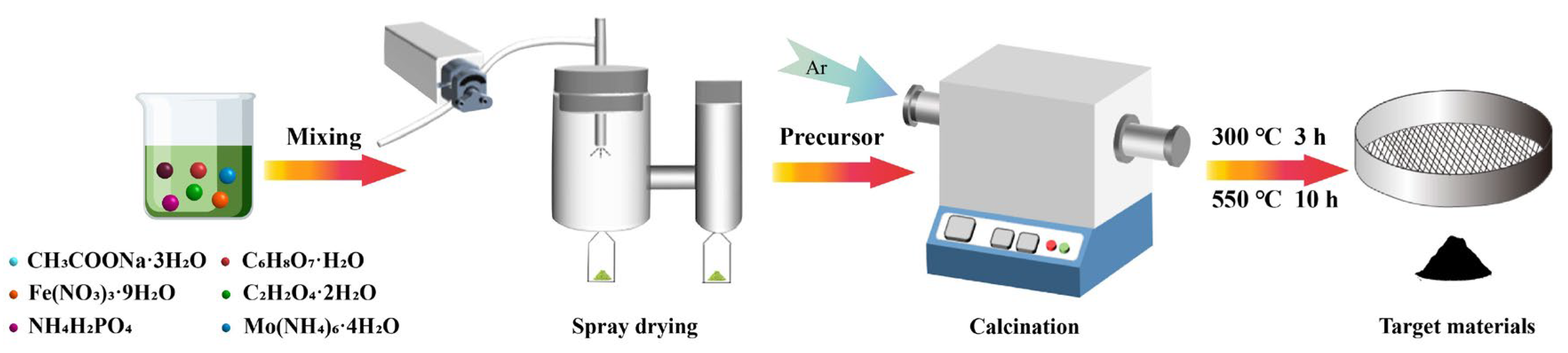
2.1. Synthesis of Mox-NFPP Materials
2.2. Microstructural Characterization
2.3. Electrochemical Measurements
2.4. First-Principles Calculation Method
3. Results and Discussion
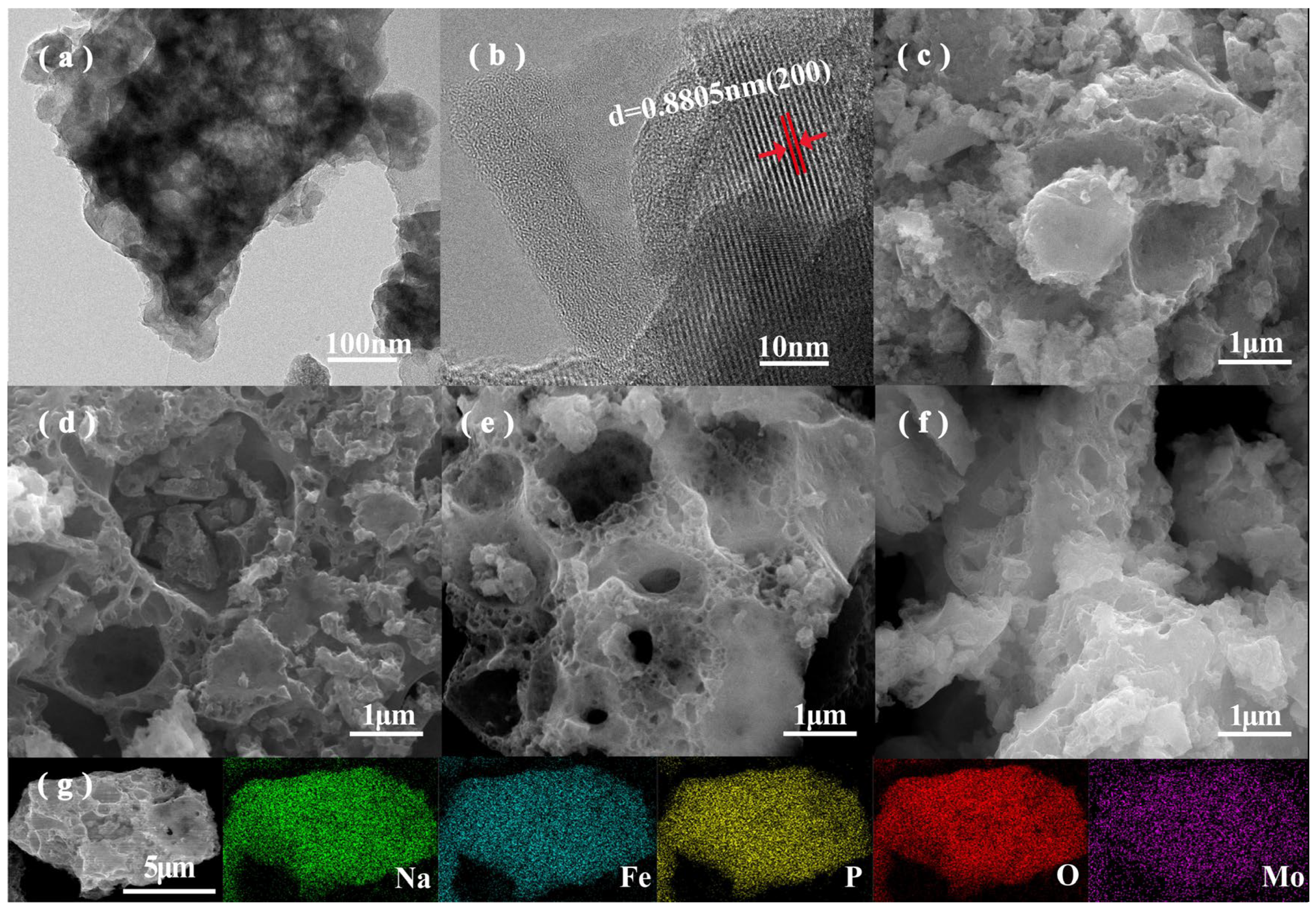
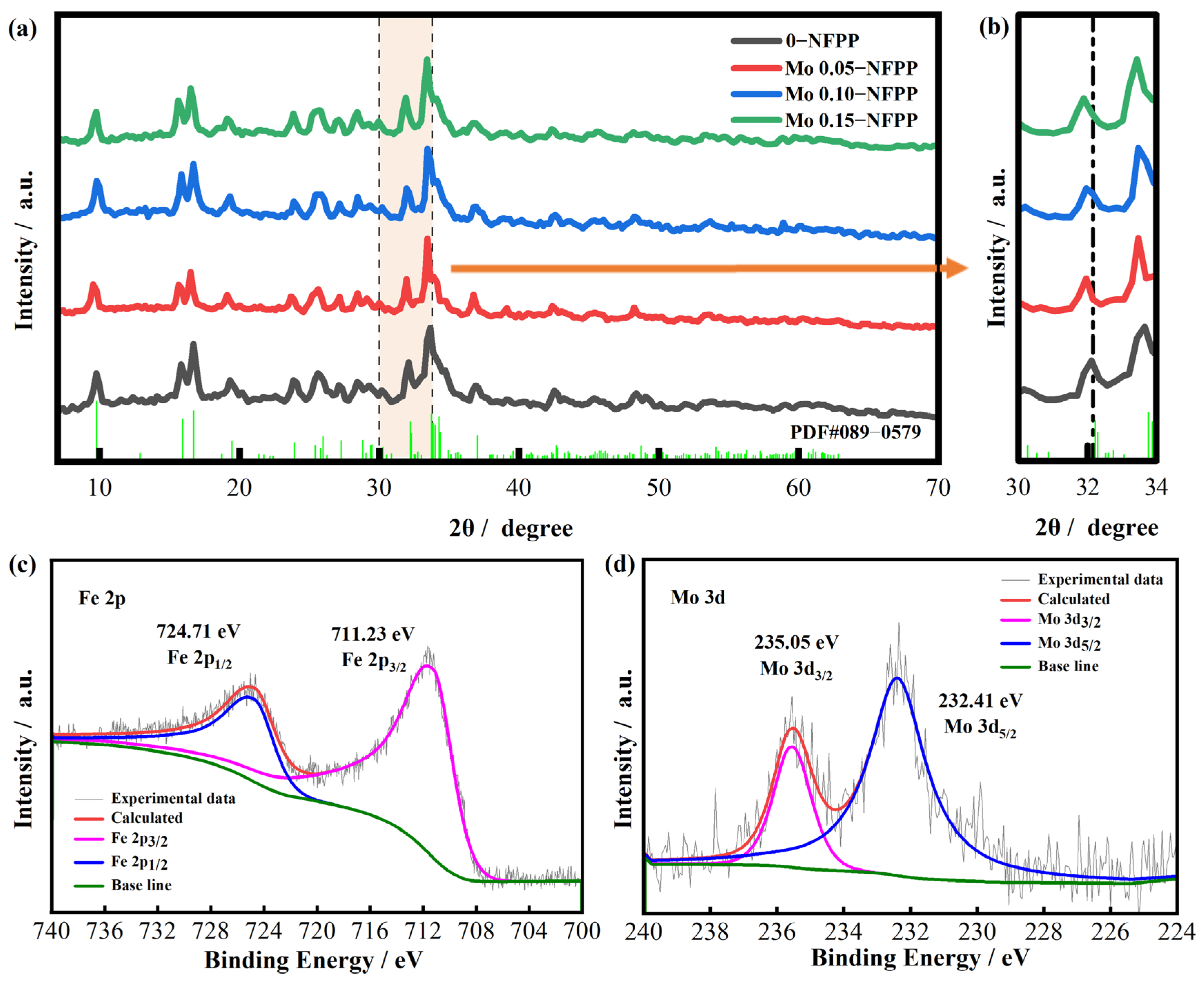
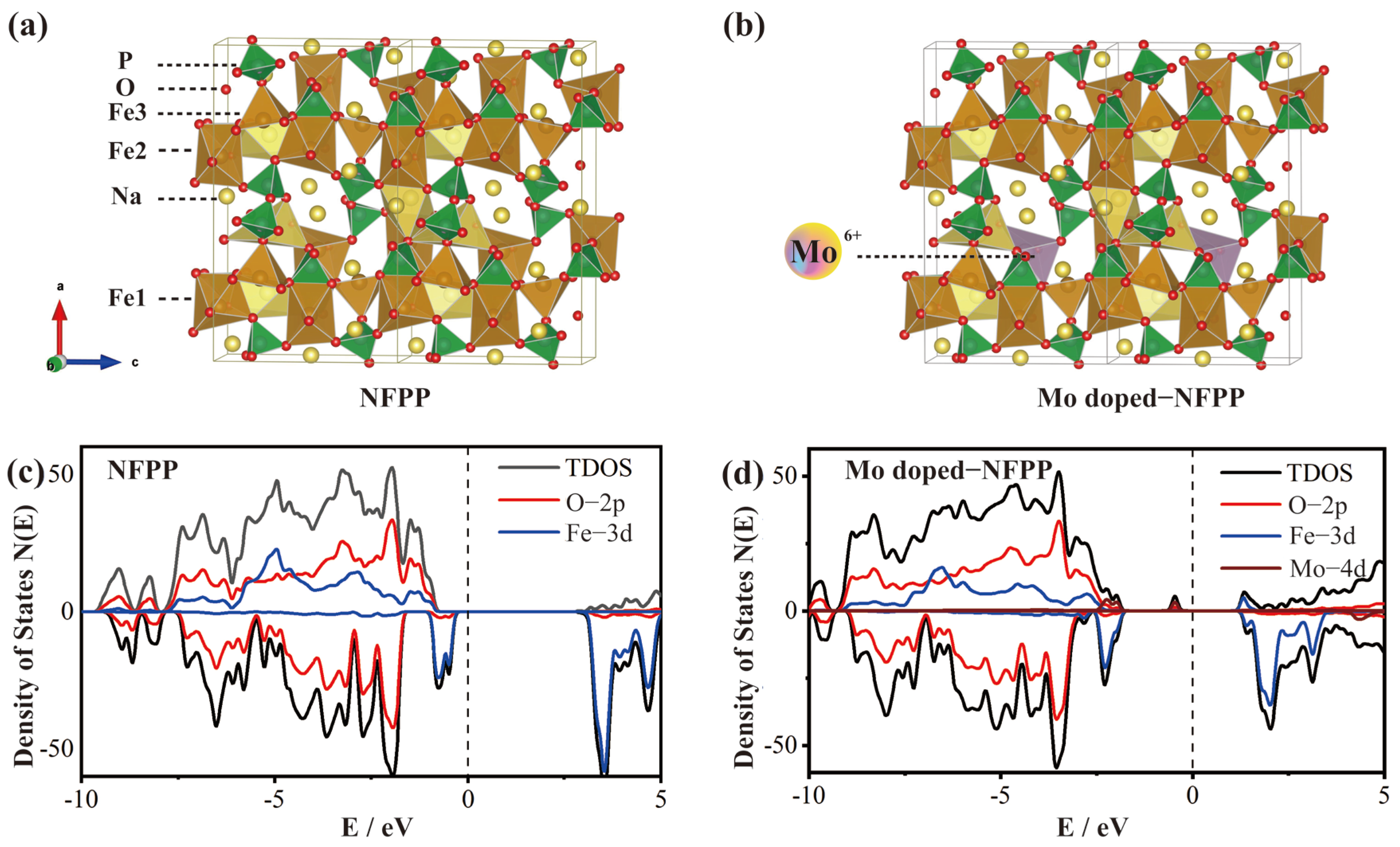
3.1. Morphology and Structural Characterization
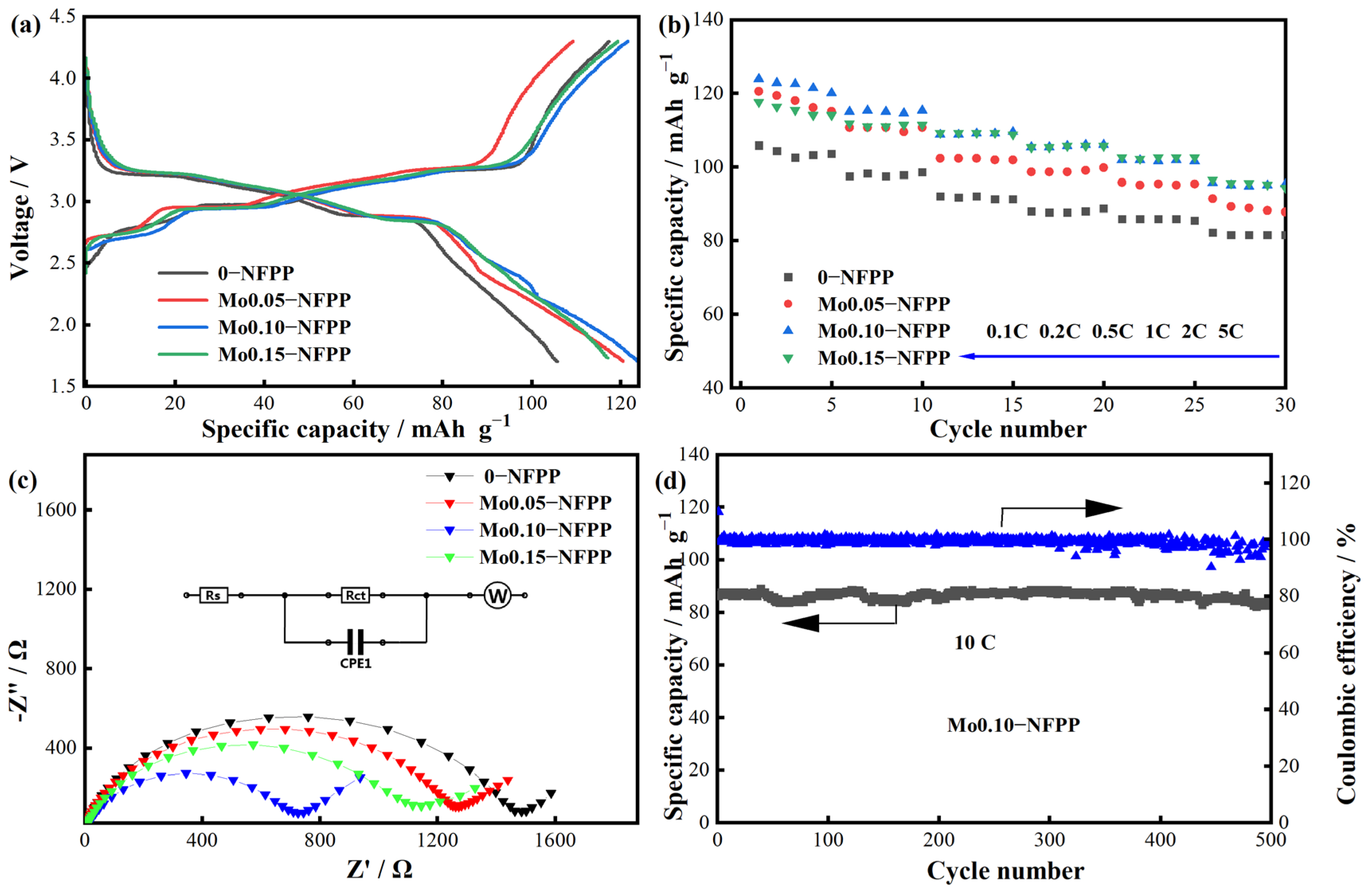
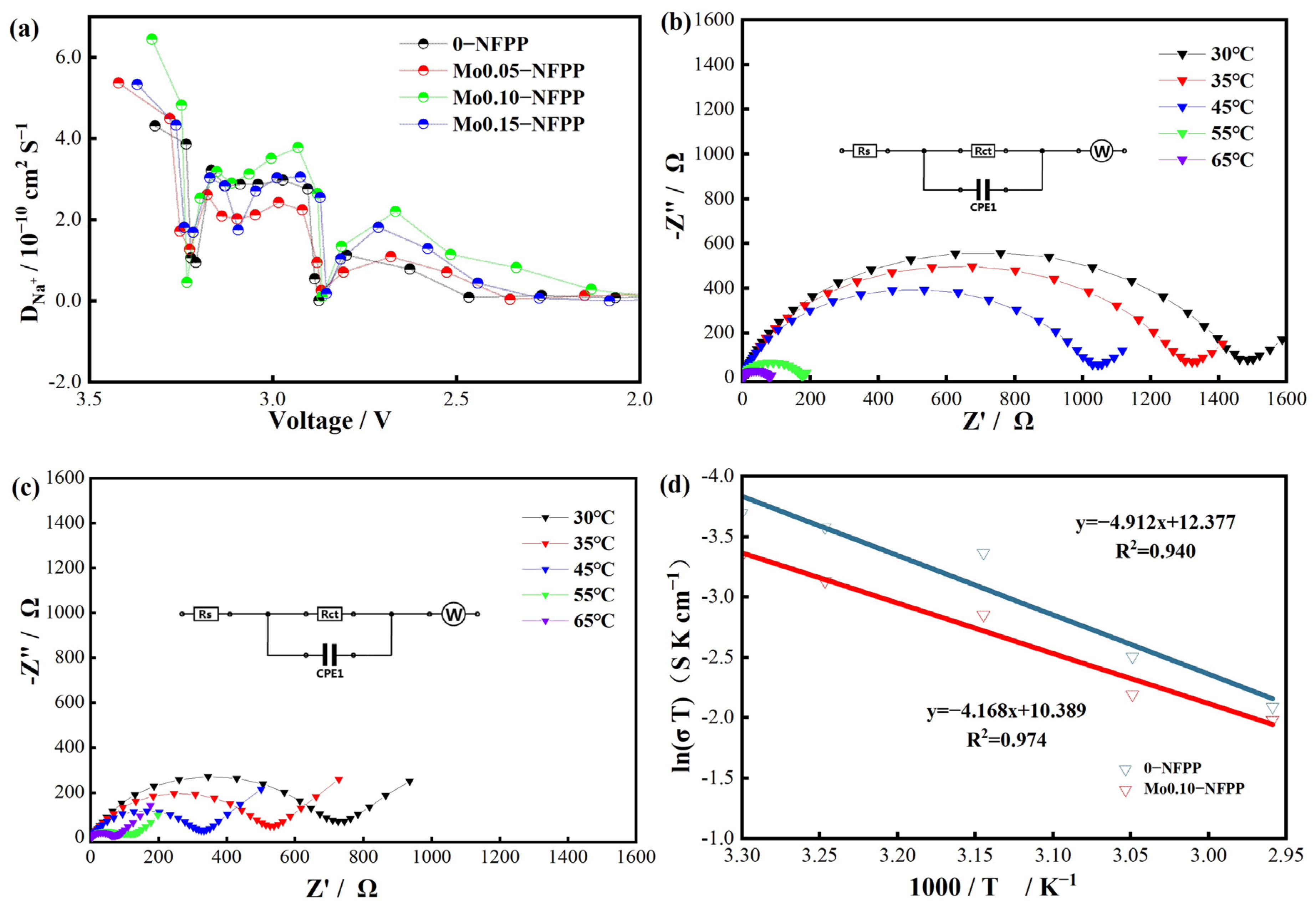
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Q.; Rehman, J.; Eid, K.; Alofi, A.S.; Laref, A.; Albaqami, M.D.; Alotabi, R.G.; Shibl, M.F. Vanadium carbide (V4C3) MXene as an efficient anode for Li-ion and Na-ion batteries. Nanomaterials 2022, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.R.; Liu, Y.X.; Huang, T.; Du, X.R.; Lu, Q.Q.; Kid, K. Facile in situ polymerization synthesis of poly (ionic liquid)-based polymer electrolyte for high-performance solid-state batteries. Energy Convers. Manag. X 2024, 22, 100570. [Google Scholar] [CrossRef]
- Cao, Y.L.; Li, M.; Lu, J.; Liu, J.; Amine, K. Bridging the academic and industrial metrics for next-generation practical batteries. Nat. Nanotechnol. 2019, 14, 200–207. [Google Scholar] [CrossRef]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the design of cathode materials for Na-ion batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Chen, Z.X.; Ai, X.P.; Yang, H.X.; Cao, Y.L. Recent developments in cathode materials for Na-ion batteries. Acta Phys. Chim. Sin. 2017, 33, 211–241. [Google Scholar] [CrossRef]
- Ma, X.W.; Yang, C.; Xu, Z.Y.; Li, R.Q.; Song, L.; Zhang, M.D.; Yang, M.; Jin, Y.C. Structural and electrochemical progress of O3-type layered oxide cathodes for Na-ion batteries. Nanoscale 2023, 15, 14737–14753. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.N.; Wang, J.Z.; Chou, S.L.; Liu, H.K.; Dou, S.X. Prussian blue analogues for sodium-ion batteries: Past, present, and future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef] [PubMed]
- Deb, D.; Gautam, G.S. Critical overview of polyanionic frameworks as positive electrodes for Na-ion batteries. J. Mater. Res. 2022, 37, 3169–3196. [Google Scholar] [CrossRef]
- Gezovic, A.; Vujkovic, M.J.; Milovic, M.; Grudic, V.; Dominko, R.; Mentus, S. Recent developments of Na4M3(PO4)2(P2O7) as the cathode material for alkaline-ion rechargeable batteries: Challenges and outlook. Energy Storage Mater. 2021, 37, 243–273. [Google Scholar] [CrossRef]
- Gao, J.Q.; Mei, Y.; Ni, L.S.; Wang, H.J.; Song, B.; Deng, W.T.; Zou, G.Q.; Hou, H.S.; Ji, X.B. Advanced NASICON-type Na4Fe3(PO4)2(P2O7) cathode for high-performance Na+/Li+ batteries. Inorg. Chem. 2023, 62, 9099–9110. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, Y.; Zhang, B.L.; Qin, K.; Liu, H.M.; Ma, Z.F. Mn-doped Na4Fe3(PO4)2(P2O7) facilitating Na+ migration at low temperature as a high performance cathode material of sodium ion batteries. J. Power Sources 2022, 521, 230922–230933. [Google Scholar] [CrossRef]
- Tao, Q.D.; Ding, H.Y.; Tang, X.; Zhang, K.B.; Teng, J.H.; Zhao, H.M.; Li, J. Mn-doped Na4Fe3(PO4)2P2O7 as a low-cost and high-performance cathode material for sodium-ion batteries. Energy Fuels 2023, 37, 6230–6239. [Google Scholar] [CrossRef]
- Xiong, F.Y.; Li, J.T.; Zuo, C.L.; Zhang, X.L.; Tan, S.S.; Jiang, Y.L.; An, Q.Y.; Chu, P.K.; Mai, L.Q. Mg-doped Na4Fe3(PO4)2(P2O7)/C composite with enhanced intercalation pseudocapacitance for ultra-stable and high-rate sodium-ion storage. Adv. Funct. Mater. 2023, 33, 2211257–2211265. [Google Scholar] [CrossRef]
- Wang, G.H.; Chen, T.T.; Chen, J.; Zhang, Z.Y.; Zhen, C.; Han, X.Y.; Li, J.G. Preparation and electrochemical properties of Na4Fe3-xCrx(PO4)2P2O7/C@CNT cathode materials for sodium ion batteries. Mod. Chem. Ind. 2024, 44, 149–154. (In Chinese) [Google Scholar]
- Huang, L.; Liu, C.J.; Bao, L.; Chen, Y.; Jiang, Y.H.; Fu, X.C. Large scalable preparation of Ti-doped Na4Fe3(PO4)2P2O7 as cathode material for high rate and long-life sodium-ion batteries. ACS Appl. Energ. Mater. 2023, 6, 11541–11549. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.J.; Liu, Z.L.; Cheng, X.S.; Li, X.L.; Xu, J.; Wang, N.; Yang, H.; Liu, Y.; Zhang, J.X. Structurally modulated Na4−xFe3−xVx(PO4)2P2O7 by vanadium doping for long-life sodium-ion batteries. ACS Sustainable Chem. Eng. 2024, 12, 5310–5318. [Google Scholar] [CrossRef]
- Xi, Y.K.; Wang, X.X.; Wang, H.; Wang, M.J.; Wang, G.J.; Peng, J.Q.; Hou, N.J.; Huang, X.; Cao, Y.Y.; Yang, Z.H.; et al. Optimizing the electron spin states of Na4Fe3(PO4)2P2O7 cathodes via Mn/F dual-doping for enhanced sodium storage. Adv. Funct. Mater. 2024, 34, 2309701. [Google Scholar] [CrossRef]
- Li, G.D.; Cao, Y.J.; Chen, J.W.; Zhang, K.; Liu, Y.J.; Zhang, X.E.; Wang, Y.G.; Wang, F.; Xia, Y.Y. Entropy-enhanced multi-doping strategy to promote the electrochemical performance of Na4Fe3(PO4)2P2O7. Small Methods 2024, 2301745. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.C.; Li, H.X.; Li, J.; Guan, C.H.; Wang, X.; He, L.; Li, S.M.; Lai, Y.Q.; Zhang, Z. High-entropy doping boosts ion/electronic transport of Na4Fe3(PO4)2(P2O7)/C cathode for superior performance sodium-ion batteries. Small 2023, 19, 2302609. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Dong, C.R.; Chen, L.; Fu, C.L.; Zeng, Y.B.; Wang, Q.; Cao, Y.L.; Chen, Z.X. “One stone two birds” design for hollow spherical Na4Fe3(PO4)2P2O7/C cathode enabled high-performance sodium-ion batteries from iron rust. EcoMat 2023, 5, 12393–12404. [Google Scholar] [CrossRef]
- Yuan, W.; Yan, J.; Tang, Z.Y.; Sha, O.; Wang, J.M.; Mao, W.F.; Ma, L. Mo-doped Li3V2(PO4)3/C cathode material with high rate capability and long term cyclic stability. Electrochim. Acta 2012, 72, 138–142. [Google Scholar] [CrossRef]
- Xue, L.L.; Li, Y.J.; Xu, B.; Chen, Y.X.; Cao, G.L.; Li, J.G.; Deng, S.Y.; Chen, Y.J.; Chen, J. Effect of Mo doping on the structure and electrochemical performances of LiNi0.6Co0.2Mn0.2O2 cathode material at high cut-off voltage. J. Alloys Compd. 2018, 748, 561–568. [Google Scholar] [CrossRef]
- Zeng, F.F.; Zhang, Y.; Shao, Z.C. Synthesis and electrochemical performance of Mo-doped LiNi0.5Mn1.5O4 cathode material. Mater. Manuf. Process. 2023, 38, 197–205. [Google Scholar] [CrossRef]
- Meghnani, D.; Singh, S.K.; Srivastava, N.; Tiwari, R.K.; Mishra, R.; Patel, A.; Tiwari, A.; Singh, R.K. Electrochemical performance of high-valence Mo6+ and low-valence Mn2+ doped- Na3V2(PO4)3@C cathode for sodium-ion batteries. ChemPhysChem 2022, 23, 202200459–202200471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Wang, W.W.; Zhu, B.C.; Qian, F.F.; Fang, Z. Mo-doped Na3V2(PO4)3@C composites for high stable sodium ion battery cathode. Front. Mater. Sci. 2018, 12, 53–63. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.Y.; Wang, J.S.; Miao, L.; Li, Y.Y.; Liu, Y.; Qiu, Y.G.; Fang, C.; Han, J.T.; Huang, Y.H. High valence Mo-doped Na3V2(PO4)3/C as a high rate and stable cycle-life cathode for sodium battery. J. Mater. Chem. A 2018, 6, 1390–1396. [Google Scholar] [CrossRef]
- Ran, P.L.; Wu, K.; Zhao, E.Y.; Wang, F.W.; Wu, Z.M. Enhancing reversible capacity and cycling stability of Li1.2Ni0.13Fe0.13Mn0.54O2 by inducing low Li/Ni misalignment through Mo doping. Acta Phys. Sin. 2024, 73, 028201. [Google Scholar] [CrossRef]
- Wen, L.Z.; Guan, Z.W.; Wang, L.; Liu, X.M.; Wen, G.Q.; Zhao, Y.; Pang, D.F.; Dou, R.Z. Synthesis and electrochemical properties of molybdenum-doped LiMn0.6Fe0.4PO4 cathode materials. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Yu, H.n.; Xue, Z.C.; Xue, Z.Y.; Luo, Z.Y.; Ding, C.X.; Hu, G.R.; Peng, Z.D.; Cao, Y.B.; Du, K. Inhibition of oxygen release and stabilization of the bulk structure of lithium-rich layered oxides by strong Mo-O covalent binding. J. Mater. Chem. A 2024, 12, 267–276. [Google Scholar] [CrossRef]
- Kumar, R.S.; Mannu, P.; Prabhakaran, S.; Nga, T.T.T.; Kim, Y.; Kim, D.H.; Chen, J.-L.; Dong, C.-L.; Yoo, D.J. Trimetallic oxide electrocatalyst for enhanced redox activity in zinc–air batteries evaluated by in situ analysis. Adv. Sci. 2023, 10, 2303525. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wang, Y.G. High-power and low-cost sodium-ion batteries with a wide operation temperature from −70 °C to 130 °C. SmartMat 2023, 4, 1191–1201. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 18–28. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Aryasetiawan, F.; Lichtenstein, A.I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA+ U method. J. Phys. Condens. Mat. 1997, 9, 767. [Google Scholar] [CrossRef]
- Coquet, R.; Willock, D.J. The (010) surface of α-MoO3, a DFT+ U study. Phys. Chem. Chem. Phys. 2005, 7, 3819–3828. [Google Scholar] [CrossRef]
- Xiao, P.H.; Deng, Z.Q.; Manthiram, A.; Henkelman, G. Calculations of oxygen stability in lithium-rich layered cathodes. J. Phys. Chem. C 2012, 116, 23201–23204. [Google Scholar] [CrossRef]
- Ma, X.D.; Wang, D.H.; Xu, R.M.; Lai, Y.J.; Yu, X.; Liu, Y. Iron-based NASICON-type Na4Fe3(PO4)2(P2O7) cathode for zinc-ion battery: Zn2+/Na+ co-intercalation enabling high capacity. ChemSusChem 2021, 14, 5424–5433. [Google Scholar] [CrossRef]
- Zhao, A.L.; Yuan, T.C.; Li, P.; Liu, C.Y.; Cong, H.J.; Pu, X.J.; Chen, Z.X.; Ai, X.P.; Yang, H.X.; Cao, Y.L. A novel Fe-defect induced pure-phase Na4Fe2.91(PO4)2P2O7 cathode material with high capacity and ultra-long lifetime for low-cost sodium-ion batteries. Nano Energy 2022, 91, 106680–106689. [Google Scholar] [CrossRef]
- Ren, W.; Qin, M.L.; Zhou, Y.F.; Zhou, H.; Zhu, J.; Pan, J.N.; Zhou, J.; Cao, X.X.; Liang, S.Q. Electrospun Na4Fe3(PO4)2(P2O7) nanofibers as free-standing cathodes for ultralong-life and high-rate sodium-ion batteries. Energy Storage Mater. 2023, 54, 776–783. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Mishra, T.P.; Mahayoni, E.; Ma, Q.L.; Tieu, A.J.K.; Guillon, O.; Chotard, J.N.; Seznec, V.; Cheetham, A.K.; Masquelier, C.; et al. Fundamental investigations on the sodium-ion transport properties of mixed polyanion solid-state battery electrolytes. Nat. Commun. 2022, 13, 4470–4484. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Han, X.; Jie, M.; Guo, Z.; Li, J.; He, X. Mo-Doped Na4Fe3(PO4)2P2O7/C Composites for High-Rate and Long-Life Sodium-Ion Batteries. Materials 2024, 17, 2679. https://doi.org/10.3390/ma17112679
Chen T, Han X, Jie M, Guo Z, Li J, He X. Mo-Doped Na4Fe3(PO4)2P2O7/C Composites for High-Rate and Long-Life Sodium-Ion Batteries. Materials. 2024; 17(11):2679. https://doi.org/10.3390/ma17112679
Chicago/Turabian StyleChen, Tongtong, Xianying Han, Mengling Jie, Zhiwu Guo, Jiangang Li, and Xiangming He. 2024. "Mo-Doped Na4Fe3(PO4)2P2O7/C Composites for High-Rate and Long-Life Sodium-Ion Batteries" Materials 17, no. 11: 2679. https://doi.org/10.3390/ma17112679
APA StyleChen, T., Han, X., Jie, M., Guo, Z., Li, J., & He, X. (2024). Mo-Doped Na4Fe3(PO4)2P2O7/C Composites for High-Rate and Long-Life Sodium-Ion Batteries. Materials, 17(11), 2679. https://doi.org/10.3390/ma17112679







