Adsorption Behavior of Co2+, Ni2+, Sr2+, Cs+, and I− by Corrosion Products α-FeOOH from Typical Metal Tanks
Abstract
:1. Introduction
2. Adsorption Experiment
2.1. Materials Preparation
2.2. Adsorption Experiment for α-FeOOH
2.3. Preliminary Experiment
2.4. Adsorption Thermodynamics
2.5. Adsorption Kinetics
- (1)
- pseudo-first-order kinetic model:
- (2)
- pseudo-second-order kinetic model:
3. Results
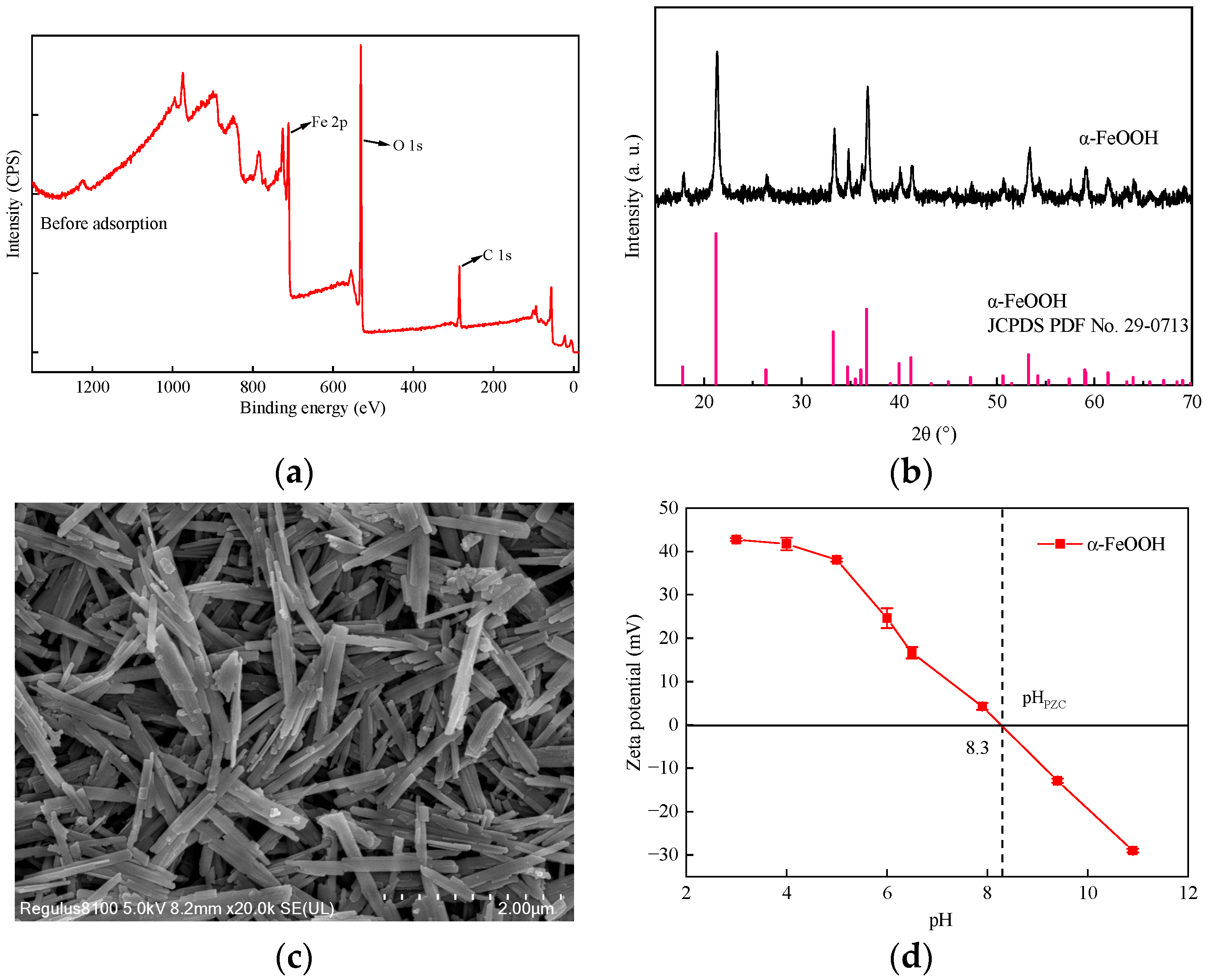
3.1. Characterization
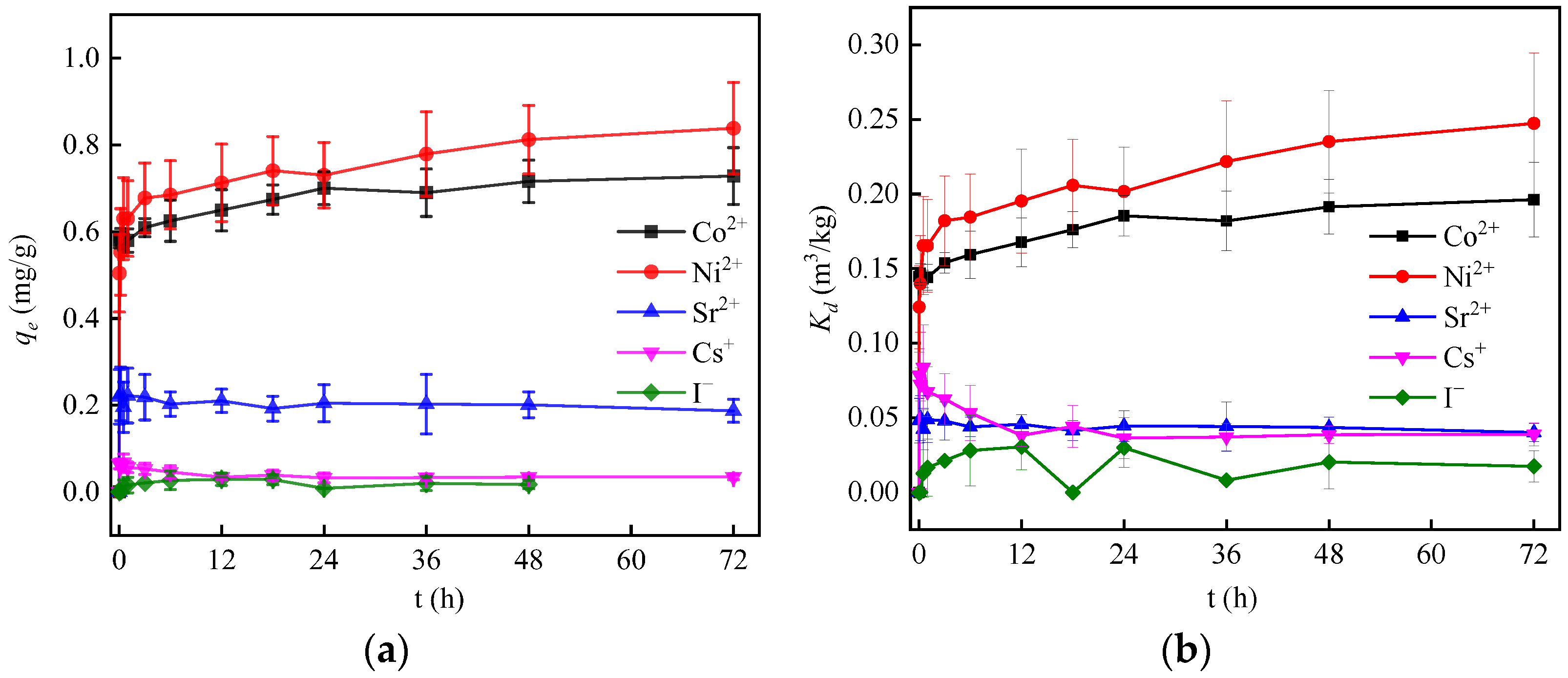
3.2. Effect of Adsorption Time
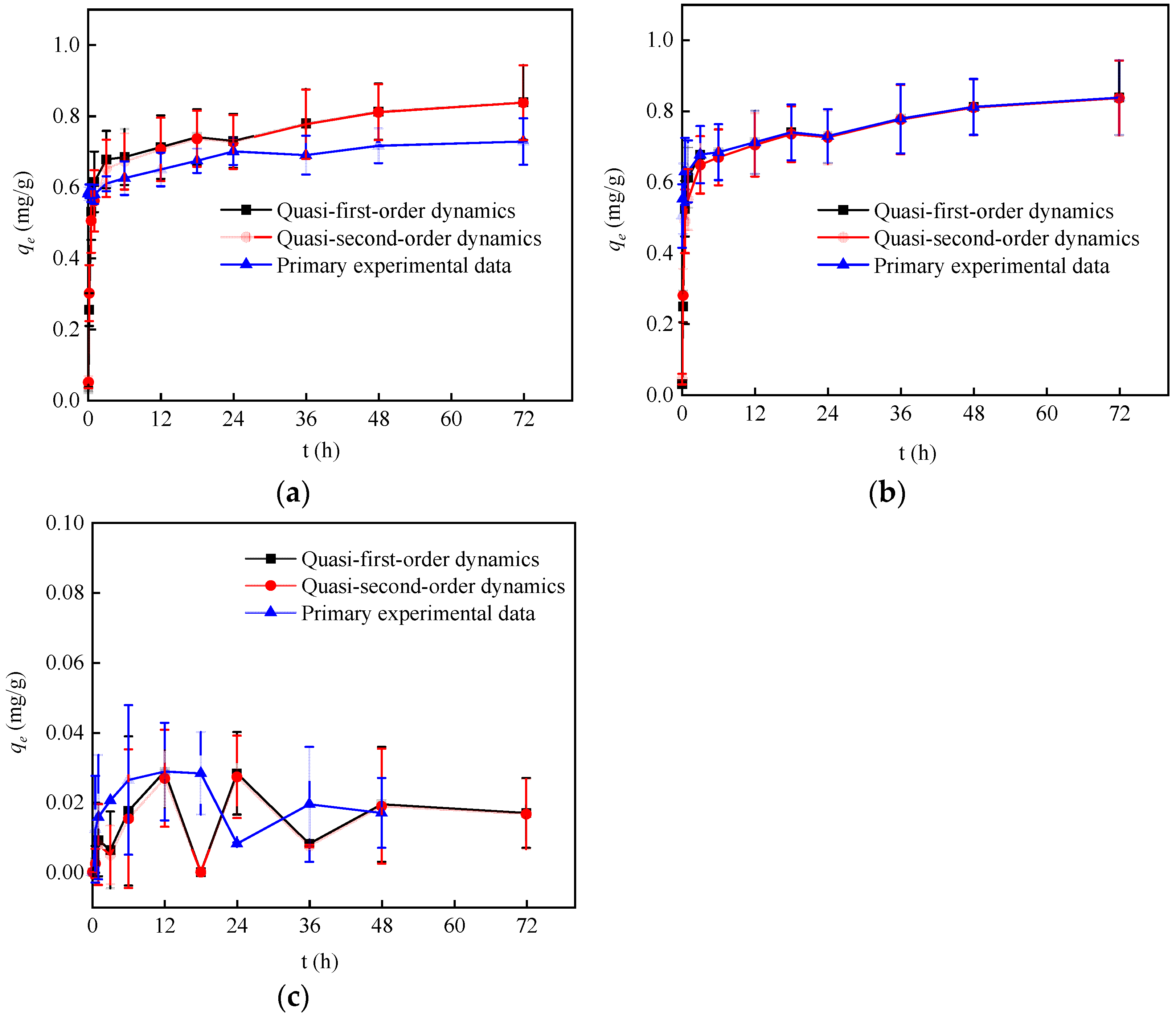
3.3. Adsorption Kinetics Study
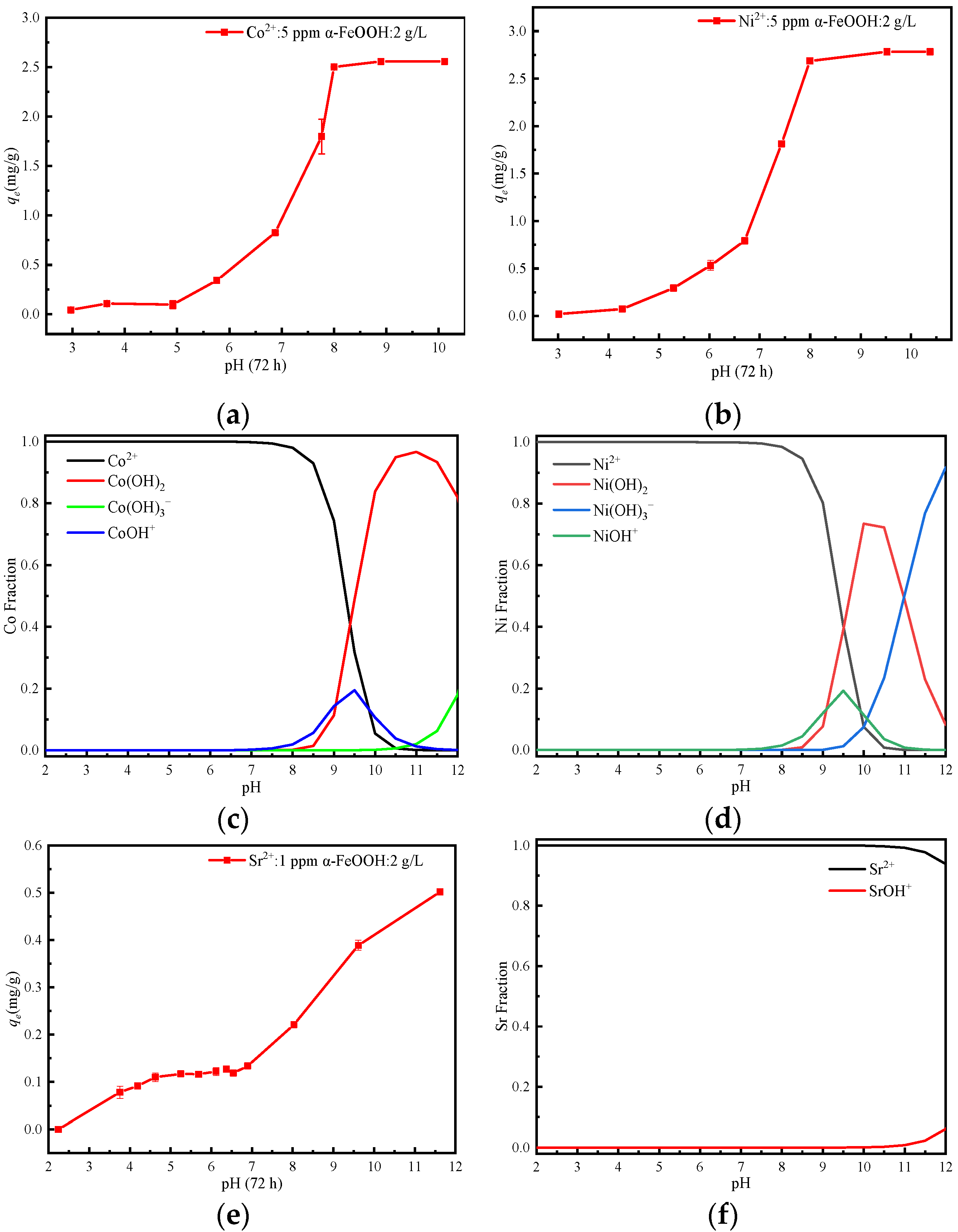
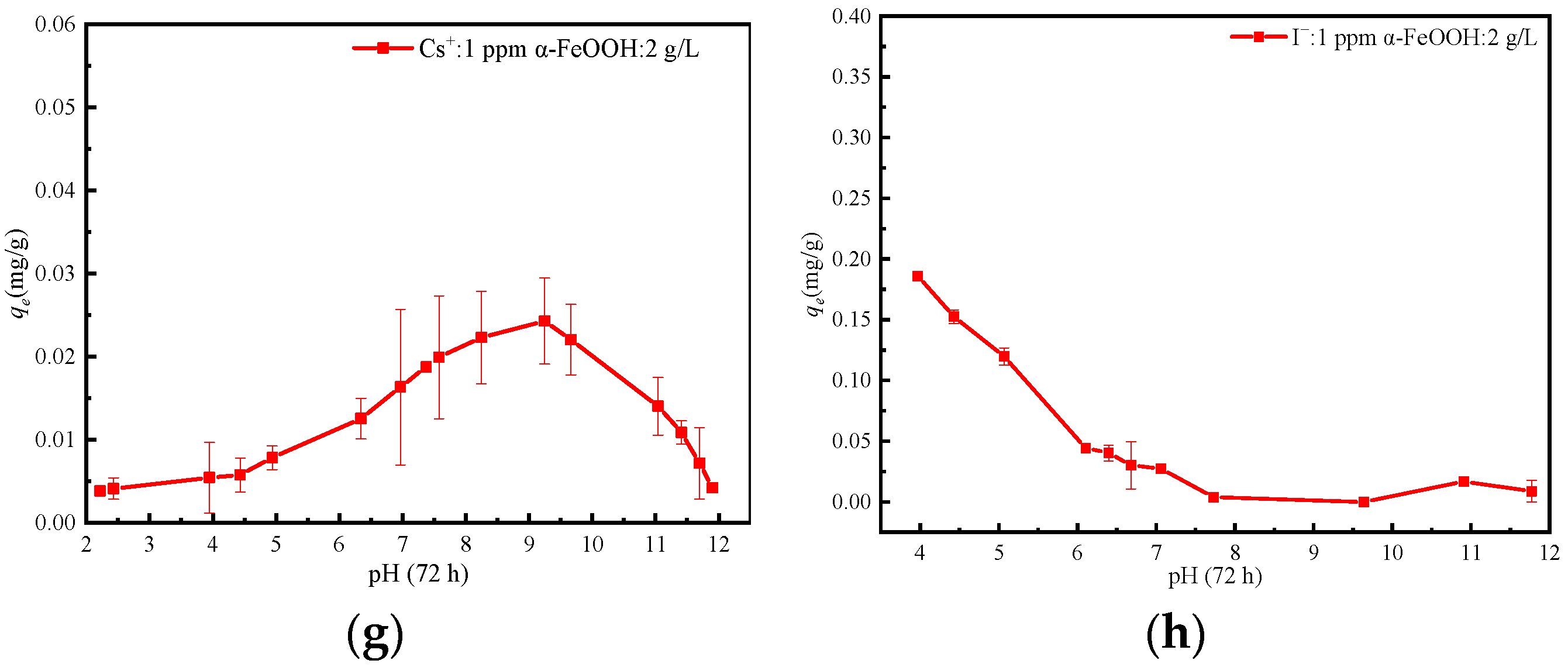
3.4. Effect of pH
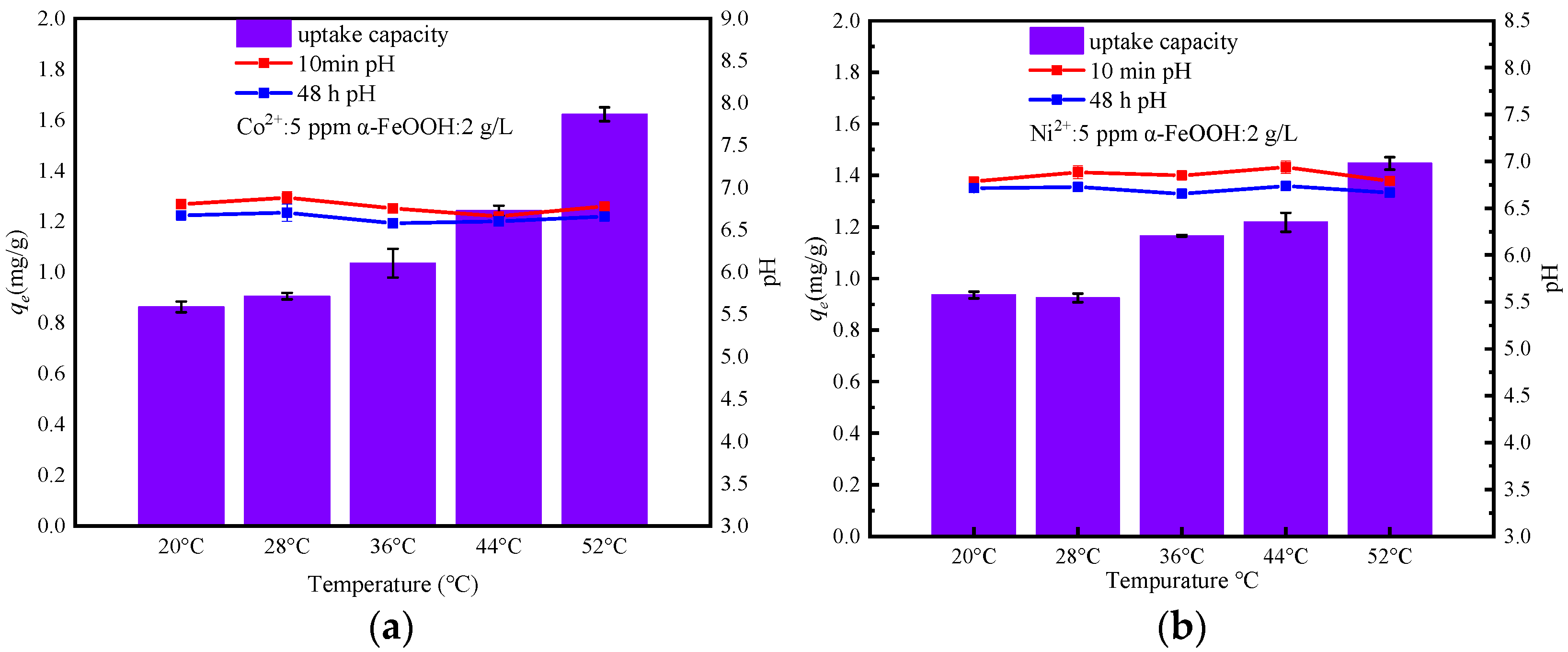
3.5. Effect of Temperature and Thermodynamic Analysis
3.6. Effect of Ionic Strength
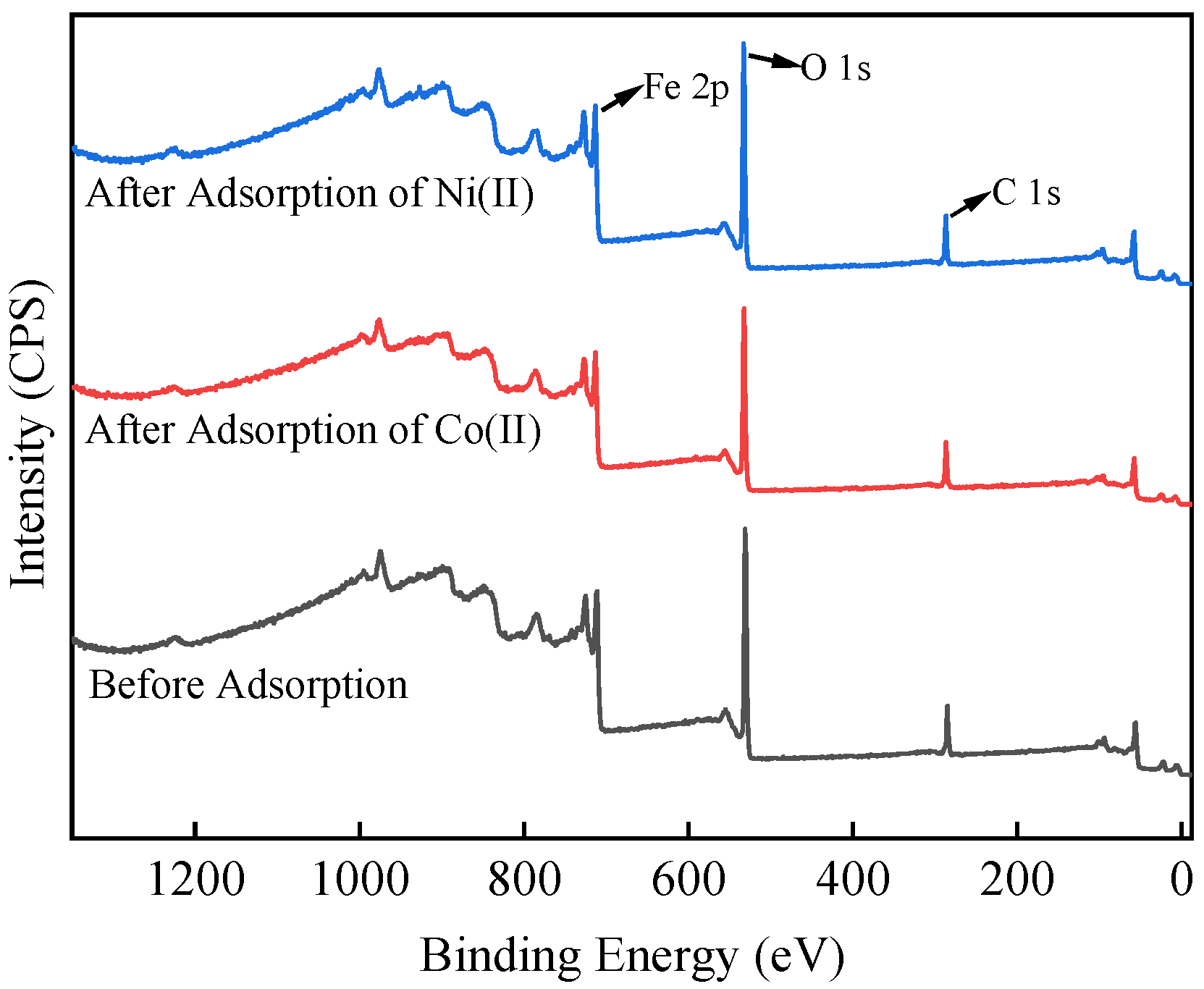
3.7. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nie, Z.; Finck, N.; Heberling, F.; Pruessmann, T.; Liu, C.; Lützenkirchen, J. Adsorption of Selenium and Strontium on Goethite: EXAFS Study and Surface Complexation Modeling of the Ternary Systems. Environ. Sci. Technol. 2017, 51, 3751–3758. [Google Scholar] [CrossRef]
- Bracke, G.; Kudla, W.; Rosenzweig, T. Status of Deep Borehole Disposal of High-Level Radioactive Waste in Germany. Energies 2019, 12, 2580. [Google Scholar] [CrossRef]
- Carter, A.; Kelly, M.; Bailey, L. Radioactive high level waste insight modelling for geological disposal facilities. Phys. Chem. Earth Parts A/B/C 2013, 64, 1–11. [Google Scholar] [CrossRef]
- Gibb, F.G.F.; Beswick, A.J. A deep borehole disposal solution for the UK’s high-level radioactive waste. Proc. Inst. Civ. Eng. Energy 2022, 175, 11–29. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, Y.-M.; Kim, J.-W.; Cho, D.-K.; Ko, N.Y.; Baik, M.H. Progress of the long-term safety assessment of a reference disposal system for high level wastes in Korea. Prog. Nucl. Energy 2016, 90, 37–45. [Google Scholar] [CrossRef]
- King, F.; Padovani, C. Review of the corrosion performance of selected canister materials for disposal of UK HLW and/or spent fuel. Corros. Eng. Sci. Technol. 2011, 46, 82–90. [Google Scholar] [CrossRef]
- Bennett, D.G.; Gens, R. Overview of European concepts for high-level waste and spent fuel disposal with special reference waste container corrosion. J. Nucl. Mater. 2008, 379, 1–8. [Google Scholar] [CrossRef]
- Ewing, R.C. Long-term storage of spent nuclear fuel. Nat. Mater. 2015, 14, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Rigonat, N.; Isnard, O.; Harley, S.L.; Butler, I.B. Monitoring long-term evolution of engineered barrier systems using magnets: Magnetic response. J. Hazard. Mater. 2018, 341, 28–35. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Dong, J.; Cao, S.; Xie, J.; Chen, N.; Xue, F.; Wang, C.; Ke, W. Study on corrosion behavior of low carbon steel under different water conditions in bentonite of China-Mock-Up. Appl. Clay Sci. 2019, 167, 23–32. [Google Scholar] [CrossRef]
- Grambow, B.; Smailos, E.; Geckeis, H.; Muller, R.; Hentschel, H. Sorption and reduction of uranium(VI) on iron corrosion products under reducing saline conditions. Radiochim. Acta 1996, 74, 149–154. [Google Scholar] [CrossRef]
- Ma, B.; Fernandez-Martinez, A.; Madé, B.; Findling, N.; Markelova, E.; Salas-Colera, E.; Maffeis, T.G.G.; Lewis, A.R.; Tisserand, D.; Bureau, S.; et al. XANES-Based Determination of Redox Potentials Imposed by Steel Corrosion Products in Cement-Based Media. Environ. Sci. Technol. 2018, 52, 11931–11940. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.; Ma, L.; Fu, H.; Lin, X.; Parker, S.C.; Molinari, M. Adsorption of phosphate and cadmium on iron (oxyhydr)oxides: A comparative study on ferrihydrite, goethite, and hematite. Geoderma 2021, 383, 114799. [Google Scholar] [CrossRef]
- Shi, M.; Min, X.; Ke, Y.; Lin, Z.; Yang, Z.; Wang, S.; Peng, N.; Yan, X.; Luo, S.; Wu, J.; et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr)oxides. Sci. Total Environ. 2021, 752, 141930. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, D.; Zhao, D.; Zhang, S.; Xu, J. Influence of pH, ionic strength, foreign ions and FA on adsorption of radiocobalt on goethite. J. Radioanal. Nucl. Chem. 2011, 287, 505–512. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, J.; Knudsen, T.Š.; Liu, J.; Zhu, X.; Ma, B.; Li, H.; Cao, Y.; Liu, B. Performance and mechanisms for Cd(II) and As(III) simultaneous adsorption by goethite-loaded montmorillonite in aqueous solution and soil. J. Environ. Manag. 2023, 330, 117163. [Google Scholar] [CrossRef]
- Goldberg, S. Chemical Modeling of Anion Competition on Goethite Using the Constant Capacitance Model. Soil Sci. Soc. Am. J. 1985, 49, 851–856. [Google Scholar] [CrossRef]
- Wang, G.; Qafoku, N.P.; Szecsody, J.E.; Strickland, C.E.; Brown, C.F.; Freedman, V.L. Time-Dependent Iodate and Iodide Adsorption to Fe Oxides. ACS Earth Space Chem. 2019, 3, 2415–2420. [Google Scholar] [CrossRef]
- Sun, P.; Binter, E.A.; Vo, T.; Benjamin, I.; Bera, M.K.; Lin, B.; Bu, W.; Schlossman, M.L. Relevance of Surface Adsorption and Aqueous Complexation for the Separation of Co(II), Ni(II), and Fe(III). J. Phys. Chem. B 2023, 127, 3505–3515. [Google Scholar] [CrossRef]
- Ugwu, I.M.; Sherman, D.M. The solubility of goethite with structurally incorporated nickel and cobalt: Implication for laterites. Chem. Geol. 2019, 518, 1–8. [Google Scholar] [CrossRef]
- Johansen, M.P.; Ruedig, E.; Tagami, K.; Uchida, S.; Higley, K.; Beresford, N.A. Radiological Dose Rates to Marine Fish from the Fukushima Daiichi Accident: The First Three Years Across the North Pacific. Environ. Sci. Technol. 2015, 49, 1277–1285. [Google Scholar] [CrossRef]
- Lin, W.; Yu, K.; Du, J.; Lin, H.; Yu, W.; Mo, M. Marine ecological environment impact and countermeasures under the scenario of Fukushima nuclear wastewater discharge in Japan. Chin. Sci. Bull. 2021, 66, 4500–4509. [Google Scholar] [CrossRef]
- Achour, S.; Amokrane, S.; Chegrouche, S.; Guedioura, B.; Nibou, D. Adsorption Mechanism Study of Radionuclide 60Co by Purified and α-Fe2O3-Supported Bentonite from Radioactive Solution. Arab. J. Sci. Eng. 2021, 47, 5629–5645. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Zhang, L.; Yu, X. Impact of environmental conditions on the adsorption behavior of radionuclide Ni(II) onto hematite. J. Radioanal. Nucl. Chem. 2010, 287, 357–365. [Google Scholar] [CrossRef]
- Casacuberta, N.; Masqué, P.; Garcia-Orellana, J.; Garcia-Tenorio, R.; Buesseler, K.O. 90Sr and 89Sr in seawater off Japan as a consequence of the Fukushima Dai-ichi nuclear accident. Biogeosciences 2013, 10, 3649–3659. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, F.; Yu, K.; Ma, H.; Du, J.; Mo, Z.; Li, Y.; He, X.; Mo, M. Assessment and enrichment of artificial radionuclides in coral reef ecosystems. Adv. Earth Sci. 2023, 38, 286–295. [Google Scholar] [CrossRef]
- Dash, B.; Dash, B.; Rath, S.S. A thorough understanding of the adsorption of Ni (II), Cd (II) and Zn (II) on goethite using experiments and molecular dynamics simulation. Sep. Purif. Technol. 2020, 240, 116649. [Google Scholar] [CrossRef]
- Collins, C.R.; Sherman, D.M.; Ragnarsdottir, K.V. The adsorption mechanism of Sr2+ on the surface of goethite. Radiochim. Acta 1998, 81, 201–206. [Google Scholar] [CrossRef]
- Fuller, A.J.; Shaw, S.; Peacock, C.L.; Trivedi, D.; Burke, I.T. EXAFS Study of Sr sorption to Illite, Goethite, Chlorite, and Mixed Sediment under Hyperalkaline Conditions. Langmuir 2016, 32, 2937–2946. [Google Scholar] [CrossRef]
- Efimova, N.V.; Krasnopyorova, A.P.; Yuhno, G.D.; Sofronov, D.S.; Rucki, M. Uptake of Radionuclides (60)Co, (137)Cs, and (90)Sr with alpha-Fe2O3 and Fe2O3 Particles from Aqueous Environment. Materials 2021, 14, 2899. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, K.; Wang, C.; Zhu, Q.; Luo, J.; Chen, F.; Xiao, F.; Peng, G. Fabrication of magnetic functionalized m-carboxyphenyl azo calix[4]arene amine oxime derivatives for highly efficient and selective adsorption of uranium (VI). J. Radioanal. Nucl. Chem. 2020, 323, 1145–1155. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Zhang, G.; Liu, S.; Dong, L.; Gu, P.; Hou, L.a. Rapid and effective removal of strontium ions from aqueous solutions by a novel layered metal sulfide NaTS-2. J. Radioanal. Nucl. Chem. 2023, 332, 2367–2378. [Google Scholar] [CrossRef]
- Datta, M.; Mathieu, H.J.; Landolt, D. AES/XPS Study of Transpassive Films on Iron in Nitrate Solution. J. Electrochem. Soc. 2019, 131, 2484. [Google Scholar] [CrossRef]
- He, X.; Li, J.; Chen, M.; Jin, Y.; Wang, Y.; Li, J. Resistance of deliquescence and caking to enhance the effective utilization of potassium nitrate: A novel surface modification method by SDS. Powder Technol. 2019, 356, 500–507. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Wang, Y.; Zhu, Z.; Tang, S.; Zhang, J.; Pan, Q.; Hu, T. Goethite Enhances Cr(VI) Reduction by S. oneidensis MR-1 under Different Conditions: Mechanistic Insights. Microorganisms 2024, 12, 754. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, J.; Yang, Y.; Ding, J.; Zhang, A. Experimental and theoretical studies of cadmium adsorption over Fe2O3 sorbent in incineration flue gas. Chem. Eng. J. 2021, 425, 131647. [Google Scholar] [CrossRef]
- Liu, H.; Wei, G.; Xu, Z.; Liu, P.; Li, Y. Quantitative analysis of Fe and Co in Co-substituted magnetite using XPS: The application of non-linear least squares fitting (NLLSF). Appl. Surf. Sci. 2016, 389, 438–446. [Google Scholar] [CrossRef]
- Wu, L.-K.; Wu, H.; Zhang, H.-B.; Cao, H.-Z.; Hou, G.-Y.; Tang, Y.-P.; Zheng, G.-Q. Graphene oxide/CuFe2O4 foam as an efficient absorbent for arsenic removal from water. Chem. Eng. J. 2018, 334, 1808–1819. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Zhang, S. Enhanced PEC performance of hematite photoanode coupled with bimetallic oxyhydroxide NiFeOOH through a simple electroless method. Appl. Catal. B Environ. 2020, 265, 118580. [Google Scholar] [CrossRef]
- Muthu, M.; Ramachandran, D.; Hasan, N.; Jeevanandam, M.; Gopal, J.; Chun, S. Unprecedented nitrate adsorption efficiency of carbon-silicon nano composites prepared from bamboo leaves. Mater. Chem. Phys. 2017, 189, 12–21. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, Z.; Li, Z.; Lin, Q.; Wu, Y.; Dou, W. Adsorption characteristics and mechanism for K2Ti4O9 whiskers removal of Pb(II), Cd(II), and Cu(II) cations in wastewater. J. Environ. Chem. Eng. 2021, 9, 106236. [Google Scholar] [CrossRef]
- Hamed, M.M.; Ahmed, I.M.; Holiel, M. Retention behavior of anionic radionuclides using metal hydroxide sludge. Radiochim. Acta 2019, 107, 1161–1172. [Google Scholar] [CrossRef]
- Hamed, M.M.; Holiel, M.; El-Aryan, Y.F. Removal of selenium and iodine radionuclides from waste solutions using synthetic inorganic ion exchanger. J. Mol. Liq. 2017, 242, 722–731. [Google Scholar] [CrossRef]
- Jordan, N.; Foerstendorf, H.; Weiß, S.; Heim, K.; Schild, D.; Brendler, V. Sorption of selenium(VI) onto anatase: Macroscopic and microscopic characterization. Geochim. Et Cosmochim. Acta 2011, 75, 1519–1530. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Lu, X.; Huangfu, X.; Zou, J. High efficient removal of molybdenum from water by Fe2(SO4)3: Effects of pH and affecting factors in the presence of co-existing background constituents. J. Hazard. Mater. 2015, 300, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Netpradit, S.; Thiravetyan, P.; Towprayoon, S. Application of ‘waste’ metal hydroxide sludge for adsorption of azo reactive dyes. Water Res. 2003, 37, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lv, R.; Fan, J.; Zhang, X.; Tao, Q. Adsorption of cesium using supermolecular impregnated XAD-7 composite: Isotherms, kinetics and thermodynamics. J. Radioanal. Nucl. Chem. 2019, 321, 473–480. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Pan, C.; Zheng, X.; Hu, F.; Xu, L.; Xu, G.; Jian, Y.; Peng, X. Prediction and optimization of adsorption properties for Cs+ on NiSiO@NiAlFe LDHs hollow spheres from aqueous solution: Kinetics, isotherms, and BBD model. J. Hazard. Mater. 2021, 401, 123374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Z.; Zhu, D.; Feng, Z.; Li, X.; Huang, J.; Shen, X. Construction of hierarchical Mn-CoO@Fe(OH)3 nanofiber array for oxygen evolution reaction. J. Alloys Compd. 2020, 847, 155560. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, Y.; Wu, X.; Sunarso, J.; Ni, M.; Zhou, W.; Shao, Z. Bifunctionality from Synergy: CoP Nanoparticles Embedded in Amorphous CoOx Nanoplates with Heterostructures for Highly Efficient Water Electrolysis. Adv. Sci. 2018, 5, 1800514. [Google Scholar] [CrossRef]
- Santaya, M.; Jimenez, C.E.; Esteban Troiani, H.; Carbonio, E.A.; Damian Arce, M.; Maria Toscani, L.; Garcia-Diez, R.; Wilks, R.G.; Knop-Gericke, A.; Baer, M.; et al. Tracking the nanoparticle exsolution/reoxidation processes of Ni-doped SrTi0.3Fe0.7O3−δ electrodes for intermediate temperature symmetric solid oxide fuel cells. J. Mater. Chem. A 2022, 10, 15554–15568. [Google Scholar] [CrossRef]
- Carley, A.F.; Jackson, S.D.; O’Shea, J.N.; Roberts, M.W. The formation and characterisation of Ni3+: An X-ray photoelectron spectroscopic investigation of potassium-doped Ni(110)-O. Surf. Sci. 1999, 440, L868–L874. [Google Scholar] [CrossRef]
- Chen, Y.S.; Kang, J.F.; Chen, B.; Gao, B.; Liu, L.F.; Liu, X.Y.; Wang, Y.Y.; Wu, L.; Yu, H.Y.; Wang, J.Y.; et al. Microscopic mechanism for unipolar resistive switching behaviour of nickel oxides. J. Phys. D-Appl. Phys. 2012, 45, 065303. [Google Scholar] [CrossRef]






| Nuclide Ions | Inorganic Salts | Mion (g∙mol−1) | Minorganic salts (g∙mol−1) | Calculated Dosage (mg) |
|---|---|---|---|---|
| Co2+ | Co(NO3)2·6H2O | 58.9 | 291.03 | 370.5815 |
| Ni2+ | Ni(NO3)2·6H2O | 58.69 | 290.79 | 371.6008 |
| Sr2+ | Sr(NO3)2 | 87.63 | 211.63 | 181.1280 |
| Cs+ | CsNO3 | 132.9 | 194.91 | 109.9944 |
| I− | KI | 126.9 | 166.0 | 98.2636 |
| Research Object | Experimental Conditions | Main Selection Condition |
|---|---|---|
| Adsorption time | 1 min, 10 min, 30 min, 1 h, 3 h, 6 h, 12 h, 18 h, 24 h, 36 h, 48 h, 72 h | 72 h |
| pH | 2.0~12.0 | 6.5~7.5 |
| Temperature | 20 °C, 28 °C, 36 °C, 44 °C, 52 °C | 28 °C |
| Ionic strength (NaClO4) | 0 mmol/L, 0.5 mmol/L, 1 mmol/L, 5 mmol/L, 10 mmol/L, 50 mmol/L, 100 mmol/L | 0 mmol/L |
| Nuclide Ions | Adsorption Equilibrium Time (h) | qe (mg∙g−1) | Kd (m3∙kg−1) |
|---|---|---|---|
| Co2+ | 48 | 0.69 | 0.196 |
| Ni2+ | 72 | 0.78 | 0.247 |
| Sr2+ | 24 | 0.2 | 0.044 |
| Cs+ | 24 | 0.34 | 0.039 |
| I− | 12 | 0.029 | 0.031 |
| Nuclide Ions | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe/(mg·g−1) | R2 | k2/(g·mg−1·min−1) | qe/(mg·g−1) | R2 | |
| Co2+ | 0.0616 | 0.660 | 0.960 | 0.2135 | 0.674 | 0.978 |
| Ni2+ | 0.0596 | 0.726 | 0.951 | 0.1831 | 0.743 | 0.971 |
| I− | 0.0145 | 0.0269 | 0.940 | 0.6605 | 0.0295 | 0.982 |
| Nuclide Ions | ΔS (J·mol−1·K−1) | ΔH (kJ·mol−1) | ∆G (kJ·mol−1) | ||||
|---|---|---|---|---|---|---|---|
| 293.15 K | 301.15 K | 309.15 K | 317.15 K | 325.15 K | |||
| Co2+ | 133.442 | 25.966 | −13.153 | −14.220 | −15.288 | −16.356 | −17.423 |
| Ni2+ | 112.297 | 19.500 | −13.420 | −14.319 | −15.217 | −16.115 | −17.014 |
| Sr2+ | 151.091 | 31.683 | −12.609 | −13.818 | −15.027 | −16.236 | −17.444 |
| Cs+ | −40.348 | −19.432 | −7.604 | −7.281 | −6.959 | −6.636 | −6.313 |
| I− | 95.839 | 21.411 | −6.684 | −7.451 | −8.218 | −8.984 | −9.751 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Li, L.; Yuan, Y.; Yin, Y.; Dai, G.; Ren, Y.; Li, S.; Lin, P. Adsorption Behavior of Co2+, Ni2+, Sr2+, Cs+, and I− by Corrosion Products α-FeOOH from Typical Metal Tanks. Materials 2024, 17, 2706. https://doi.org/10.3390/ma17112706
Du Y, Li L, Yuan Y, Yin Y, Dai G, Ren Y, Li S, Lin P. Adsorption Behavior of Co2+, Ni2+, Sr2+, Cs+, and I− by Corrosion Products α-FeOOH from Typical Metal Tanks. Materials. 2024; 17(11):2706. https://doi.org/10.3390/ma17112706
Chicago/Turabian StyleDu, Yingzhe, Lili Li, Yukun Yuan, Yufaning Yin, Genggeng Dai, Yaqing Ren, Shiying Li, and Peng Lin. 2024. "Adsorption Behavior of Co2+, Ni2+, Sr2+, Cs+, and I− by Corrosion Products α-FeOOH from Typical Metal Tanks" Materials 17, no. 11: 2706. https://doi.org/10.3390/ma17112706




