CaAl-Layered Double Hydroxides-Modified Biochar Composites Mitigate the Toxic Effects of Cu and Pb in Soil on Pea Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. CaAl-LDH/BC Preparation
2.3. Characterization Method
2.4. Experimental Soil Remediation in Mining Areas
2.5. Planting Experiments
2.6. Chao1 and Shannon Index
2.7. Statistical Analysis
3. Results and Discussion
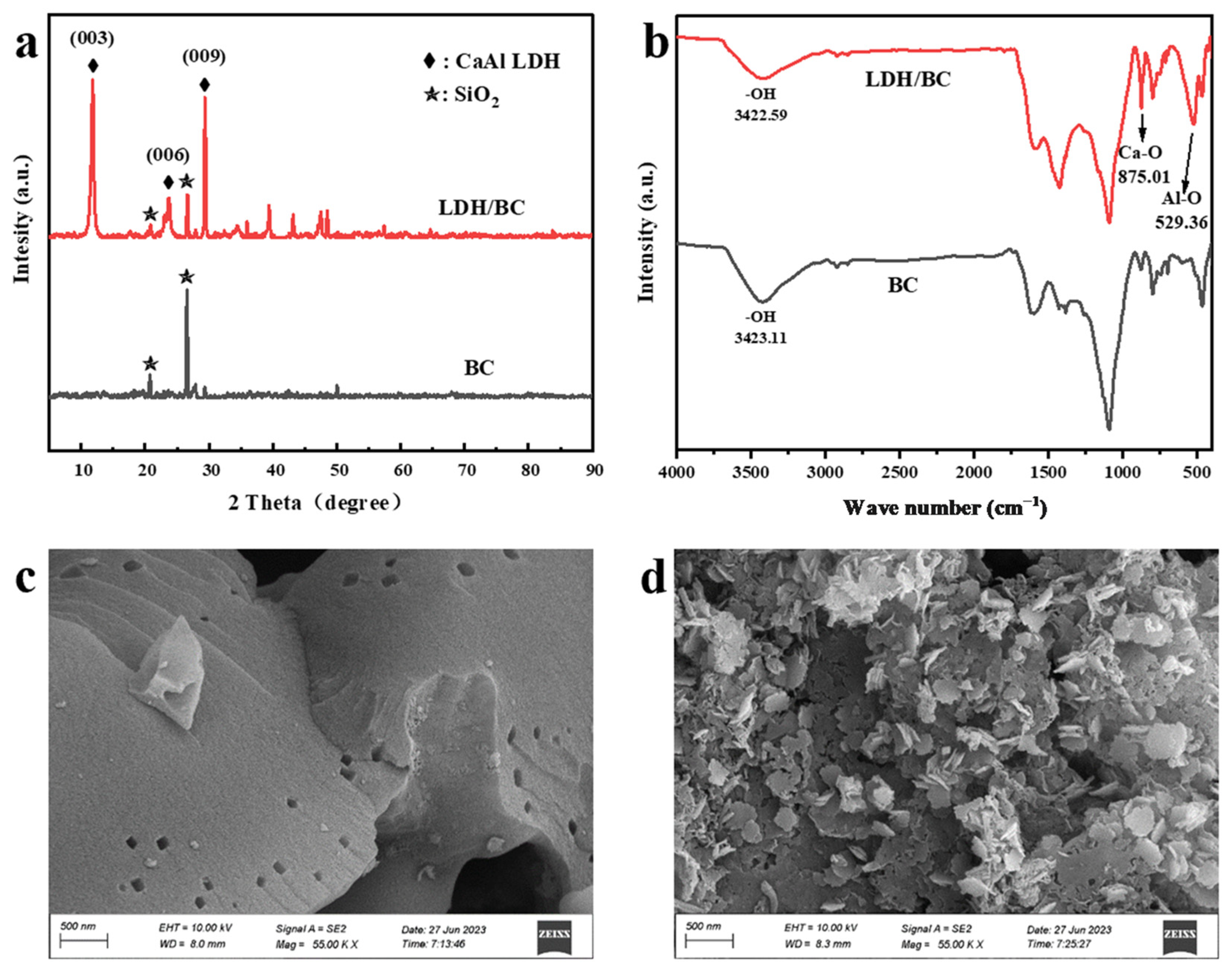
3.1. Material Characterization
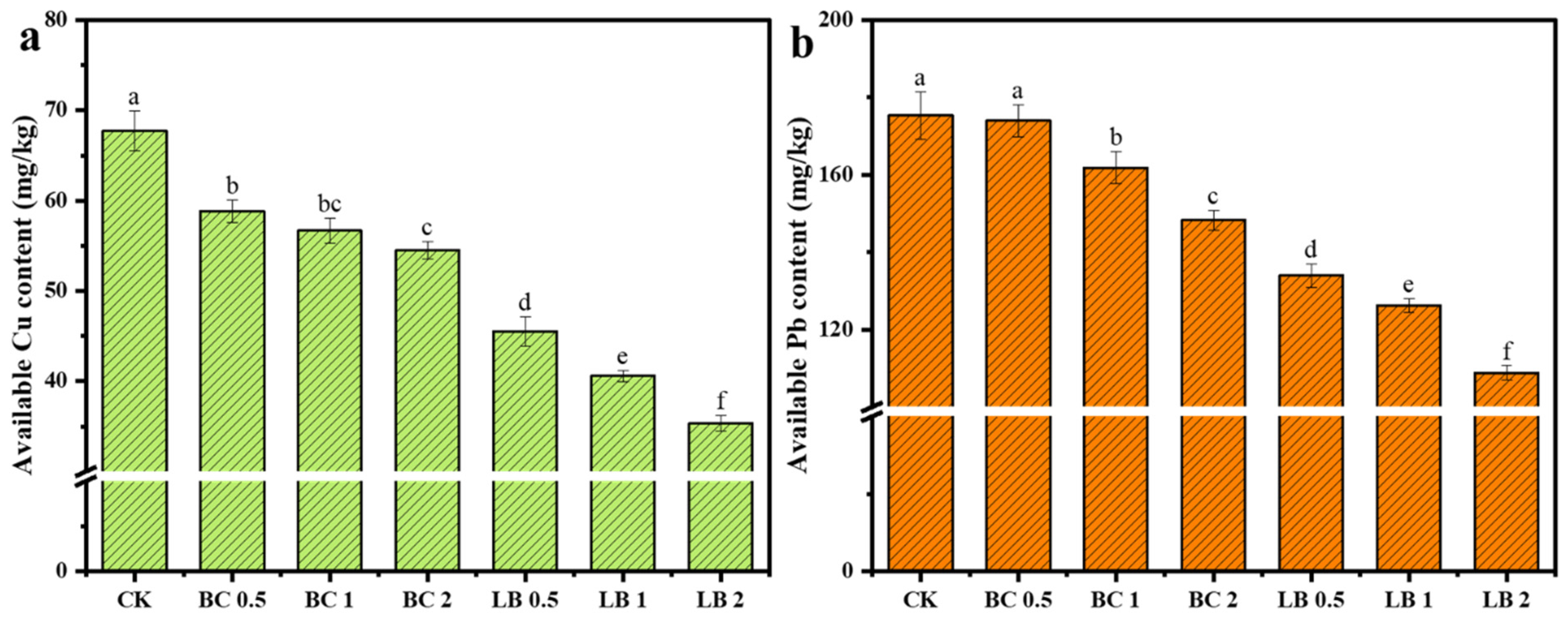
3.2. Effect of CaAl-LDH/BC Composites on Soil Physicochemical Properties
3.3. Effects of CaAl-LDH/BC on the Diversity of Soil Microbial Communities
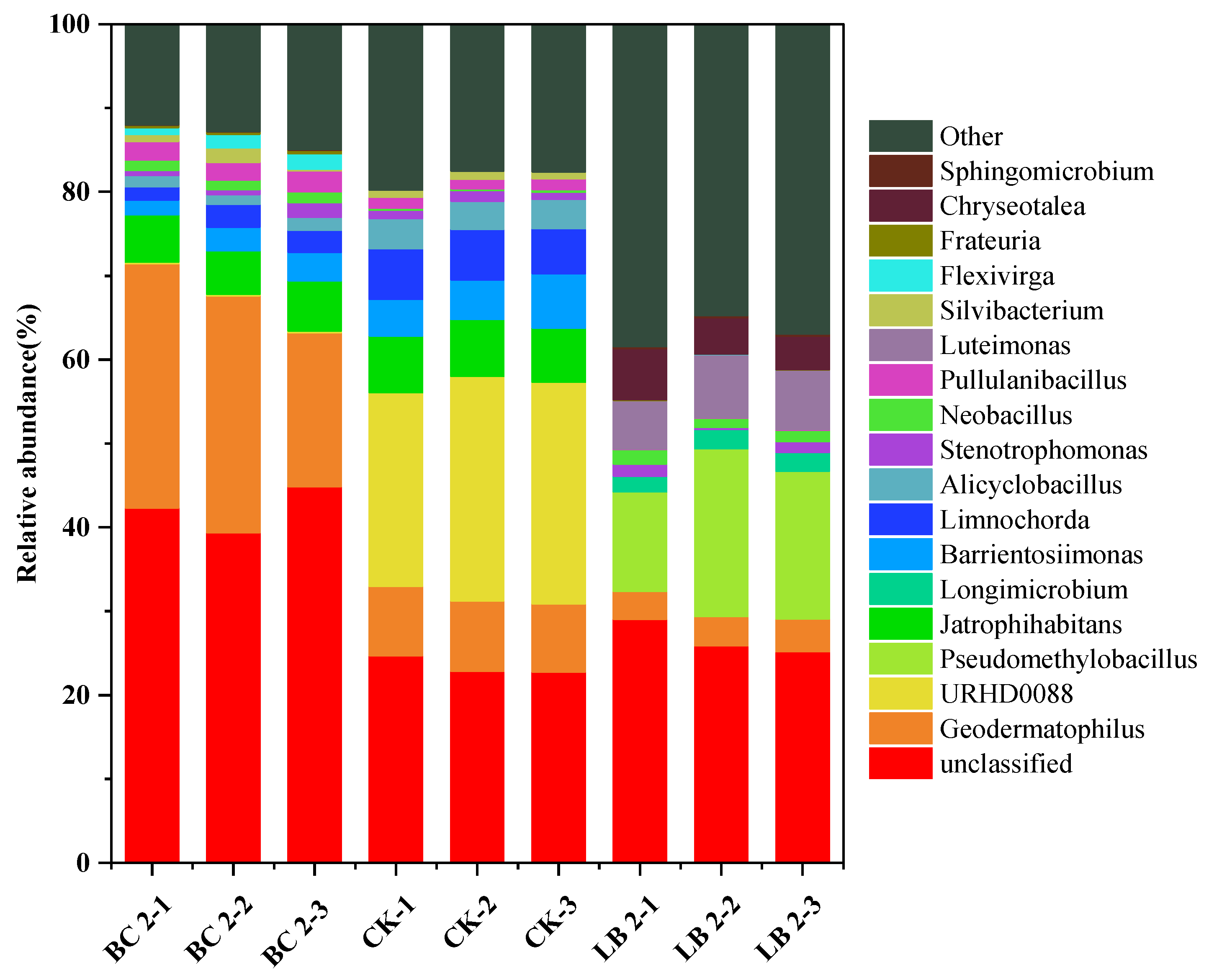
3.4. Effects of CaAl-LDH/BC on the Structural Composition of Soil Microbial Communities
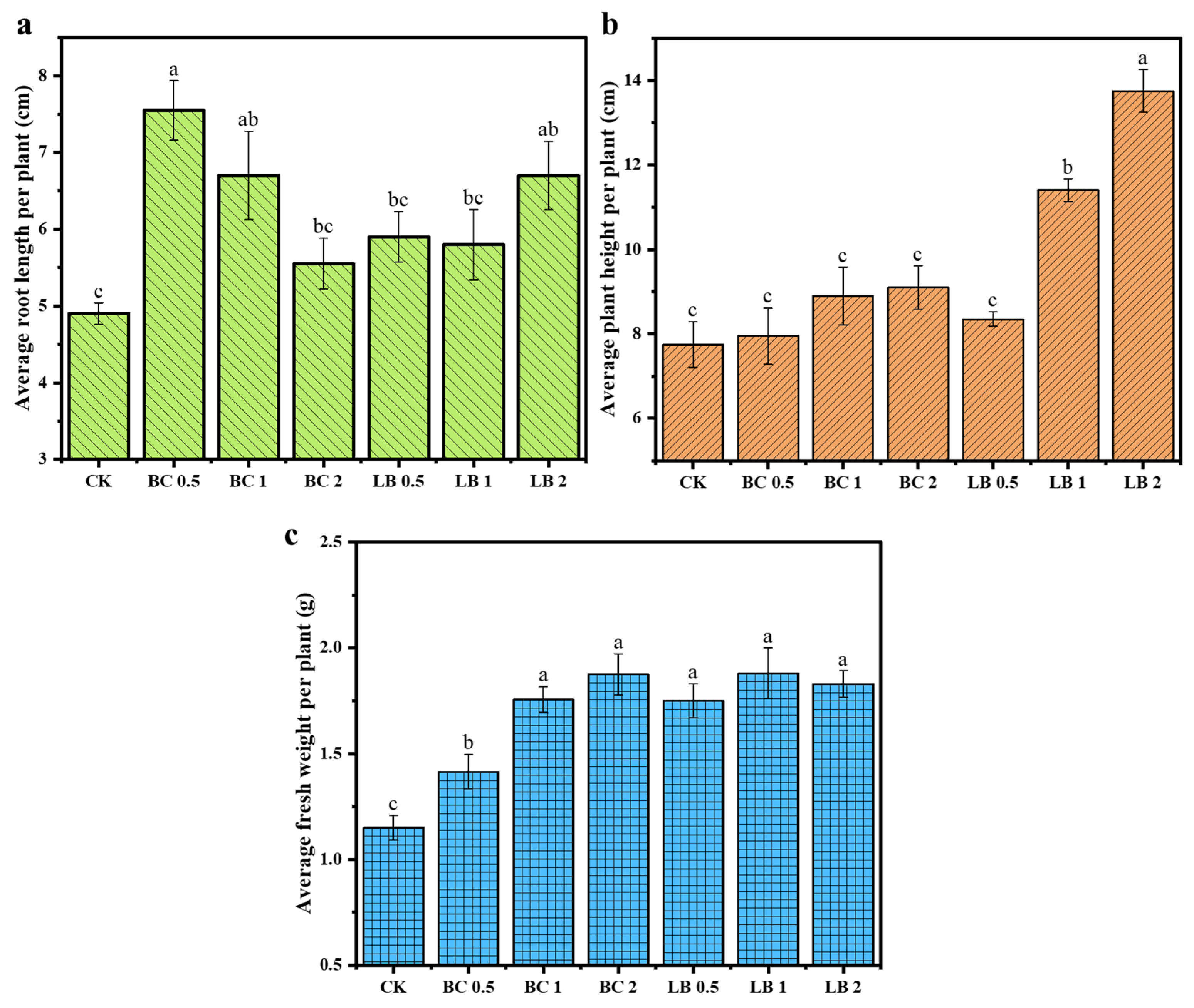
3.5. Changes in the Growth of Pea Seedlings
3.6. Changes in CAT Activity and Protein Content in Pea Seedlings
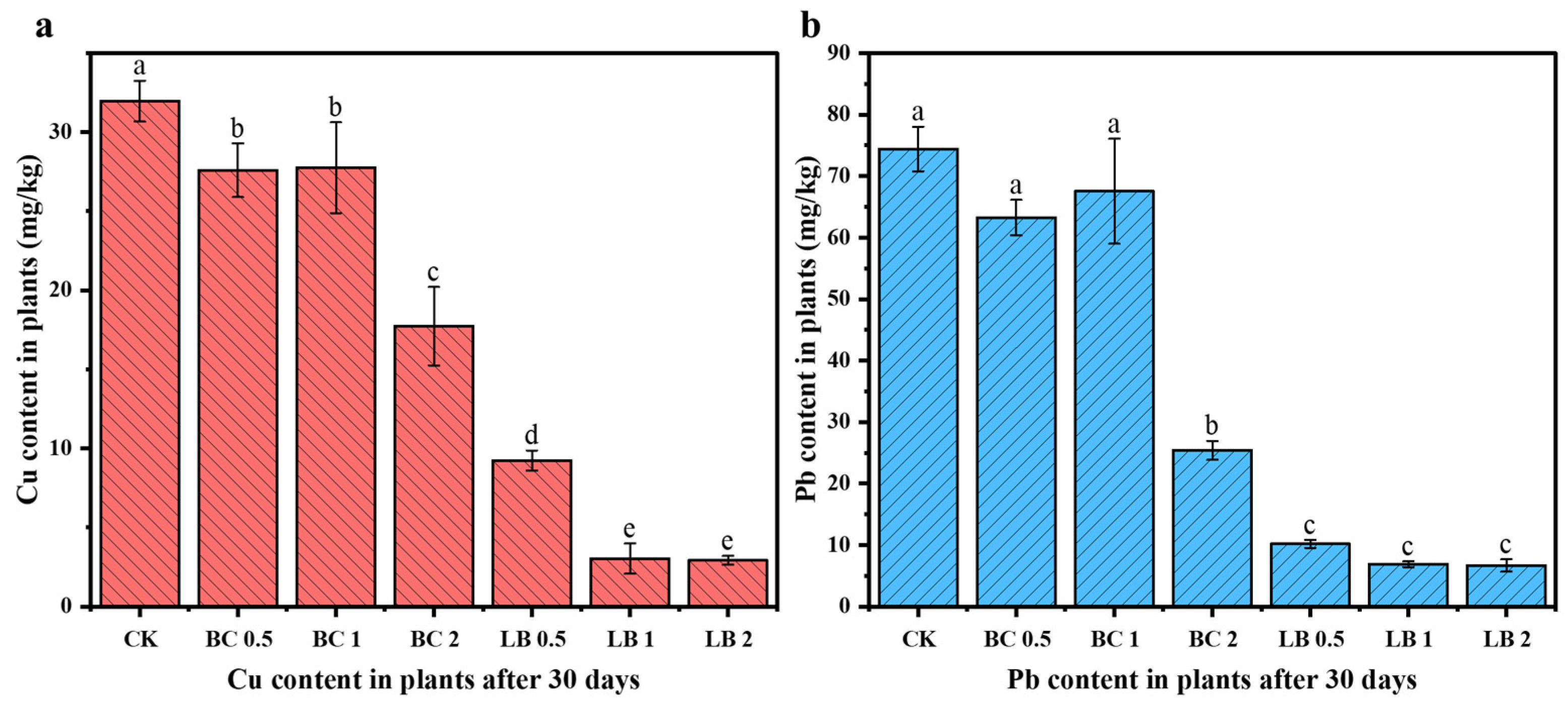
3.7. Changes in Cu and Pb Content in Pea Seedlings
4. Conclusions
- CaAl-LDH/BC could increase the content of SOC, AKC, and APC in the soil, and compared with BC, CaAl-LDH/BC could better increase the soil pH and cation exchange, which indicated that CaAl-LDH/BC could more effectively promote soil improvement and fertility enhancement. Furthermore, CaAl-LDH/BC significantly reduced the available content of Cu and Pb in the soil, of which the 2% dosage of CaAl-LDH/BC showed the best effect, and its passivation of Cu and Pb in soil reached 47.85% and 37.95%, respectively.
- Based on the results of 16S rRNA gene sequencing, the Chao1 index and Shannon index values of microbial communities in CaAl-LDH/BC-restored soils were significantly increased compared with those of BC, indicating that CaAl-LDH/BC had a significant effect on enhancing the diversity and abundance of soil microorganisms. CaAl-LDH/BC could significantly enhance the average relative abundance of beneficial microorganisms such as Actinobacteriota, Gemmatimonadota, Luteimonas, and Longimicrobium in the soil, which could help to enhance the tolerance of plants to heavy metals, reduce the enrichment capacity of plants for heavy metals, and enhance the nitrogen fixation capacity of the soil, It indicated that CaAl-LDH/BC had a positive effect on improving the soil microbial environment and inducing microbial communities to reduce the damage of heavy metals to plants.
- CaAl-LDH/BC can increase the fresh weight, root length, and plant height of pea seedlings, promote the growth of pea seedlings, reduce the content of Cu and Pb in pea seedlings, and enhance the CAT activity and protein content in pea seedlings. The lowest Cu and Pb contents in pea seedlings were 2.92 mg/kg and 6.67 mg/kg, which were reduced by 90.84% and 91.03%, respectively, after 2% CaAl-LDH/BC treatment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baran, H.A.; Nalbantcilar, M.T.; Koktan, N. Pollution and Health Risk Assessment of Heavy Metals in Waters around Mine Sites of Elazig (Eastern Turkey). J. Mt. Sci. 2023, 20, 1293–1306. [Google Scholar] [CrossRef]
- Fazle Bari, A.S.M.; Lamb, D.; MacFarlane, G.R.; Rahman, M.M. Soil Washing of Arsenic from Mixed Contaminated Abandoned Mine Soils and Fate of Arsenic after Washing. Chemosphere 2022, 296, 134053. [Google Scholar] [CrossRef] [PubMed]
- Kavehei, A.; Hose, G.C.; Chariton, A.A.; Gore, D.B. Application of Environmental DNA for Assessment of Contamination Downstream of a Legacy Base Metal Mine. J. Hazard. Mater. 2021, 416, 125794. [Google Scholar] [CrossRef] [PubMed]
- Mandal, J.; Bakare, W.A.; Rahman, M.M.; Rahman, M.A.; Siddique, A.B.; Oku, E.; Wood, M.D.; Hutchinson, S.M.; Mondal, D. Varietal Differences Influence Arsenic and Lead Contamination of Rice Grown in Mining Impacted Agricultural Fields of Zamfara State, Nigeria. Chemosphere 2022, 305, 135339. [Google Scholar] [CrossRef] [PubMed]
- Igwe, O.; Una, C.O.; Abu, E.; Adepehin, E.J. Environmental Risk Assessment of Lead–Zinc Mining: A Case Study of Adudu Metallogenic Province, Middle Benue Trough, Nigeria. Environ. Monit. Assess. 2017, 189, 492. [Google Scholar] [CrossRef] [PubMed]
- Ayari, J.; Barbieri, M.; Agnan, Y.; Sellami, A.; Braham, A.; Dhaha, F.; Charef, A. Trace Element Contamination in the Mine-Affected Stream Sediments of Oued Rarai in North-Western Tunisia: A River Basin Scale Assessment. Environ. Geochem. Health 2021, 43, 4027–4042. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.; Wood, P.J.; Reid, I. The Impairment of River Systems by Metal Mine Contamination: A Review Including Remediation Options. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2017–2077. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R.; Khan, M.A.; Bansal, M.; Garg, V.K. Utilization of Biosynthesized Silica-Supported Iron Oxide Nanocomposites for the Adsorptive Removal of Heavy Metal Ions from Aqueous Solutions. Environ. Sci. Pollut. Res. 2023, 30, 81319–81332. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.C.J.; Maggi, P.; Pirard, C.; Charlier, C.; Ruttens, A.; Liénard, A.; Colinet, G.; Remy, S. Human Biomonitoring Survey (Pb, Cd, As, Cu, Zn, Mo) for Urban Gardeners Exposed to Metal Contaminated Soils. Environ. Pollut. 2022, 312, 120028. [Google Scholar] [CrossRef]
- Azhar, U.; Ahmad, H.; Shafqat, H.; Babar, M.; Shahzad Munir, H.M.; Sagir, M.; Arif, M.; Hassan, A.; Rachmadona, N.; Rajendran, S.; et al. Remediation Techniques for Elimination of Heavy Metal Pollutants from Soil: A Review. Environ. Res. 2022, 214, 113918. [Google Scholar] [CrossRef]
- Liang, X.; Su, Y.; Wang, X.; Liang, C.; Tang, C.; Wei, J.; Liu, K.; Ma, J.; Yu, F.; Li, Y. Insights into the Heavy Metal Adsorption and Immobilization Mechanisms of CaFe-Layered Double Hydroxide Corn Straw Biochar: Synthesis and Application in a Combined Heavy Metal-Contaminated Environment. Chemosphere 2023, 313, 137467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of Heavy Metal-Contaminated Soils by Biochar: Challenges and Recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shin, H.-S. Competitive Adsorption of Heavy Metals onto Modified Biochars: Comparison of Biochar Properties and Modification Methods. J. Environ. Manag. 2021, 299, 113651. [Google Scholar] [CrossRef] [PubMed]
- Jijoe, P.S.; Yashas, S.R.; Shivaraju, H.P. Fundamentals, Synthesis, Characterization and Environmental Applications of Layered Double Hydroxides: A Review. Environ. Chem. Lett. 2021, 19, 2643–2661. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, G.; Huang, Y.; He, S.; Li, Y.; Jin, L.; Han, J. Surface-Modified LDH Nanosheets with High Dispersibility in Oil for Friction and Wear Reduction. ACS Appl. Mater. Interfaces 2024, 16, 5316–5325. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.; Huang, J.; Ye, J.; Zhu, C.; Xie, J.; Wang, Z.; Kang, M.; Yan, A. Enhancing Sorption of Layered Double Hydroxide-Based Magnetic Biochar for Arsenic and Cadmium through Optimized Preparation Protocols. Bioresour. Technol. 2023, 388, 129756. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Zhao, J.; Cheng, J.; Yu, Q.; Zhang, S.; Zhao, J.; Qiu, X. Remediation of Chromium-Contaminated Soil Using Delaminated Layered Double Hydroxides with Different Divalent Metals. Chemosphere 2020, 254, 126879. [Google Scholar] [CrossRef]
- Huang, W.-H.; Chang, Y.-J.; Lee, D.-J. Layered Double Hydroxide Loaded Pinecone Biochar as Adsorbent for Heavy Metals and Phosphate Ion Removal from Water. Bioresour. Technol. 2024, 391, 129984. [Google Scholar] [CrossRef]
- Veselska, V.; Sillerova, H.; Hudcova, B.; Ratie, G.; Lacina, P.; Lalinska-Volekova, B.; Trakal, L.; Sottnik, P.; Jurkovic, L.; Pohorely, M.; et al. Innovative in Situ Remediation of Mine Waters Using a Layered Double Hydroxide-Biochar Composite. J. Hazard. Mater. 2022, 424, 127136. [Google Scholar] [CrossRef]
- Puissant, J.; Jones, B.; Goodall, T.; Mang, D.; Blaud, A.; Gweon, H.S.; Malik, A.; Jones, D.L.; Clark, I.M.; Hirsch, P.R.; et al. The pH Optimum of Soil Exoenzymes Adapt to Long Term Changes in Soil pH. Soil Biol. Biochem. 2019, 138, 107601. [Google Scholar] [CrossRef]
- Aran, D.; Maul, A.; Masfaraud, J.-F. A Spectrophotometric Measurement of Soil Cation Exchange Capacity Based on Cobaltihexamine Chloride Absorbance. Comptes Rendus Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Adhikari, S.; Moon, E.; Timms, W. Identifying Biochar Production Variables to Maximise Exchangeable Cations and Increase Nutrient Availability in Soils. J. Clean. Prod. 2024, 446, 141454. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, S.; Yuan, L.; Li, Y.; Zhao, L.; Wen, Y.; Xu, J.; Hu, S.; Zhao, B. Effects of Humic Acid–Enhanced Phosphate Fertilizer on Wheat Yield, Phosphorus Uptake, and Soil Available Phosphorus Content. Crop Sci. 2023, 63, 956–966. [Google Scholar] [CrossRef]
- Liu, T.; Guo, L.; Cao, C.; Tan, W.; Li, C. Long-Term Rice-Oilseed Rape Rotation Increases Soil Organic Carbon by Improving Functional Groups of Soil Organic Matter. Agric. Ecosyst. Environ. 2021, 319, 107548. [Google Scholar] [CrossRef]
- Dai, J.; Becquer, T.; Henri Rouiller, J.; Reversat, G.; Bernhard-Reversat, F.; Nahmani, J.; Lavelle, P. Heavy Metal Accumulation by Two Earthworm Species and Its Relationship to Total and DTPA-Extractable Metals in Soils. Soil Biol. Biochem. 2004, 36, 91–98. [Google Scholar] [CrossRef]
- Bonanno, G.; Borg, J.A.; Di Martino, V. Levels of Heavy Metals in Wetland and Marine Vascular Plants and Their Biomonitoring Potential: A Comparative Assessment. Sci. Total Environ. 2017, 576, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, X.; Luo, X.; Wen, S.; Chen, W.; Huang, Q. Nitrite-Oxidizing Bacteria Community Composition and Diversity Are Influenced by Fertilizer Regimes, but Are Independent of the Soil Aggregate in Acidic Subtropical Red Soil. Front. Microbiol. 2018, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, Y.; Sun, R.; Zhou, Y.; Tsang, D.C.W.; Chen, Q. Optimizing the Synthesis of Fe/Al (Hydr)Oxides-Biochars to Maximize Phosphate Removal via Response Surface Model. J. Clean. Prod. 2019, 237, 117770. [Google Scholar] [CrossRef]
- Li, D.; Yan, W.; Guo, X.; Tian, Q.; Xu, Z.; Zhu, L. Removal of Selenium from Caustic Solution by Adsorption with CaAl Layered Double Hydroxides. Hydrometallurgy 2020, 191, 105231. [Google Scholar] [CrossRef]
- Wang, J.; Kang, Y.; Duan, H.; Zhou, Y.; Li, H.; Chen, S.; Tian, F.; Li, L.; Drosos, M.; Dong, C.; et al. Remediation of Cd2+ in Aqueous Systems by Alkali-Modified (Ca) Biochar and Quantitative Analysis of Its Mechanism. Arab. J. Chem. 2022, 15, 103750. [Google Scholar] [CrossRef]
- He, X.; Jiang, J.; Hong, Z.; Pan, X.; Dong, Y.; Xu, R. Effect of Aluminum Modification of Rice Straw–Based Biochar on Arsenate Adsorption. J. Soils Sediments 2020, 20, 3073–3082. [Google Scholar] [CrossRef]
- Wang, F.; Jin, L.; Guo, C.; Min, L.; Zhang, P.; Sun, H.; Zhu, H.; Zhang, C. Enhanced Heavy Metals Sorption by Modified Biochars Derived from Pig Manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Y.; Liu, T.; Kong, X.; Pan, T.; Zhang, F.; Lei, X.; Duan, X. Super-Stable Mineralization of Cu, Cd, Zn and Pb by CaAl-Layered Double Hydroxide: Performance, Mechanism, and Large-Scale Application in Agriculture Soil Remediation. J. Hazard. Mater. 2023, 447, 130723. [Google Scholar] [CrossRef]
- Huang, C.; Wang, W.; Yue, S.; Adeel, M.; Qiao, Y. Role of Biochar and Eisenia fetida on Metal Bioavailability and Biochar Effects on Earthworm Fitness. Environ. Pollut. 2020, 263, 114586. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial Effects of Biochar Application to Contaminated Soils on the Bioavailability of Cd, Pb and Zn and the Biomass Production of Rapeseed (Brassica napus L.). Biomass Bioenergy 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Qin, C.; Yuan, X.; Xiong, T.; Tan, Y.Z.; Wang, H. Physicochemical Properties, Metal Availability and Bacterial Community Structure in Heavy Metal-Polluted Soil Remediated by Montmorillonite-Based Amendments. Chemosphere 2020, 261, 128010. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Kim, Y.-K. Stabilization of Heavy Metal Contaminated Marine Sediments with Red Mud and Apatite Composite. J. Soils Sediments 2016, 16, 726–735. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar Applications Influence Soil Physical and Chemical Properties, Microbial Diversity, and Crop Productivity: A Meta-Analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Zhang, R.; Chen, Y.; Lei, L.; Qiu, C.; Xu, H. Effect of Slow-Released Biomass Alkaline Amendments Oyster Shell on Microecology in Acidic Heavy Metal Contaminated Paddy Soils. J. Environ. Manag. 2022, 319, 115683. [Google Scholar] [CrossRef]
- Luo, J.; Tao, Q.; Jupa, R.; Liu, Y.; Wu, K.; Song, Y.; Li, J.; Huang, Y.; Zou, L.; Liang, Y.; et al. Role of Vertical Transmission of Shoot Endophytes in Root-Associated Microbiome Assembly and Heavy Metal Hyperaccumulation in Sedum Alfredii. Environ. Sci. Technol. 2019, 53, 6954–6963. [Google Scholar] [CrossRef]
- Cui, H.; Liu, L.-L.; Dai, J.-R.; Yu, X.-N.; Guo, X.; Yi, S.-J.; Zhou, D.-Y.; Guo, W.-H.; Du, N. Bacterial Community Shaped by Heavy Metals and Contributing to Health Risks in Cornfields. Ecotoxicol. Environ. Saf. 2018, 166, 259–269. [Google Scholar] [CrossRef]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional Action of Bacteroidota and Firmicutes in Decomposing Straw Polymers in a Paddy Soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef]
- Oshiki, M.; Toyama, Y.; Suenaga, T.; Terada, A.; Kasahara, Y.; Yamaguchi, T.; Araki, N. N2O Reduction by Gemmatimonas Aurantiaca and Potential Involvement of Gemmatimonadetes Bacteria in N2O Reduction in Agricultural Soils. Microbes Environ. 2022, 37, ME21090. [Google Scholar] [CrossRef]
- Touceda-González, M.; Kidd, P.S.; Smalla, K.; Prieto-Fernández, A. Bacterial Communities in the Rhizosphere of Different Populations of the Ni-Hyperaccumulator Alyssum Serpyllifolium and the Metal-Excluder Dactylis Glomerata Growing in Ultramafic Soils. Plant Soil 2018, 431, 317–332. [Google Scholar] [CrossRef]
- Sheu, C.; Cai, C.-Y.; Sheu, S.-Y.; Li, Z.-H.; Chen, W.-M. Pseudomethylobacillus Aquaticus Gen. Nov., Sp. Nov., a New Member of the Family Methylophilaceae Isolated from an Artificial Reservoir. Int. J. Syst. Evol. Microbiol. 2019, 69, 3551–3559. [Google Scholar] [CrossRef]
- Pitt, A.; Lienbacher, S.; Schmidt, J.; Neumann-Schaal, M.; Wolf, J.; Hahn, M.W. Description of a New Freshwater Bacterium Aquirufa Regiilacus Sp. Nov., Classification of the Genera Aquirufa, Arundinibacter, Sandaracinomonas, and Tellurirhabdus to the Family Spirosomataceae, Classification of the Genus Chryseotalea to the Family Fulvivirgaceae and Litoribacter to the Family Cyclobacteriaceae, as Well as Classification of Litoribacter Alkaliphilus as a Later Heterotypic Synonym of Litoribacter Ruber. Arch. Microbiol. 2024, 206, 79. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Z.; Wang, K.; Zhao, J.; Fang, J.; Liu, G.; Yao, H.; Pan, J. Comparison Analysis of Microbial Agent and Different Compost Material on Microbial Community and Nitrogen Transformation Genes Dynamic Changes during Pig Manure Compost. Bioresour. Technol. 2024, 395, 130359. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Luo, J.; Cui, X.; Xu, J.; Hou, Y.; Hao, B.; Guo, W. Arbuscular Mycorrhizal Fungi and Nitrilotriacetic Acid Regulated Suaeda Salsa Growth in Cd-Contaminated Saline Soil by Driving Rhizosphere Bacterial Assemblages. Environ. Exp. Bot. 2022, 193, 104669. [Google Scholar] [CrossRef]


| pH | AKC | APC | SOCC | SOC | |
|---|---|---|---|---|---|
| mg/kg | mg/kg | mmol/kg | mg/kg | ||
| CK | 4.47 | 327.63 | 30.88 | 32.59 | 11.75 |
| BC 0.5 | 4.54 | 346.50 | 31.45 | 37.43 | 15.73 |
| BC 1 | 4.66 | 376.05 | 31.63 | 39.54 | 18.66 |
| BC 2 | 4.92 | 402.00 | 31.73 | 36.59 | 25.92 |
| LB 0.5 | 5.30 | 343.03 | 34.50 | 38.71 | 14.80 |
| LB 1 | 5.75 | 384.16 | 34.71 | 49.75 | 17.22 |
| LB 2 | 6.33 | 444.11 | 35.72 | 62.91 | 24.65 |
| Feed Material | Dosage | Soil Heavy Metal Contaminants | Processing Times | Raw Soil Heavy Metal Content | Reference |
|---|---|---|---|---|---|
| Cornstalk biochar | 2% | The available contents of Cd, Cu, Zn, and Pb were reduced by 23.8%, 11.9%, 5.27%, and 14.3%, respectively. | 28 d | The available contents of Cd, Cu, Zn, and Pb were 3.2, 17, 108, and 145 mg/kg, respectively. | [35] |
| Miscanthus × giganteus biochar | 1% | CaCl2-extractable Cd, Zn, and Pb concentrations were reduced by 14%, 15%, and 29%, respectively. | 84 d | CaCl2-extractable Cd, Zn, and Pb concentrations were 24.0, 2980, and 3110 mg/kg. | [36] |
| Nano-Montmorillonite | 1% | Cd available content was reduced by 5.59%. | 60 d | Cd available content was 2.51 mg/kg. | [37] |
| Red mud/Apatite/Red mud + Apatite | 5%/5%/2.5% + 2.5% | Bioavailable and potentially bioavailable fraction of lead reduced from 92.1% to 80.2%, 86.4%, and 82.2%, respectively. | 120 d | The total content of Pb was 44.2 mg/kg. | [38] |
| CaAl-LDH/BC | 2% | The available contents of Pb and Cu were reduced by 37.95% and 47.85%. | 80 d | The available contents of Pb and Cu were 175.3 and 67.7 mg/kg. | This paper |
| Chao1 | ACE | Shannon | Simpson | Coverage | |
|---|---|---|---|---|---|
| BC 2-1 | 515.447 | 518.8916 | 6.072507 | 0.964053 | 0.999863 |
| BC 2-2 | 592.8255 | 594.9367 | 6.208726 | 0.967411 | 0.999871 |
| BC 2-3 | 571.3871 | 572.7808 | 6.300023 | 0.971114 | 0.999887 |
| CK-1 | 402.8966 | 405.1257 | 5.154584 | 0.918712 | 0.999919 |
| CK-2 | 402.3636 | 404.6076 | 5.052613 | 0.90576 | 0.999902 |
| CK-3 | 448.561 | 452.0917 | 5.049857 | 0.906209 | 0.999784 |
| LB 2-1 | 817.1224 | 820.1697 | 6.564774 | 0.970319 | 0.999827 |
| LB 2-2 | 808.5941 | 819.0188 | 6.224698 | 0.953588 | 0.999676 |
| LB 2-3 | 808.9655 | 807.51 | 6.564133 | 0.963213 | 0.999857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cai, Y.; Wu, Y.; Yan, C.; Dang, Z.; Yin, H. CaAl-Layered Double Hydroxides-Modified Biochar Composites Mitigate the Toxic Effects of Cu and Pb in Soil on Pea Seedlings. Materials 2024, 17, 2763. https://doi.org/10.3390/ma17112763
Wang Y, Cai Y, Wu Y, Yan C, Dang Z, Yin H. CaAl-Layered Double Hydroxides-Modified Biochar Composites Mitigate the Toxic Effects of Cu and Pb in Soil on Pea Seedlings. Materials. 2024; 17(11):2763. https://doi.org/10.3390/ma17112763
Chicago/Turabian StyleWang, Yuanzheng, Yuhao Cai, Yuxuan Wu, Caiya Yan, Zhi Dang, and Hua Yin. 2024. "CaAl-Layered Double Hydroxides-Modified Biochar Composites Mitigate the Toxic Effects of Cu and Pb in Soil on Pea Seedlings" Materials 17, no. 11: 2763. https://doi.org/10.3390/ma17112763
APA StyleWang, Y., Cai, Y., Wu, Y., Yan, C., Dang, Z., & Yin, H. (2024). CaAl-Layered Double Hydroxides-Modified Biochar Composites Mitigate the Toxic Effects of Cu and Pb in Soil on Pea Seedlings. Materials, 17(11), 2763. https://doi.org/10.3390/ma17112763





