Study of the Rolling Effect on MoS2–Carbon Fiber Density and Its Consequences for the Functionality of Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Method
2.1. Electrode Slurry Fabrication by Solid-Phase Synthesis
2.2. Thick Film Deposited by Blade-Coating
2.3. Electrodes Sheet Fabrication
2.4. Coin Cell Assembly
- Inside the glove box, use a lithium metal disc as the positive electrode.
- Sequentially stack the following components: cell bottom cover, lithium metal (99.9%, FMC), and pre-wetted separator membrane (Celgard 2500) with electrolyte (1 M LiPF6 in EC/DEC/EMC (3:2:5) solution) applied.
- Use a dropper to add a few drops of electrolyte into the assembly.
- Proceed by placing the MoS2–carbon fiber negative electrode plate, spacer, and cell top cover.
- Seal and lock the assembly using a capping machine (provided by Hao-Ju Industrial Co., Ltd.).
- Once sealed, transfer the assembly out of the glove box for storage and subsequent charge–discharge testing.
2.5. Characterization Techniques
3. Results and Discussion
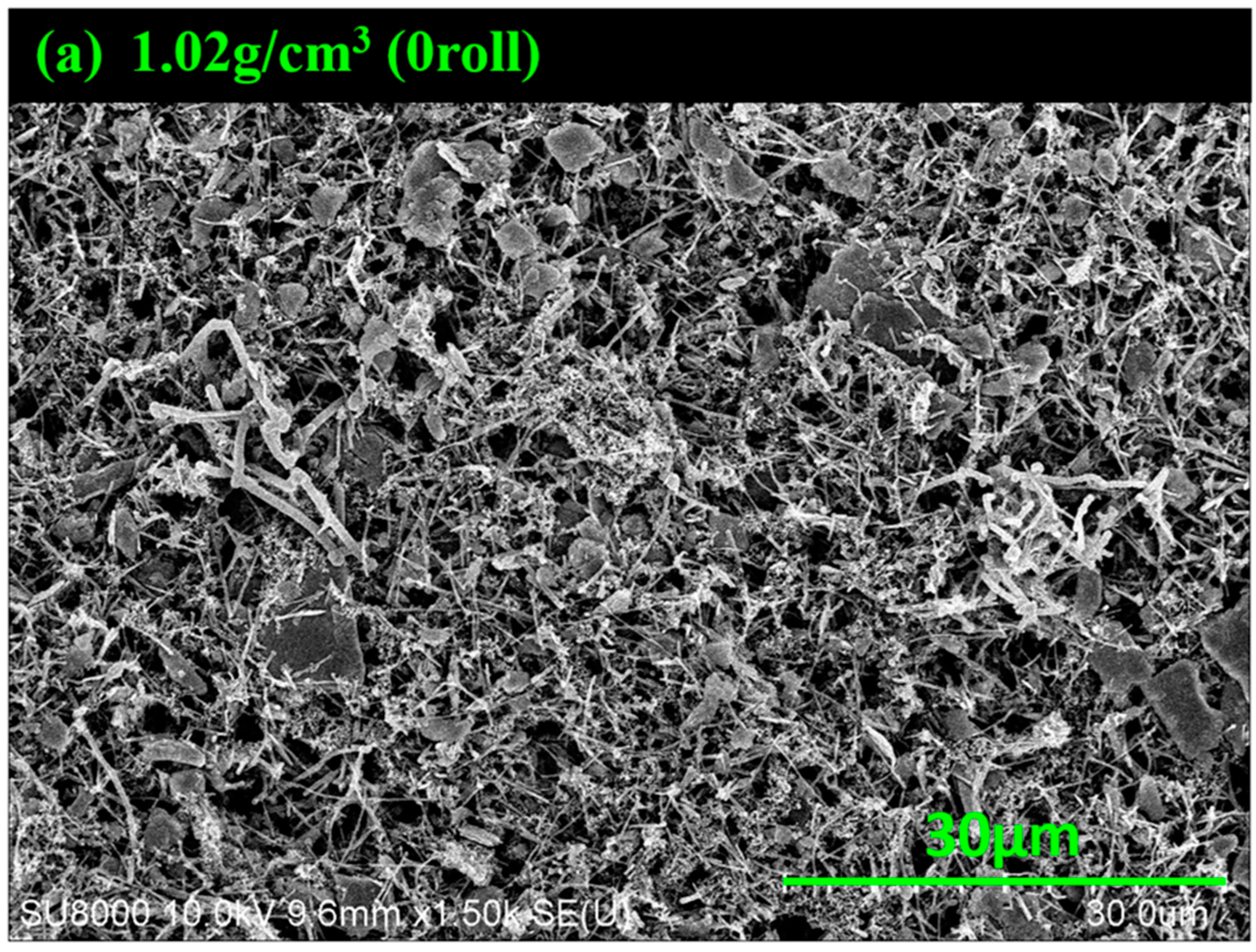
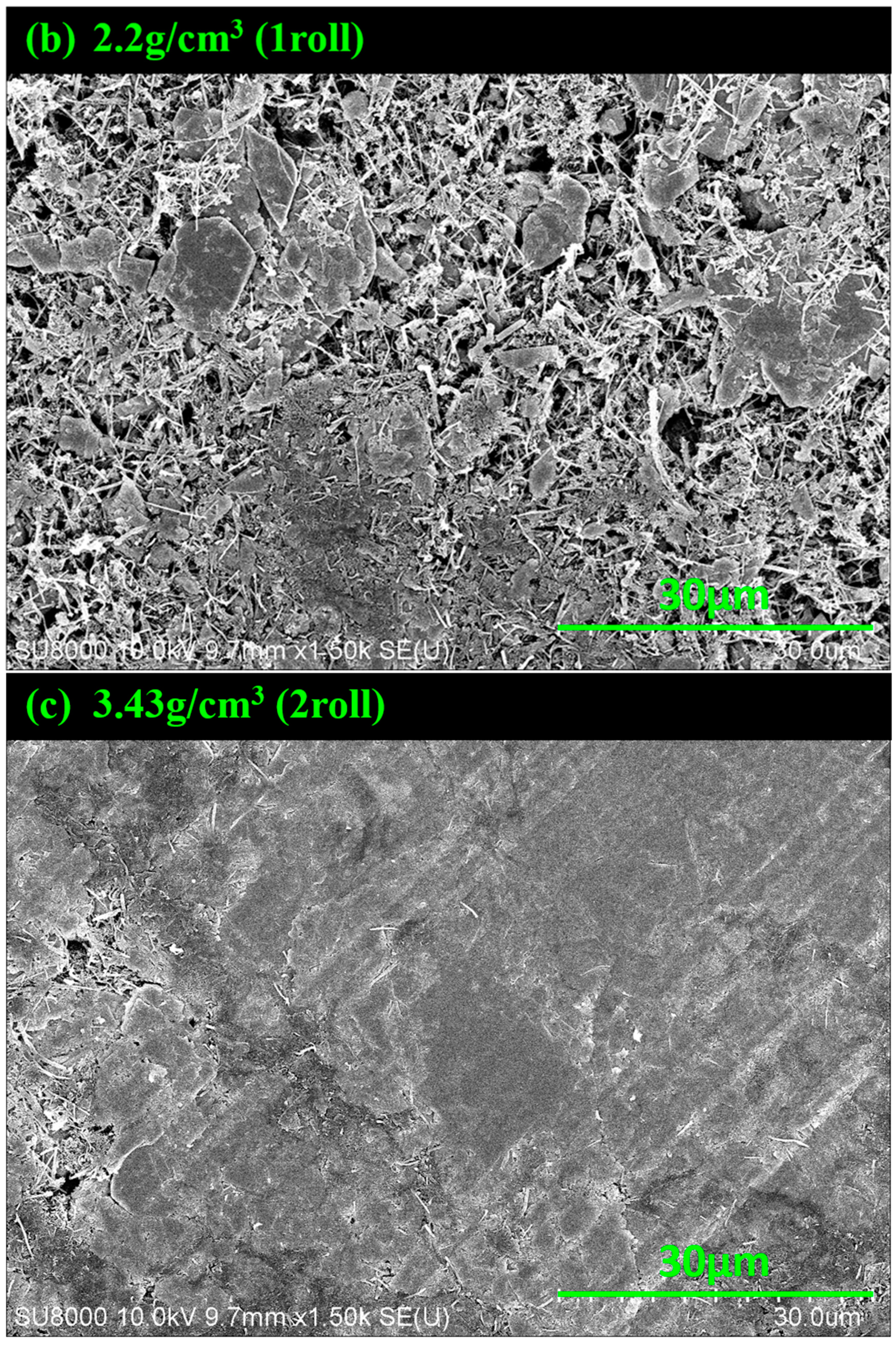
3.1. SEM Images of MoS2–Carbon Fiber Anodes with Different Material Density
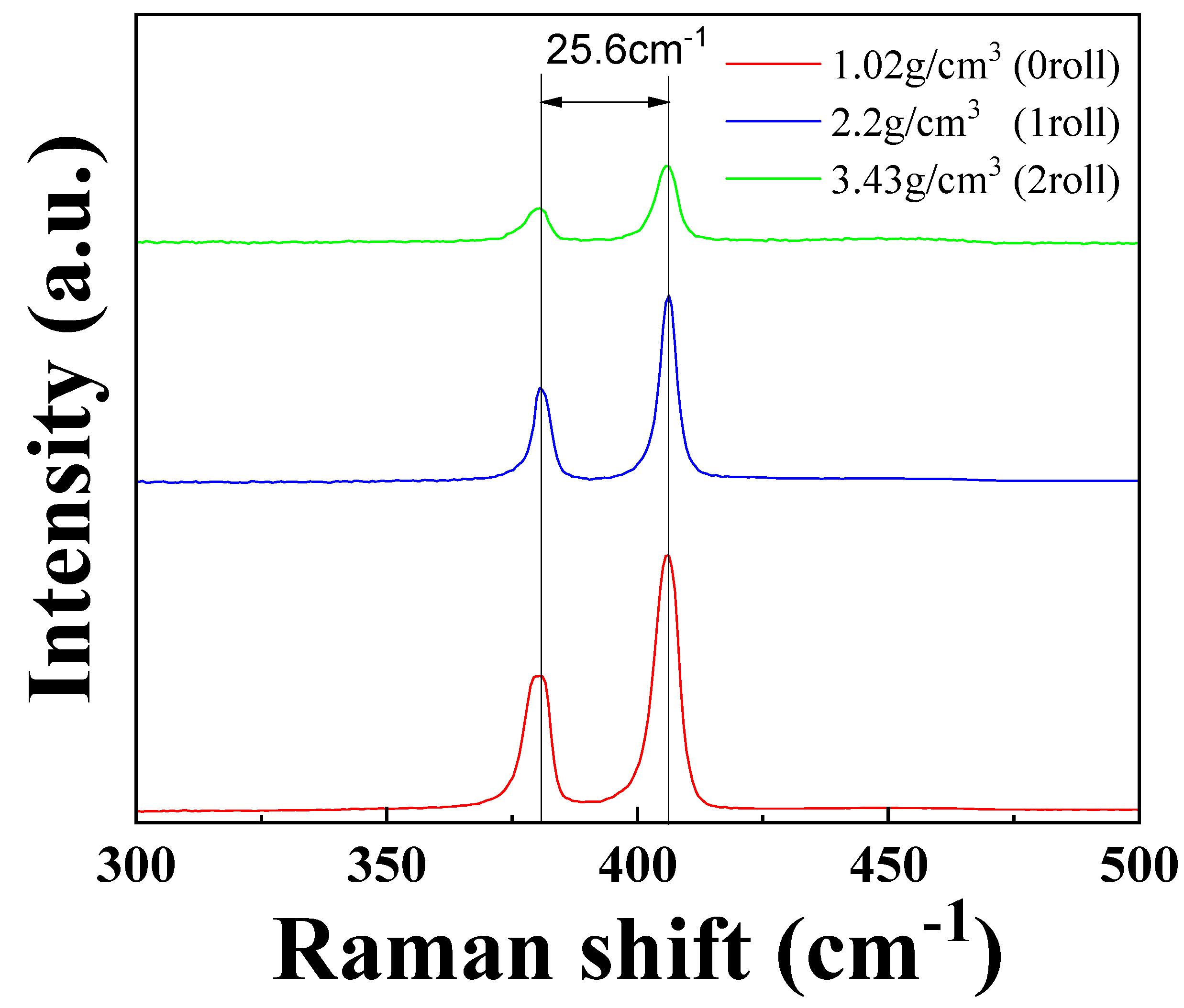
3.2. Raman Spectra Results of MoS2–Carbon Fiber Anodes with Different Material Density
3.3. X-ray Photoelectron Spectroscopy Results of MoS2–Carbon Fiber Anodes with Different Material Density
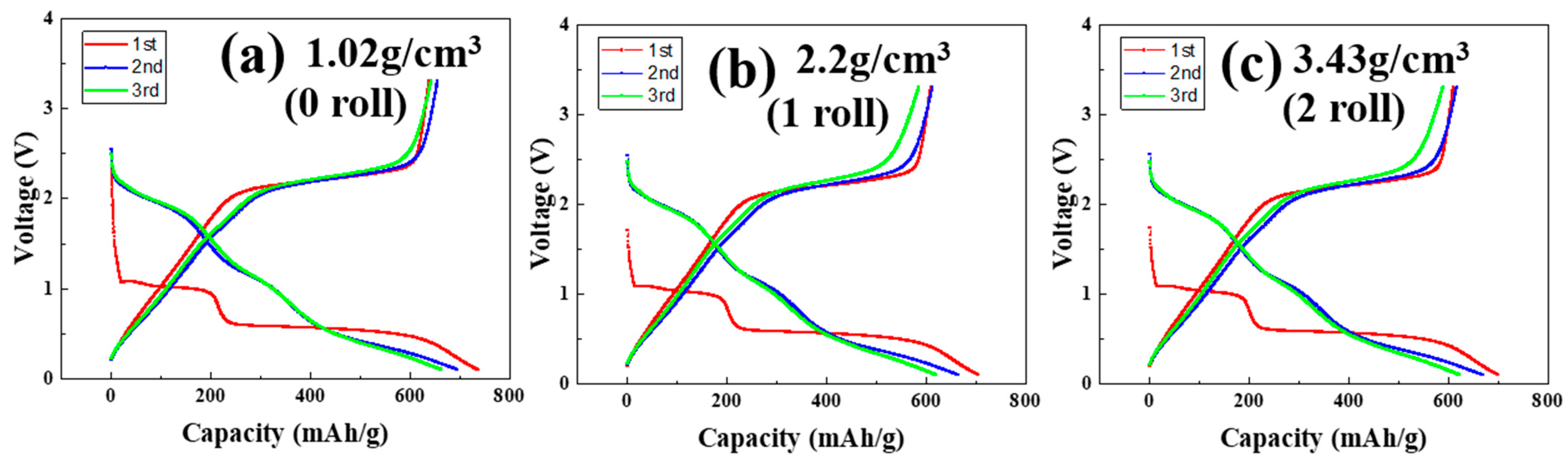
3.4. Formation Results of MoS2–Carbon Fiber Anodes with Different Material Density
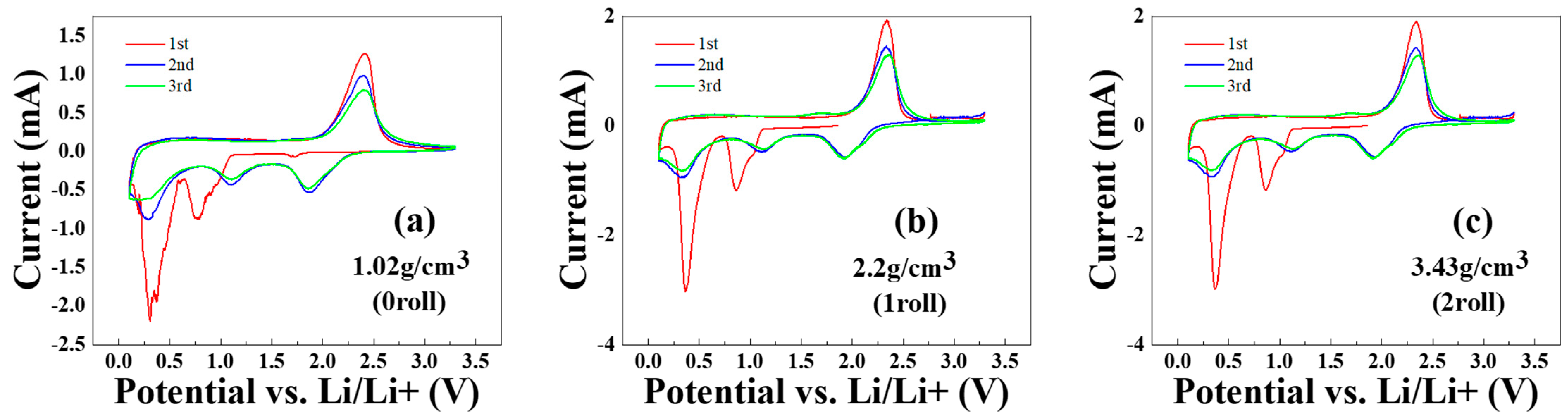
3.5. Cyclic Voltammetry Test Results of MoS2–Carbon Fiber Anodes with Different Material Density
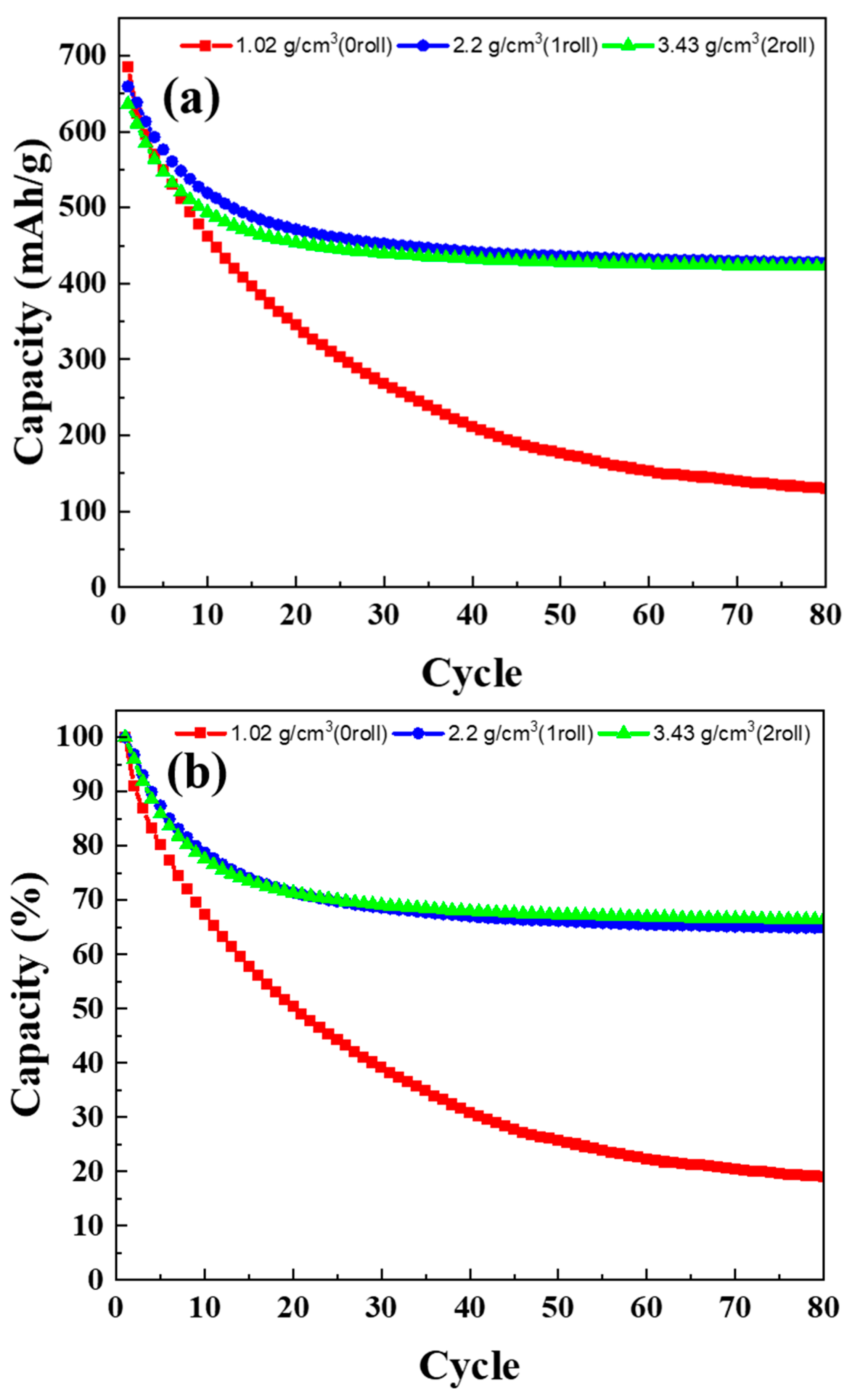
3.6. Cycle Life Test Results of MoS2–Carbon Fiber Anodes with Different Material Density
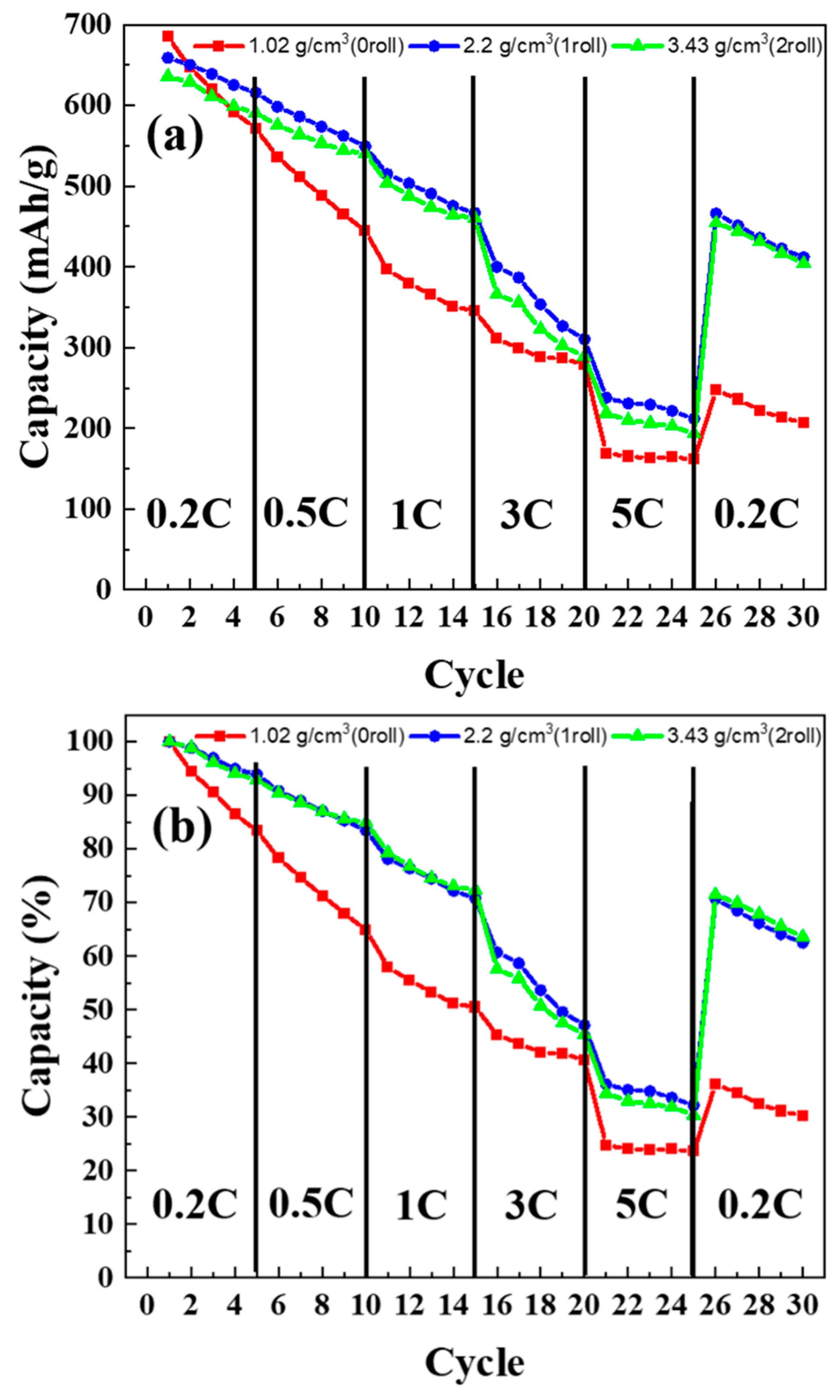
3.7. C-Rate Test Results of MoS2–Carbon Fiber Anodes with Different Material Density
3.8. Compared to Other Reported Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Zhou, Z.; Yan, X.; Hou, F.; Wen, L.; Luo, W.; Liang, J.; Dou, S.X. Engineering of lithium-metal anodes towards a safe and stable battery. Energy Storage Mater. 2018, 14, 22. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High rate capability of graphite negative electrodes for lithium-ion batteries. J. Electrochem. Soc. 2005, 152, A474. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66. [Google Scholar] [CrossRef]
- Hu, J.; Zhong, S.; Yan, T. Using carbon black to facilitate fast charging in lithium-ion batteries. J. Power Sources 2021, 508, 230342. [Google Scholar] [CrossRef]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676. [Google Scholar] [CrossRef]
- Varzi, A.; Täubert, C.; Wohlfahrt-Mehrens, M.; Kreis, M.; Schütz, W. Study of multi-walled carbon nanotubes for lithium-ion battery electrodes. J. Power Sources 2011, 196, 3303. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Qiu, X.; Ma, Y.; Kong, D.; Müllen, K.; Li, X.; Zhi, L. Stable high-capacity and high-rate silicon-based lithium battery anodes upon two-dimensional covalent encapsulation. Nat. Commun. 2020, 11, 3826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, A. Nanostructured transition metal oxides as advanced anodes for lithium-ion batteries. Sci. Bull. 2015, 60, 823. [Google Scholar] [CrossRef]
- Fan, S.; Zou, X.; Du, H.; Gan, L.; Xu, C.; Lv, W.; He, Y.-B.; Yang, Q.-H.; Kang, F.; Li, J. Theoretical investigation of the intercalation chemistry of lithium/sodium ions in transition metal dichalcogenides. J. Phys. Chem. C 2017, 121, 13599. [Google Scholar] [CrossRef]
- Bozheyev, F.; Zhexembekova, A.; Zhumagali, S.; Molkenova, A.; Bakenov, Z. MoS2 nanopowder as anode material for lithium-ion batteries produced by self-propagating high-temperature synthesis. Mater. Today Proc. 2017, 4, 4567. [Google Scholar]
- Shi, Y.; Wang, Y.; Wong, J.; Tan, A.; Hsu, C.-L.; Li, L.; Lu, Y.-C.; Yang, H.Y. Self-assembly of hierarchical MoSx/CNT nanocomposites (2< x< 3): Towards high performance anode materials for lithium ion batteries. Sci. Rep. 2013, 3, 2169. [Google Scholar]
- Zhang, C.; Wu, H.B.; Guo, Z. Facile synthesis of carbon-coated MoS2 nanorods with enhanced lithium storage properties. Electrochem. Commun. 2012, 20, 7. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, F.; Ou, X.; Yang, C.; Xiong, X.; Liu, M. MoS2 encapsulated SnO2-SnS/C nanosheets as a high performance anode material for lithium ion batteries. Chem. Eng. J. 2017, 316, 393. [Google Scholar] [CrossRef]
- Shokhen, V.; Miroshnikov, Y.; Gershinsky, G.; Gotlib, N.; Stern, C.; Naveh, D.; Zitoun, D. On the impact of Vertical Alignment of MoS2 for Efficient Lithium Storage. Sci. Rep. 2017, 7, 3280. [Google Scholar] [CrossRef] [PubMed]
- Shyyko, L.O.; Kotsyubynsky, V.O.; Budzulyak, I.M.; Sagan, P. MoS2/C multilayer nanospheres as an electrode base for lithium power sources. Nanoscale Res. Lett. 2016, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thakur, P.; Sharma, R.; Puthirath, A.; Ajayan, P.; Narayanan, T. Photo Rechargeable Li-Ion Batteries Using Nanorod Heterostructure Electrodes. Small 2021, 17, 2105029. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chen, W. L-cysteine-assisted synthesis of layered MoS2/graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 5, 4720. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.N.; Vuong, V.-D.; Phong, M.T.; Le, T.V. Fabrication of MoS2 nanoflakes supported on carbon nanotubes for high performance anode in lithium-ion batteries (LIBs). J. Nanomater. 2019, 2019, 8364740. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, X.; Fan, X.; Su, X. Fabrication of flower-like MoS2/TiO2 hybrid as an anode material for lithium ion batteries. RSC Adv. 2017, 7, 38119. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, R.; Tay, B.K.; Teo, E.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385. [Google Scholar] [CrossRef]
- Tumino, F.; Casari, C.; Passoni, M.; Russo, V.; Bassi, A. Pulsed laser deposition of single-layer MoS2 on Au (111): From nanosized crystals to large-area films. Nanoscale Adv. 2019, 1, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, Q.; Liu, S.; Kang, Z.; Zhang, Z.; Du, J.; Li, F.; Zhang, S.; Xiao, J.; Liu, B.; et al. Poly (4-styrenesulfonate)-induced sulfur vacancy self-healing strategy for monolayer MoS2 homojunction photodiode. Nat. Commun. 2017, 8, 15881. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Momma, T.; Ahn, S.; Yokoshima, T.; Nara, H.; Osaka, T. On-site chemical pre-lithiation of S cathode at room temperature on a 3D nano-structured current collector. J. Power Sources 2017, 366, 65. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209. [Google Scholar] [CrossRef]
- Yang, F.; Feng, X.; Glans, P.-A.; Guo, J. MoS2 for beyond lithium-ion batteries. APL Mater. 2021, 9, 050903. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Giebeler, L. Nanosized Li2S-based cathodes derived from MoS2 for high-energy density Li–S cells and Si–Li2S full cells in carbonate-based electrolyte. Energy Storage Mater. 2017, 8, 209. [Google Scholar] [CrossRef]
- Faizan, M.; Hussain, S.; Vikraman, D.; Ali, B.; Kim, H.-S.; Jung, J.; Nam, K.-W. MoS2@ Mo2C hybrid nanostructures formation as an efficient anode material for lithium-ion batteries. J. Mater. Res. Technol. 2021, 14, 2382. [Google Scholar]
- Fu, Y.; Zu, C.; Manthiram, A. In situ-formed Li2S in lithiated graphite electrodes for lithium–sulfur batteries. J. Am. Chem. Soc. 2013, 135, 18044. [Google Scholar] [CrossRef] [PubMed]
- Carey, T.; Alhourani, A.; Tian, R.; Seyedin, S.; Arbab, A.; Maughan, J.; Šiller, L.; Horvath, D.; Kelly, A.; Kaur, H.; et al. Cyclic production of biocompatible few-layer graphene ink with in-line shear-mixing for inkjet-printed electrodes and Li-ion energy storage. npj 2D Mater. Appl. 2022, 6, 3. [Google Scholar] [CrossRef]
- Hsu, C.-J.; Chou, C.-Y.; Yang, C.-H.; Lee, T.-C.; Chang, J.-K. MoS2/graphene cathodes for reversibly storing Mg2+ and Mg2+/Li+ in rechargeable magnesium-anode batteries. Chem. Commun. 2016, 52, 1701. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Yuan, H.; Takeshita, H.T.; Sakai, T. Electrochemical hydrogen storage in MoS2 nanotubes. J. Am. Chem. Soc. 2001, 123, 11813. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, Y.; Hernandez, F.C.R.; Yoo, H.D.; An, Q.; Yao, Y. Enhancing sodium-ion battery performance with interlayer-expanded MoS2–PEO nanocomposites. Nano Energy 2015, 15, 453. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-S.; Park, J.-W.; Nam, T.-H.; Kim, K.-W.; Ahn, J.-H.; Wang, G.; Ahn, H.-J. Discharge mechanism of MoS2 for sodium ion battery: Electrochemical measurements and characterization. Electrochim. Acta 2013, 92, 427. [Google Scholar] [CrossRef]




| Material | Chemical Formula | Purity | Manufacturer |
|---|---|---|---|
| Molybdenum disulfide powder | MoS2 | 98% | Sigma-Aldrich, St. Louis, MO, USA |
| Vapor-grown carbon fiber (VGCF) powder | C | >98% | Showa Denko K.K., Oyama City, Japan |
| Super P (conductive carbon) | C | >99.5% | TIMCAL, Westlake, OH, USA |
| N-Methyl-2-pyrrolidone (NMP) | C5H9NO | 99% | Emperor Chemical Co., Ltd., Hangzhou, China |
| Polyvinylidene difluoride (PVDF) | -(C2H2F2)n- | 99% | KUREHA, Louisville, KY, USA |
| Electrolyte: EC/DEC/EMC 3:2:5 (w:w:w) + 1 M LiPF6 | - | - | Taiwan Hopax Chemicals Mfg. Co., Ltd., Kaohsiung, Taiwan |
| Copper foil | Cu | 99.9% | UACJ Co., Tokyo, Japan |
| Lithium metal | Li | 99.9 | FMC Co., Philadelphia, PA, USA |
| Separator (Celegard2300) (polypropylene/polyethylene/polypropylene) | - | - | Celegard LLC, Charlotte, NC, USA |
| STEPS | Action | Speed (100 rpm) | Time(min) |
|---|---|---|---|
| STEP1 | Add PVDF in NMP | 3/6/3/7/3 | 6/6/6/6/6 |
| STEP2 | Continue milling | 3/6/3/7/3 | 6/6/6/6/6 |
| STEP3 | Continue milling | 3/6/3/7/3 | 6/6/6/6/6 |
| STEP4 | Add SP and VGCF | 3/6/3/7/3 | 6/6/6/6/6 |
| STEP5 | Add MoS2 | 3/6/3/7/3 | 6/6/6/6/6 |
| STEP6 | Defoaming | 1.5 | 30 |
| 0 Roll (1.02 g/cm3) | 1 Roll (2.2 g/cm3) | 2 Roll (3.43 g/cm3) | |
|---|---|---|---|
| 1st discharge (mAh/g) | 736.6 | 704.9 | 700.7 |
| 1st charge (mAh/g) | 638.7 | 610.1 | 610.2 |
| 1st coulombic efficiency | 86.7% | 86.6% | 87.1% |
| 2nd discharge (mAh/g) | 695.6 | 664.2 | 669.3 |
| 2nd charge (mAh/g) | 655.6 | 613.3 | 616.8 |
| 2nd coulombic efficiency | 94.2% | 92.3% | 92.2% |
| 3rd discharge (mAh/g) | 663.3 | 620.7 | 623.7 |
| 3rd charge (mAh/g) | 642.5 | 585.7 | 589.7 |
| 3rd coulombic efficiency (mAh/g) | 96.8% | 94.4% | 94.5% |
| 3rd retention | 90.0% | 88.1% | 89.0% |
| Material | Capacity (mAh/g) | Cycles (retention%) | Ref. |
|---|---|---|---|
| MoS2/graphene | 225 | 200(90%) (@1000 mA/g) | [30] |
| MoS2 nanotubes | 260 | 30(98%) (@50 mA/g) | [31] |
| MoS2/PEO | 225 | 70(66%) (@50 mA/g) | [32] |
| MoS2 | 85 | 100(64%) (@50 mA/g) | [33] |
| MoS2–carbon fiber | 427 | 80(64.8%) (@134 mA/g) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-Y.; Li, X.-R.; Chen, B.-C.; Wang, L.-W.; Wang, J.-H.; Chu, S.-Y.; Chang, C.-C. Study of the Rolling Effect on MoS2–Carbon Fiber Density and Its Consequences for the Functionality of Li-Ion Batteries. Materials 2024, 17, 2825. https://doi.org/10.3390/ma17122825
Wu T-Y, Li X-R, Chen B-C, Wang L-W, Wang J-H, Chu S-Y, Chang C-C. Study of the Rolling Effect on MoS2–Carbon Fiber Density and Its Consequences for the Functionality of Li-Ion Batteries. Materials. 2024; 17(12):2825. https://doi.org/10.3390/ma17122825
Chicago/Turabian StyleWu, Tai-Yu, Xiao-Ru Li, Bo-Chun Chen, Li-Wen Wang, Jia-Hao Wang, Sheng-Yuan Chu, and Chia-Chin Chang. 2024. "Study of the Rolling Effect on MoS2–Carbon Fiber Density and Its Consequences for the Functionality of Li-Ion Batteries" Materials 17, no. 12: 2825. https://doi.org/10.3390/ma17122825





