Microwave-Induced Processing of Free-Standing 3D Printouts: An Effortless Route to High-Redox Kinetics in Electroanalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. 3D Printing Electrodes
3. Results and Discussion
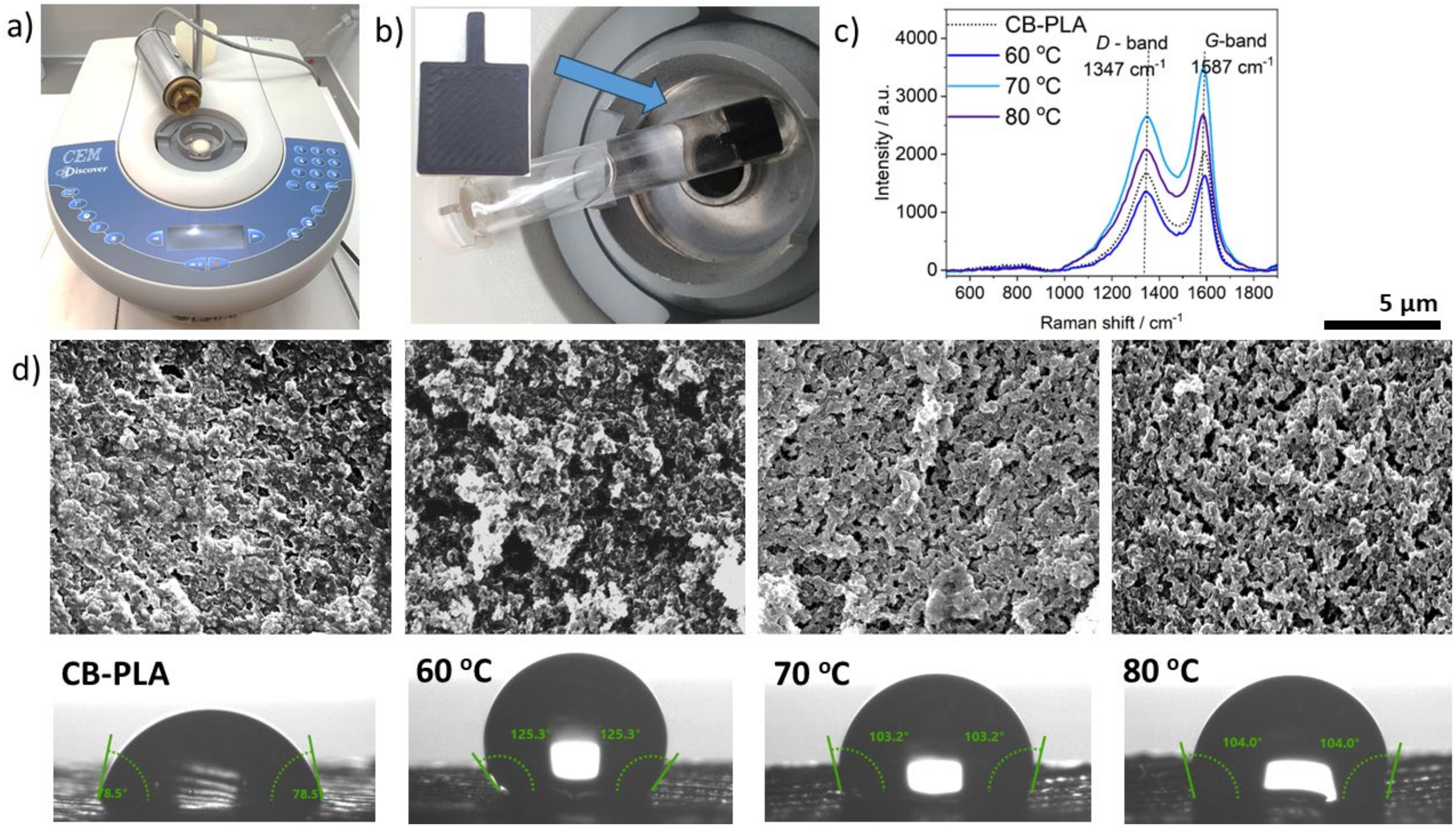
3.1. The Microwave-Induced Activation of the CB-PLA Electrode
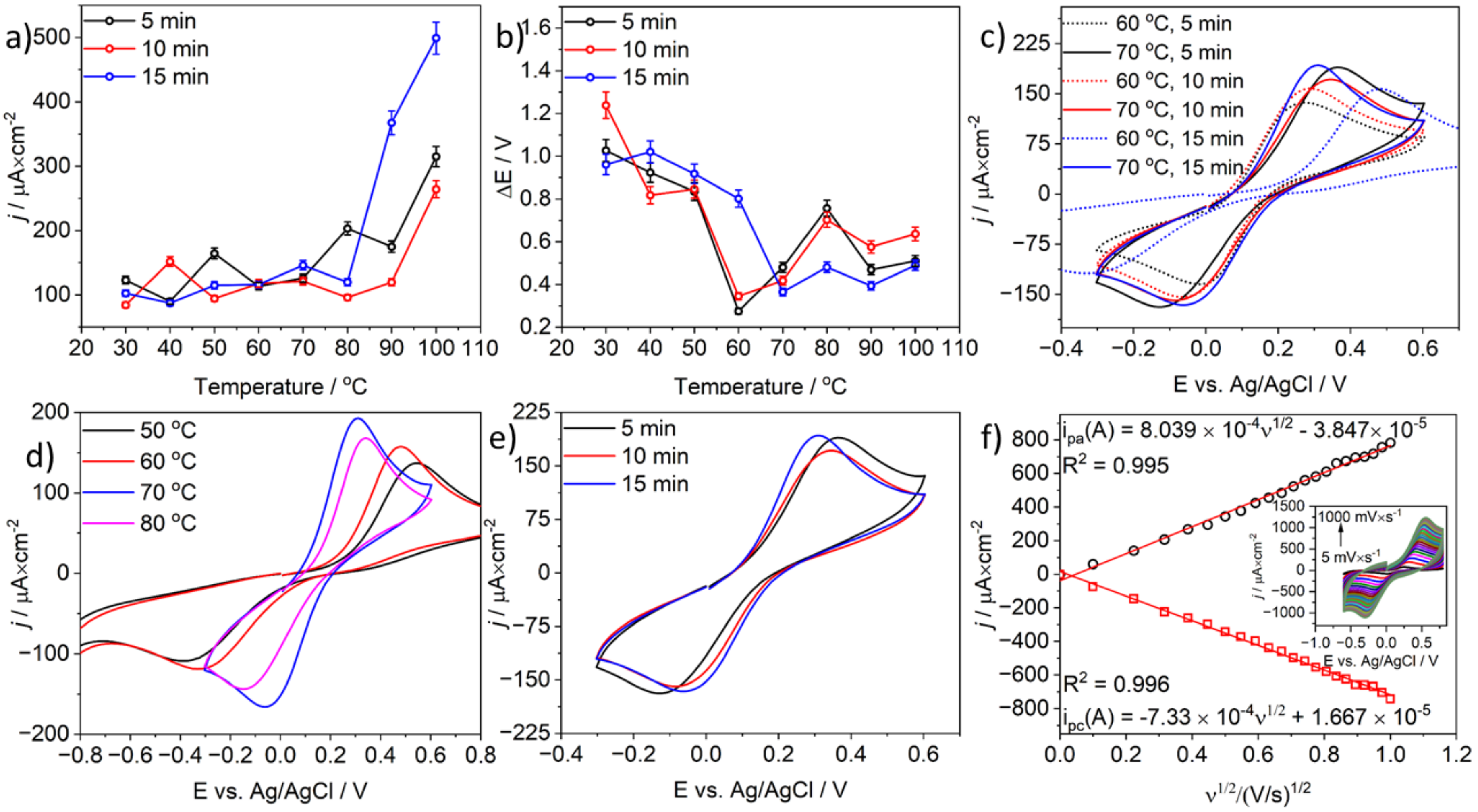
3.2. The Microwave Activation of Model CB-PLA Electrode—Electrochemical Investigations
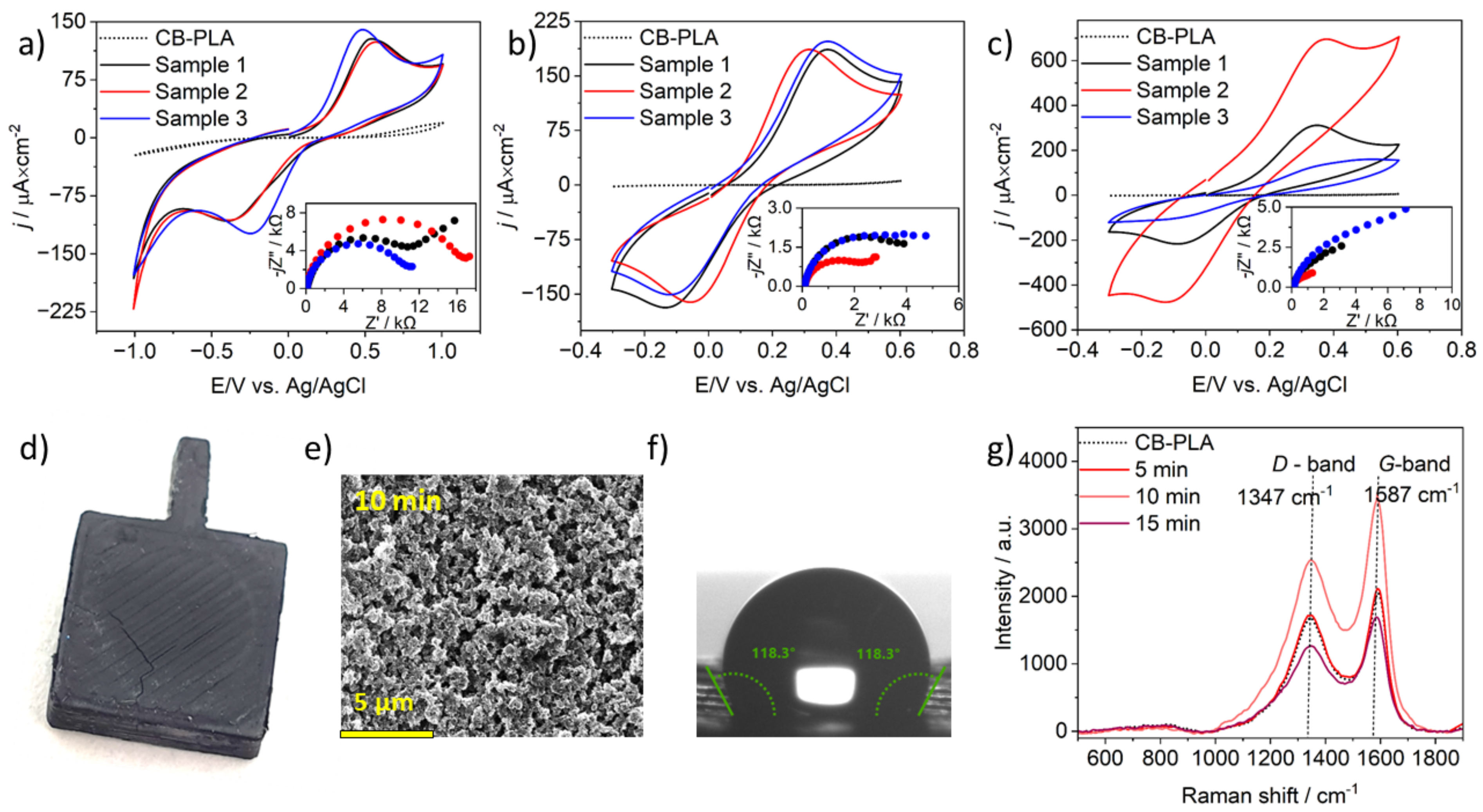
3.3. CB-PLA Electrodes MW Activation in a Kitchen Microwave Oven
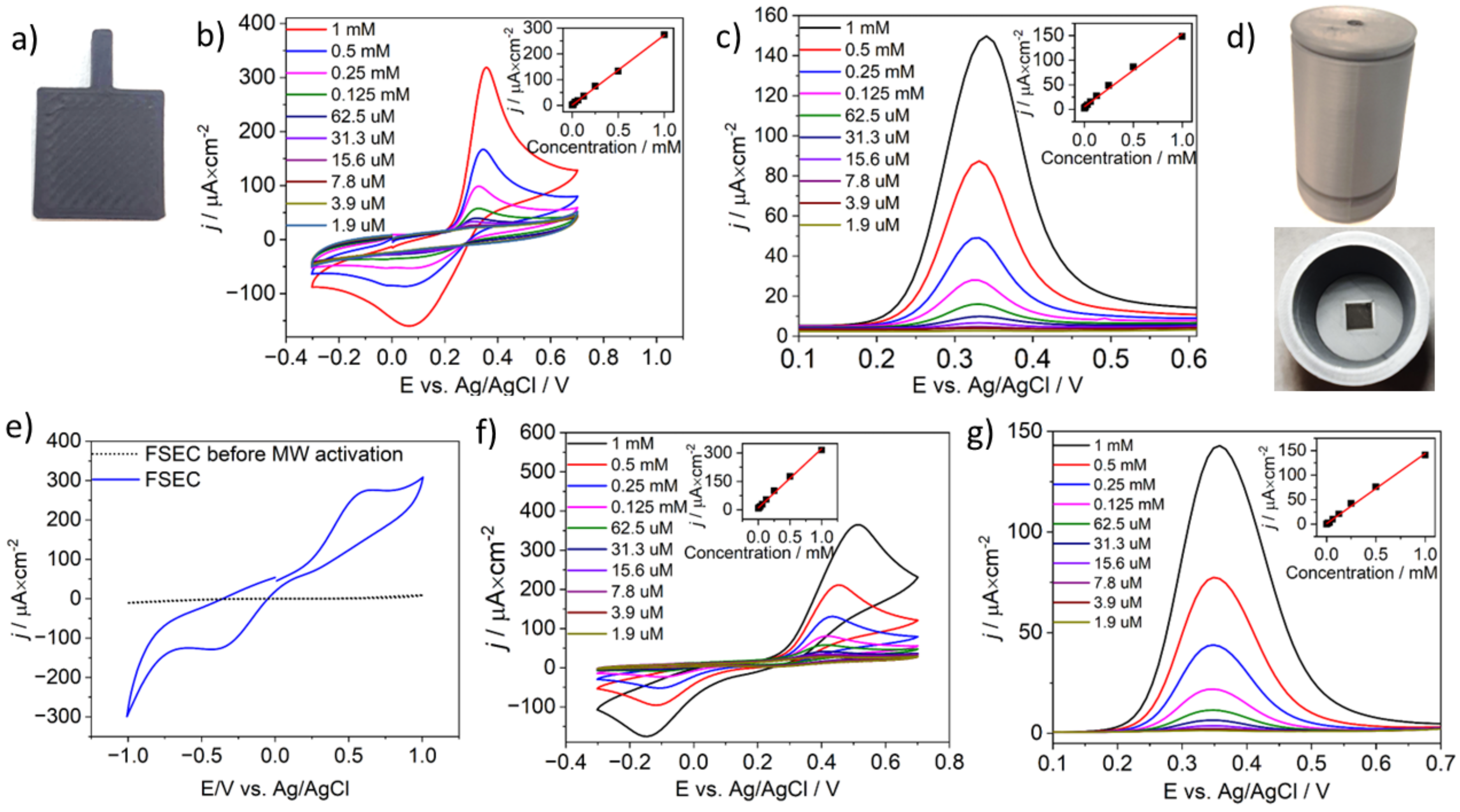
3.4. Paracetamol Electroanalysis with MW-Activated, CB-PLA Electrodes and Free-Standing Electrochemical Cells (FSEC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, A.A.; Harcen, C.S.; Haque, M. Graphene (GNP) Reinforced 3D Printing Nanocomposites: An Advanced Structural Perspective. Heliyon 2024, 10, e28771. [Google Scholar] [CrossRef] [PubMed]
- Agrawaal, H.; Thompson, J.E. Additive Manufacturing (3D Printing) for Analytical Chemistry. Talanta Open 2021, 3, 100036. [Google Scholar] [CrossRef]
- Aquino Monteiro, S.; Scheid, C.; Deon, M.; Merib, J. Fundamentals, Recent Applications, and Perspectives of 3D Printing in Sample Preparation Approaches. Microchem. J. 2023, 195, 109385. [Google Scholar] [CrossRef]
- Sultana, N.; Ali, A.; Waheed, A.; Aqil, M. 3D Printing in Pharmaceutical Manufacturing: Current Status and Future Prospects. Mater. Today Commun. 2024, 38, 107987. [Google Scholar] [CrossRef]
- Wang, L.; Pumera, M. Recent Advances of 3D Printing in Analytical Chemistry: Focus on Microfluidic, Separation, and Extraction Devices. TrAC Trends Anal. Chem. 2021, 135, 116151. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Tan, H.W.; Patel, D.C.; Choong, W.T.N.; Chen, C.-H.; Low, H.Y.; Tan, M.J.; Patel, C.D.; Chua, C.K. The Global Rise of 3D Printing during the COVID-19 Pandemic. Nat. Rev. Mater. 2020, 5, 637–639. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Jiang, C.; Liu, D.; Zhang, L.; Lang, G.; Mao, T.; Effrem, D.B.; Iimaa, T.; Surenjav, U.; et al. 3-Dimentional Printing of Polysaccharides for Water-Treatment: A Review. Int. J. Biol. Macromol. 2024, 265, 131117. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, W.; Liu, X.; Gu, Z. Recent Advances in 3D-Printing-Based Organ-on-a-Chip. EngMedicine 2024, 1, 100003. [Google Scholar] [CrossRef]
- Browne, M.P.; Redondo, E.; Pumera, M. 3D Printing for Electrochemical Energy Applications. Chem. Rev. 2020, 120, 2783–2810. [Google Scholar] [CrossRef] [PubMed]
- Žujović, M.; Obradović, R.; Rakonjac, I.; Milošević, J. 3D Printing Technologies in Architectural Design and Construction: A Systematic Literature Review. Buildings 2022, 12, 1319. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef] [PubMed]
- Swetham, T.; Reddy, K.M.M.; Huggi, A.; Kumar, M.N. A Critical Review on of 3D Printing Materials and Details of Materials Used in FDM. Int. J. Sci. Res. Sci. Eng. Technol. 2017, 3, 353–361. [Google Scholar]
- Cieślik, M.; Susik, A.; Banasiak, M.; Bogdanowicz, R.; Formela, K.; Ryl, J. Tailoring Diamondised Nanocarbon-Loaded Poly(Lactic Acid) Composites for Highly Electroactive Surfaces: Extrusion and Characterisation of Filaments for Improved 3D-Printed Surfaces. Microchim. Acta 2023, 190, 370. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Additive-Manufactured (3D-Printed) Electrochemical Sensors: A Critical Review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef]
- Glowacki, M.J.; Cieslik, M.; Sawczak, M.; Koterwa, A.; Kaczmarzyk, I.; Jendrzejewski, R.; Szynkiewicz, L.; Ossowski, T.; Bogdanowicz, R.; Niedzialkowski, P.; et al. Helium-Assisted, Solvent-Free Electro-Activation of 3D Printed Conductive Carbon-Polylactide Electrodes by Pulsed Laser Ablation. Appl. Surf. Sci. 2021, 556, 149788. [Google Scholar] [CrossRef]
- Pal, A.; Amreen, K.; Dubey, S.K.; Goel, S. Highly Sensitive and Interference-Free Electrochemical Nitrite Detection in a 3D Printed Miniaturized Device. IEEE Trans. NanoBioscience 2021, 20, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Katseli, V.; Thomaidis, N.; Economou, A.; Kokkinos, C. Miniature 3D-Printed Integrated Electrochemical Cell for Trace Voltammetric Hg(II) Determination. Sens. Actuators B Chem. 2020, 308, 127715. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Neumsteir, N.V.; Henriques, B.F.; de Oliveira Aparecido, G.; Loureiro, H.C.; Janegitz, B.C.; Bonacin, J.A. Influence of Filament Aging and Conductive Additive in 3D Printed Sensors. Anal. Chim. Acta 2022, 1191, 339228. [Google Scholar] [CrossRef] [PubMed]
- Koterwa, A.; Kaczmarzyk, I.; Mania, S.; Cieslik, M.; Tylingo, R.; Ossowski, T.; Bogdanowicz, R.; Niedziałkowski, P.; Ryl, J. The Role of Electrolysis and Enzymatic Hydrolysis Treatment in the Enhancement of the Electrochemical Properties of 3D-Printed Carbon Black/Poly(Lactic Acid) Structures. Appl. Surf. Sci. 2022, 574, 151587. [Google Scholar] [CrossRef]
- Browne, M.P.; Novotný, F.; Sofer, Z.; Pumera, M. 3D Printed Graphene Electrodes’ Electrochemical Activation. ACS Appl. Mater. Interfaces 2018, 10, 40294–40301. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.G.; Stefano, J.S.; Cardoso, R.M.; Zambiazi, P.J.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Electrochemical Synthesis of Prussian Blue from Iron Impurities in 3D-Printed Graphene Electrodes: Amperometric Sensing Platform for Hydrogen Peroxide. Talanta 2020, 219, 121289. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.G.; Cardoso, R.M.; Zambiazi, P.J.; Castro, S.V.F.; Ferraz, T.V.B.; Aparecido, G.d.O.; Bonacin, J.A.; Munoz, R.A.A.; Richter, E.M. Production of 3D-Printed Disposable Electrochemical Sensors for Glucose Detection Using a Conductive Filament Modified with Nickel Microparticles. Anal. Chim. Acta 2020, 1132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; Neumsteir, N.V.; de Oliveira Aparecido, G.; de Barros Ferraz, T.V.; dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of Activation Processes for 3D Printed PLA-Graphene Electrodes: Electrochemical Properties and Application for Sensing of Dopamine. Analyst 2020, 145, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.M.; Castro, S.V.F.; Silva, M.N.T.; Lima, A.P.; Santana, M.H.P.; Nossol, E.; Silva, R.A.B.; Richter, E.M.; Paixão, T.R.L.C.; Muñoz, R.A.A. 3D-Printed Flexible Device Combining Sampling and Detection of Explosives. Sens. Actuators B Chem. 2019, 292, 308–313. [Google Scholar] [CrossRef]
- Manzanares Palenzuela, C.L.; Novotný, F.; Krupička, P.; Sofer, Z.; Pumera, M. 3D-Printed Graphene/Polylactic Acid Electrodes Promise High Sensitivity in Electroanalysis. Anal. Chem. 2018, 90, 5753–5757. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Chen, W.; Chandrasekaran, S.; Yao, B.; Song, Y.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; et al. 3D Printed Functional Nanomaterials for Electrochemical Energy Storage. Nano Today 2017, 15, 107–120. [Google Scholar] [CrossRef]
- Wang, Y.; Su, S.; Cai, L.; Qiu, B.; Wang, N.; Xiong, J.; Yang, C.; Tao, X.; Chai, Y. Monolithic Integration of All-in-One Supercapacitor for 3D Electronics. Adv. Energy Mater. 2019, 9, 1900037. [Google Scholar] [CrossRef]
- Gusmão, R.; Browne, M.P.; Sofer, Z.; Pumera, M. The Capacitance and Electron Transfer of 3D-Printed Graphene Electrodes Are Dramatically Influenced by the Type of Solvent Used for Pre-Treatment. Electrochem. Commun. 2019, 102, 83–88. [Google Scholar] [CrossRef]
- Sato, S.; Gondo, D.; Wada, T.; Kanehashi, S.; Nagai, K. Effects of Various Liquid Organic Solvents on Solvent-Induced Crystallization of Amorphous Poly(Lactic Acid) Film. J. Appl. Polym. Sci. 2013, 129, 1607–1617. [Google Scholar] [CrossRef]
- Richter, E.M.; Rocha, D.P.; Cardoso, R.M.; Keefe, E.M.; Foster, C.W.; Munoz, R.A.A.; Banks, C.E. Complete Additively Manufactured (3D-Printed) Electrochemical Sensing Platform. Anal. Chem. 2019, 91, 12844–12851. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.E.; Perez-Gonzalez, V.H.; Mata-Gómez, M.A.; Aguilar, O. 3D-Printed Hybrid-Carbon-Based Electrodes for Electroanalytical Sensing Applications. Electrochem. Commun. 2021, 130, 107098. [Google Scholar] [CrossRef]
- Manzanares-Palenzuela, C.L.; Hermanova, S.; Sofer, Z.; Pumera, M. Proteinase-Sculptured 3D-Printed Graphene/Polylactic Acid Electrodes as Potential Biosensing Platforms: Towards Enzymatic Modeling of 3D-Printed Structures. Nanoscale 2019, 11, 12124–12131. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.P.; Rocha, R.G.; Castro, S.V.F.; Trindade, M.A.G.; Munoz, R.A.A.; Richter, E.M.; Angnes, L. Posttreatment of 3D-Printed Surfaces for Electrochemical Applications: A Critical Review on Proposed Protocols. Electrochem. Sci. Adv. 2022, 2, e2100136. [Google Scholar] [CrossRef]
- Novotný, F.; Urbanová, V.; Plutnar, J.; Pumera, M. Preserving Fine Structure Details and Dramatically Enhancing Electron Transfer Rates in Graphene 3D-Printed Electrodes via Thermal Annealing: Toward Nitroaromatic Explosives Sensing. ACS Appl. Mater. Interfaces 2019, 11, 35371–35375. [Google Scholar] [CrossRef] [PubMed]
- Redondo, E.; Muñoz, J.; Pumera, M. Green Activation Using Reducing Agents of Carbon-Based 3D Printed Electrodes: Turning Good Electrodes to Great. Carbon 2021, 175, 413–419. [Google Scholar] [CrossRef]
- Veloso, W.B.; Ataide, V.N.; Rocha, D.P.; Nogueira, H.P.; de Siervo, A.; Angnes, L.; Muñoz, R.A.A.; Paixão, T.R.L.C. 3D-Printed Sensor Decorated with Nanomaterials by CO2 Laser Ablation and Electrochemical Treatment for Non-Enzymatic Tyrosine Detection. Microchim. Acta 2023, 190, 63. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.P.; Ataide, V.N.; de Siervo, A.; Gonçalves, J.M.; Muñoz, R.A.A.; Paixão, T.R.L.C.; Angnes, L. Reagentless and Sub-Minute Laser-Scribing Treatment to Produce Enhanced Disposable Electrochemical Sensors via Additive Manufacture. Chem. Eng. J. 2021, 425, 130594. [Google Scholar] [CrossRef]
- Cieslik, M.; Sawczak, M.; Jendrzejewski, R.; Celej, J.; Nogala, W.; Ryl, J. Locally Sculptured Modification of the Electrochemical Response of Conductive Poly(Lactic Acid) 3D Prints by Femtosecond Laser Processing. Electrochim. Acta 2022, 416, 140288. [Google Scholar] [CrossRef]
- Pereira, J.F.S.; Rocha, R.G.; Castro, S.V.F.; João, A.F.; Borges, P.H.S.; Rocha, D.P.; de Siervo, A.; Richter, E.M.; Nossol, E.; Gelamo, R.V.; et al. Reactive Oxygen Plasma Treatment of 3D-Printed Carbon Electrodes towards High-Performance Electrochemical Sensors. Sens. Actuators B Chem. 2021, 347, 130651. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave Assisted Organic Synthesis—A Review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Marken, F.; Sur, U.K.; Coles, B.A.; Compton, R.G. Focused Microwaves in Electrochemical Processes. Electrochim. Acta 2006, 51, 2195–2203. [Google Scholar] [CrossRef]
- Sur, U.K.; Marken, F.; Seager, R.; Foord, J.S.; Chatterjee, A.; Coles, B.A.; Compton, R.G. Microwave Activation of Electrochemical Processes at Glassy Carbon and Boron-Doped Diamond Electrodes. Electroanalysis 2005, 17, 385–391. [Google Scholar] [CrossRef]
- Sur, U.K.; Marken, F.; Compton, R.G.; Coles, B.A. Microwave Effects on the Electrochemical Deposition of Copper. New J. Chem. 2004, 28, 1544–1549. [Google Scholar] [CrossRef]
- Shang, X.; Chi, J.-Q.; Wang, Z.-B.; Dong, B.; Zhao, J.-C.; Li, X.-H.; Yan, K.-L.; Wang, L.; Chai, Y.-M.; Liu, C.-G. Microwave Annealing Promoted In-Situ Electrochemical Activation of Ni3S2 Nanowires for Water Electrolysis. J. Catal. 2018, 368, 112–119. [Google Scholar] [CrossRef]
- Lee, E.J.; Bae, J.; Choi, K.M.; Jeong, N.C. Exploiting Microwave Chemistry for Activation of Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 35155–35161. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Coles, B.A.; Compton, R.G.; Marken, F. Microwave Activation of Electrochemical Processes: Enhanced Electrodehalogenation in Organic Solvent Media. J. Am. Chem. Soc. 2002, 124, 9784–9788. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.A.; Compton, R.G.; Coles, B.A.; Psillakis, E.; Kulandainathan, M.A.; Marken, F. Microwave Activation of Electrochemical Processes: High Temperature Phenol and Triclosan Electro-Oxidation at Carbon and Diamond Electrodes. Electrochim. Acta 2007, 53, 1092–1099. [Google Scholar] [CrossRef]
- Cieślik, M.; Rodak, A.; Susik, A.; Wójcik, N.; Szociński, M.; Ryl, J.; Formela, K. Multiple Reprocessing of Conductive PLA 3D-Printing Filament: Rheology, Morphology, Thermal and Electrochemical Properties Assessment. Materials 2023, 16, 1307. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.P.; Squissato, A.L.; da Silva, S.M.; Richter, E.M.; Munoz, R.A.A. Improved Electrochemical Detection of Metals in Biological Samples Using 3D-Printed Electrode: Chemical/Electrochemical Treatment Exposes Carbon-Black Conductive Sites. Electrochim. Acta 2020, 335, 135688. [Google Scholar] [CrossRef]
- Zappielo, C.D.; Nanicuacua, D.M.; dos Santos, W.N.L.; da Silva, D.L.F.; Dall’Antônia, L.H.; de Oliveira, F.M.; Clausen, D.N.; Tarley, C.R.T. Solid Phase Extraction to On-Line Preconcentrate Trace Cadmium Using Chemically Modified Nano-Carbon Black with 3-Mercaptopropyltrimethoxysilane. J. Braz. Chem. Soc. 2016, 27, 1715–1726. [Google Scholar] [CrossRef]
- Youssry, M.; Kamand, F.Z.; Magzoub, M.I.; Nasser, M.S. Aqueous Dispersions of Carbon Black and Its Hybrid with Carbon Nanofibers. RSC Adv. 2018, 8, 32119–32131. [Google Scholar] [CrossRef] [PubMed]
- Paydayesh, A.; Pashaei Soorbaghi, F.; Aref Azar, A.; Jalali-Arani, A. Electrical Conductivity of Graphene Filled PLA/PMMA Blends: Experimental Investigation and Modeling. Polym. Compos. 2019, 40, 704–715. [Google Scholar] [CrossRef]
- Guo, J.; Tsou, C.-H.; Yu, Y.; Wu, C.-S.; Zhang, X.; Chen, Z.; Yang, T.; Ge, F.; Liu, P.; Guzman, M.R.D. Conductivity and Mechanical Properties of Carbon Black-Reinforced Poly(Lactic Acid) (PLA/CB) Composites. Iran. Polym. J. 2021, 30, 1251–1262. [Google Scholar] [CrossRef]
- Kwaczyński, K.; Szymaniec, O.; Bobrowska, D.M.; Poltorak, L. Solvent-Activated 3D-Printed Electrodes and Their Electroanalytical Potential. Sci. Rep. 2023, 13, 22797. [Google Scholar] [CrossRef]
- Stefano, J.S.; Silva, L.R.G.e.; Janegitz, B.C. New Carbon Black-Based Conductive Filaments for the Additive Manufacture of Improved Electrochemical Sensors by Fused Deposition Modeling. Microchim. Acta 2022, 189, 414. [Google Scholar] [CrossRef] [PubMed]
- Ubuo, E.E.; Udoetok, I.A.; Tyowua, A.T.; Ekwere, I.O.; Al-Shehri, H.S. The Direct Cause of Amplified Wettability: Roughness or Surface Chemistry? J. Compos. Sci. 2021, 5, 213. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite. Minerals 2020, 10, 114. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Niedziałkowski, P.; Białobrzeska, W.; Burnat, D.; Sezemsky, P.; Stranak, V.; Wulff, H.; Ossowski, T.; Bogdanowicz, R.; Koba, M.; Śmietana, M. Electrochemical Performance of Indium-Tin-Oxide-Coated Lossy-Mode Resonance Optical Fiber Sensor. Sens. Actuators B Chem. 2019, 301, 127043. [Google Scholar] [CrossRef]
- Martins, G.; Gogola, J.L.; Budni, L.H.; Janegitz, B.C.; Marcolino-Junior, L.H.; Bergamini, M.F. 3D-Printed Electrode as a New Platform for Electrochemical Immunosensors for Virus Detection. Anal. Chim. Acta 2021, 1147, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Niedziałkowski, P.; Cebula, Z.; Malinowska, N.; Białobrzeska, W.; Sobaszek, M.; Ficek, M.; Bogdanowicz, R.; Anand, J.S.; Ossowski, T. Comparison of the Paracetamol Electrochemical Determination Using Boron-Doped Diamond Electrode and Boron-Doped Carbon Nanowalls. Biosens. Bioelectron. 2019, 126, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Joshi, H.; Ghosh, B.K.; Ahmed, A.; Gibson, T.; Millner, P.; Ghosh, N.N. Development of a Novel and Efficient H2O2 Sensor by Simple Modification of a Screen Printed Au Electrode with Ru Nanoparticle Loaded Functionalized Mesoporous SBA15. RSC Adv. 2015, 5, 34390–34397. [Google Scholar] [CrossRef]
- Katseli, V.; Economou, A.; Kokkinos, C. A Novel All-3D-Printed Cell-on-a-Chip Device as a Useful Electroanalytical Tool: Application to the Simultaneous Voltammetric Determination of Caffeine and Paracetamol. Talanta 2020, 208, 120388. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, T.P.; de Faria, L.V.; de Oliveira, W.B.V.; Oliveira, R.S.; de Souza, C.C.; Matos, M.A.C.; Dornellas, R.M.; Matos, R.C. Simultaneous Monitoring of Amoxicillin and Paracetamol in Synthetic Biological Fluids Using a 3D Printed Disposable Electrode with a Lab-Made Conductive Filament. Anal. Bioanal. Chem. 2024, 416, 215–226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, K.; Cieślik, M.; Koterwa, A.; Formela, K.; Ryl, J.; Niedziałkowski, P. Microwave-Induced Processing of Free-Standing 3D Printouts: An Effortless Route to High-Redox Kinetics in Electroanalysis. Materials 2024, 17, 2833. https://doi.org/10.3390/ma17122833
Kozłowska K, Cieślik M, Koterwa A, Formela K, Ryl J, Niedziałkowski P. Microwave-Induced Processing of Free-Standing 3D Printouts: An Effortless Route to High-Redox Kinetics in Electroanalysis. Materials. 2024; 17(12):2833. https://doi.org/10.3390/ma17122833
Chicago/Turabian StyleKozłowska, Kornelia, Mateusz Cieślik, Adrian Koterwa, Krzysztof Formela, Jacek Ryl, and Paweł Niedziałkowski. 2024. "Microwave-Induced Processing of Free-Standing 3D Printouts: An Effortless Route to High-Redox Kinetics in Electroanalysis" Materials 17, no. 12: 2833. https://doi.org/10.3390/ma17122833






