Electron-Deficient Multicenter Bonding in Phase Change Materials: A Chance for Reconciliation
Abstract
1. Introduction
2. Hypervalent vs. Metavalent Bonding Models in PCMs
3. A New Perspective: The Chance for Reconciliation
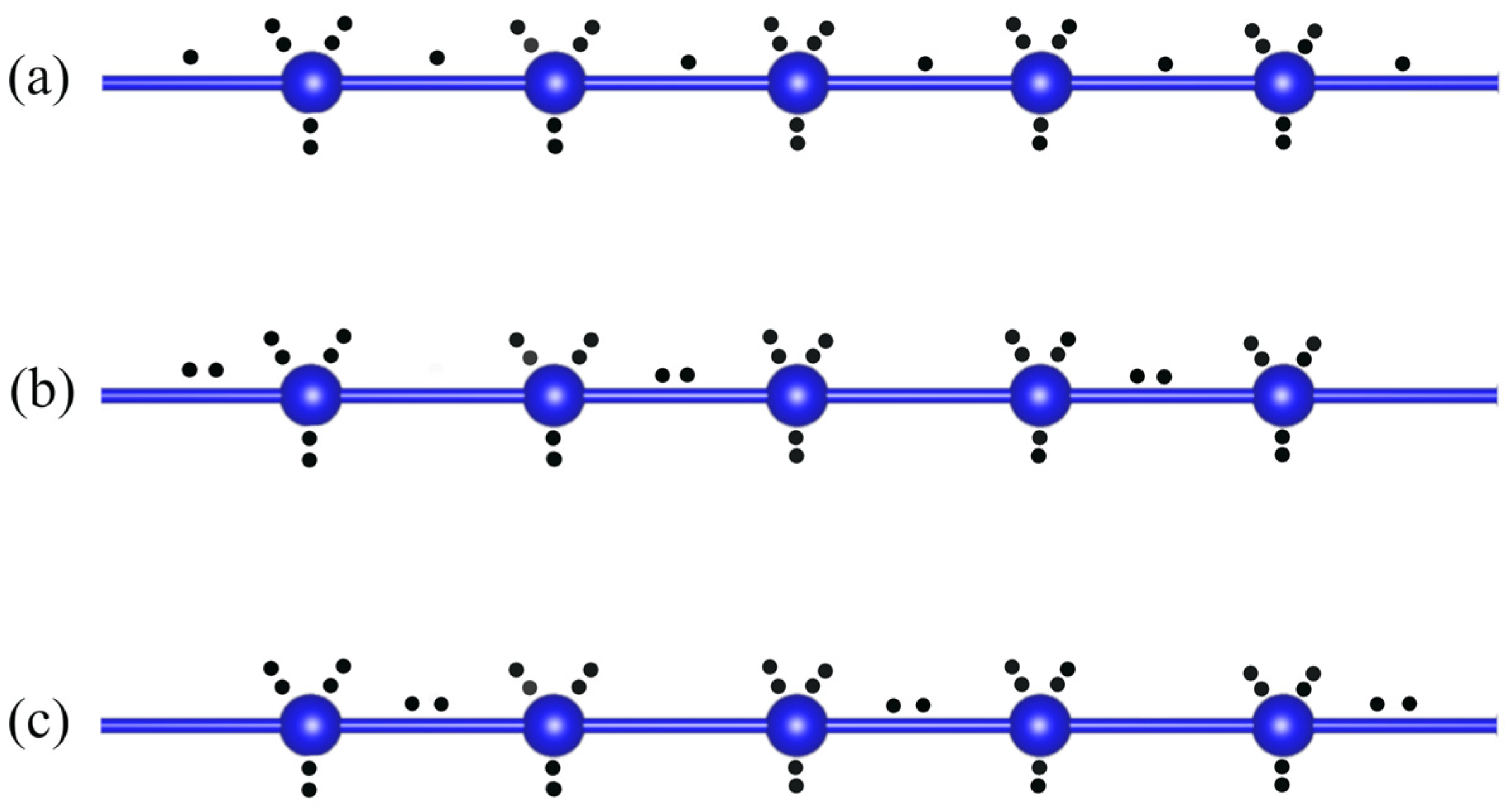
4. ERMBs and EDMBs in Polyiodides and Its Relation to PCMs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wuttig, M.; Deringer, V.L.; Gonze, X.; Bichara, C.; Raty, J.-Y. Incipient Metals: Functional Materials with a Unique Bonding Mechanism. Adv. Mater. 2018, 30, 1803777. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cojocaru-Mirédin, O.; Keutgen, J.; Yu, Y.; Küpers, M.; Schumacher, M.; Golub, P.; Raty, J.-Y.; Dronskowski, R.; Wuttig, M. Understanding the Structure and Properties of Sesqui-Chalcogenides (i.e., V2VI3 or Pn2Ch3 (Pn = Pnictogen, Ch = Chalcogen) Compounds) from a Bonding Perspective. Adv. Mater. 2019, 31, 1904316. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cagnoni, M.; Cojocaru-Mirédin, O.; Wuttig, M. Chalcogenide Thermoelectrics Empowered by an Unconventional Bonding Mechanism. Adv. Funct. Mater. 2020, 30, 1904862. [Google Scholar] [CrossRef]
- Wuttig, M.; Schön, C.-F.; Lötfering, J.; Golub, P.; Gatti, C.; Raty, J.-Y. Revisiting the Nature of Chemical Bonding in Chalcogenides to Explain and Design their Properties. Adv. Mater. 2023, 35, 2208485. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H. Der Einfluss homöopolarer Bindungsanteile auf die Struktur anorganischer Salze. II. Halbleiter und legierungsartige Phasen. Acta Cryst. 1956, 9, 95–108. [Google Scholar] [CrossRef]
- Krebs, H. Der Einfluss homöopolarer Bindungsanteile auf die Struktur anorganischer Salze. III. Z. Elektrochem. Ber. Bunsenges. Phys. 1957, 61, 925–934. [Google Scholar]
- Lucovsky, G.; White, R.M. Effects of resonance bonding on the properties of crystalline and amorphous semiconductors. Phys. Rev. B 1973, 8, 660–667. [Google Scholar] [CrossRef]
- Littlewood, P.B. Structure and bonding in narrow gap semiconductors. Crit. Rev. Solid State Mater. Sci. 1983, 11, 229–285. [Google Scholar] [CrossRef]
- Jones, R.O.; Elliott, S.R.; Dronskowski, R. The Myth of “Metavalency” in Phase-Change Materials. Adv. Mater. 2023, 35, 2300836. [Google Scholar] [CrossRef]
- Arora, R.; Waghmare, U.V.; Rao, C.N.R. Metavalent bonding origins of unusual properties of group IV chalcogenides. Adv. Mater. 2023, 35, 2208724. [Google Scholar] [CrossRef]
- Wuttig, M.; Schön, C.-F.; Kim, D.; Golub, P.; Gatti, C.; Raty, J.-Y.; Kooi, B.J.; Martín Pendás, Á.; Arora, R.; Waghmare, U. Metavalent or Hypervalent Bonding: Is There a Chance for Reconciliation? Adv. Sci. 2023, 35, 2308578. [Google Scholar] [CrossRef]
- Osman, H.H.; Otero-de-la-Roza, A.; Rodríguez-Hernandez, P.; Muñoz, A.; Manjón, F.J. Metavalent multicenter bonding in pnictogens and chalcogens: Nature and mechanism of formation. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Osman, H.H.; Otero-de-la-Roza, A.; Rodríguez-Hernandez, P.; Muñoz, A.; Manjón, F.J. Electron-deficient multicenter bonding in pnictogens and chalcogens: Mechanism of formation. J. Mater. Chem. C 2024. accepted manuscript. [Google Scholar] [CrossRef]
- Hempelmann, J.; Müller, P.C.; Reitz, L.; Dronskowski, R. Quantum Chemical Similarities of Bonding in Polyiodides and Phase-Change Materials. Inorg. Chem. 2023, 62, 20162–20171. [Google Scholar] [CrossRef] [PubMed]
- Raty, J.-Y.; Schumacher, M.; Golub, P.; Deringer, V.L.; Gatti, C.; Wuttig, M. A quantum-mechanical map for bonding and properties in solids. Adv. Mater. 2019, 31, 1806280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wahl, S.; Wuttig, M. Metavalent bonding in solids: Characteristic representatives, their properties, and design options. Phys. Status Solidi RRL 2021, 15, 2000482. [Google Scholar] [CrossRef]
- Guarneri, L.; Jakobs, S.; von Hoegen, A.; Maier, S.; Xu, M.; Zhu, M.; Wahl, S.; Teichrib, C.; Zhou, Y.; Cojocaru-Mirédin, O.; et al. Metavalent bonding in crystalline solids: How does it collapse? Adv. Mater. 2021, 33, 2102356. [Google Scholar] [CrossRef]
- Raty, J.-Y.; Gatti, C.; Schön, C.-F.; Wuttig, M. How to Identify Lone Pairs, Van der Waals Gaps, and Metavalent Bonding Using Charge and Pair Density Methods: From Elemental Chalcogens to Lead Chalcogenides and Phase-Change Materials. Phys. Status Solidi RRL 2021, 15, 2000534. [Google Scholar] [CrossRef]
- Wuttig, M.; Schön, C.-F.; Schumacher, M.; Robertson, J.; Golub, P.; Bousquet, E.; Gatti, C.; Raty, J.-Y. Halide perovskites: Advanced photovoltaic materials empowered by a unique bonding mechanism. Adv. Funct. Mater. 2022, 32, 2110166. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Fons, P.; Tominaga, J.; Ovshinsky, S.R. Vacancy-mediated three-center four-electron bonds in GeTe-SbTe phase-change memory alloys. Phys. Rev. B 2013, 87, 165206. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Fons, P.; Tominaga, J. Understanding phase-change memory alloys from a chemical perspective. Sci. Rep. 2015, 5, 13698. [Google Scholar] [CrossRef]
- Hempelmann, J.; Müller, P.C.; Konze, P.M.; Stoffel, R.P.; Steinberg, S.; Dronskowski, R. Long-Range Forces in Rock-Salt-Type Tellurides and How they Mirror the Underlying Chemical Bonding. Adv. Mater. 2021, 33, 2100163. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.C.; Ertural, C.; Hempelmann, J.; Dronskowski, R. Crystal orbital bond index: Covalent bond orders in solids. J. Phys. Chem. C 2021, 125, 7959–7970. [Google Scholar] [CrossRef]
- Hempelmann, J.; Müller, P.C.; Ertural, C.; Dronskowski, R. The Orbital Origins of Chemical Bonding in Ge−Sb−Te Phase-Change Materials. Angew. Chem. Int. Ed. 2022, 61, e202115778. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Elliott, S.R. Chemical bonding in chalcogenides: The concept of multicenter hyperbonding. Adv. Mater. 2020, 32, 2000340. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Elliott, S.R. Multi-Center Hyperbonding in Phase-Change Materials. Phys. Status Solidi RRL 2021, 15, 2000516. [Google Scholar] [CrossRef]
- Lee, T.H.; Elliott, S.R. Hypervalency in amorphous chalcogenides. Nat. Commun. 2022, 13, 1458. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.O. The chemical bond in solids—Revisited. J. Phys. Condens. Matter 2022, 34, 343001. [Google Scholar] [CrossRef] [PubMed]
- Sidgwick, N.V. The Electronic Theory of Valency; Oxford University Press: Oxford, UK, 1929. [Google Scholar]
- Hund, F. Zur deutung der molekelspektren. iv. Z. Phys. 1928, 51, 759–795. [Google Scholar] [CrossRef]
- Bloch, F. Über die quantenmechanik der elektronen in kristallgittern. Z. Phys. 1929, 52, 555–600. [Google Scholar] [CrossRef]
- Hückel, E. Quantentheoretische Beiträge zum Benzolproblem. I. Die Elektronenkonfiguration des Benzols und werwandter Verbindungen. Z. Phys. 1931, 70, 204–286. [Google Scholar] [CrossRef]
- Hach, R.J.; Rundle, R.E. The Structure of Tetramethylammonium Pentaiodide1,1a. J. Am. Chem. Soc. 1951, 73, 4321–4324. [Google Scholar] [CrossRef]
- Pimentel, G.C. The bonding of trihalide and bifluoride ions by the molecular orbital method. J. Chem. Phys. 1951, 19, 446–448. [Google Scholar] [CrossRef]
- Papoian, G.A.; Hoffmann, R. Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl–Klemm Concept to Nonclassical Electron-Rich Networks. Angew. Chem. Int. Ed. 2000, 39, 2408–2448. [Google Scholar] [CrossRef]
- Rundle, R.E. Electron Deficient Compounds1. J. Am. Chem. Soc. 1947, 69, 1327–1331. [Google Scholar] [CrossRef]
- Rundle, R.E. Electron Deficient Compounds. II. Relative Energies of “Half-Bonds”. J. Chem. Phys. 1949, 17, 671–675. [Google Scholar] [CrossRef]
- Rundle, R.E. Electron deficient compounds. J. Phys. Chem. 1957, 61, 45–50. [Google Scholar] [CrossRef]
- Longuet-Higgins, H.C. The structures of electron-deficient molecules. Q. Rev. Chem. Soc. 1957, 11, 121–133. [Google Scholar] [CrossRef]
- Wade, K. Electron Deficient Compounds (Studies in Modern Chemistry); Waddington, T.C., Ed.; Nelson: London, UK, 1971. [Google Scholar]
- Available online: https://www.nobelprize.org/uploads/2018/06/lipscomb-lecture.pdf (accessed on 5 June 2024).
- Hoffmann, R. (Cornell University, Ithaca, NY, USA). Private communication.
- Landrum, G.A.; Goldberg, N.; Hoffmann, R. Bonding in the trihalides (X3−, mixed trihalides (X2Y−) and hydrogen bihalides (X2H−). The connection between hypervalent, electron-rich three-center, donor-acceptor and strong hydrogen bonding. J. Chem. Soc. Dalton Trans. 1997, 19, 3605–3613. [Google Scholar] [CrossRef]
- Grabowski, S.J. [FHF]−-The Strongest Hydrogen Bond under the Influence of External Interactions. Crystals 2016, 6, 3. [Google Scholar] [CrossRef]
- Crabtree, R.H. Hypervalency, secondary bonding and hydrogen bonding: Siblings under the skin. Chem. Soc. Rev. 2017, 46, 1720–1729. [Google Scholar] [CrossRef]
- Vegas, A.; Notario, R.; Chamorro, E.; Pérez, P.; Liebman, J.F. Isoelectronic and isolobal O, CH2, CH3+, and BH3 as electron pairs; similarities between molecular and solid-state chemistry. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2013, 69, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Vegas, A. Structural Models of Inorganic Crystals: From the Elements to the Compounds, 1st ed.; Universitat Politècnica de València: Valencia, Spain, 2018; p. 15. [Google Scholar]
- Munzarová, M.L.; Hoffmann, R. Electron-rich three-center bonding: Role of s, p interactions across the p-block. J. Am. Chem. Soc. 2002, 124, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Wuttig, M. (RWTH Aachen University, Aachen, Germany). Private communication.
- Müller, P.C.; Elliott, S.R.; Dronskowski, R.; Jones, R.O. Chemical bonding in phase-change chalcogenides. J. Phys. Condens. Matter 2024, 36, 325706. [Google Scholar] [CrossRef]
- Gaspard, J.-P. Phonons of Phase-Change Materials. Phys. Status Solidi RRL 2022, 16, 2200111. [Google Scholar] [CrossRef]
- Cuenca-Gotor, V.P.; Sans, J.Á.; Gomis, O.; Mujica, A.; Radescu, S.; Muñoz, A.; Rodríguez-Hernández, P.; Lora da Silva, E.; Popescu, C.; Ibañez, J.; et al. Orpiment under compression: Metavalent bonding at high pressure. Phys. Chem. Chem. Phys. 2020, 22, 3352–3369. [Google Scholar] [CrossRef] [PubMed]
- Sans, J.A.; Vilaplana, R.; Lora da Silva, E.; Popescu, C.; Cuenca-Gotor, V.P.; Andrada-Chacón, A.; Sánchez-Benitez, J.; Gomis, O.; Pereira, A.L.J.; Rodríguez-Hernández, P.; et al. Characterization and Decomposition of the Natural van der Waals SnSb2Te4 under Compression. Inorg. Chem. 2020, 59, 9900–9918. [Google Scholar] [CrossRef]
- Liang, S.A.; Rahman, P.; Rodríguez-Hernández, A.; Muñoz, F.J.; Manjón, G. Nenert, and D.; Errandonea, High-pressure Raman study of Fe(IO3)3: Soft-mode behavior driven by coordination changes of iodine atoms. J. Phys. Chem. C 2020, 124, 21329. [Google Scholar] [CrossRef]
- Liang, A.; Popescu, C.; Manjón, F.J.; Rodriguez-Hernandez, P.; Muñoz, A.; Hebboul, Z.; Errandonea, D. Structural and vibrational study of Zn(IO3)2 combining high-pressure experiments and density-functional theory. Phys. Rev. B 2021, 103, 054102. [Google Scholar] [CrossRef]
- Lora da Silva, E.; Leonardo, A.; Yang, T.; Santos, M.C.; Vilaplana, R.; Gallego-Parra, S.; Bergara, A.; Manjón, F.J. β-As2Te3: Pressure-induced three-dimensional Dirac semimetal with ultralow room-pressure lattice thermal conductivity. Phys. Rev. B 2021, 104, 024103. [Google Scholar] [CrossRef]
- Osman, H.H.; Manjón, F.J. Metavalent Bonding in Chalcogenides: DFT-Chemical Pressure Approach. Phys. Chem. Chem. Phys. 2022, 24, 9936. [Google Scholar] [CrossRef]
- Vilaplana, R.; Gallego-Parra, S.; Lora da Silva, E.; Martínez-García, D.; Delaizir, G.; Muñoz, A.; Rodríguez-Hernández, P.; Cuenca-Gotor, V.P.; Sans, J.A.; Popescu, C.; et al. Experimental and theoretical study of β-As2Te3 under hydrostatic pressure. J. Mater. Chem. C 2023, 11, 1037. [Google Scholar] [CrossRef]
- Errandonea, D.; Osman, H.H.H.; Turnbull, R.; Diaz-Anichtchenko, D.; Liang, A.; Sanchez-Martin, J.; Popescu, C.; Jiang, D.; Song, H.; Wang, Y.; et al. Pressure-induced hypercoordination of iodine and dimerization of I2O6H in strontium di-iodate hydrogen-iodate (Sr(IO3)2HIO3). Mat. Today Adv. 2024, 22, 100495. [Google Scholar] [CrossRef]
- Loa, I.; Husband, R.J.; Downie, R.A.; Popuri, S.R.; Bos, J.-W.G. Structural changes in thermoelectric SnSe at high pressures. J. Phys. Condens. Matter 2015, 27, 072202. [Google Scholar] [CrossRef]
- Xu, M.; Jakobs, S.; Mazzarello, R.; Cho, J.-Y.; Yang, Z.; Hollermann, H.; Shang, D.; Miao, X.; Yu, Z.; Wang, L.; et al. Impact of pressure on the resonant bonding in chalcogenides. J. Phys. Chem. C 2017, 121, 25447–25454. [Google Scholar] [CrossRef]
- Grochala, W.; Hoffmann, R.; Feng, J.; Ashcroft, N.W. The Chemical Imagination at Work in very Tight Places. Angew. Chem. Int. Ed. 2007, 46, 3620–3642. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.C.; Ho, V.; Lubchenko, V. The chemical bond as an emergent phenomenon. J. Chem. Phys. 2017, 146, 174502. [Google Scholar] [CrossRef] [PubMed]
- Mori-Sánchez, P.; Pendás, A.M.; Luaña, V. A classification of covalent, ionic, and metallic solids based on the electron density. J. Am. Chem. Soc. 2002, 124, 14721–14723. [Google Scholar] [CrossRef]
- Wuttig, M. Phase change materials: Chalcogenides with remarkable properties due to an unconventional bonding mechanism. Phys. Status Solidi B 2012, 249, 1843–1850. [Google Scholar] [CrossRef]
- Dong, X.; Oganov, A.R.; Cui, H.; Wang, H.-T. Electronegativity and chemical hardness of elements under pressure. Proc. Natl. Acad. Sci. USA 2022, 119, e2117416119. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–HF–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Schleyer, P.R. Hypervalent Compounds. Chem. Eng. News 1984, 62, 4. [Google Scholar]
- Suidan, L.; Badenhoop, J.K.; Glendening, E.D.; Weinhold, F. Common Textbook and Teaching Misrepresentations of Lewis Structures. J. Chem. Educ. 1995, 72, 583. [Google Scholar] [CrossRef]
- Curnow, O.J. A Simple Qualitative Molecular-Orbital/Valence-Bond Description of the Bonding in Main Group “Hypervalent” Molecules. J. Chem. Educ. 1998, 75, 910. [Google Scholar] [CrossRef]
- Gillespie, R.J.; Popelier, P.L.A. Chemical Bonding and Molecular Geometry: From Lewis to Electron Densities; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Noury, S.; Silvi, B.; Gillespie, R.J. Chemical Bonding in Hypervalent Molecules: Is the Octet Rule Relevant? Inorg. Chem. 2002, 41, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Jensen, W.B. The Origin of the Term “Hypervalent”. J. Chem. Educ. 2006, 83, 1751. [Google Scholar] [CrossRef]
- Galbraith, J.M. On the role of d orbital hybridization in the chemistry curriculum. J. Chem. Educ. 2007, 84, 783. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen bonds, and σ-hole and σ-hole bonds–mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.A.; Harshman, J.; Miliordos, E. Addressing the hypervalent model: A straightforward explanation of traditionally hypervalent molecules. J. Chem. Educ. 2020, 97, 3638–3646. [Google Scholar] [CrossRef]
- Grabowski, S.J. Classification of so-called non-covalent interactions based on VSEPR model. Molecules 2021, 26, 4939. [Google Scholar] [CrossRef]
- Norman, N.C.; Pringle, P.G. Hypervalence: A useful concept or one that should be gracefully retired? Chemistry 2022, 4, 1226–1249. [Google Scholar] [CrossRef]
- Sæthre, L.J.; Gropen, O.; Sletten, J. Structure and Bonding in Linear Polyiodine Compounds. A Theoretical Investigation. Acta Chem. Scandinavica. Ser. A Phys. Inorg. Chem. 1988, 42, 16–26. [Google Scholar] [CrossRef]
- Available online: https://www.basissetexchange.org (accessed on 5 June 2024).
- Stromberg, A.; Gropen, O.; Wahlgren, U. Gaussian Basis Sets for the Fourth-Row Main Group Elements, In-Xe. J. Comput. Chem. 1983, 4, 181. [Google Scholar] [CrossRef]
- Svenson, H.; Kloo, L. Synthesis, structure, and bonding in polyiodide and metal iodide− iodine systems. Chem. Revs. 2003, 103, 1649–1684. [Google Scholar] [CrossRef] [PubMed]
- Takahama, T.; Saharin, S.M.; Tashiro, K. Details of the intermolecular interactions in poly(vinyl alcohol)-iodine complexes as studied by quantum chemical calculations. Polymer 2016, 99, 566–579. [Google Scholar] [CrossRef]
- Groessl, M.; Fei, Z.F.; Dyson, P.J.; Katsyuba, S.A.; Vikse, K.L.; McIndoe, J. S, Mass Spectrometric and Theoretical Study of Polyiodides: The Connection between Solid State, Solution, and Gas Phases. Inorg. Chem. 2011, 50, 9728–9733. [Google Scholar] [CrossRef] [PubMed]
- Thanthiriwatte, K.S.; Spruell, J.M.; Dixon, D.A.; Christe, K.O.; Jenkins, H.D.B. Structures, Vibrational Frequencies, and Stabilities of Halogen Cluster Anions and Cations, Xn+/−, n = 3, 4, and 5. Inorg. Chem. 2014, 53, 8136–8146. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, F.; Esterhuysen, C.; Dillen, J. Extensive theoretical investigation: Influence of the electrostatic environment on the I3−···I3− anion–anion interaction. Theor. Chem. Acc. 2012, 131, 1281. [Google Scholar] [CrossRef]
- Otsuka, M.; Mori, H.; Kikuchi, H.; Takano, K. Density functional theory calculations of iodine cluster anions: Structures, chemical bonding nature, and vibrational spectra. Comp. Theor. Chem. 2011, 973, 69–75. [Google Scholar] [CrossRef]
- Sharp, S.B.; Gellene, G.I. Ab initio Calculations of the Ground Electronic States of Polyiodide Anions. J. Phys. Chem. A 1997, 101, 2192–2197. [Google Scholar] [CrossRef]
- Savastano, M. Words in supramolecular chemistry: The ineffable advances of polyiodide chemistry. Dalton Trans. 2021, 50, 1142–1165. [Google Scholar] [CrossRef]
- Datta, S.N.; Ewig, C.S.; Van Wazer, J.R. The geometric and electronic structures of I3− and I5− from effective-potential calculations. J. Mol. Struct. 1978, 48, 407–416. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Bent, H.A. Structural chemistry of donor-acceptor interactions. Chem. Rev. 1968, 68, 587–648. [Google Scholar] [CrossRef]
- Savastano, M. Ye Olde supramolecular chemistry, its modern rebranding and overarching trends in chemistry. Dalton Trans. 2024, 53, 1373–1392. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Aerogen Bond, Halogen Bond, Chalcogen Bond, Pnictogen Bond, Tetrel Bond, Triel Bond ... Why So Many Names? Cryst. Growth Des. 2024, 24, 4003–4012. [Google Scholar] [CrossRef]
- Zhang, W.W.; Oganov, A.R.; Goncharov, A.F.; Zhu, Q.; Boulfelfel, S.E.; Lyakhov, A.O.; Stavrou, E.; Somayazulu, M.; Prakapenka, V.B.; Konôpková, Z. Unexpected stable stoichiometries of sodium chlorides. Science 2013, 342, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.B.; Wang, J.Y.; Deng, S.Y.; Zhang, S.T.; Li, Q. Hypervalent iodine with linear chain at high pressure. Sci. Rep. 2015, 5, 14393. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Sunder, M.; Garg, A.B.; Poswal, H.K. Pressure-induced polymorphism in hypervalent CsI3. Phys. Rev. B 2017, 96, 174114. [Google Scholar] [CrossRef]
- Yin, Y.Q.; Aslandukova, A.; Jena, N.; Trybel, F.; Abrikosov, I.A.; Winkler, B.; Khandarkhaeva, S.; Fedotenko, T.; Bykova, E.; Laniel, D.; et al. Unraveling the Bonding Complexity of Polyhalogen Anions: High-Pressure Synthesis of Unpredicted Sodium Chlorides Na2Cl3 and Na4Cl5 and Bromide Na4Br5. J. Am. Chem. Soc. Au 2023, 3, 1634–1641. [Google Scholar] [CrossRef]
- Madhu, S.; Evans, H.A.; Doan-Nguyen, V.V.T.; Labram, J.G.; Wu, G.; Chabinyc, M.L.; Seshadri, R.; Wudl, F. Infinite Polyiodide Chains in the Pyrroloperylene–Iodine Complex: Insights into the Starch–Iodine and Perylene–Iodine Complexes. Angew. Chem. Int. Ed. 2016, 55, 8032–8035. [Google Scholar] [CrossRef]
- Poręba, T.; Świątkowski, M.; Kruszyński, R. Molecular self-assembly of 1D infinite polyiodide helices in a phenanthrolinium salt. Dalton Trans. 2021, 50, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Stoewe, K. The phase transition of TlTe: Crystal structure. J. Solid State Chem. 2000, 149, 123–132. [Google Scholar] [CrossRef]
- Gérardin, R.; Aubry, J. Préparation et identification d’un nouveau composé binaire Li2Sb. C.R. Seances Acad. Sci. Ser. C Sci. Chim. 1974, 278, 1097–1098. [Google Scholar]
- Burdett, J.K.; Lee, S. Peierls distortions in two and three dimensions and the structures of AB solids. J. Am. Chem. Soc. 1983, 105, 1079–1083. [Google Scholar] [CrossRef]
- Kertesz, M.; Vonderviszt, F. Electronic structure of long polyiodide chains. J. Am. Chem. Soc. 1982, 104, 5889–5893. [Google Scholar] [CrossRef]
- Komsa, H.-P.; Senga, R.; Suenaga, K.; Krasheninnikov, A.V. Structural Distortions and Charge Density Waves in Iodine Chains Encapsulated inside Carbon Nanotubes. Nano Lett. 2017, 17, 3694–3700. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.J. Fifty years of the VSEPR model. Coord. Chem. Rev. 2008, 252, 1315–1327. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry; Cornell University Press: Ithaca, NY, USA, 1960; Chapter 10. [Google Scholar]
- Liu, Z.D.; Yao, Z.; Yao, M.G.; Lv, J.Y.; Chen, S.L.; Li, Q.J.; Lv, H.; Wang, T.Y.; Lu, S.C.; Liu, R.; et al. High-pressure behavior of bromine confined in the one-dimensional channels of zeolite AlPO4-5 single crystals. J. Chem. Phys. 2016, 145, 124319. [Google Scholar] [CrossRef]
- Yao, M.G.; Wang, T.Y.; Yao, Z.; Duan, D.F.; Chen, S.L.; Liu, Z.D.; Liu, R.; Lu, S.C.; Yuan, Y.; Zou, B.; et al. Pressure-Driven Topological Transformations of Iodine Confined in One-Dimensional Channels. J. Chem. Phys. C 2013, 117, 25052–25058. [Google Scholar] [CrossRef]
- Tonkikh, A.A.; Rybkovskiy, D.V.; Obraztsova, E.D. Charge-Induced Structure Variations of 1D-Iodine Inside Thin SWCNTs. J. Phys. Chem. C 2023, 127, 3005–3012. [Google Scholar] [CrossRef]
- Sengupta, A.; Quitevis, E.L.; Holtz, M.W. Effect of High Pressure on Vibrational Modes of Polyiodides in Poly(vinyl alcohol) Films. J. Phys. Chem. B 1997, 101, 11092–11098. [Google Scholar] [CrossRef]
- Eberhardt, W.H.; Crawford, B., Jr.; Lipscomb, W.N. The valence structure of the boron hydrides. J. Chem. Phys. 1954, 22, 989–1001. [Google Scholar] [CrossRef]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Merlo, F.; Fornasini, M.L. CrB-type equiatomic compounds of europium, ytterbium and alkaline-earth metals with Si, Ge, Sn, Pb. J. Less-Common Met. 1967, 13, 603–610. [Google Scholar] [CrossRef]
- Brechtel, E.; Cordier, G.; Schaefer, H. Neue ternäre erdalkali-übergangselement-pnictide. J. Less-Common Met. 1981, 79, 131–138. [Google Scholar] [CrossRef]
- Noel, H. Crystal structure of the low-dimensional uranium pentatelluride: UTe5. Inorg. Chim. Acta 1985, 109, 205–207. [Google Scholar] [CrossRef]
- Bragg, W.L. Beyond Reductionism, New Perspectives in the Life Sciences: Proceedings of the Alpbach Symposium 1968; Koestler, A., Smythies, J.R., Eds.; Hutchison: London, UK, 1969. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manjón, F.J.; Osman, H.H.; Savastano, M.; Vegas, Á. Electron-Deficient Multicenter Bonding in Phase Change Materials: A Chance for Reconciliation. Materials 2024, 17, 2840. https://doi.org/10.3390/ma17122840
Manjón FJ, Osman HH, Savastano M, Vegas Á. Electron-Deficient Multicenter Bonding in Phase Change Materials: A Chance for Reconciliation. Materials. 2024; 17(12):2840. https://doi.org/10.3390/ma17122840
Chicago/Turabian StyleManjón, Francisco Javier, Hussien H. Osman, Matteo Savastano, and Ángel Vegas. 2024. "Electron-Deficient Multicenter Bonding in Phase Change Materials: A Chance for Reconciliation" Materials 17, no. 12: 2840. https://doi.org/10.3390/ma17122840
APA StyleManjón, F. J., Osman, H. H., Savastano, M., & Vegas, Á. (2024). Electron-Deficient Multicenter Bonding in Phase Change Materials: A Chance for Reconciliation. Materials, 17(12), 2840. https://doi.org/10.3390/ma17122840




