Comparative Effect of Antioxidant and Antibacterial Potential of Zinc Oxide Nanoparticles from Aqueous Extract of Nepeta nepetella through Different Precursor Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Extracts
Total Polyphenol Content (TPC)
2.3. Green Synthesis of Zinc Oxide Nanoparticles
2.4. Characterization of Zinc Oxide Nanoparticles
2.5. Antioxidant Activity
2.6. Antibacterial Activity of Zinc Oxide Nanoparticles
2.7. Statistical Analysis
3. Results and Discussion
3.1. Total Polyphenols Content
3.2. Characterization of the Zinc Oxide Nanoparticles
3.2.1. XRD
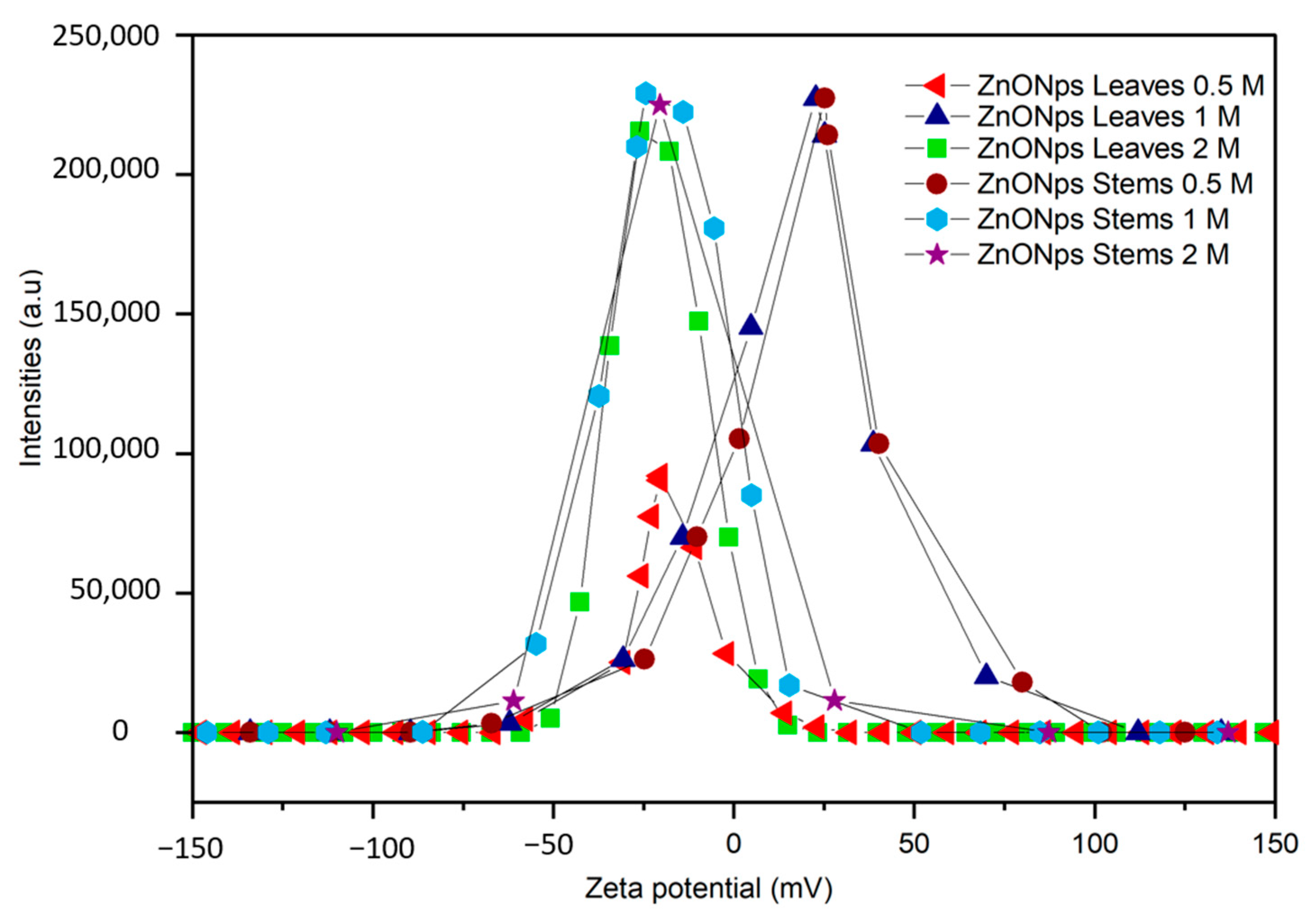
3.2.2. Zeta Potential
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.4. Scanning Electron Microscopy (SEM)
3.2.5. Transmission Electron Microscopy (TEM)
3.3. Antioxidant Activity
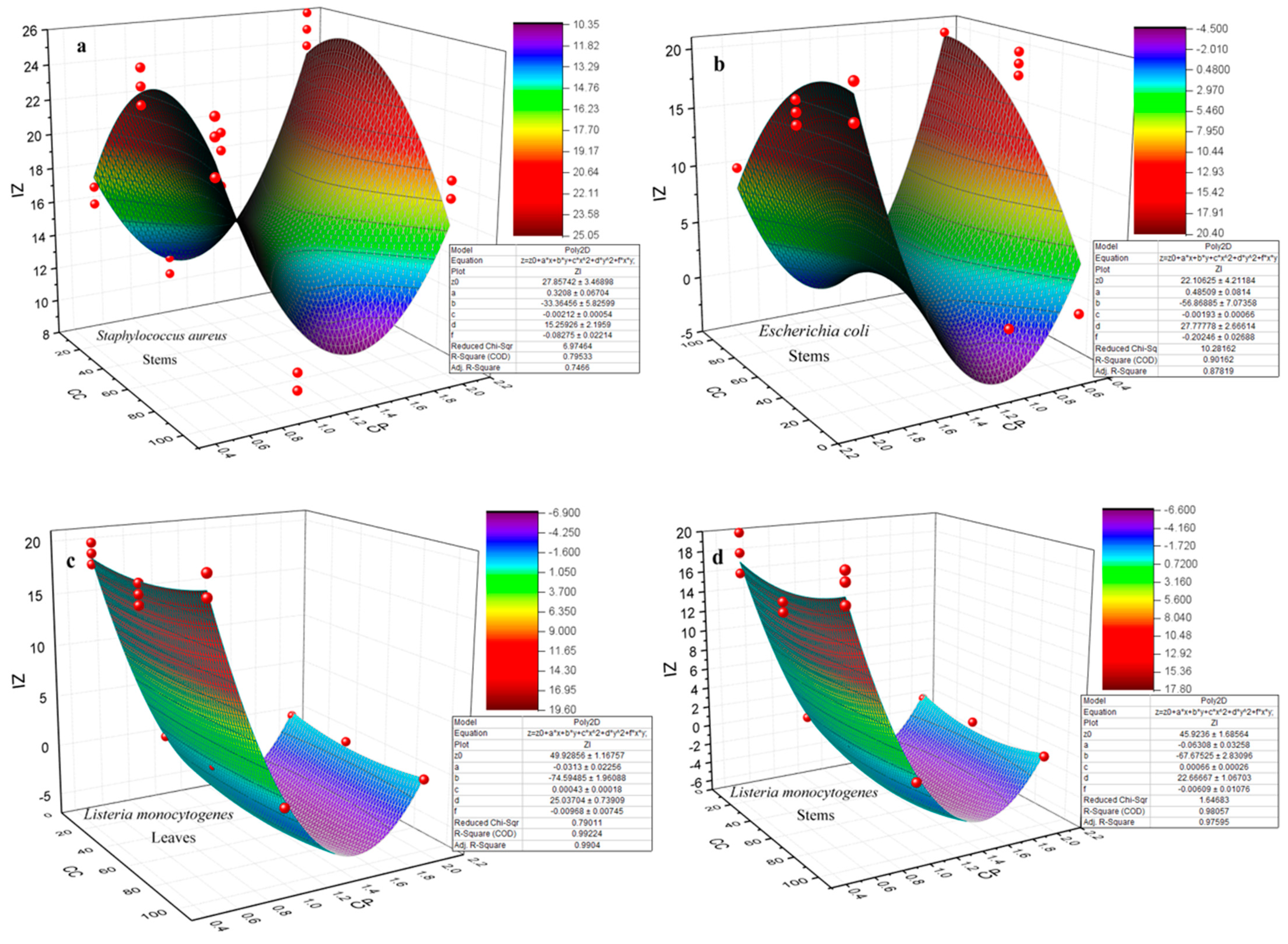
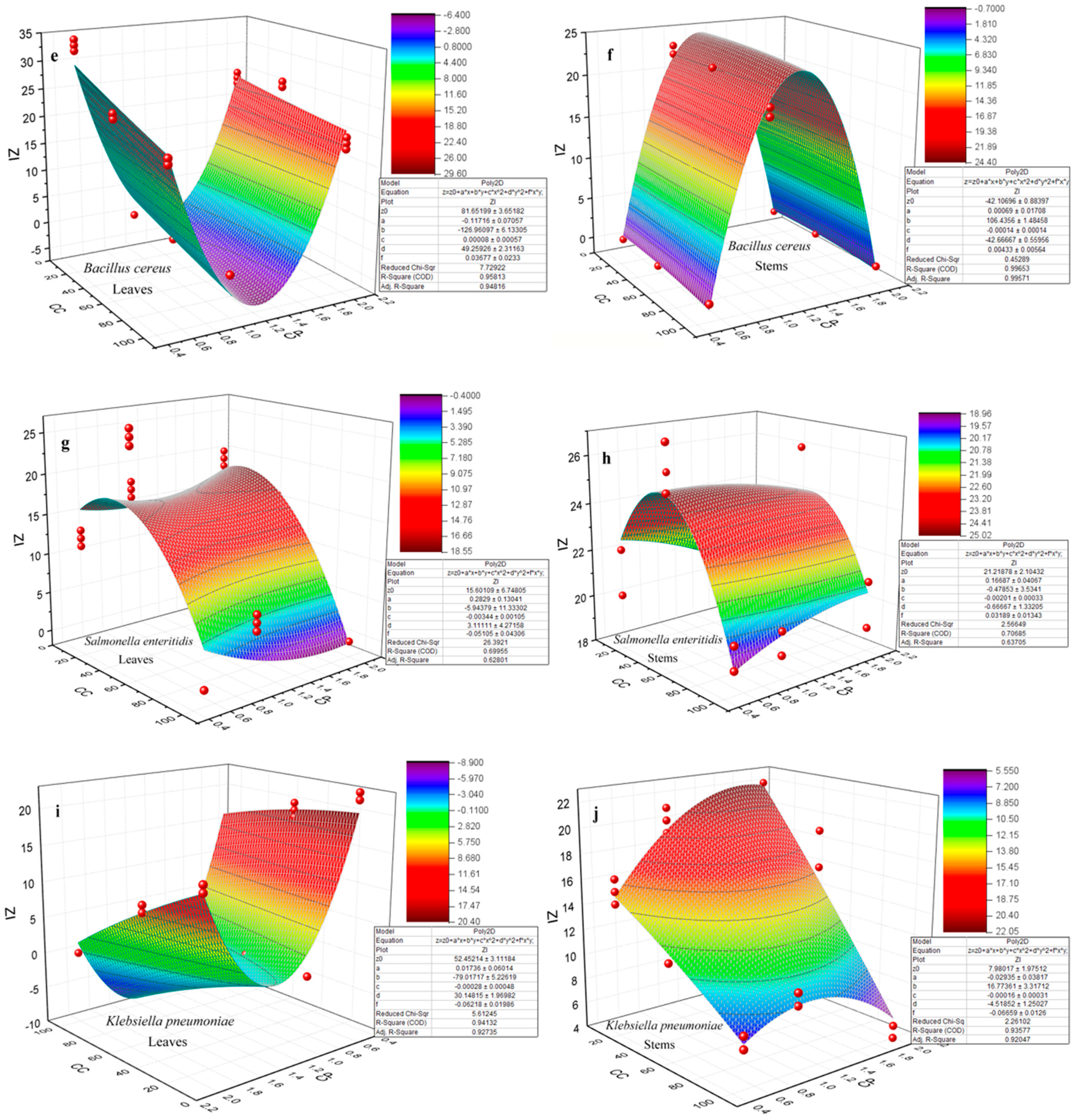
3.4. Antibacterial Activity
- (i)
- Particle size and size distribution. The influence of NP size and size distribution on antibacterial activity was highlighted by the observation, as measured by TEM, that NPs sourced from the 1 M precursor concentration with sizes ranging between 23.3 ± 1.6 nm and 32.1 ± 1.6 nm showed the most significant effect. This contrasted with ZnO NPs derived from the 2 M precursor, which exhibited sizes between 27.7 ± 2.3 nm and 37.1 ± 3.3 nm. Wu et al. [70] achieved a size-controlled synthesis of Ag NPs, ranging from 2 nm to 32 nm, by adjusting the pH to 11, 9, and 7, respectively, using sodium borohydride as a reducer and sodium citrate as a stabilizer. Antibacterial tests against both Gram-negative E. coli and Gram-positive S. aureus showed enhanced effects with smaller NPs. Particularly, 2 nm particles exhibited the most potent antibacterial activity. Yamamoto et al. [71] also found that the size of ZnO NPs (100–800 nm) affected their antibacterial activity against S. aureus and E. coli. By measuring electrical conductivity associated with bacterial growth, they concluded that a reduction in particle size increased antibacterial activity. Raghupathi et al.’s [72] research on E. coli and S. aureus, using NPs of various sizes, aligns with the findings that smaller NPs exhibited enhanced antibacterial properties. On the other hand, the impact of NPs’ size on the electrochemical gradient, established by the movement of hydrogen ions through the cell membrane, facilitating the diffusion of metal ions, was significant. Smaller NPs exhibited enhanced electrostatic interactions [73]. The study of antibacterial activity conducted by Abdullah et al. [33] on ZnO NPs revealed that smaller-sized NPs, measuring 18.6 nm, exhibited a more effective antibacterial effect than larger-sized ones, measuring 28.5 nm. Specifically, for Staphylococcus aureus, the 18.6 nm NPs demonstrated an antibacterial effect of 19.4 nm, while for Escherichia coli, this effect was 21 nm. Conversely, the 28.5 nm NPs showed less pronounced antibacterial effects, with values of 18.2 nm for Staphylococcus aureus and 17.4 nm for Escherichia coli. These results underscore the significant impact of ZnO NPs’ size on their antibacterial activity, indicating a trend toward increased efficacy with smaller sizes.
- (ii)
- The surface-to-volume ratio. The surface area of a nanoparticle is influenced by its shape, size, and material composition. Smaller NPs and those with various shapes, such as spheres, rods, and cubes, have a higher surface-to-volume ratio, affecting their surface interactions differently. This characteristic significantly impacts their chemical reactivity and biological interactions, including antibacterial efficacy [74,75,76]. The reasons why an increased surface area enhances toxicity include, firstly, the facilitation of adsorption and binding of compounds to surfaces and, secondly, the correlation between an increased surface area and the heightened production of reactive oxygen species (ROS) [74,77,78].
- (iii)
- The shape of NPs. The research conducted by Woźniak et al. [79] found that the cytotoxicity of gold NPs (Au NPs) depends on their size and shape, which influences cellular membrane integrity and cell viability. Specifically, nanospheres and nanorods were more toxic compared to star-, flower-, and prism-shaped structures due to their smaller size and propensity to aggregate. During this study, it was observed that the NPs primarily exhibited a spherical shape, yet the emergence of nanorods became noticeable as the precursor concentration increased, leading to aggregation [80]. Moreover, the surface properties of NPs, such as being hydrophobic, hydrophilic, lipophilic, or lipophobic, are determined by their surface characteristics. These properties significantly influence how NPs interact with biological systems, including their solubility, stability, and ability to interact with cell membranes, impacting their biomedical applications and effectiveness [62]. In the study published by Motelica et al. [81], the shape of the NPs was influenced by the solvent used in their synthesis. The use of butanol led to the formation of the smallest NPs, with a rod shape, exhibiting the highest antibacterial activity. This shape is believed to be responsible for the effective penetration of the NPs into the cell membranes.
| Stems = 37.5 µg/mL, 2 M |
| Stems = 20.02 µg/mL, 2 M |
| Leaves = 10 µg/mL, 0.5 M Stems = 21.02 µg/mL, 1.2 M |
| Leaves = 26.5 µg/mL, 2 M Stems = 46.7 µg/mL, 0.8 M |
| Leaves = 100 µg/mL, 0.5 M Stems = 100 µg/mL 0.5 M |
| Leaves =11.83 µg/mL, 0.5 M Stems = 10 µg/mL, 1.7 M |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. After 2015: Infectious Diseases in a New Era of Health and Development. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130426. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The Global Threat of Antimicrobial Resistance: Science for Intervention. N. Microbes N. Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm Formation by Clinical Isolates and the Implications in Chronic Infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef]
- Avnish, P.; Mohd, Z.; Ramkumar, L.; Sanket, J.J. Nanotechnology for Green Applications: How Far on the Anvil of Machine Learning! In Biobased Nanotechnology for Green Applications; Hemen, S., Sanket, J.J., Ram, P., Jampilek, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–38. ISBN 978-3-030-61985-5. [Google Scholar]
- Hassan, A.A.; Sayed-ElAhl, R.M.H.; El Hamaky, A.M.; Mansour, M.K.; Oraby, N.H.; Barakat, M.H. Green Synthesis of Metallic Nanoparticles and Their Biomedical Applications. In Handbook of Green and Sustainable Nanotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 47–71. [Google Scholar]
- Singh, A.V.; Bhardwaj, P.; Upadhyay, A.K.; Pagani, A.; Upadhyay, J.; Bhadra, J.; Tisato, V.; Thakur, M.; Gemmati, D.; Mishra, R.; et al. Navigating Regulatory Challenges in Molecularly Tailored Nanomedicine. Explor. BioMat-X 2024, 1, 124–134. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green Approach for Synthesis of Zinc Oxide Nanoparticles from Andrographis Paniculata Leaf Extract and Evaluation of Their Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities. Bioprocess. Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.W.; Chen, H.L.; Liao, Z.X.; Sureshbabu, R.; Hsiao, H.C.; Lin, S.J.; Chang, Y.; Sung, H.W. Effective Photothermal Killing of Pathogenic Bacteria by Using Spatially Tunable Colloidal Gels with Nano-Localized Heating Sources. Adv. Funct. Mater. 2015, 25, 721–728. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel Concept of the Smart NIR-Light-Controlled Drug Release of Black Phosphorus Nanostructure for Cancer Therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Ullah, F.; Hussain, H.; Tasleem Hussain, S.; Raza Shah, M. Nepetolide: A New Diterpene from Nepeta Suavis. Z. Naturforschung B 2008, 63, 591–594. [Google Scholar] [CrossRef]
- Sharma, A.; Cooper, R.; Bhardwaj, G.; Cannoo, D.S. The Genus Nepeta: Traditional Uses, Phytochemicals and Pharmacological Properties. J. Ethnopharmacol. 2021, 268, 113679. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.; Öztürk, G.; Çiçek, M.; Demirci, B. Phytochemical Characterization and Biological Activities of Nepeta Cadmea Boiss. Int. J. Second. Metab. 2020, 7, 28–34. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Nestorović, J.; Mišić, D.; Šiler, B.; Soković, M.; Glamočlija, J.; Ćirić, A.; Maksimović, V.; Grubišić, D. Nepetalactone Content in Shoot Cultures of Three Endemic Nepeta Species and the Evaluation of Their Antimicrobial Activity. Fitoterapia 2010, 81, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Smiljkovic, M.; Dias, M.I.; Stojkovic, D.; Barros, L.; Bukvički, D.; Ferreira, I.C.F.R.; Sokovic, M. Characterization of Phenolic Compounds in Tincture of Edible Nepeta Nuda: Development of Antimicrobial Mouthwash. Food Funct. 2018, 9, 5417–5425. [Google Scholar] [CrossRef]
- Kumar, V.; Mathela, C.S.; Tewari, A.K.; Bisht, K.S. In Vitro Inhibition Activity of Essential Oils from Some Lamiaceae Species against Phytopathogenic Fungi. Pestic. Biochem. Physiol. 2014, 114, 67–71. [Google Scholar] [CrossRef]
- Salehi, P.; Sonboli, A.; Allahyari, L. Antibacterial and Antioxidant Properties of the Essential Oil and Various Extracts of Nepeta Ispahanica from Iran. J. Essent. Oil-Bear. Plants 2007, 10, 324–331. [Google Scholar] [CrossRef]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and Antimicrobial Activity of the Essential Oil and Methanol Extracts of Achillea millefolium Subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Shaddel, R.; Asili, J. Volatile Composition, Antimicrobial, Cytotoxic and Antioxidant Evaluation of the Essential Oil from Nepeta Sintenisii Bornm. Ind. Crops Prod. 2016, 84, 224–229. [Google Scholar] [CrossRef]
- Ali, T.; Javan, M.; Sonboli, A.; Semnanian, S. Evaluation of the Antinociceptive and Anti-Inflammatory Effects of Essential Oil of Nepeta Pogonosperma Jamzad et Assadi in Rats. Daru 2012, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, A.S.; Soelberg, J.; Jäger, A.K. Antibacterial and COX-1 Inhibitory Effect of Medicinal Plants from the Pamir Mountains, Afghanistan. Plants 2012, 1, 74–81. [Google Scholar] [CrossRef]
- Huang, S.; Tan, M.; Guo, F.; Dong, L.; Liu, Z.; Yuan, R.; Dongzhi, Z.; Lee, D.S.; Wang, Y.; Li, B. Nepeta angustifolia C. Y. Wu Improves Renal Injury in HFD/STZ-Induced Diabetic Nephropathy and Inhibits Oxidative Stress-Induced Apoptosis of Mesangial Cells. J. Ethnopharmacol. 2020, 255, 112771. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F. Chemical Constituents and Biological Activities of Nepeta Species. Chem. Biodivers. 2011, 8, 1783–1818. [Google Scholar] [PubMed]
- Pachaiappan, R.; Rajendran, S.; Ramalingam, G.; Vo, D.V.N.; Priya, P.M.; Soto-Moscoso, M. Green Synthesis of Zinc Oxide Nanoparticles by Justicia Adhatoda Leaves and Their Antimicrobial Activity. Chem. Eng. Technol. 2021, 44, 551–558. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Gomez-Zavaglia, A.; Guerrero, A.; Romero, A. Green Synthesis of ZnO Nanoparticles Using Polyphenol Extracts from Pepper Waste (Capsicum annuum). J. Clean. Prod. 2022, 350, 131541. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Guerrero, A.; Romero, A. Efficient and Sustainable Synthesis of Zinc Salt-Dependent Polycrystal Zinc Oxide Nanoparticles: Comprehensive Assessment of Physicochemical and Functional Properties. Appl. Sci. 2024, 14, 1815. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Rosado, M.J.; Guerrero, A.; Romero, A. Eco-Friendly Synthesis of ZnO-Nanoparticles Using Phoenix dactylifera L., Polyphenols: Physicochemical, Microstructural, and Functional Assessment. New J. Chem. 2023, 47, 4409–4417. [Google Scholar] [CrossRef]
- Dediu, V.; Busila, M.; Tucureanu, V.; Bucur, F.I.; Iliescu, F.S.; Brincoveanu, O.; Iliescu, C. Synthesis of ZnO/Au Nanocomposite for Antibacterial Applications. Nanomaterials 2022, 12, 3832. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Zinc Oxide Nanoparticles with Potential Synergistic Activity with Common Antifungal Agents against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Green Synthesis of FexOy Nanoparticles with Potential Antioxidant Properties. Nanomaterials 2022, 12, 2449. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.M.; Ghasemi, N. Influence of Temperature and Concentration on Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Cherry Extract. J. Nanostructure Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef]
- Pholnak, C.; Sirisathitkul, C.; Suwanboon, S.; Harding, D.J. Effects of Precursor Concentration and Reaction Time on Sonochemically Synthesized ZnO Nanoparticles. Mater. Res. 2014, 17, 405–411. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Azeez, H.H. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Eucalyptus Globulus Labill. Leaf Extract and Zinc Nitrate Hexahydrate Salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Mesa, A.; Mythatha, G.S.S.; Lodi, R.S.; Ravuri, S.; Balli, R. Chitosan Nanoparticles: An Overview on Preparation, Characterization and Biomedical Applications. In Nanotechnology for Advances in Medical Microbiology; Springer: Singapore, 2021; pp. 393–427. [Google Scholar]
- Kumar, V.; Bhatt, P.C.; Rahman, M.; Kaithwas, G.; Choudhry, H.; Aal-Abbasi, F.; Anwar, F.; Verma, A. Fabrication, Optimization, and Characterization of Umbelliferone β-D-Galactopyranoside-Loaded PLGA Nanoparticles in Treatment of Hepatocellular Carcinoma: In Vitro and in Vivo Studies. Int. J. Nanomed. 2017, 12, 6747–6758. [Google Scholar] [CrossRef] [PubMed]
- Sizochenko, N.; Mikolajczyk, A.; Syzochenko, M.; Puzyn, T.; Leszczynski, J. Zeta Potentials (ζ) of Metal Oxide Nanoparticles: A Meta-Analysis of Experimental Data and a Predictive Neural Networks Modeling. NanoImpact 2021, 22, 100317. [Google Scholar] [CrossRef] [PubMed]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 313–353. ISBN 9780128141311. [Google Scholar]
- Ikbal, A.M.A.; Rajkhowa, A.; Singh, W.S.; Manna, K. Green Synthesis of Zinc Oxide Nanoparticles Using Croton Joufra Leaf Extract, Characterization and Antidiabetic Activity. Int. Nano Lett. 2023, 13, 251–260. [Google Scholar] [CrossRef]
- Merlano, A.S.; Hoyos, L.M.; Gutiérrez, G.J.; Valenzuela, M.A.; Salazar, Á. Effect of Zn Precursor Concentration in the Synthesis of RGO/ZnO Composites and Their Photocatalytic Activity. N. J. Chem. 2020, 44, 19858–19867. [Google Scholar] [CrossRef]
- Hosseini Largani, S.; Akbarzadeh Pasha, M. The Effect of Concentration Ratio and Type of Functional Group on Synthesis of CNT–ZnO Hybrid Nanomaterial by an in Situ Sol–Gel Process. Int. Nano Lett. 2017, 7, 25–33. [Google Scholar] [CrossRef]
- Ahmad, N.; Ali, S.; Abbas, M.; Fazal, H.; Saqib, S.; Ali, A.; Ullah, Z.; Zaman, S.; Sawati, L.; Zada, A.; et al. Antimicrobial Efficacy of Mentha Piperata-Derived Biogenic Zinc Oxide Nanoparticles against UTI-Resistant Pathogens. Sci. Rep. 2023, 13, 14972. [Google Scholar] [CrossRef] [PubMed]
- Paudel, N.; Rai, M.; Adhikari, S.; Thapa, A.; Bharati, S.; Maharjan, B.; Rav, K.; Singh , A.V. Green Extraction, Phytochemical Profiling, and Biological Evaluation of Dysphania Ambrosioides: An In Silico and In Vitro Medicinal Investigation. J. Herbs Spices Med. Plants 2024, 30, 97–114. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Azizi, S.; Sintwa, N.; Mokalane, K.; Mohale, K.C.; Mudau, F.N.; Maaza, M. Effect of Optimized Precursor Concentration, Temperature, and Doping on Optical Properties of ZnO Nanoparticles Synthesized via a Green Route Using Bush Tea (Athrixia phylicoides DC.) Leaf Extracts. ACS Omega 2022, 7, 31658–31666. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, G.M.; Ahmed, A.B.; Al-Zubaidi, A.B. Synthesis, Characterization, and the Influence of Energy of Irradiation on Optical Properties of ZnO Nanostructures. Sci. Rep. 2022, 12, 20016. [Google Scholar] [CrossRef] [PubMed]
- Gurgur, E.; Oluyamo, S.S.; Adetuyi, A.O.; Omotunde, O.I.; Okoronkwo, A.E. Green Synthesis of Zinc Oxide Nanoparticles and Zinc Oxide–Silver, Zinc Oxide–Copper Nanocomposites Using Bridelia Ferruginea as Biotemplate. SN Appl. Sci. 2020, 2, 911. [Google Scholar] [CrossRef]
- Chan, Y.B.; Selvanathan, V.; Tey, L.H.; Akhtaruzzaman, M.; Anur, F.H.; Djearamane, S.; Watanabe, A.; Aminuzzaman, M. Effect of Calcination Temperature on Structural, Morphological and Optical Properties of Copper Oxide Nanostructures Derived from Garcinia mangostana L. Leaf Extract. Nanomaterials 2022, 12, 3589. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, P.N.; Moloto, M.J. Effect of Precursor Concentration and pH on the Shape and Size of Starch Capped Silver Selenide (Ag 2 Se). Nanoparticles 2014, 11, 577–588. [Google Scholar]
- Huang, S.J.; Hsu, Y.T.; Lee, H.; Chen, Y.C.; Volosniev, A.G.; Zinner, N.T.; Wang, D.W. Field-Induced Long-Lived Supermolecules. Phys. Rev. A 2012, 85, 055601. [Google Scholar] [CrossRef]
- Fagundes, A.P.; da Silva Júnior, A.H.; Macuvele, D.L.P.; Riella, H.G.; Padoin, N.; Soares, C. Plant-Mediated Synthesis of Nanoscale Hydroxyapatite: Morphology Variability and Biomedical Applications. In Handbook of Green and Sustainable Nanotechnology; Springer International Publishing: Chem, Switzerland, 2023; pp. 537–562. [Google Scholar]
- Dowlath, M.J.H.; Musthafa, S.A.; Mohamed Khalith, S.B.; Varjani, S.; Karuppannan, S.K.; Ramanujam, G.M.; Arunachalam, A.M.; Arunachalam, K.D.; Chandrasekaran, M.; Chang, S.W.; et al. Comparison of Characteristics and Biocompatibility of Green Synthesized Iron Oxide Nanoparticles with Chemical Synthesized Nanoparticles. Env. Environ. Res. 2021, 201, 111585. [Google Scholar] [CrossRef]
- Bibi, I.; Kamal, S.; Ahmed, A.; Iqbal, M.; Nouren, S.; Jilani, K.; Nazar, N.; Amir, M.; Abbas, A.; Ata, S.; et al. Nickel Nanoparticle Synthesis Using Camellia Sinensis as Reducing and Capping Agent: Growth Mechanism and Photo-Catalytic Activity Evaluation. Int. J. Biol. Macromol. 2017, 103, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Fe Nanoparticles Using Eucalyptus Leaf Extracts for Treatment of Eutrophic Wastewater. Sci. Total Environ. 2014, 466–467, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Luque, P.A.; Nava, O.; Soto-Robles, C.A.; Vilchis-Nestor, A.R.; Garrafa-Galvez, H.E.; Castro-Beltran, A. Effects of Daucus Carota Extract Used in Green Synthesis of Zinc Oxide Nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 17638–17643. [Google Scholar] [CrossRef]
- Zayed, M.F.; Eisa, W.H. Phoenix Dactylifera L. Leaf Extract Phytosynthesized Gold Nanoparticles; Controlled Synthesis and Catalytic Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, C.; Lee, K.S.; Suh, S.J. Effect of NaOH and Precursor Concentration on Size and Magnetic Properties of FeCo Nanoparticles Synthesized Using the Polyol Method. AIP Adv. 2020, 10, 115220. [Google Scholar] [CrossRef]
- Yousuf, S.; Shabir, S.; Kauts, S.; Minocha, T.; Obaid, A.A.; Khan, A.A.; Mujalli, A.; Jamous, Y.F.; Almaghrabi, S.; Baothman, B.K.; et al. Appraisal of the Antioxidant Activity, Polyphenolic Content, and Characterization of Selected Himalayan Herbs: Anti-Proliferative Potential in HepG2 Cells. Molecules 2022, 27, 8629. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Cuartas-Gómez, E.; Dynes, S.; Utomo, E.; Anjani, Q.K.; Detamornrat, U.; Donnelly, R.F.; Moreno-Castellanos, N.; Larrañeta, E. Poly(Caprolactone)/Lignin-Based 3D-Printed Dressings Loaded with a Novel Combination of Bioactive Agents for Wound-Healing Applications. Sustain. Mater. Technol. 2023, 35, e00581. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Stewart, S.A.; Rendl, A.; González, Z.; Donnelly, R.F.; Larrañeta, E. Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Farswan, T.S.; Pandey, M.M. Seasonal Variation in the Phytoconstituents and Antioxidant Activity in Moringa oleifera Lam. Leaves of North India. S. Afr. J. Bot. 2024, 166, 492–502. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A Facile Method to Prepare Size-Tunable Silver Nanoparticles and Its Antibacterial Mechanism. Adv. Powder Technol. 2018, 29, 407–415. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of Particle Size on the Antibacterial Activity of Zinc Oxide. Nanomicro Lett. 2001, 3, 219–242. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (ZnO Nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO Size and Shape Effect on Antibacterial Activity and Cytotoxicity Profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial Activity of Silver Nanoparticles of Different Particle Size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and Shape-Dependent Cytotoxicity Profile of Gold Nanoparticles for Biomedical Applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B. Size and Shape Effect on Biomedical Applications of Nanomaterials. In Biomedical Engineering—Technical Applications in Medicine; InTech: London, UK, 2012. [Google Scholar]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Tejada Jacob, G.; Castro, G.R.; Alvarez, V.A. Nanotechnology Applied to Personalized 3D Dressings for Diabetic Feet. In Handbook of Consumer Nanoproducts; Springer Nature: Singapore, 2022; pp. 525–547. [Google Scholar]
- Mahsa, Y.; Hamed, A.; Amir, A.A. Antibacterial Activity of Silver-Nanoparticles against Staphylococcus aureus. Afr. J. Microbiol. Res. 2016, 10, 850–855. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic Investigation into Antibacterial Behaviour of Suspensions of ZnO Nanoparticles against E. coli. J. Nanoparticle Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Kottappara, R.; Pillai, S.C.; Vijayan, B.K. Copper- and Iron-Based Bio-Nanocomposites for Green Applications. In Nanotechnology in the Life Sciences; Springer Science and Business Media B.V.: Dordrecht, The Netherlands, 2021; pp. 41–72. [Google Scholar]
- Guo, B.L.; Han, P.; Guo, L.C.; Cao, Y.Q.; Li, A.D.; Kong, J.Z.; Zhai, H.F.; Wu, D. The Antibacterial Activity of Ta-Doped ZnO Nanoparticles. Nanoscale Res. Lett. 2015, 10, 336. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative Eco-Toxicity of Nanoscale TiO2, SiO2, and ZnO Water Suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Hirota, K.; Sugimoto, M.; Kato, M.; Tsukagoshi, K.; Tanigawa, T.; Sugimoto, H. Preparation of Zinc Oxide Ceramics with a Sustainable Antibacterial Activity under Dark Conditions. Ceram. Int. 2010, 36, 497–506. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles to Escherichia coli: Mechanism and the Influence of Medium Components. Env. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Bolzinger, M.A. Zinc Oxide as a New Antimicrobial Preservative of Topical Products: Interactions with Common Formulation Ingredients. Int. J. Pharm. 2015, 479, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E. Investigation of Nanoparticle Metallic Core Antibacterial Activity: Gold and Silver Nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905. [Google Scholar] [CrossRef] [PubMed]
- Ilbeigi, G.; Kariminik, A.; Moshafi, M.H. The Antibacterial Activities of NiO Nanoparticles against Some Gram-Positive and Gram-Negative Bacterial Strains. Int. J. Basic. Sci. Med. 2019, 4, 69–74. [Google Scholar] [CrossRef]
- Manyasree, D.; Kiranmayi, P.; Kumar, R. Synthesis, Characterization and Antibacterial Activity of Aluminium Oxide Nanoparticles. Int. J. Pharm. Pharm. Sci. 2018, 10, 32. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Grinberg, M.A.; Sukhov, V.; Vodeneev, V. Effect of Ionizing Radiation on Physiological and Molecular Processes in Plants. J. Env. Environ. Radioact. 2019, 202, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Salas Orozco, M.F.; Niño-Martínez, N.; Martínez-Castañón, G.A.; Méndez, F.T.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Graves, J.L.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid Evolution of Silver Nanoparticle Resistance in Escherichia coli. Front. Genet. 2015, 5, 42. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Sanusi, N.; Ismail, H.; Othman, N.; Ariffin, A. Application of Response Surface Methodology (RSM) for Optimization of Cassava Starch Grafted PolyDADMAC Synthesis for Cationic Properties. Starch/Staerke 2012, 64, 935–943. [Google Scholar] [CrossRef]



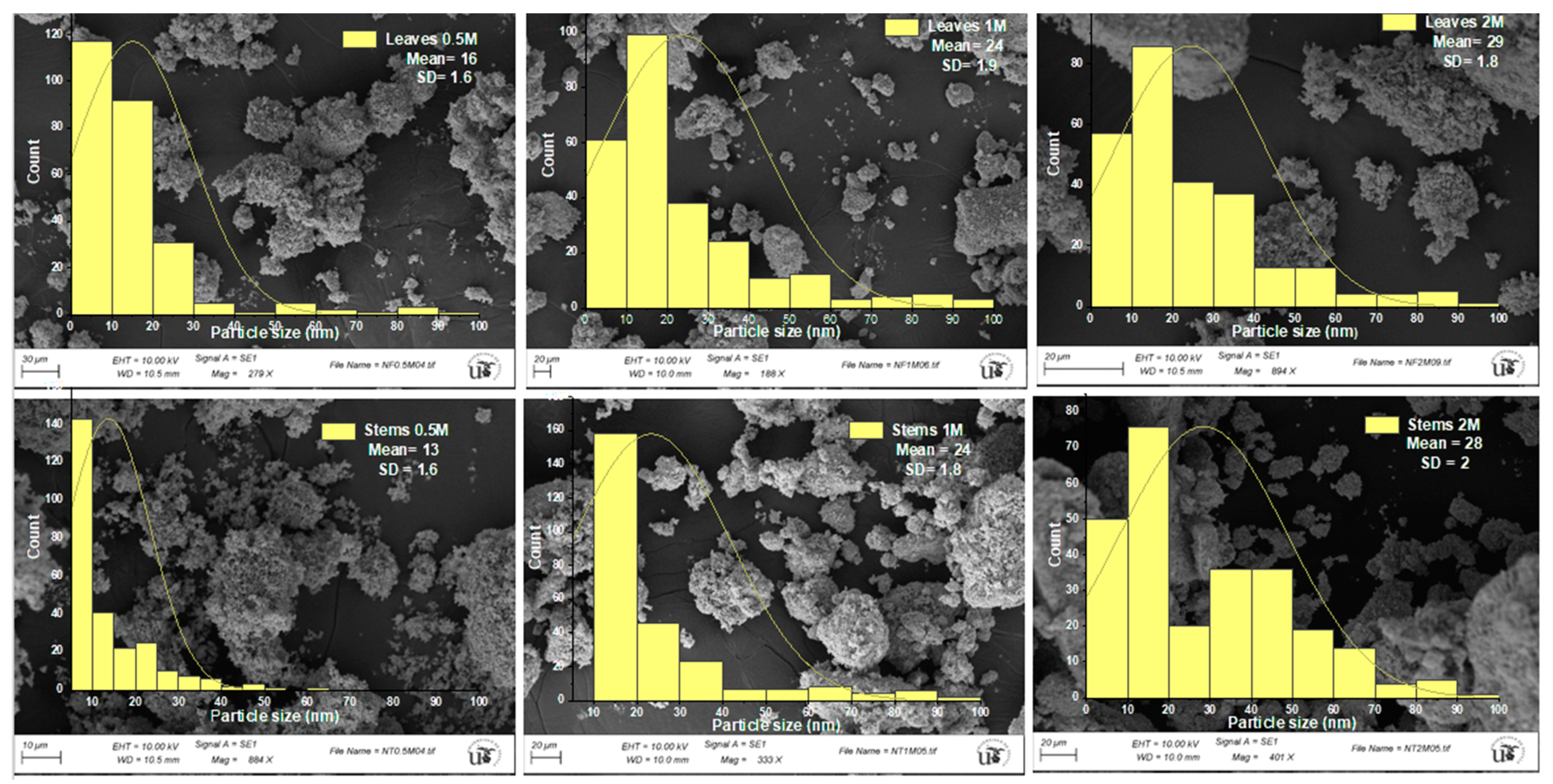
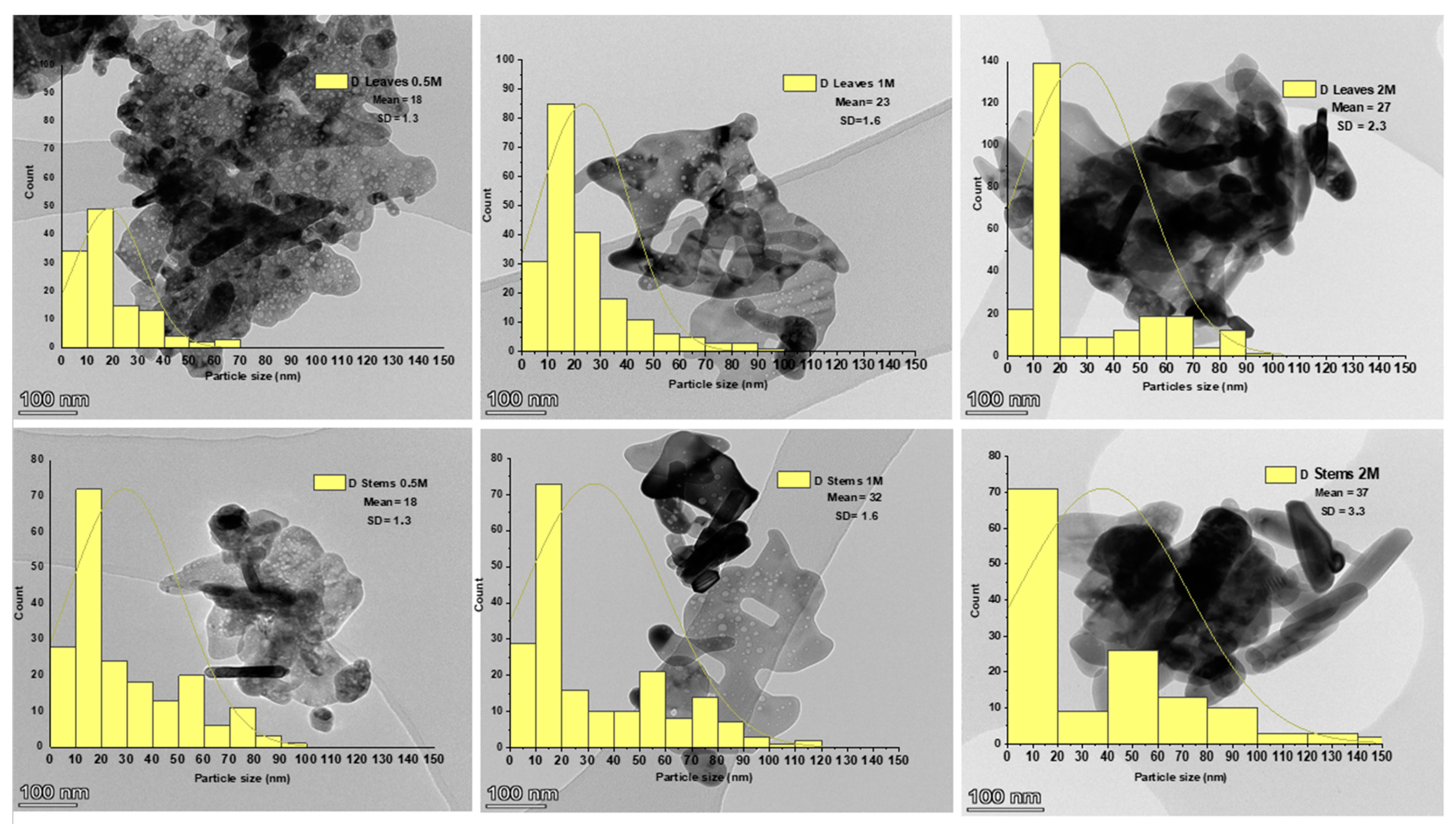
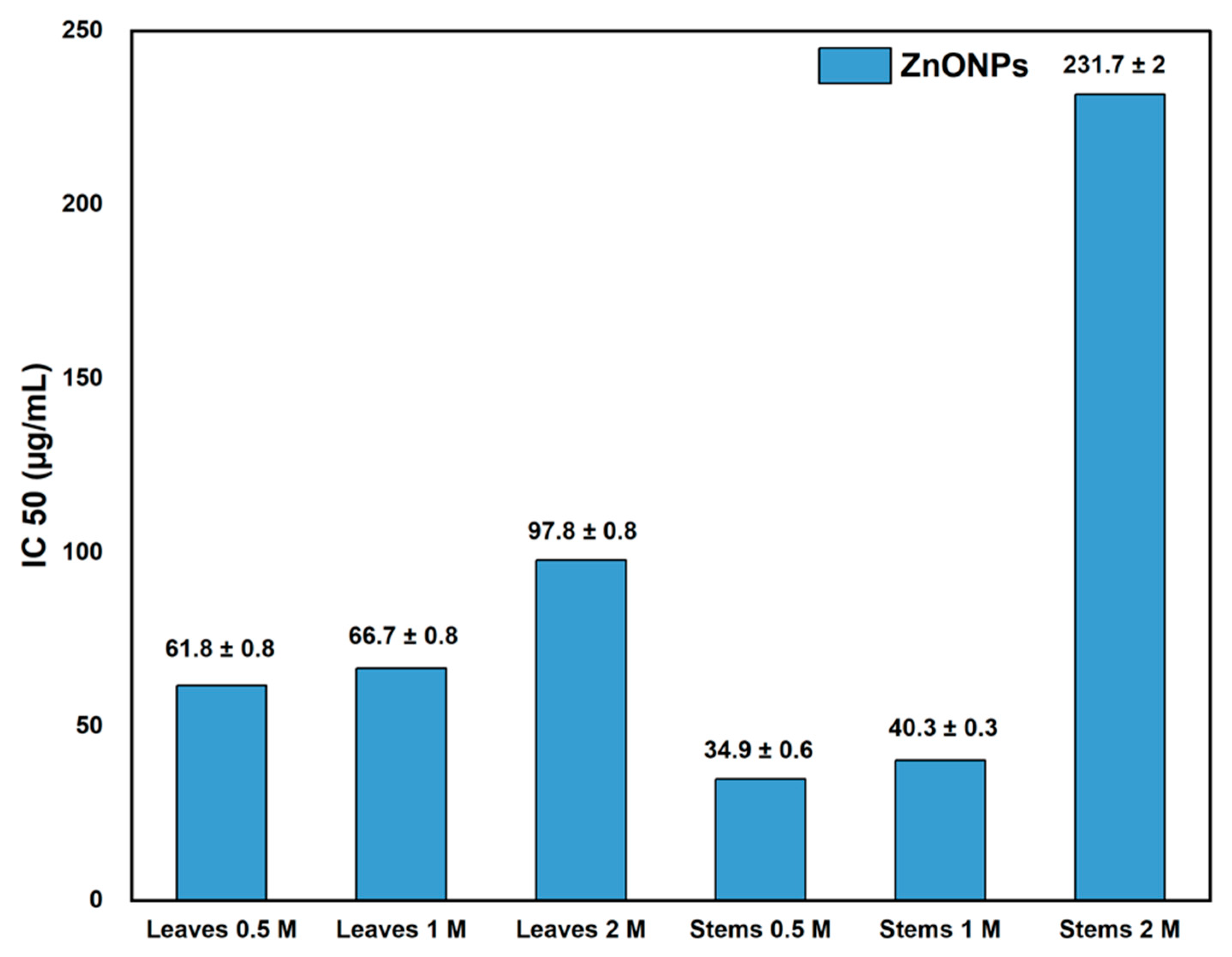
| Concentration of Precursor | Part of the Plant | D (XRD) (nm) | D (TEM) (nm) | D (SEM) (nm) | Crystallinity (%) | IC50 (µg/mL) | TAC (µg AAE/mg ZnO NPs) |
|---|---|---|---|---|---|---|---|
| 0.5 M | Leaves | 16.9 ± 0.3 b | 18.5 ± 1.3 b | 16.3 ± 1.6 b | 91.0 b | 61.8 ± 0.8 c | 19.95 ± 0.7 ab |
| Stems | 16.3 ± 0.1 a | 18.1 ± 1.3 a | 13.7 ± 1.6 a | 89.8 a | 34.9 ± 0.6 a | 19.96 ± 0.9 ab | |
| 1 M | Leaves | 17.4 ± 0.3 c | 23.3 ± 1.6 c | 24.2 ± 1.9 d | 91.1 b | 66.7 ± 0.8 d | 20.57 ± 0.7 b |
| Stems | 20.3 ± 0.1 d | 32.1 ± 1.6 e | 23.5 ± 1.8 c | 96.3 c | 40.3 ± 0.3 b | 22.70 ± 0.9 c | |
| 2 M | Leaves | 20.3 ± 0.9 d | 27.7 ± 2.3 d | 29.3 ± 1.8 f | 93.6 d | 97.8 ± 0.8 d | 18.54 ± 1.1 a |
| Stems | 21.6 ± 0.4 e | 37.1 ± 3.3 f | 28.4 ± 2.0 e | 97.2 e | 231.7 ± 2 e | 19.28 ± 0.5 ab |
| Nanoparticles | Leaves 0.5 M | Leaves 1 M | Leaves 2 M | Stems 0.5 M | Stems 1 M | Stems 2 M |
|---|---|---|---|---|---|---|
| Zeta potential (mV) | −20.4 ± 0.1 b | +25.2 ± 1.5 a | −20.5 ± 1.8 b | +25.2 ± 0 a | −24.3 ± 1.2 c | −20.5 ± 1.6 b |
| Functional Groups | Stems 0.5 M | Leaves 0.5 M | Stems 1 M | Leaves 1 M | Stems 2 M | Leaves 2 M |
|---|---|---|---|---|---|---|
| COOH | 3714 | 3734 | 3732 | 3720 | 3727 | 3724 |
| O-H stretching | 3348 | / | / | 3492 | / | / |
| C-H stretching | 3009 | 3003 | 3006 | 2992 | 3003 | 3006 |
| C-O-H bending | 1528 | 1525 | 1517 | 1523 | 1520 | 1528 |
| C-O stretching | 1129 | 1140 | 1137 | 1134 | 1132 | 1129 |
| C-C bending | 878 | 878 | 897 | 873 | 878 | 865 |
| ZnO vibration | 573 | 556 | 551 | 548 | 544 | 548 |
| 429 | 456 | 432 | 440 | 439 | 428 |
| Parameters | S. aureus | L. monocytogenes | B. cereus | E. coli | S. enteritidis | K. pneumoniae | ||
|---|---|---|---|---|---|---|---|---|
| Precursor C (M) | Part | ZnO NPs C (µg/mL) | ||||||
| 0.5 | Leaves | 10 | 0.0 n | 14.3 ± 0.5 e | 32.8 ± 1 j | 09.7 ± 0.5 ce | 12.0 ± 1 n | 22.6 ± 0.5 fk |
| 0.5 | Leaves | 50 | 11.0 ± 1.4 f | 10.0 ±1.0 c | 23.3 ± 0.5 bd | 00.00 a | 26.0 ± 1 d | 19.0 ± 1 bcef |
| 0.5 | Leaves | 100 | 17.5 ± 0.7 de | 13.3 ± 1.5 e | 20.6 ± 0.5 ef | 00.00 a | 0.0 f | 14.0 ± 0 bd |
| 0.5 | Leaves | Gentamicin | 30.0 ± 1.4 ag | 38.3 ± 0.5 f | 39.3 ± 0.5 g | 33.5 ± 0.7 f | 31.0 ± 1 hi | 30.0 ± 1 i |
| 0.5 | Stems | 10 | 16.5 ± 0.7 d | 19.0 ± 1.0 di | 0.0 a | 00.00 a | 21.3 ± 1.1 ckl | 15.0 ± 1 bcd |
| 0.5 | Stems | 50 | 24.0 ± 1.4 lm | 17.0 ± 1.0 i | 0.0 a | 19.0 ± 1 hi | 25.6 ± 1.1 dm | 11.0 ± 0 dg |
| 0.5 | Stems | 100 | 22.5 ± 2.1 jlm | 20.3 ±1.1 bd | 0.0 a | 20.0 ± 0 h | 19.3 ± 0.5 ac | 07.6 ± 0.5 gh |
| 0.5 | Stems | Gentamicin | 33.0 ± 2.8 ab | 39.7 ± 0.5 f | 37.6 ± 0.5 g | 30.0 ± 1 gj | 35.0 ± 1 j | 27.0 ± 0 ik |
| 1 | Leaves | 10 | 31.5 ± 0.7 ab | 0.0 a | 0.0 a | 0.0 a | 17.0 ± 1 ab | 0.0 a |
| 1 | Leaves | 50 | 28.0 ± 1.4 gh | 0.0 a | 0.0 a | 11.0 ± 0 cde | 09.0 ± 1 e | 0.0 a |
| 1 | Leaves | 100 | 05.5 ± 0.7 k | 0.0 a | 0.0 a | 12.0 ± 1 bd | 06.0 ± 1 g | 0.0 a |
| 1 | Leaves | Gentamicin | 34.0 ± 0.0 b | 29.3 ±1.1 g | 29.3 ± 0.5 h | 28.7 ± 0.5 g | 29.0 ± 0 h | 36.0 ± 1 j |
| 1 | Stems | 10 | 11.5 ± 0.7 f | 0.0 a | 21.6 ± 1.5 bd | 0.0 a | 24.0 ± 1 dkm | 20.0 ± 1 cef |
| 1 | Stems | 50 | 19.5 ± 2.1 ceij | 0.0 a | 22.0 ± 0 df | 0.0 a | 23.0 ± 0 klm | 13.6 ± 0.5 bd |
| 1 | Stems | 100 | 09.5 ± 0.7 f | 0.0 a | 19.6 ± 0.5 e | 0.0 a | 19.6 ± 0.5 ac | 09.6 ± 0.5 dgh |
| 1 | Stems | Gentamicin | 30.5 ± 0.7 ag | 35.0 ± 1.0 h | 34.0 ± 0 j | 35.0 ± 0 f | 33.0 ± 0 ij | 27.6 ± 0.7 dg |
| 2 | Leaves | 10 | 17.5 ± 0.7 cde | 22.0 ±1.0 b | 24.3 ± 1.1 bc | 14.0 ± 1 b | 19.0 ± 1 ac | 14.6 ± 0.5 bcd |
| 2 | Leaves | 50 | 20.5 ± 2.1 cij | 20.7 ± 1.1.0 bd | 25.5 ± 0.5 c | 11.6 ± 0.5 cd | 15.0 ± 1 b | 09.6 ± 0.5 dgh |
| 2 | Leaves | 100 | 18.5 ± 2.1 cdei | 20.0 ± 1.0 bd | 19.0 ± 1 e | 09.3 ± 0.5 e | 0.0 f | 0.0 a |
| 2 | Leaves | Gentamicin | 30.0 ± 1.4 ag | 35.0 ± 0.0 h | 42.0 ± 0 i | 34.6 ± 0.5 f | 34.6 ± 0.5 j | 31.0 ± 0 ij |
| 2 | Stems | 10 | 25.0 ± 1.4 hl | 0.0 a | 0.0 a | 20.0 ± 1 h | 18.6 ± 0.5 ac | 20.6 ± 1.5 ef |
| 2 | Stems | 50 | 21.0 ± 1.4 ijm | 0.0 a | 0.0 a | 17.0 ± 1 i | 25.3 ± 1.1 dm | 17.0 ± 1.7 bce |
| 2 | Stems | 100 | 18.5 ± 0.7 cdei | 0.0 a | 0.0 a | 10.0 ± 0 cde | 20.3 ± 1.1 cl | 04.6 ± 0.5 ah |
| 2 | Stems | Gentamicin | 34.0 ± 0.0 b | 34.7 ± 0.5 h | 37.6 ± 0.5 g | 31.0 ± 1 j | 35.3 ± 1.1 j | 26.3 ± 2.8 ik |
| 0.5 M Stems | 0.5 M Leaves | 1 M Stems | 1 M Leaves | 2 M Stems | 2 M Leaves | |
|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | 10 µg/mL | |||||
| Listeria monocytogenes ATCC 19115 | 10 µg/mL | |||||
| Bacillus cereus ATCC 11768 | 10 µg/mL | |||||
| Escherichia coli ATCC 25922 | 10 µg/mL | |||||
| Salmonella enteritidis ATCC 13076 | 50 µg/mL | |||||
| Klebsiella pneumoniae ATCC 70603 | 10 µg/mL |
| S. aureus | L. monocytogenes | B. cereus | E. coli | S. enteritidis | K. pneumoniae | |
|---|---|---|---|---|---|---|
| CC | *** | *** | *** | *** | *** | *** |
| CP | *** | *** | ** | *** | *** | *** |
| PP | *** | *** | *** | *** | *** | *** |
| CC:CP | *** | *** | *** | *** | *** | *** |
| CC:PP | *** | *** | *** | *** | *** | *** |
| CP:PP | *** | *** | *** | *** | *** | *** |
| CC:CP:PP | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodil, N.; Serra, D.; Abdullah, J.A.A.; Domínguez-Robles, J.; Romero, A.; Abdelilah, A. Comparative Effect of Antioxidant and Antibacterial Potential of Zinc Oxide Nanoparticles from Aqueous Extract of Nepeta nepetella through Different Precursor Concentrations. Materials 2024, 17, 2853. https://doi.org/10.3390/ma17122853
Fodil N, Serra D, Abdullah JAA, Domínguez-Robles J, Romero A, Abdelilah A. Comparative Effect of Antioxidant and Antibacterial Potential of Zinc Oxide Nanoparticles from Aqueous Extract of Nepeta nepetella through Different Precursor Concentrations. Materials. 2024; 17(12):2853. https://doi.org/10.3390/ma17122853
Chicago/Turabian StyleFodil, Nouzha, Djaaboub Serra, Johar Amin Ahmed Abdullah, Juan Domínguez-Robles, Alberto Romero, and Amrouche Abdelilah. 2024. "Comparative Effect of Antioxidant and Antibacterial Potential of Zinc Oxide Nanoparticles from Aqueous Extract of Nepeta nepetella through Different Precursor Concentrations" Materials 17, no. 12: 2853. https://doi.org/10.3390/ma17122853
APA StyleFodil, N., Serra, D., Abdullah, J. A. A., Domínguez-Robles, J., Romero, A., & Abdelilah, A. (2024). Comparative Effect of Antioxidant and Antibacterial Potential of Zinc Oxide Nanoparticles from Aqueous Extract of Nepeta nepetella through Different Precursor Concentrations. Materials, 17(12), 2853. https://doi.org/10.3390/ma17122853










