Development and Experimental Verification of Inorganic Electromagnetic Pulse Shielding Paint for Building Interiors Using Carbon-Based Materials
Abstract
1. Introduction
1.1. Background and Purpose
1.2. Objectives
1.3. Research Trends
2. Experiments and Methods
2.1. Experimental Overview
2.2. Used Materials
2.3. Paint Manufacturing and Experimental Details
3. Mixing Experiments and Results of Inorganic EMP Shielding Paint
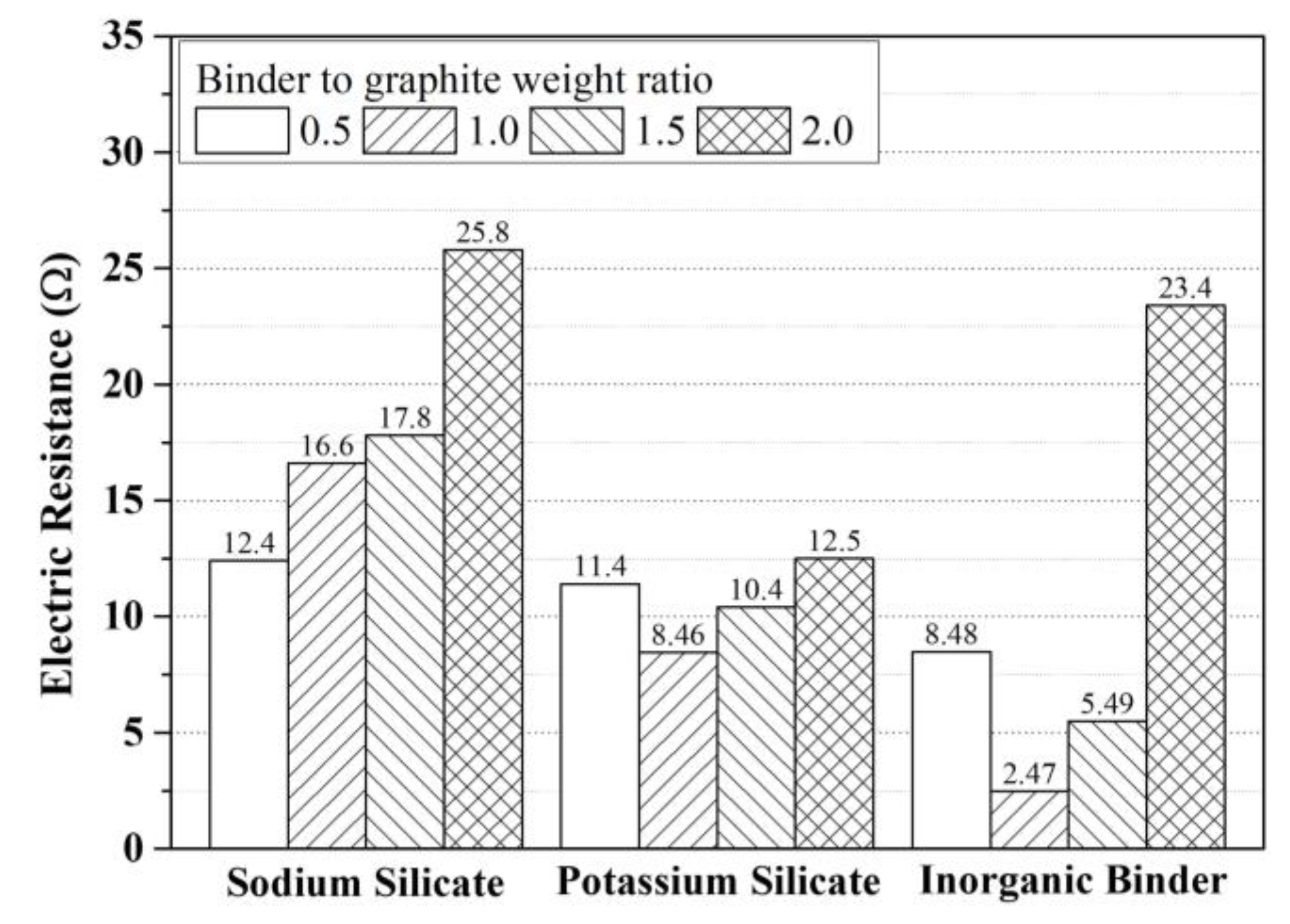
3.1. First Mixing Experiment and Results
3.2. Second Mixing Experiment and Results
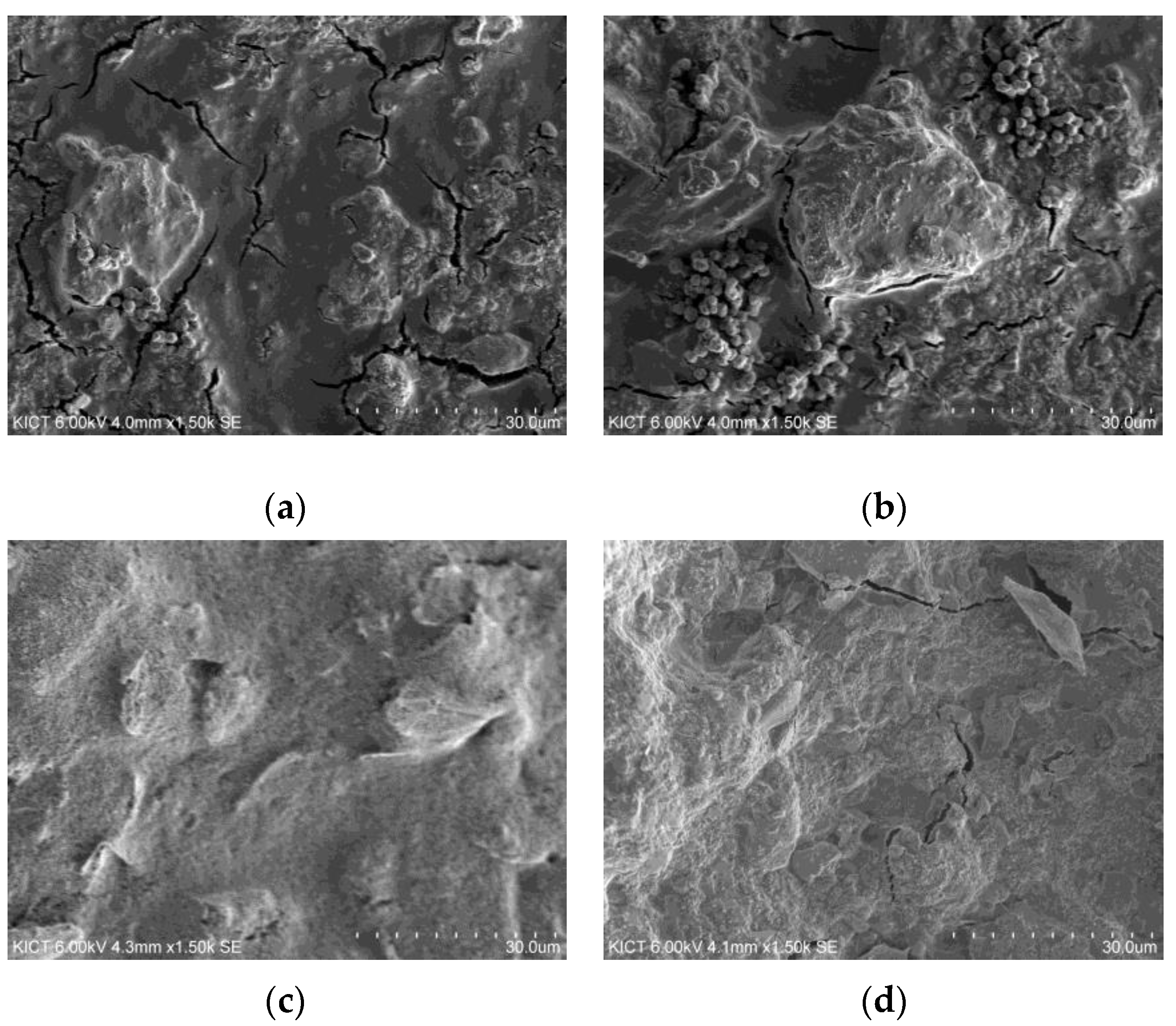
3.3. Microstructural Analysis of EMP Shielding Paints
3.4. Evaluation of the Shielding Effectiveness of Inorganic EMP Shielding Paint
3.5. Evaluation of the Adhesion Strength and the Resistance to Environmental Factors
4. Shielding Effectiveness Verification Experiment for the Inorganic EMP Shielding Paint
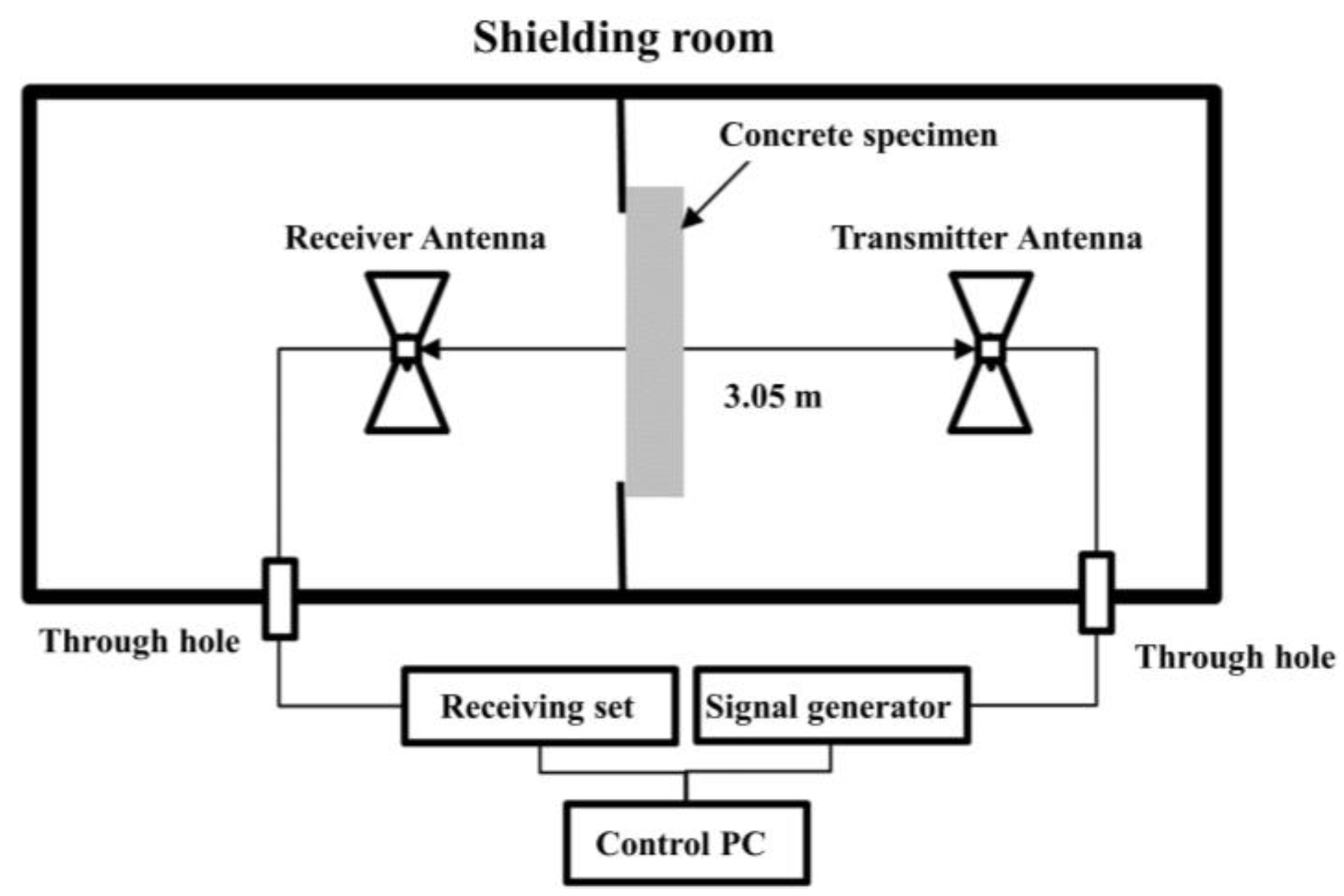
4.1. Fabrication of Specimens
4.2. Experimental Method
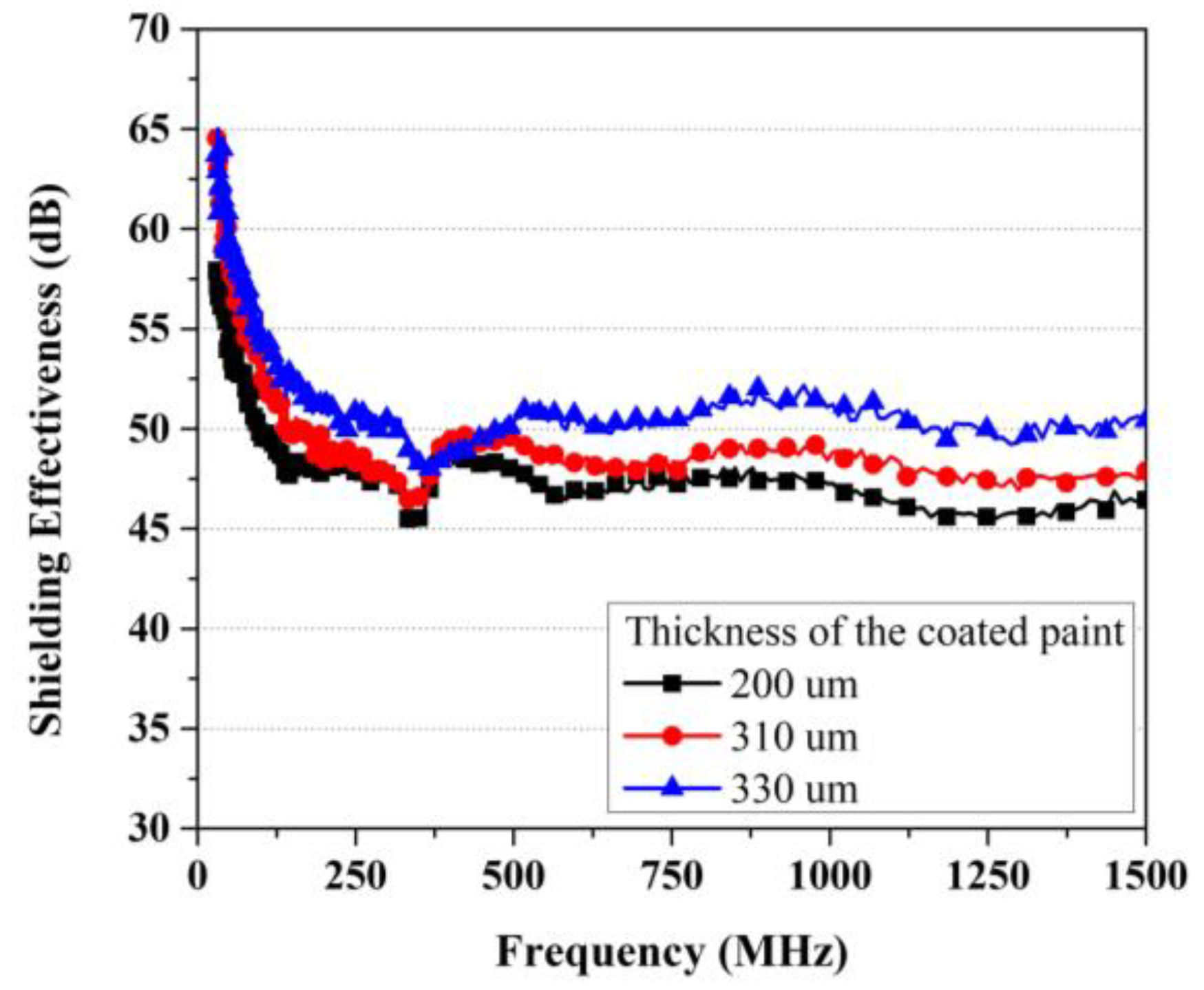
4.3. EMP Shielding Effectiveness Evaluation Results
5. Conclusions
- To select the most suitable binder for EMP shielding inorganic paint, mixture experiments were conducted using four types of binder. It was confirmed that an inorganic binder produced by modifying sodium-based water glass and potassium-based water glass, rather than using them alone, was effective in improving the shielding performance of the paint.
- As a result of measuring the resistance value of the shielding paint according to the type of additive, the resistance value of carbon black was significantly lower than that of acetylene black. The electrical resistance value of a mixture using the same dosage of acetylene black was measured to be up to 15 times higher than that of a mixture using carbon black.
- According to the measured electrical resistance values based on the weight ratio of carbon black in graphite, the resistance value increased as the carbon black content increased for contents ≥ 20%. The ratio of carbon black used in inorganic EMP shielding paint was estimated to be in the range of 15–20% by weight of graphite.
- To verify the shielding effectiveness improvement effect of EMP shielding paint for building interiors, it was applied to shielding concrete. As a result, the application of EMP shielding inorganic paint improved the shielding effectiveness by approximately 25 dB and up to 43 dB.
- The adhesion strength and moisture resistance evaluation of the EMP shielding paint were evaluated. The average adhesive strength of the EMP shielding paint was 1.26 MPa. As a result of a moisture resistance test left at a temperature of 50 ± 3 °C and a relative humidity of 95% or higher for more than 120 h, no cracks or peeling were observed on the painted surface.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.H.; Kim, S.H.; Park, T.Y. Concept of EMP (Electromagnetic Pulse) and Way of Solutions. In Proceedings of the Korean Institute of Information and Communication Sciences Conference, Busan, Republic of Korea, 30 October 2015. [Google Scholar]
- Shurenkov, V.V.; Pershenkov, V.S. Electromagnetic pulse effects and damage mechanism on the semiconductor electronics. Electron. Energetics 2016, 29, 621–629. [Google Scholar] [CrossRef]
- Hyun, S.Y.; Du, J.K.; Kim, W.J.; Yook, J.G. Estimation of Damage in Electric Power Networks due to High Power Electromagnetic Pulse. J. Korean Inst. Electromagn. Eng. Sci. 2014, 25, 757–766. [Google Scholar] [CrossRef]
- Lee, N.K.; Park, G.J.; Park, J.J.; Kim, S.W. Study on the Electrical Conductivity and Electromagnetic Shielding of High Performance Fiber Reinforced Cementitious Composites (HPFRCC). J. Korea Inst. Struct. Maint. Insp. 2019, 23, 37–43. [Google Scholar]
- Wu, J.; Chung, D.L. Increasing the electromagnetic interference shielding effectiveness of carbon fiber polymer-matrix composite by using activated carbon fibers. Carbon 2002, 40, 445–447. [Google Scholar]
- Chung, D.L. Electromagnetic interference shielding effectiveness of carbon materials. Carbon 2001, 39, 279–285. [Google Scholar]
- Yang, S.; Lozano, K.; Lomeli, A.; Foltz, H.D.; Jones, R. Electromagnetic interference shielding effectiveness of carbon nanofiber/LCP composites. Compos. Part A Appl. Sci. Manuf. 2005, 36, 691–697. [Google Scholar] [CrossRef]
- Sau, K.P.; Chaki, T.K.; Chakraborty, A.; Khastgir, D. Electromagnetic interference shielding by carbon black and carbon fiber filled rubber composites. Plast. Rubber Compos. Process. Appl. 1997, 26, 291–297. [Google Scholar]
- Jang, K.P.; Kim, S.H. Evaluation of Electromagnetic Pulse Shielding Effectiveness and Bonding Performance of Inorganic Paint based on Carbon Material. J. Korea Acad.-Ind. Coop. Soc. 2021, 22, 801–807. [Google Scholar] [CrossRef]
- KTL L 378-2022; Electromagnetic Pulse Shielding Inorganic Paint for Building Interiors. Korea Testing Laboratory: Jinsu-si, Gyeongsangnam-do, Republic of Korea, 28 March 2022.
- Zhu, R.Q.; Li, Z.Y.; Deng, G.; Yu, Y.H.; Shui, J.G.; Yu, R.H.; Pan, C.F.; Liu, X.F. Anisotropic magnetic liquid metal film for wearable wireless electromagnetic sensing and smart electromagnetic interference shielding. Nano Energy 2022, 92, 106700. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Gu, J.W. A Perspective for Developing Polymer-Based Electromagnetic Interference Shielding Composites. Nano-Micro Lett. 2022, 14, 89. [Google Scholar] [CrossRef]
- He, Q.M.; Tao, J.R.; Yang, Y.; Yang, D.; Zhang, K.; Wang, M. Effect surface micro-wrinkled and micro-cracks on microwave shielding performance of copper-coated carbon nanotubes/polydimethyl1siloxane composites. Carbon 2023, 213, 118216. [Google Scholar] [CrossRef]
- Min, T.B. An Experimental Study on the Development of Electro Magnetic Pulse Shielding Cement Using Milled Carbon Fiber. J. Korean Recycl. Constr. Resour. Inst. 2020, 8, 429–435. [Google Scholar]
- Shi, S.H.; Jiang, Y.H.; Ren, H.; Deng, S.W.; Sun, J.P.; Cheng, F.C.; Jing, J.J.; Chen, Y.H. 3D-Printed Carbon-Based Conformal Electromagnetic Interference Shielding Module for Integrated Electronics. Nano-Micro Lett. 2024, 16, 85. [Google Scholar] [CrossRef]
- Azim, S.S.; Satheesh, A.; Ramu, K.K.; Ramu, S.; Venkatachari, G. Studies on graphite based conductive paint coatings. Prog. Org. Coat. 2006, 55, 1–4. [Google Scholar] [CrossRef]
- Vovchenko, K.; Perets, Y.; Ovsienko, I.; Matqui, L.; Oliynyk, V.; Launetz, V. Shielding coatings based on carbon-polymer composites. Surf. Coat. Technol. 2012, 211, 196–199. [Google Scholar] [CrossRef]
- Na, K.H.; Jang, K.P.; Kim, S.W.; Choi, W.Y. Fabrication of Electrospun Ni0.5Zn0.5Fe2O4 Nanofibers Using Polyvinyl Pyrrolidone Precursors and Electromagnetic Wave Absorption Performance Improvement. Polymers 2021, 13, 4247. [Google Scholar] [CrossRef] [PubMed]
- Zecchi, S.; Cristoforo, G.; Bartoli, M.; Tagliaferro, A.; Torsello, D.; Rosso, C.; Boccaccio, M.; Acerra, F. A Comprehensive Review of Electromagnetic Interference Shielding Composite Materials. Micromachines 2024, 15, 187. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, J.; Ou, J.P. Superdurable fiber-reinforced composite enabled by synergistic bridging effects of MXene and carbon nanotubes. Carbon 2022, 190, 104–114. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, J.H.; Singh, J.K.; Choi, J.H.; Mandal, S.; Jang, J.M.; Yang, H.M. Electromagnetic Shielding Performance of Carbon Black Mixed Concrete with Zn-Al Metal Thermal Spray Coating. Materials 2020, 13, 895. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, H.; Kim, Y.J. Literature Review on Material Development and Performance Evaluation Method for EMP Shielding Concrete. J. Korea Acad.-Ind. Coop. Soc. 2020, 21, 67–76. [Google Scholar]
- Min, T.B. An Experimental Study on the Development of EMP Shielding Concrete According to the Types of Aggregate of Industrial By-products. J. Korean Recycl. Constr. Resour. Inst. 2020, 8, 310–316. [Google Scholar]
- Min, T.B.; Kim, H.C.; Choi, H.K.; Roh, J.H.; Kim, K.J.; Park, Y.J. Experimental Study on the Development of EMP Shielded Concrete Using Industrial By-products. J. Korea Inst. Build. Constr. 2019, 19, 477–484. [Google Scholar]
- Min, T.B.; Cho, H.K. Evaluation of Electromagnetic Pulse Shielding Performance of Carbon Fiber-Mixed Cement Paste. Int. J. Concr. Struct. Mater. 2022, 16, 27. [Google Scholar] [CrossRef]
- ASTM D 4935-10; Standard Test Method for Measuring the Electromagnetic Shielding Effectiveness of Planar Materials. American Society of Testing Materials: West Conshohocken, PA, USA, 2010; p. 11.
- Saini, P.; Arora, M. Microwave Absorption and EMI Shielding Behavior of Nanocomposites Based on Intrinsically Conducting Polymers, Graphene and Carbon Nanotubes. In New Polymers for Special Applications; Gomes, A.D.S., Ed.; InTech: London, UK, 2012; pp. 71–112. [Google Scholar] [CrossRef]
- JIS K 7194; Testing Method for Resistivity of Conductive Plastics with a Four-Point Probe Array. Japanese Standards Association: Tokyo, Japan, 1994.
- Tudos, I.V.; Mouratis, K.; Ionescu, O.N.; Romanitan, C.; Pachiu, C.; Pricop, E.; Khomenko, V.H.; Butenko, O.; Chernysh, O.; Barsukov, V.Z.; et al. Carbon Allotropes-Based Paints and Their Composite Coatings for Electromagnetic Shielding Applications. Nanomaterials 2022, 12, 1839. [Google Scholar] [CrossRef] [PubMed]
- KS M 6040; Synthetic Resin emulsion Paints. Korea Agency for Technology and Standards: Eumseong-gun, Chungcheongbuk-do, Republic of Korea, 2014.
- KS D 8502; Liquid Epoxy Resin Paints for Water Works and Method of Coating. Korea Agency for Technology and Standards: Eumseong-gun, Chungcheongbuk-do, Republic of Korea, 2021.
- MIL-STD-188-125; High-Altitude Electromagnetic Pulse (HEMP) Protection for Ground-Based C4I Facilities Performing Critical, Tine-urgent Missions, Part 1 Fixed Facilities (Volume 1). Department of Defense: Arlington, VA, USA, 2005.









| Content | Graphite | Acetylene Black | Carbon Black |
|---|---|---|---|
| carbon content (%) | 96–99.9 | >95 | >95 |
| particle size (μm) | 2.0–7.0 | 0.035 | 6.5–12.5 |
| bulk density (g/L) | 2230 | 250 | 112 |
| Product | PH | Specific Gravity (20 ± 1 °C) | Nonvolatile Content (wt %, 20 ± 1 °C, 1 h) | Viscosity (cps, 20 ± 1 °C) |
|---|---|---|---|---|
| sodium silicate | 12–13 | 1.38 | - | 100 |
| potassium silicate | 11–12 | 1.33 | - | 50 |
| inorganic binder | 11–12 | 1.3–1.4 | 37 ± 1 | 200–400 |
| nanohybrid resins | 9–10 | 1.1–1.2 | 43 ± 1 | 50 |
| Mixture Weight (g) | |||||||
|---|---|---|---|---|---|---|---|
| Mixture (mix) | Graphite | Water | Sodium Silicate | Potassium Silicate | Inorganic Binder | Nanohybrid Resin | Electric Resistance (Ω) |
| mix 1-1 | 200 | 200 | - | - | - | - | 5.54 |
| mix 1-2 | 200 | - | 200 | - | - | - | 7.91 |
| mix 1-3 | 200 | - | - | 200 | - | - | 3.43 |
| mix 1-4 | 200 | - | - | - | 200 | - | 2.22 |
| mix 1-5 | 200 | - | - | - | - | 200 | 64.5 |
| mix 1-6 | 200 | 140 | 100 | - | - | - | 12.4 |
| mix 1-7 | 200 | 140 | 200 | - | - | - | 16.6 |
| mix 1-8 | 200 | 140 | 300 | - | - | - | 17.8 |
| mix 1-9 | 200 | 140 | 400 | - | - | - | 25.8 |
| mix 1-10 | 200 | 140 | - | 100 | - | - | 11.4 |
| mix 1-11 | 200 | 140 | - | 200 | - | - | 8.46 |
| mix 1-12 | 200 | 140 | - | 300 | - | - | 10.4 |
| mix 1-13 | 200 | 140 | - | 400 | - | - | 12.5 |
| mix 1-14 | 200 | 140 | - | - | 100 | - | 8.48 |
| mix 1-15 | 200 | 140 | - | - | 200 | - | 2.47 |
| mix 1-16 | 200 | 140 | - | - | 300 | - | 5.49 |
| mix 1-17 | 200 | 140 | - | - | 400 | - | 23.4 |
| Mixture Weight (g) | ||||||
|---|---|---|---|---|---|---|
| Mixture | Graphite | Acetylene Black | Carbon Black | Inorganic Binder | Water | Electric Resistance (Ω) |
| mix 2-1 | 200 | 20 | - | 200 | 400 | 29.6 |
| mix 2-2 | 200 | 30 | - | 200 | 440 | 21.3 |
| mix 2-3 | 200 | 40 | - | 200 | 460 | 29.1 |
| mix 2-4 | 200 | 50 | - | 200 | 480 | 55.3 |
| mix 2-5 | 200 | 60 | - | 200 | 500 | 66.4 |
| mix 2-6 | 200 | - | 20 | 200 | 400 | 5.25 |
| mix 2-7 | 200 | - | 30 | 200 | 440 | 4.31 |
| mix 2-8 | 200 | - | 40 | 200 | 460 | 2.64 |
| mix 2-9 | 200 | - | 50 | 200 | 480 | 7.21 |
| mix 2-10 | 200 | - | 60 | 200 | 500 | 7.94 |
| Mix | Adhesion Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | Average | |
| Mix 2-8 | 0.92 | 1.72 | 1.65 | 1.41 | 1.07 | 1.32 | 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, K.-P. Development and Experimental Verification of Inorganic Electromagnetic Pulse Shielding Paint for Building Interiors Using Carbon-Based Materials. Materials 2024, 17, 2863. https://doi.org/10.3390/ma17122863
Jang K-P. Development and Experimental Verification of Inorganic Electromagnetic Pulse Shielding Paint for Building Interiors Using Carbon-Based Materials. Materials. 2024; 17(12):2863. https://doi.org/10.3390/ma17122863
Chicago/Turabian StyleJang, Kyong-Pil. 2024. "Development and Experimental Verification of Inorganic Electromagnetic Pulse Shielding Paint for Building Interiors Using Carbon-Based Materials" Materials 17, no. 12: 2863. https://doi.org/10.3390/ma17122863
APA StyleJang, K.-P. (2024). Development and Experimental Verification of Inorganic Electromagnetic Pulse Shielding Paint for Building Interiors Using Carbon-Based Materials. Materials, 17(12), 2863. https://doi.org/10.3390/ma17122863


_Low.png)



