The Recent Applications of Magnetic Nanoparticles in Biomedical Fields
Abstract
1. Introduction
2. Characteristics of MNPs
2.1. Functional Characteristics of MNPs
2.2. Biocompatibility of MNPs
3. Functional Surface Coatings on MNPs
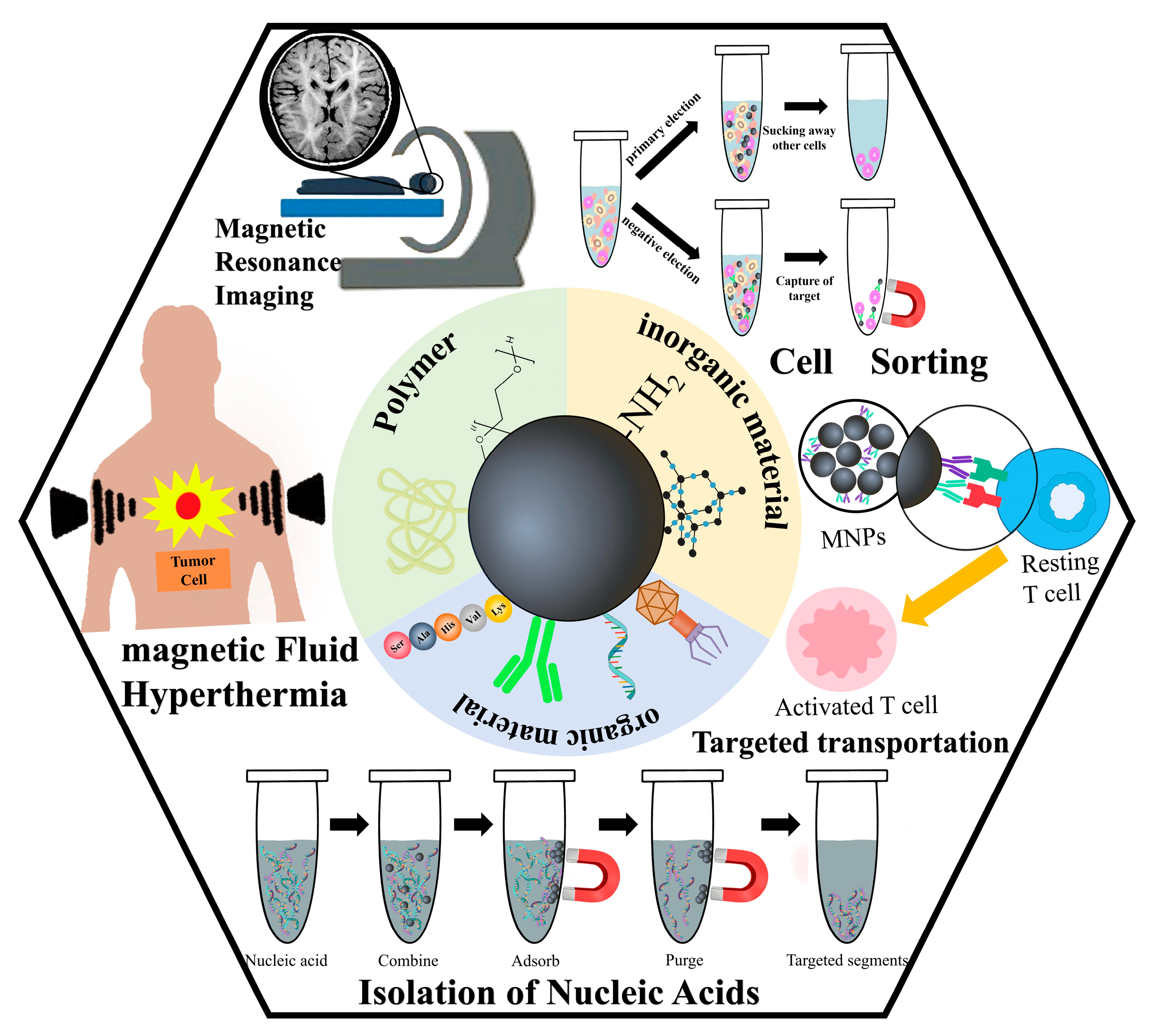
3.1. Inorganic Material
3.2. Multifunctional Organic Material
3.3. Polymer Material
4. Applications of MNPs in Biomedical Fields
4.1. Applications of MNPs in Medical Imaging Technology
4.2. Applications of MNPs in the Treatment of Diseases
4.2.1. Applications of MNPs in Magnetic Fluid Hyperthermia
4.2.2. Drug Delivery and Targeting
4.3. Applications of MNPs in Biomedical Assays
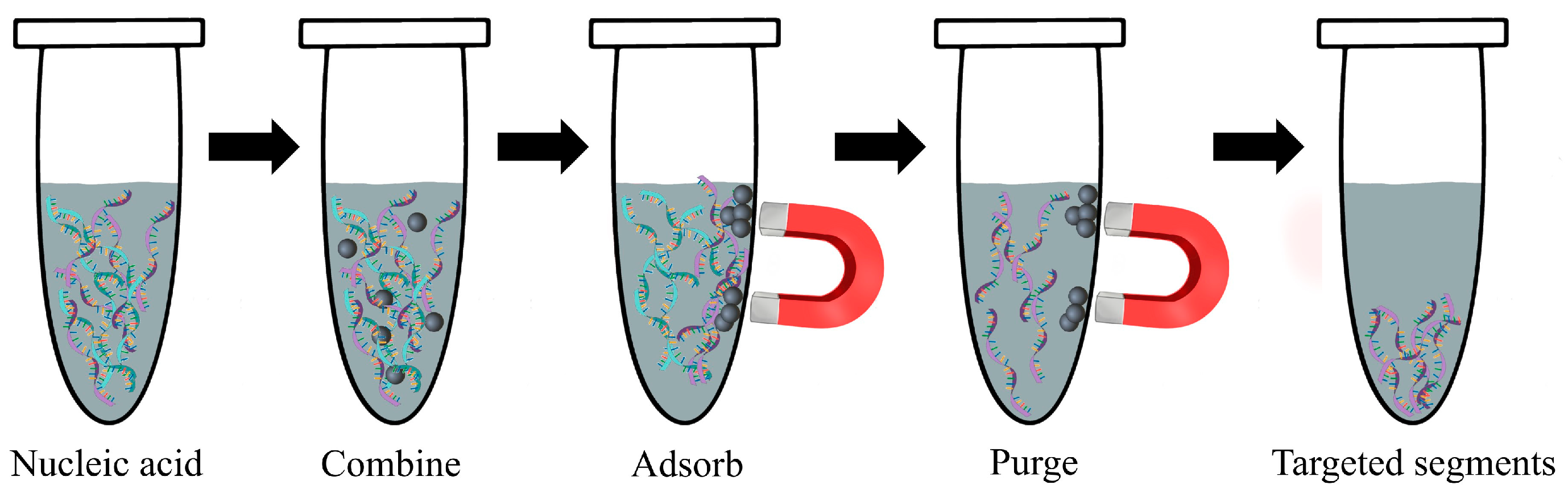
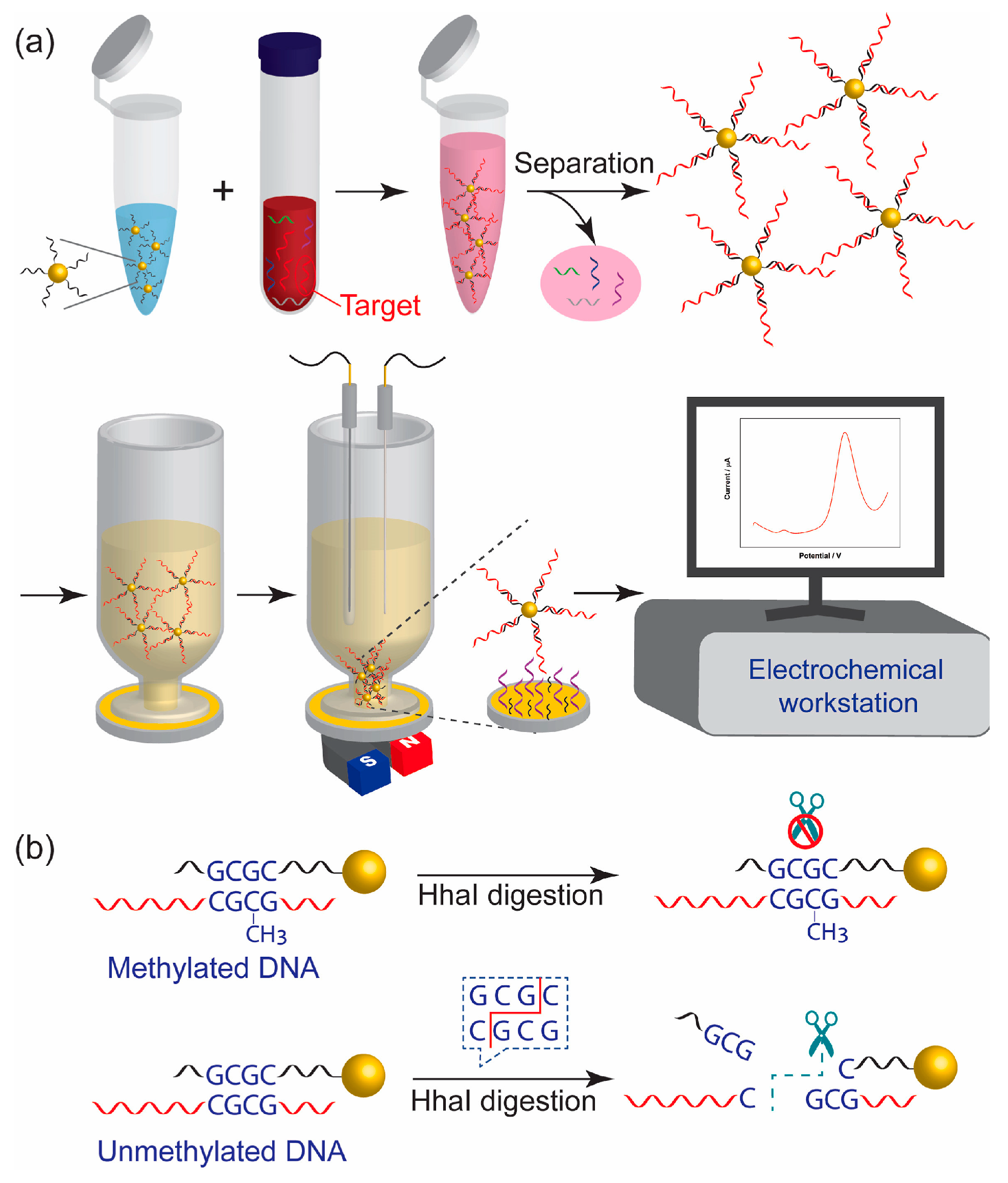
4.3.1. Detection of Nucleic Acid
4.3.2. Cell Separation
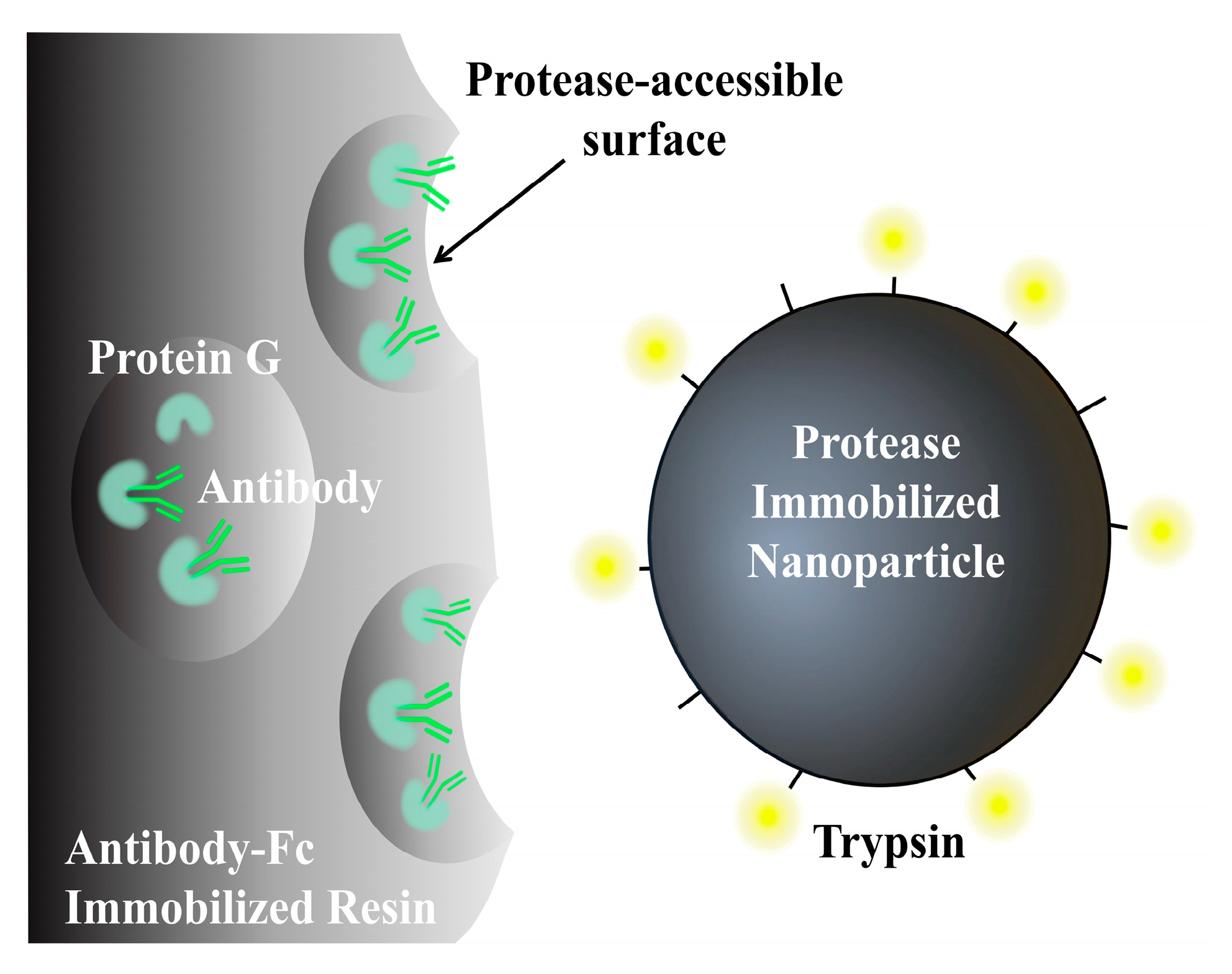
4.3.3. Determination of Other Biomolecules
4.4. Other Applications of MNPs in the Treatment of Diseases
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kianfar, E. Magnetic nanoparticles in targeted drug delivery: A review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Hosu, O.; Tertis, M.; Cristea, C. Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry 2019, 5, 55. [Google Scholar] [CrossRef]
- Gessner, I.; Park, J.H.; Lin, H.Y. Magnetic gold nanoparticles with idealized coating for enhanced point-of-care sensing. Adv. Healthc. Mater. 2022, 11, 2102035. [Google Scholar] [CrossRef]
- Daya, R.; Xu, C.; Nguyen, N.Y.T. Angiogenic hyaluronic acid hydrogels with curcumin-coated magnetic nanoparticles for tissue repair. ACS Appl. Mater. Interfaces 2022, 14, 11051–11067. [Google Scholar] [CrossRef] [PubMed]
- Katz, E. Magnetic nanoparticles. Magnetochemistry 2020, 6, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Zhang, A. Multifunctional co-loaded magnetic nanocapsules for enhancing targeted MR imaging and in vivo photodynamic therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102047. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Jing, W. Dual-modality and Noninvasive Diagnostic of MNP–PEG–Mn Nanoprobe for Renal Fibrosis Based on Photoacoustic and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2023, 15, 12797–12808. [Google Scholar] [CrossRef]
- Ma, R.; Hou, Y.; Wang, S. Imaging-Guided Cancer Therapy Based on Multifunctional Magnetic Nanoparticles. Chin. J. Chem. 2023, 41, 2557–2573. [Google Scholar] [CrossRef]
- Wong, J.; Prout, J.; Seifalian, A. Magnetic nanoparticles: New perspectives in drug delivery. Curr. Pharm. Des. 2017, 23, 2908–2917. [Google Scholar] [CrossRef]
- Wang, N.; Guan, Y.; Yang, L. Magnetic nanoparticles (MNPs) covalently coated by PEO–PPO–PEO block copolymer for drug delivery. J. Colloid Interface Sci. 2013, 395, 50–57. [Google Scholar] [CrossRef]
- Demessie, A.A.; Park, Y.; Singh, P. An Advanced Thermal Decomposition Method to Produce Magnetic Nanoparticles with Ultrahigh Heating Efficiency for Systemic Magnetic Hyperthermia. Small Methods 2022, 6, 2200916. [Google Scholar] [CrossRef]
- Kaushik, S.; Thomas, J.; Panwar, V.A. drug-free strategy to combat bacterial infections with magnetic nanoparticles biosynthesized in bacterial pathogens. Nanoscale 2022, 14, 1713–1722. [Google Scholar] [CrossRef]
- Willis, A.J.; Pernal, S.P.; Gaertner, Z.A. Rotating magnetic nanoparticle clusters as microdevices for drug delivery. Int. J. Nanomed. 2020, 15, 4105–4123. [Google Scholar] [CrossRef]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E. Magnetic targeting of nanotheranostics enhances cerenkov radiation-induced photodynamic therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef] [PubMed]
- Ostroverkhov, P.; Semkina, A.; Naumenko, V. HSA—Coated magnetic nanoparticles for MRI-guided photodynamic cancer therapy. Pharmaceutics 2018, 10, 284. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Li, L. Bacterial magnetic nanoparticles for photothermal therapy of cancer under the guidance of MRI. Biomaterials 2016, 104, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, I.B.; Hatekah, S.W.; Yaya, A. Photothermally-heated superparamagnetic polymeric nanocomposite implants for interstitial thermotherapy. Nanomaterials 2022, 12, 955. [Google Scholar] [CrossRef]
- Kang, S.; Baskaran, R.; Ozlu, B. T1-positive Mn2+-doped multi-stimuli responsive poly (L-DOPA) nanoparticles for photothermal and photodynamic combination cancer therapy. Biomedicines 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, W.; Xu, H. Advances in magnetic nanoparticles for the separation of foodborne pathogens: Recognition, separation strategy, and application. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4478–4504. [Google Scholar] [CrossRef]
- Azadpour, B.; Aharipour, N.; Paryab, A. Magnetically-assisted viral transduction (magnetofection) medical applications: An update. Biomater. Adv. 2023, 154, 213657. [Google Scholar] [CrossRef]
- Guo, Y.; Li, S.; Wang, Y. Diagnosis–Therapy integrative systems based on magnetic RNA nanoflowers for Co-drug delivery and targeted therapy. Anal. Chem. 2017, 89, 2267–2274. [Google Scholar] [CrossRef]
- Farooq, A.; Tosheva, L.; Azzawi, M. Real-time observation of aortic vessel dilation through delivery of sodium nitroprusside via slow release mesoporous nanoparticles. J. Colloid Interface Sci. 2016, 478, 127–135. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Wang, Z. Automated detection system of single nucleotide polymorphisms using two kinds of functional magnetic nanoparticles. Appl. Surf. Sci. 2008, 255, 600–603. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible iron oxide nanoparticles for targeted cancer gene therapy: A review. Nanomaterials 2022, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Brestovac, B.; Harnett, G.B.; Smith, D.W. Multiplex nested PCR (MNP) assay for the detection of 15 high risk genotypes of human papillomavirus. J. Clin. Virol. 2005, 33, 116–122. [Google Scholar] [CrossRef]
- Jun, Y.; Seo, J.; Cheon, J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef]
- Bai, S.; Hou, S.; Chen, T. Magnetic nanoparticle-mediated hyperthermia: From heating mechanisms to cancer theranostics. Innov. Mater. 2024, 2, 100051-1–100051-16. [Google Scholar] [CrossRef]
- Fallows, T.W.; McGrath, A.J.; Silva, J. High-throughput chemical and chemoenzymatic approaches to saccharide-coated magnetic nanoparticles for MRI. Nanoscale Adv. 2019, 1, 3597–3606. [Google Scholar] [CrossRef]
- Quan, K.; Zhang, Z.; Ren, Y. Possibilities and impossibilities of magnetic nanoparticle use in the control of infectious biofilms. J. Mater. Sci. Technol. 2021, 69, 69–78. [Google Scholar] [CrossRef]
- Bruschi, M.L.; de Toledo, L.A.S. Pharmaceutical applications of iron-oxide magnetic nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S. Principles of magnetic hyperthermia: A focus on using multifunctional hybrid magnetic nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M. Magnetic nanynchronous disintegration of ferroptosis defense axis via engineered exosome-conjugated magnetic nanopaapplications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, X.; Qiu, W. Synchronous disintegration of ferroptosis defense axis via engineered exosome-conjugated magnetic nanoparticles for glioblastoma therapy. Adv. Sci. 2022, 9, 2105451. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Mehmood, S.; Rasheed, T. Bio-catalysis and biomedical perspectives of magnetic nanoparticles as versatile carriers. Magnetochemistry 2019, 5, 42. [Google Scholar] [CrossRef]
- Slimani, Y.; Hannachi, E.; Tombuloglu, H. Magnetic nanoparticles based nanocontainers for biomedical application. In Smart Nanocontainers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 229–250. [Google Scholar]
- Bao, S.; Yang, W.; Wang, Y. One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd (II) and Pb (II) removal from aqueous solution. J. Hazard. Mater. 2020, 381, 120914. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.V.; Ngo, N.M.; Medhi, R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Abd Elrahman, A.A.; Mansour, F.R. Targeted magnetic iron oxide nanoparticles: Preparation, functionalization and biomedical application. J. Drug Deliv. Sci. Technol. 2019, 52, 702–712. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Hepel, M. Magnetic Nanoparticles for Nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef]
- Sabale, S.; Kandesar, P.; Jadhav, V. Recent developments in the syn-thesis, properties, and biomedical applications of core/shell superparamagnetic iron oxide nanoparticles with gold. Biomater. Sci. 2017, 5, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, P.; Wu, X. Multifunctional biosensor constructed by Ag-coating magnetic-assisted unique urchin core porous shell structure for dual SERS enhancement, enrichment, and quantitative detection of multi-components inflammatory markers. Biosens. Bioelectron. 2022, 210, 114257. [Google Scholar] [CrossRef] [PubMed]
- Alyassin, Y.; Sayed, E.G.; Mehta, P. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov. Today 2020, 25, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.L.; Shahed-Al-Mahmud, M.; Selvaprakash, K. Tail fiber protein-immobilized magnetic nanoparticle-based affinity approaches for detection of Acinetobacter baumannii. Anal. Chem. 2019, 91, 10335–10342. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J. Bio-medical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Yang, D.; Yang, G.; Yang, P. Assembly of Au Plasmonic Photothermal Agent and Iron Oxide Nanoparticles on Ultrathin Black Phosphorus for Targeted Photothermal and Photodynamic Cancer Therapy. Adv. Funct. Mater. 2017, 27, 1700371. [Google Scholar] [CrossRef]
- Bai, Y.; Roncancio, D.; Suo, Y. A method based on amino-modified magnetic nanoparticles to extract DNA for PCR-based analysis. Colloids Surf. B Biointerfaces 2019, 7, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Zhang, F. High-efficient nucleic acid separation from animal tissue samples via surface modified magnetic nanoparticles. Sep. Purif. Technol. 2021, 262, 118348. [Google Scholar] [CrossRef]
- Zhou, Z.; Kadam, U.S.; Irudayaraj, J. One-stop genomic DNA extraction by salicylic acid-coated magnetic nanoparticles. Anal. Biochem. 2013, 442, 249–252. [Google Scholar] [CrossRef]
- Sahoo, S.L.; Liu, C.H. Adsorption behaviors of DNA by modified magnetic nanoparticles: Effect of spacer and salt. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 184–194. [Google Scholar] [CrossRef]
- Maeda, Y.; Toyoda, T.; Mogi, T. DNA recovery from a single bacterial nanoparticles. Colloids Surf. B Biointerfaces 2016, 139, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, K.; Barr, J.R.; Kalb, S.R. Detection of ricin activity and structure by using novel galactose-terminated magnetic bead extraction coupled with mass spectrometric detection. Anal. Biochem. 2021, 631, 114364. [Google Scholar] [CrossRef]
- Abdelaziz, M.M.; Hefnawy, A.; Anter, A. Respirable spray dried vancomycin coated magnetic nanoparticles for localized lung delivery. Int. J. Pharm. 2022, 611, 121318. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Fang, H.; Chen, Q. M13 bacteriophage as biometric component for orderly assembly of dynamic light scattering immunosensor. Biosens. Bioelectron. 2022, 217, 114693. [Google Scholar] [CrossRef] [PubMed]
- Antal, I.; Strbak, O.; Khmara, I. MRI Relaxivity Changes of the Magnetic Nanoparticles Induced by Different Amino Acid Coatings. Nanomaterials 2020, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Kuan, W.C.; Lai, J.W.; Lee, W.C. Covalent binding of glutathione on magnetic nanoparticles: Application for immobilizing small fragment ubiquitin-like-specific protease 1. Enzym. Microb. Technol. 2021, 143, 109697. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. Magnetic Nanoparticle-Containing Supports as Carriers of Immobilized Enzymes: Key Factors Influencing the Biocatalyst Performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A. DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378, 13. [Google Scholar] [CrossRef] [PubMed]
- Millart, E.; Lesieur, S.; Faivre, V. Superparamagnetic lipid-based hybrid nanosystems for drug delivery. Expert Opin. Drug Deliv. 2018, 15, 523–540. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Bao, L. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat. Biomed. Eng. 2020, 4, 1063–1075. [Google Scholar] [CrossRef]
- Sizikov, A.A.; Kharlamova, M.V.; Nikitin, M.P. Nonviral locally injected magnetic vectors for in vivo gene delivery: A review of studies on magnetofection. Nanomaterials 2021, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- You, S.M.; Jeong, K.B.; Luo, K. based colorimetric detection of pathogenic bacteria in food through magnetic separation and enzyme-mediated signal amplification on paper disc. Anal. Chim. Acta 2021, 1151, 338252. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Wang, Y.; Chen, G. Poly-l-lysine-functionalized magnetic beads combined with polymerase chain reaction for the detection of Staphylococcus aureus and Escherichia coli O157: H7 in milk. Dairy Sci 2021, 104, 12342–12352. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yao, X.; Zhong, Z. Rapid and sensitive detection of Staphylococcus aureus assisted by polydopamine modified magnetic nanoparticles. Talanta 2018, 186, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.J.; Agliardi, G.; Lin, F.Y. Potential of magnetic hyperthermia to stimulate localized immune activation. Small 2021, 17, 2005241. [Google Scholar] [CrossRef]
- Veloso, S.R.; Tiryaki, E.; Spuch, C. Tuning the drug multimodal release through a co-assembly strategy based on magnetic gels. Nanoscale 2022, 14, 5488–5500. [Google Scholar] [CrossRef]
- Cremin, K.; Jones, B.A.; Teahan, J. Scanning ion conductance microscopy reveals differences in the ionic environments of gram-positive and negative bacteria. Anal. Chem. 2020, 92, 16024–16032. [Google Scholar] [CrossRef]
- Etemadi, H.; Buchanan, J.K.; Kandile, N.G. Iron oxide nanoparticles: Physicochemical characteristics and historical developments to commercialization for potential technological applications. ACS Biomater. Sci. Eng. 2021, 7, 5432–5450. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 287–307. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Jiao, J. A hepatocyte-targeting nanoparticle for enhanced hepatobiliary magnetic resonance imaging. Nat. Biomed. Eng. 2023, 7, 221–235. [Google Scholar] [CrossRef]
- Thomas, G.; Boudon, J.; Maurizi, L. Innovative magnetic nanoparticles for PET/MRI bimodal imaging. ACS Omega 2019, 4, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Myrovali, E.; Maniotis, N.; Samaras, T. Spatial focusing of magnetic particle hyperthermia. Nanoscale Adv. 2020, 2, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, A.; Sidhu, N.; Hu, C.A.A. Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. Am. J. Roentgenol. 2015, 204, W302–W313. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W. Superparamagnetic iron oxides as MPI tracers: A primer and review of early applications. Adv. Drug Deliv. Rev. 2019, 138, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Wang, M.; Xiong, C. Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J. Mater. Res. 2019, 34, 1828–1844. [Google Scholar] [CrossRef]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W. Clinical magnetic hyperthermia requires integrated magnetic particle imaging. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef] [PubMed]
- Marchal, S.; Hor, A.E.; Millard, M. Anticancer drug delivery: An update on clinically applied nanotherapeutics. Drugs 2015, 75, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D.; Lindner, L.H.; Verweij, J. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: The EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- van Landeghem, F.K.; Maier-Hauff, K.; Jordan, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef]
- Toraya-Brown, S.; Sheen, M.R.; Zhang, P. Local hyperthermia treatment of tumors induces CD8+ T cell-mediated resistance against distal and secondary tumors. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1273–1285. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Pan, J. Magneto-based synergetic therapy for im-plant-associated infections via biofilm disruption and innate immunity regulation. Adv. Sci. 2021, 8, 2004010. [Google Scholar] [CrossRef]
- Atluri, V.S.R.; Jayant, R.D.; Pilakka-Kanthikeel, S. Development of TIMP1 magnetic nanoformulation for regulation of synaptic plasticity in HIV-1 infection. Int. J. Nanomed. 2016, 11, 4287–4298. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.P.; Le, T.A.; Yoon, J. A magnetic particle imaging-based navigation platform for magnetic nano-particles using interactive manipulation of a virtual field free point to ensure targeted drug delivery. IEEE Trans. Ind. Electron. 2020, 68, 12493–12503. [Google Scholar] [CrossRef]
- Gul, S.; Khan, S.B.; Rehman, I.U. A comprehensive review of magnetic nanomaterials modern day theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, F.; Sun, B. Magnetic nanoparticles: A new diagnostic and treatment platform for rheumatoid arthritis. J. Leucoc. Biol. 2021, 109, 415–424. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T. Identification of a primary target of thalidomide teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.M.; McLean, G.R. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, T.; Ullah, R. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Han, X.; Cao, M.; Zhou, B.; Yu, C. Specifically immobilizing His-tagged allergens to magnetic nanoparticles for fast and quantitative detection of allergen-specific IgE in serum samples. Talanta 2022, 19, 121301. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Paca, A.M. Tris (dithiocarbamato) iron (III) complexes as precursors for iron sulfide nano-crystals and iron sulfide-hydroxyethyl cellulose composites. J. Sulfur Chem. 2019, 40, 52–64. [Google Scholar] [CrossRef]
- Pires, F.; Silva, J.C.; Ferreira, F.C. Heparinized Acellular Hydrogels for Magnetically In-duced Wound Healing Applications. ACS Appl. Mater. Interfaces 2024, 16, 9908–9924. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Zhang, A.; Wang, R. Using single antigen specificity magnetic beads for the isolation of specific antibodies against HLA antigens. HLA 2024, 103, e15490. [Google Scholar] [CrossRef] [PubMed]
- Källsten, M.; Ghorasaini, M.; Hartmann, R. Magnetic beads for desalting of monoclonal antibodies and antibody–drug Conjugates. Anal. Chem. 2020, 92, 9001–9007. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, F.; Zhao, S. Immunomagnetic separation method integrated with the Strep-Tag II system for rapid enrichment and mild release of exosomes. Anal. Chem. 2023, 95, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Wu, P. Development of a highly sensitive detection method for TTX based on a magnetic bead-aptamer competition system under triple cycle amplification. Anal. Chim. Acta 2020, 1119, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Khadsai, S.; Seeja, N.; Rutnakornpituk, M. Selective enrichment of zein gene of maize from cereal products using magnetic support having pyrrolidinyl peptide nucleic acid probe. Food Chem. 2021, 338, 127812. [Google Scholar] [CrossRef]
- Nan, K.; He, M.; Chen, B. Histidine tag modified magnetic beads for analysis of arsenic binding proteins. Anal. Chim. Acta 2024, 1304, 342554. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Leu, C.C.; Lin, C.C. Gold-decorated magnetic nanoparticles modified with hairpin-shaped DNA for fluorometric discrimination of single-base mismatch DNA. Microchim. Acta 2019, 186, 80. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Shi, Y. Magnetic particles for integrated nucleic acid purification, amplification and detection without pipetting. TrAC Trends Anal. Chem. 2020, 127, 115912. [Google Scholar] [CrossRef]
- Xuhong, Y.; Sinong, Z.; Jianping, L. A PCR-lateral flow assay system based on gold magnetic nanoparticles for CYP2C19 genotyping and its clinical applications. Artif. Cells Nanomed. Biotechnol. 2019, 47, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Kelkar, R.K.; Pise, P.V. The un-tapped potential of magnetic nanoparticles for forensic investigations: A comprehensive review. Talanta 2021, 230, 122297. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, J.; Ma, W. Low background cascade signal amplification electrochemical sensing platform for tumor-related mRNA quantification by target-activated hybridization chain reaction and electroactive cargo release. Anal. Chem. 2018, 90, 12544–12552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Y.; Chen, H. Design and application of automatic and rapid nucleic acid extractor using magnetic nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 6998–7004. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Jia, Y. An automatic high-throughput single nucleotide polymorphism genotyping approach based on universal tagged arrays and magnetic nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 689–698. [Google Scholar] [CrossRef]
- Wang, L.; Yao, M.; Fang, X. Novel competitive chemiluminescence DNA assay based on Fe3O4@ SiO2@Au-functionalized magnetic nanoparticles for sensitive detection of p53 tumor suppressor gene. Appl. Biochem. Biotechnol. 2019, 187, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, Y.; Tilley, R.D. Rapid and ultrasensitive electrochemical detection of DNA methylation for ovarian cancer diagnosis. Biosens. Bioelectron. 2022, 206, 114126. [Google Scholar] [CrossRef]
- Iwamoto, N.; Shimada, T.; Umino, Y. Selective detection of complementarity-determining regions of monoclonal antibody by limiting protease access to the substrate: Nano-surface and molecular-orientation limited proteolysis. Analyst 2014, 139, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jung, S.H.; Seo, H. Magnetic activated cell sorting (MACS) pipette tip for immunomagnetic bacteria separation. Sens. Actuators B Chem. 2018, 272, 324–330. [Google Scholar] [CrossRef]
- González-Ravina, C.; Castro, A.P.; Palomino, M.C. P–081 Microfluidic sorting does not improve clinical outcomes compared to magnetic activated cell sorting (MACS) in Assisted Re-production. Hum. Reprod. 2021, 36, deab130-080. [Google Scholar] [CrossRef]
- Adams, J.D.; Kim, U.; Soh, H.T. Multitarget magnetic activated cell sorter. Proc. Natl. Acad. Sci. USA 2008, 105, 18165–18170. [Google Scholar] [CrossRef]
- Wang, L.; Lin, H.; Zhang, J. Phage long tail fiber protein-immobilized magnetic nanoparticles for rapid and ultrasensitive detection of Salmonella. Talanta 2022, 248, 123627. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V. Human Schwann Cells in vitro II. Passaging, Purification, Banking, and Labeling of Established Cultures. Bio-Protocol 2023, 13, e4882. [Google Scholar] [CrossRef]
- Yang, G.; Huang, M.; Wang, Y. Streptavidin-exposed magnetic nanoparticles for lectin magnetic separation (LMS) of Staphylococcus aureus prior to three quantification strategies. Microchim. Acta 2019, 186, 813. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, S.Y.; Xie, P. Rapid Assessment of Surface Markers on Cancer Cells Using Immuno-Magnetic Separation and Multi-frequency Impedance Cytometry for Targeted Therapy. Sci. Rep. 2020, 10, 3015. [Google Scholar] [CrossRef]
- Wang, L.; Balasubramanian, P.; Chen, A.P. Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin. Oncol. 2016, 43, 464–475. [Google Scholar] [CrossRef]
- FDA Approves CliniMACS CD34 Reagent System for the Prevention of Graft-vs-Host Disease in AML. Available online: https://www.ascopost.com/issues/february-15-2014/fda-approves-clinimacs-cd34-reagent-system-for-the-prevention-of-graft-vs-host-disease-in-aml/ (accessed on 14 November 2020).
- Manousi, N.; Rosenberg, E.; Deliyanni, E. Magnetic solid-phase extraction of organic compounds based on graphene oxide nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef] [PubMed]
- Mylkie, K.; Nowak, P.; Rybczynski, P. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Nitsche, C.; Orton, H. Paramagnetic Chemical Probes for Studying Biological Macromolecules. Chem. Rev. 2022, 122, 9571–9642. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Zhang, X. Novel aerosol detection platform for SARS-CoV-2: Based on specific magnetic nanoparticles adsorption sampling and digital droplet PCR detection. Chin. Chem. Lett. 2023, 34, 107701. [Google Scholar] [CrossRef]
- Kavetskyy, T.; Alipour, M.; Smutok, O. Magneto-immunoassay of cancer biomarkers: Recent progress and challenges in biomedical analysis. Microchem. J. 2021, 167, 106320. [Google Scholar] [CrossRef]
- Gheorghiu, E. Detection of pathogenic bacteria by magneto-immunoassays: A review. J. Biomed. Res. 2021, 35, 277. [Google Scholar] [CrossRef] [PubMed]
- Chikkaveeraiah, B.V.; Mani, V.; Patel, V. Microfluidic electrochemical immunoarray for ultrasensitive detection of two cancer biomarker proteins in serum. Biosens. Bioelectron. 2011, 26, 4477–4483. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Canales, A.; Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2017, 2, 16093. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, J.G.; Spizzo, F.; Morales, M.P. Nanoparticles for Magnetic Heating: When Two (or More) Is Better Than One. Materials 2021, 14, 6416. [Google Scholar] [CrossRef]
- Van de Walle, A.; Perez, J.E.; Abou-Hassan, A. Magnetic nanoparticles in regenerative medicine: What of their fate and impact in stem cells? Mater. Today Nano 2020, 11, 100084. [Google Scholar] [CrossRef]

| Organic Molecules | Mechanism of Action | Appliance | Reference |
|---|---|---|---|
| amino group | The positively charged surfaces of MNPs facilitate electrostatic interactions and hydrogen bonding | Attachment of groups, binding of DNA, or capture of bacteria | Bai et al. [48] |
| carboxyl group (-COOH) | The carboxyl group facilitates the formation of ionic bridges between sodium ions in solution and the phosphate groups of nucleic acid molecules | Linkage groups, specific adsorption of nucleic acids | Li et al. [49] |
| Salicylic acid (SA) | Introducing carboxylic acid and phenolic functional groups onto MNPs | Make MNPs have good adsorption properties | Zhou et al. [50] |
| Acridine Orange, (ACO) | ACO is a cell-permeable fluorescent and water-soluble stain, while MNPs@ACO exhibits the ability to interact with DNA and RNA through embedding or electrostatic attraction | binding nucleic acid | Sahoo et al. [51] |
| Imidazole (IMI) | The charge of MNPs@IMI can reach neutrality and exhibits reversible charge behavior upon pH modification | adsorbing DNA by electrostatic action | Maeda et al. [52] |
| agglutinin | This sugar-binding protein possesses one or more glycosyl binding sites within its three-dimensional structure, enabling it to interact with peptidoglycan and lipopolysaccharide present on the surface of diverse cell types, thereby inducing agglutination or glycoconjugate precipitation in a wide range of cellular contexts | Binding bacteria in a broad spectrum | Kaitlin et al. [53] |
| antibiotics | Antibiotic-modified MNPs exhibit antibacterial activity through specific binding interactions with bacterial surface structures | Specific recognition of ligands, target drugs | Abdelaziz et al. [54] |
| bacteriophage | The phage tail fibers exhibit a recognition and binding capability toward bacteria | Biometric ligands, specific isolation of target pathogens | Zhan et al. [55] |
| amino acids | Numerous side-chain amino acids possess plentiful carboxyl, hydroxyl, and sulfhydryl groups, thereby offering a significant quantity of binding sites for nanoparticles | Functionalized modification, capture of bacteria | Antal et al. [56] |
| polypeptides | Selective and potent signaling molecules that bind to specific cell surface receptors (e.g., G protein-coupled receptors or ion channels) to trigger intracellular effects | Specific recognition of ligands and target drugs | Kuan et al. [57] |
| enzymes | MNPs@enzyme serves to safeguard enzyme activity while concurrently functioning as a magnetic separation and recovery tool | Enzyme-carrier complexes with high stability and selectivity | Matveeva et al. [58] |
| streptomycin (antibiotic) | Streptavidin demonstrates a high degree of specificity and a robust affinity for tetrameric biotin binding | Commonly used as affinity-adsorbed MNPs for biological use | Sosa-Acosta et al. [59] |
| liposome | Magnetic-fluid-loaded liposomes (MFLs) possess a positively charged surface that enables them to interact with phosphorylates in DNA. MFLs have the ability to adsorb to cell membranes, which are negatively charged, and subsequently enter the cell through membrane depressions, thereby leveraging the benefits of both magnetic materials and liposomes | Carriers of targeted drugs | Millart et al. [60] |
| antibodies | Antibody-modified MNPs, commonly referred to as immunomagnetic beads, exhibit specific binding capabilities to antigens | Specific binding ligands and targeting drugs | Liu et al. [61] |
| aptamers | 1. The recognition of ligands occurs through the mutual alignment of spatial conformations, resulting in high selectivity and affinity for their respective targets. 2. The termini of aptamer sequences may be adorned with a variety of functional groups or molecules to facilitate chemical modification and sensing, including but not limited to amino, carboxyl, biotin, and fluorescein | Acting as affinity adsorption and specific binding, applying in the fields of magnetic transfection and gene therapy | Sizikov et al. [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Wang, L.; Zheng, Q.; Cai, C.; Yang, X.; Liao, Z. The Recent Applications of Magnetic Nanoparticles in Biomedical Fields. Materials 2024, 17, 2870. https://doi.org/10.3390/ma17122870
Hong J, Wang L, Zheng Q, Cai C, Yang X, Liao Z. The Recent Applications of Magnetic Nanoparticles in Biomedical Fields. Materials. 2024; 17(12):2870. https://doi.org/10.3390/ma17122870
Chicago/Turabian StyleHong, Jiaqi, Linhao Wang, Qikai Zheng, Changyu Cai, Xiaohua Yang, and Zhenlin Liao. 2024. "The Recent Applications of Magnetic Nanoparticles in Biomedical Fields" Materials 17, no. 12: 2870. https://doi.org/10.3390/ma17122870
APA StyleHong, J., Wang, L., Zheng, Q., Cai, C., Yang, X., & Liao, Z. (2024). The Recent Applications of Magnetic Nanoparticles in Biomedical Fields. Materials, 17(12), 2870. https://doi.org/10.3390/ma17122870





