Preparation and Characterization of Graphite–SiO2 Composites for Thermal Storage Cement-Based Materials
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Materials
2.2. Experiments
2.2.1. Preparation of MPCMs and C@SiO2
2.2.2. Preparation of Thermal Storage Cement Mortars
2.3. Characterization Methods
2.3.1. Microscopic Morphology
2.3.2. Phase Change Properties
2.3.3. Workability
2.3.4. Mechanical Property Test
2.3.5. Thermal Conductivity
3. Results and Discussion
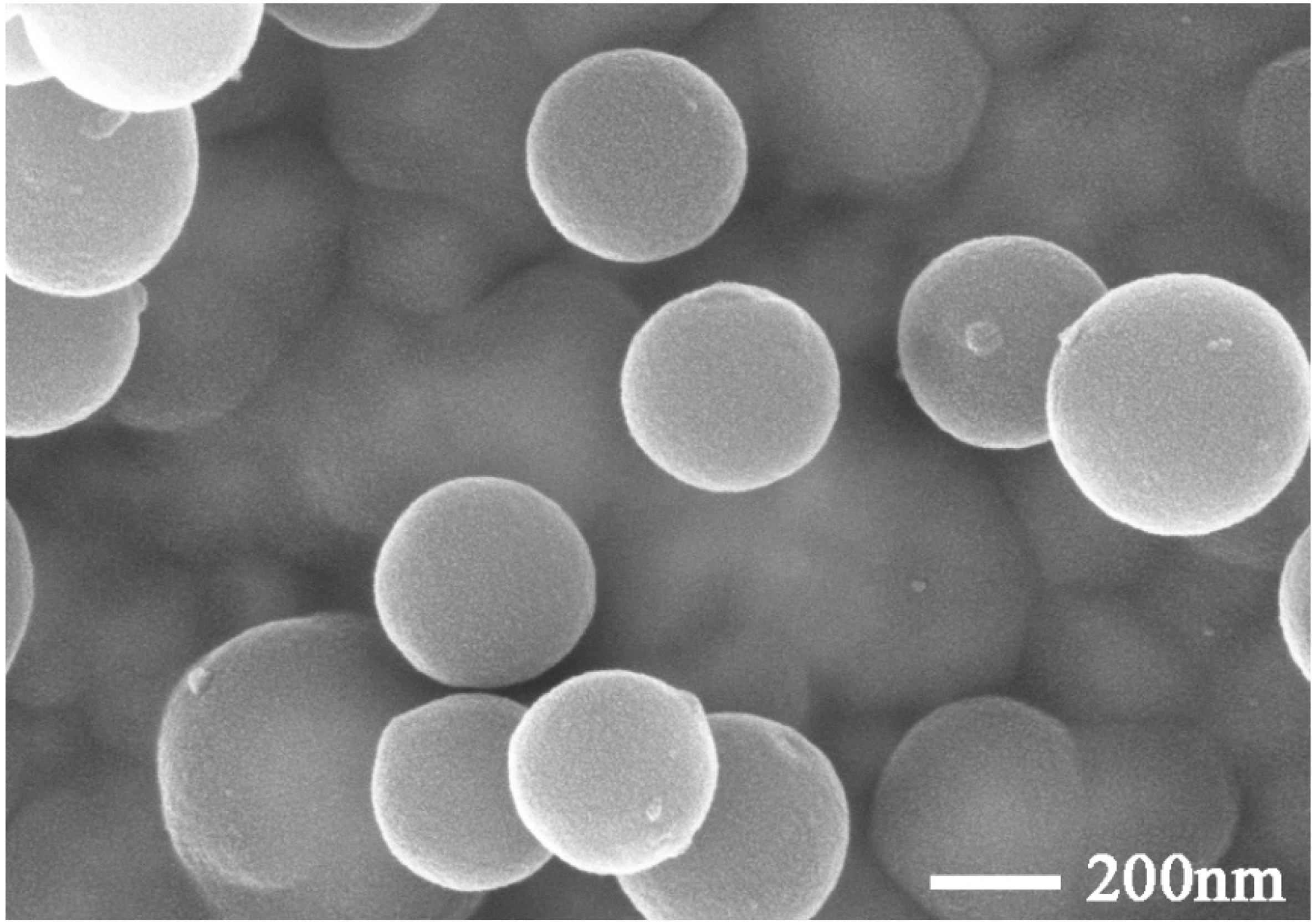
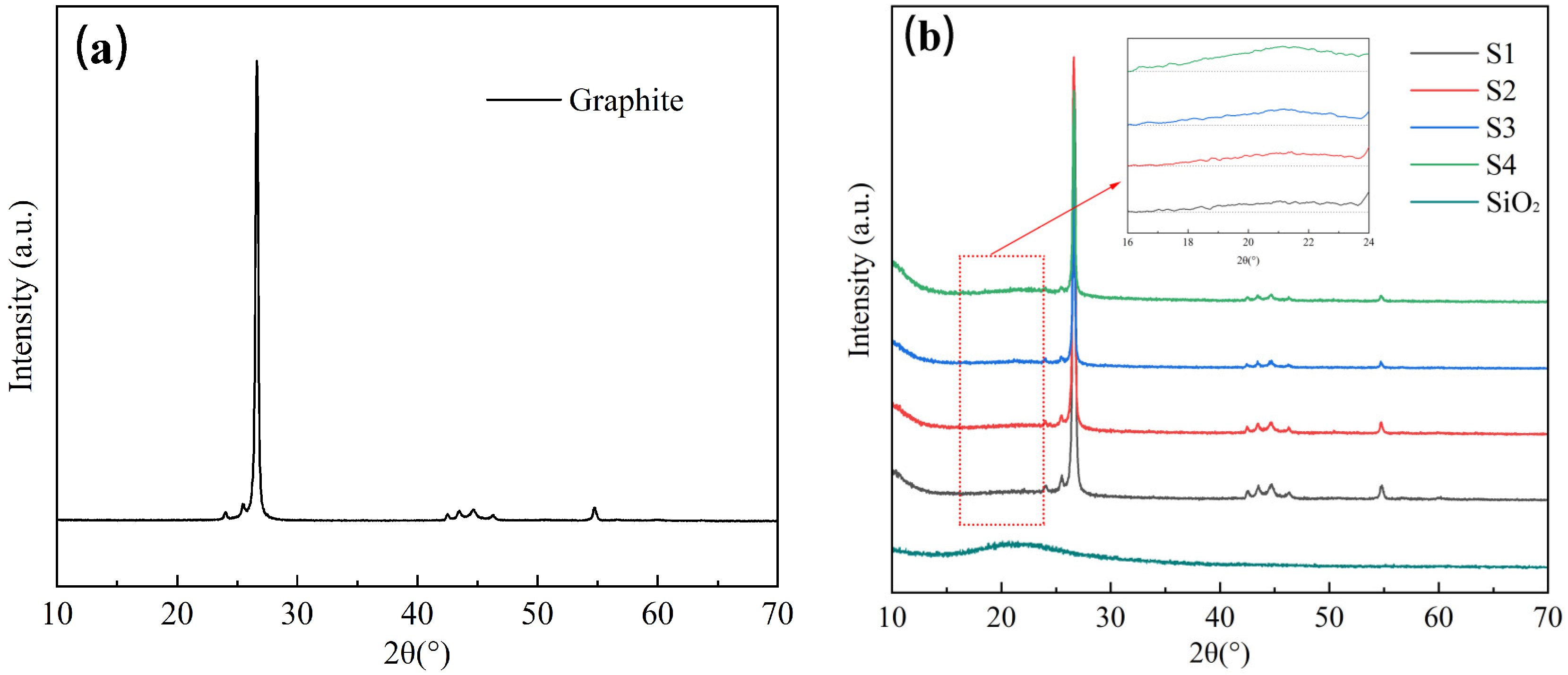
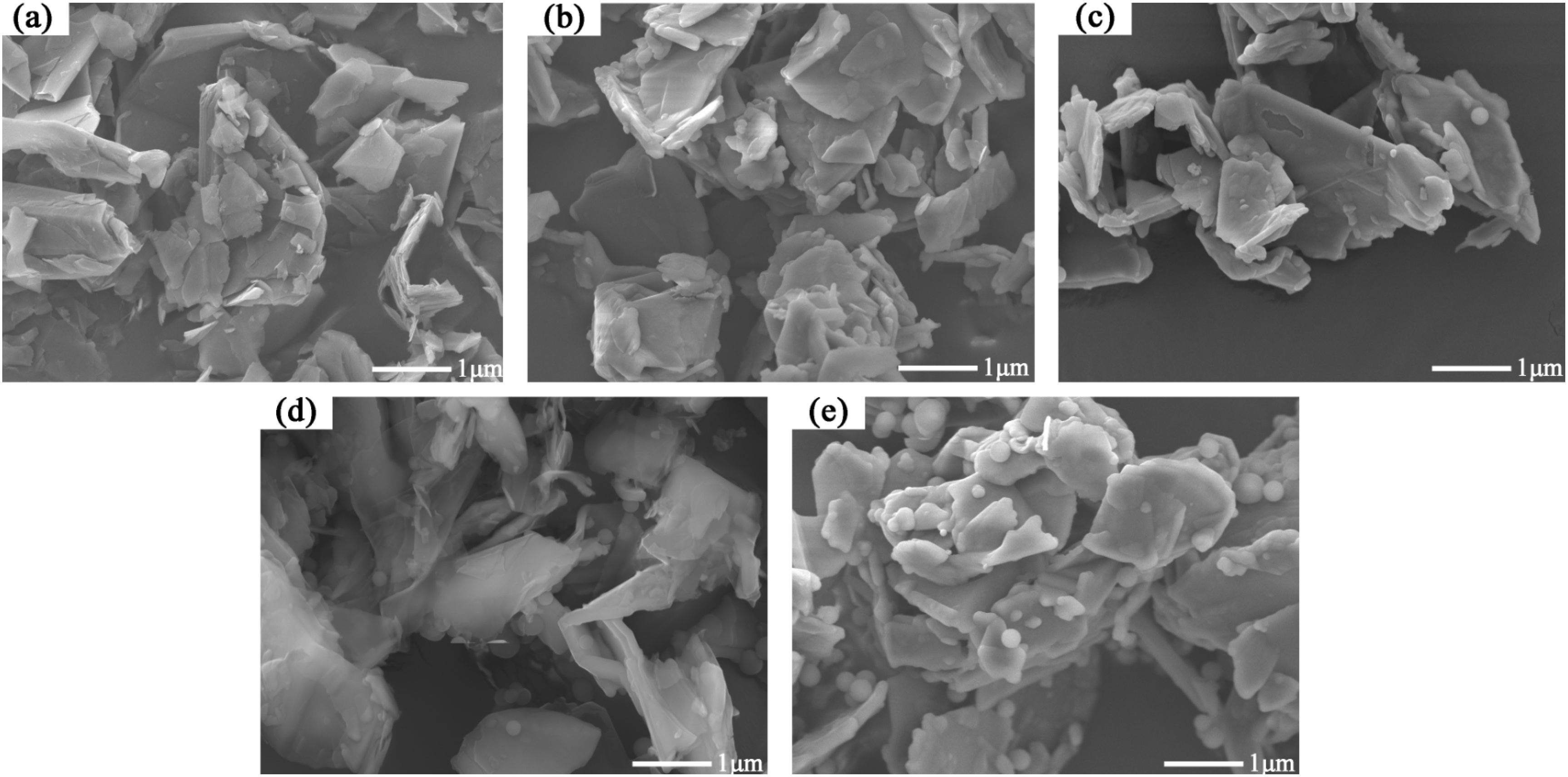
3.1. Preparation and Properties of C@SiO2
3.2. Effect of C@SiO2 on Workability of TSCM
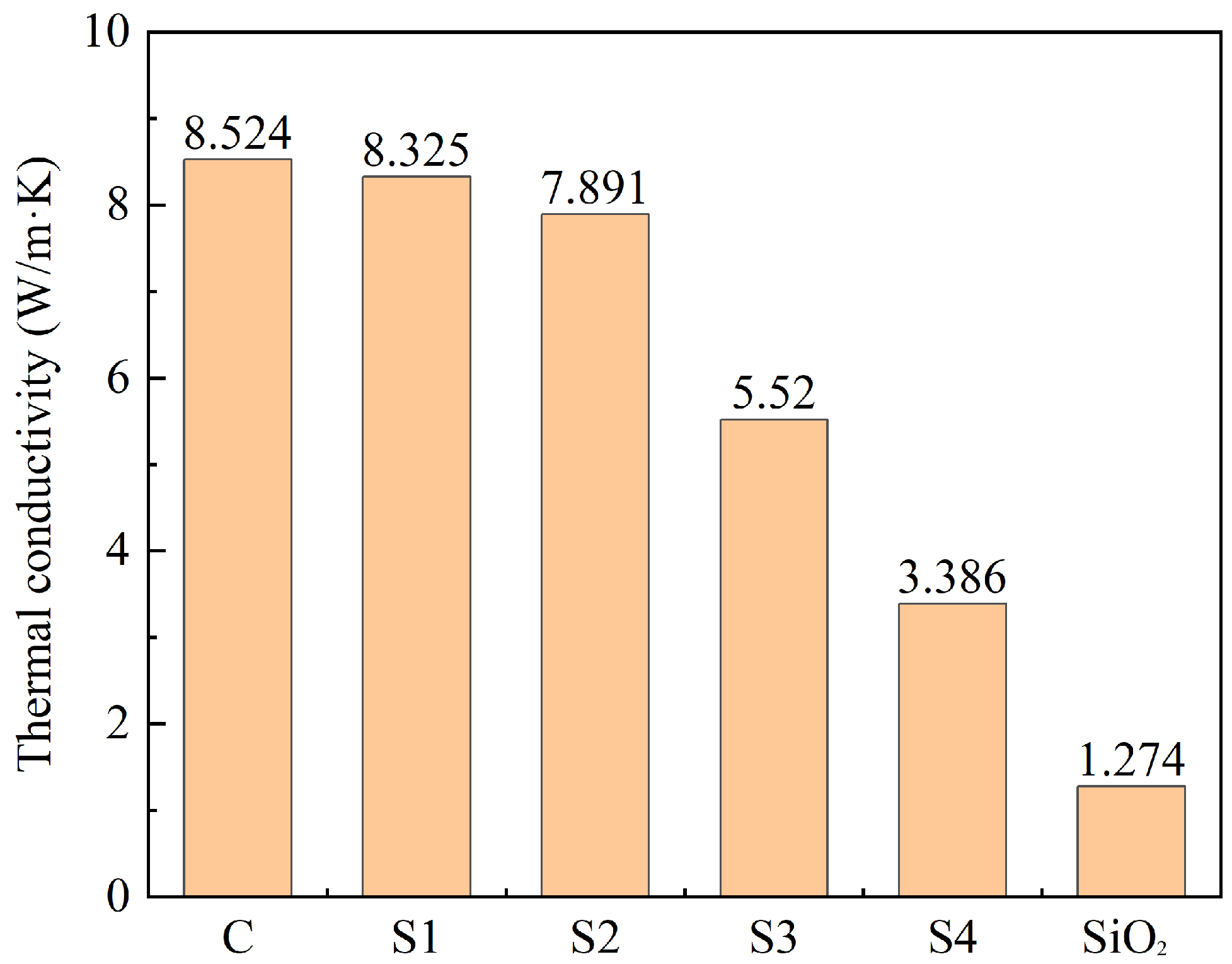
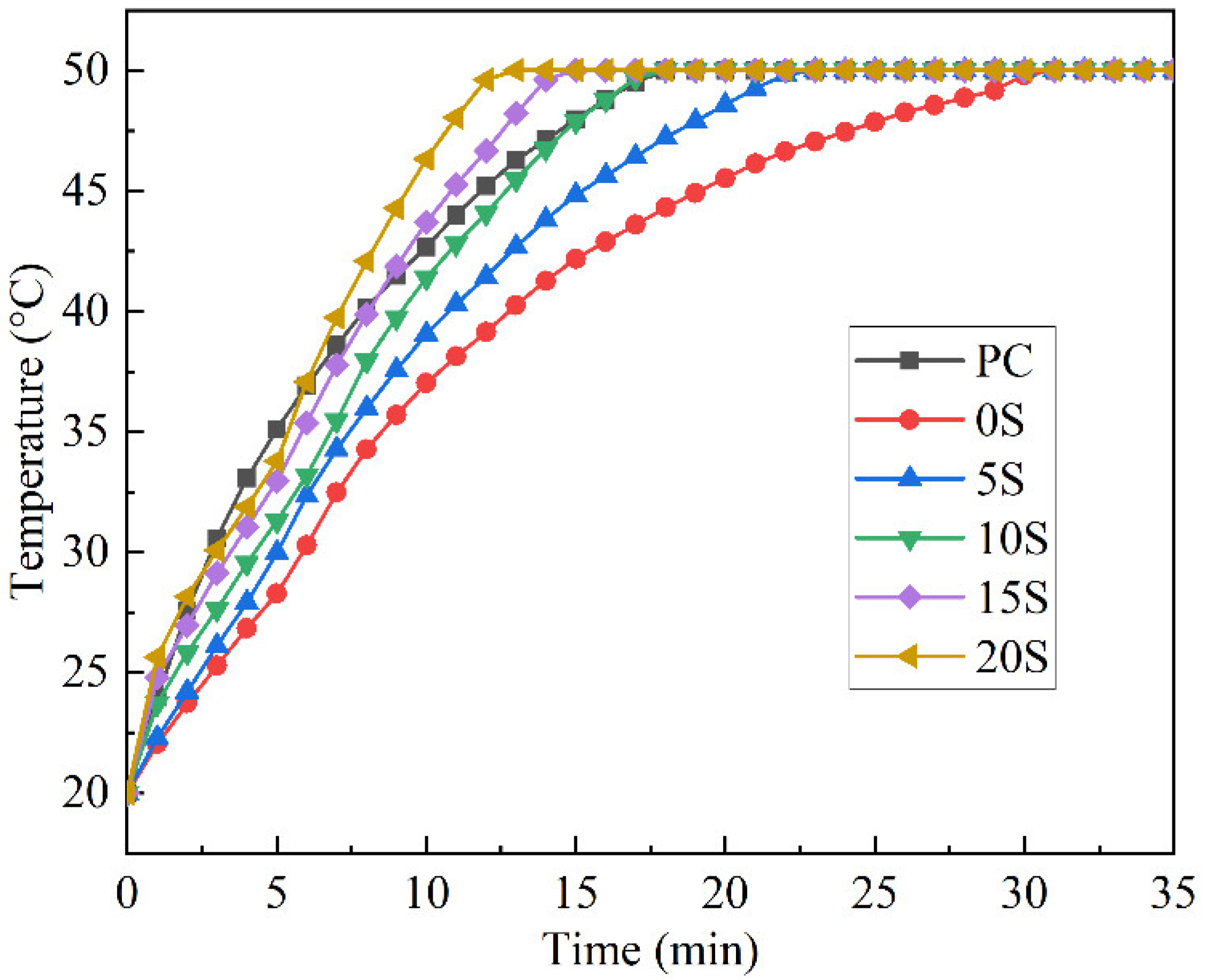
3.3. Effect of C@SiO2 on Thermal Storage Performance of TSCM
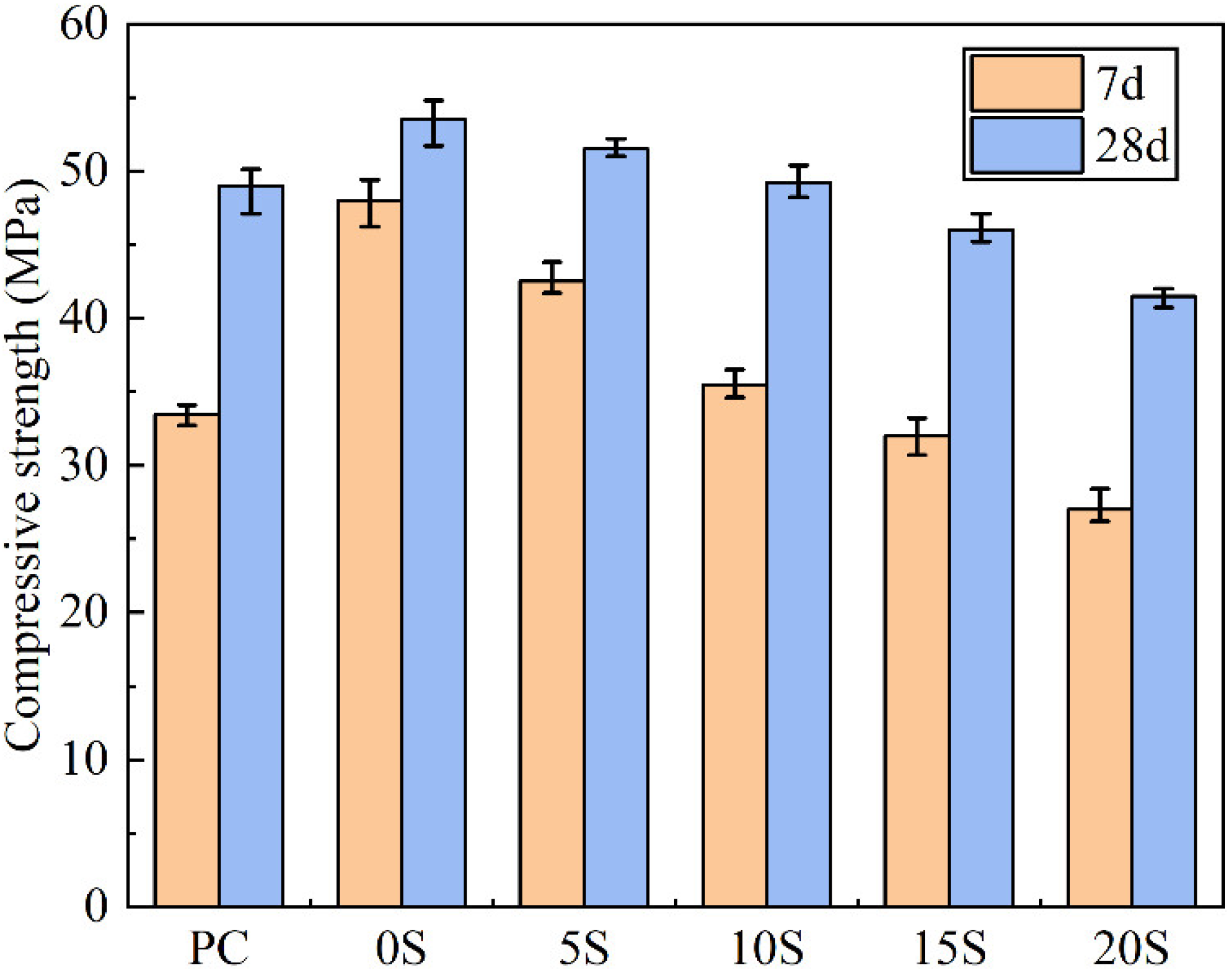
3.4. Effect of C@SiO2 on Compressive Strength of TSCMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Liu, L.; Li, R.; Guo, M.; Wang, L.; Zhang, M. One step synthesis of novel CuS-ZnS/SiO2@n-octadecane nanocapsules with full spectral absorption range and high photothermal conversion efficiency. Thermochim. Acta 2023, 726, 179539. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailor-made for thermal energy storage cement-based composites. Energy 2014, 72, 371–380. [Google Scholar] [CrossRef]
- Woo, H.Y.; Chae, D.; Son, S.; Hwang, H.; Yoon, T.; Lim, H.; Wooh, S.; Lee, H.; Paik, T. Passive daytime radiative cooling with thermal energy storage using phase change n-octadecane/SiO2 nanobeads. Opt. Mater. 2023, 139, 113812. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Bui, T.Q.; Carmona, M.; Rodriguez, J.F.; Kjøniksen, A.-L. Thermal performance and numerical simulation of geopolymer concrete containing different types of thermoregulating materials for passive building applications. Energy Build. 2018, 173, 678–688. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Valentini, L.; Carmona, M.; Rodriguez, J.F.; Kjøniksen, A.-L. Influence of microcapsule size and shell polarity on thermal and mechanical properties of thermoregulating geopolymer concrete for passive building applications. Energy Convers. Manag. 2018, 164, 198–209. [Google Scholar] [CrossRef]
- Aguayo, M.; Das, S.; Maroli, A.; Kabay, N.; Mertens, J.C.E.; Rajan, S.D.; Sant, G.; Chawla, N.; Neithalath, N. The influence of microencapsulated phase change material (PCM) characteristics on the microstructure and strength of cementitious composites: Experiments and finite element simulations. Cem. Concr. Compos. 2016, 73, 29–41. [Google Scholar] [CrossRef]
- Šavija, B.; Zhang, H.; Schlangen, E.J.M. Influence of microencapsulated phase change material (PCM) addition on (micro) mechanical properties of cement paste. Materials 2017, 10, 863. [Google Scholar] [CrossRef]
- Yu, B.; Li, S.; Zhu, H.; Jiang, Q.; Wang, D.; Chen, Y. A composite phase change material for improving the freeze–thaw resistance performance of cement mortars. Constr. Build. Mater. 2023, 387, 131657. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Karaipekli, A.; Al-Sulaiman, F.A. Preparation, characterization and thermal regulation performance of cement based-composite phase change material. Sol. Energy Mater. Sol. Cells 2018, 174, 523–529. [Google Scholar] [CrossRef]
- Sarı, A.; Nas, M.; Yeşilata, B.; Ustaoğlu, A.; Erdoğmuş, E.; Torlaklı, H.; Hekimoğlu, G.; Gencel, O. A novel cement mortar comprising natural zeolite/dodecyl alcohol shape stable composite phase change material for energy effective buildings. J. Energy Storage 2024, 87, 111266. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Zhang, C.; Yang, H.; Liu, B. Thermal performance and mechanical properties of phase change cement paste with Nano-SiO2 grafted straw-paraffin. Constr. Build. Mater. 2024, 419, 135551. [Google Scholar] [CrossRef]
- Cunha, S.; Sarcinella, A.; Aguiar, J.; Frigione, M. Perspective on the Development of Energy Storage Technology Using Phase Change Materials in the Construction Industry: A Review. Energies 2023, 16, 4806. [Google Scholar] [CrossRef]
- Sharma, R.; Jang, J.G.; Hu, J.W. Phase-Change Materials in Concrete: Opportunities and Challenges for Sustainable Construction and Building Materials. Materials 2022, 15, 335. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, B.; Gong, K.C. Preparation and performance of shape stabilized phase change thermal storage materials with high thermal conductivity. Energy Convers. Manag. 2002, 43, 103–108. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Impregnation of porous material with phase change material for thermal energy storage. Mater. Chem. Phys. 2009, 115, 846–850. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, Y.; Xu, X.; Di, H.; Yang, R.; Qin, P. Experimental study of under-floor electric heating system with shape-stabilized PCM plates. Energy Build. 2005, 37, 215–220. [Google Scholar] [CrossRef]
- Lai, C.-m.; Chen, R.H.; Lin, C.-Y. Heat transfer and thermal storage behaviour of gypsum boards incorporating micro-encapsulated PCM. Energy Build. 2010, 42, 1259–1266. [Google Scholar] [CrossRef]
- Wu, M.Q.; Li, T.X.; Wang, P.F.; Wu, S.; Wang, R.Z.; Lin, J. Dual-Encapsulated Highly Conductive and Liquid-Free Phase Change Composites Enabled by Polyurethane/Graphite Nanoplatelets Hybrid Networks for Efficient Energy Storage and Thermal Management. Small 2022, 18, 2105647. [Google Scholar] [CrossRef]
- Ju, S.; Luo, Q.; Lu, Z.; Wang, F.; Shi, J.; Wang, L.; Liu, Z.; Jiang, J. A novel thermal-tailored strategy to mitigate thermal cracking of cement-based materials by carbon fibers and liquid-metal-based microencapsulated phase change materials. Constr. Build. Mater. 2024, 428, 136338. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Brown, R.C.; Rasberry, J.D.; Overmann, S.P. Microencapsulated phase-change materials as heat transfer media in gas-fluidized beds. Powder Technol. 1998, 98, 217–222. [Google Scholar] [CrossRef]
- Lu, S.; Shen, T.; Xing, J.; Song, Q.; Shao, J.; Zhang, J.; Xin, C. Preparation and characterization of cross-linked polyurethane shell microencapsulated phase change materials by interfacial polymerization. Mater. Lett. 2018, 211, 36–39. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Chen, L.J.; Song, G.L.; Tang, G.Y. Facile and low energy consumption synthesis of microencapsulated phase change materials with hybrid shell for thermal energy storage. J. Phys. Chem. Solids 2017, 111, 207–213. [Google Scholar] [CrossRef]
- Pilehvar, S.; Cao, V.D.; Szczotok, A.M.; Valentini, L.; Salvioni, D.; Magistri, M.; Pamies, R.; Kjoniksen, A.L. Mechanical properties and microscale changes of geopolymer concrete and Portland cement concrete containing micro-encapsulated phase change materials. Cem. Concr. Res. 2017, 100, 341–349. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Assessing the feasibility of integrating form-stable phase change material composites with cementitious composites and prevention of PCM leakage. Mater. Lett. 2017, 192, 88–91. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Lu, Z.; Li, Z. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl. Energy 2015, 160, 358–367. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Mankel, C.; Caggiano, A.; Ukrainczyk, N.; Koenders, E. Thermal energy storage characterization of cement-based systems containing microencapsulated-PCMs. Constr. Build. Mater. 2019, 199, 307–320. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Ansu, A.K.; Goyal, R. Study of thermal properties of organic phase change materials for energy storage. Mater. Today Proc. 2020, 28, 2353–2357. [Google Scholar] [CrossRef]
- Huang, X.; Alva, G.; Jia, Y.T.; Fang, G.Y. Morphological characterization and applications of phase change materials in thermal energy storage: A review. Renew. Sustain. Energy Rev. 2017, 72, 128–145. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Soliman, F.S.; El Maghraby, H.; Moustfa, Y.M. Thermal conductivity enhancement of treated petroleum waxes, as phase change material, by α nano alumina: Energy storage. Renew. Sustain. Energy Rev. 2017, 70, 1052–1058. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Li, Y.; Yin, X. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–435. [Google Scholar] [CrossRef]
- Raj, C.R.; Suresh, S.; Bhavsar, R.R.; Kumar Singh, V.; Reddy, S. Effect of nano-gallium capsules on thermal energy storage characteristics of manganese organometallic SS-PCM. Thermochim. Acta 2019, 680, 178341. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Ramadan, M.; Atiqure Rahman, S.M.; Abdelkareem, M.A. Recent progress on Carbon-based nanomaterial for phase change materials: Prospects and challenges. Therm. Sci. Eng. Prog. 2021, 23, 100920. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, G.; Wang, S.; Fang, X.; Liu, X. Thermal energy storage cement mortar containing n-octadecane/expanded graphite composite phase change material. Renew. Energy 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rao, Z. Experimental study on the thermal performance of graphene and exfoliated graphite sheet for thermal energy storage phase change material. Thermochim. Acta 2017, 647, 15–21. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Fleischer, A.S. Improved heat recovery from paraffin-based phase change materials due to the presence of percolating graphene networks. Int. J. Heat Mass Transf. 2014, 79, 314–323. [Google Scholar] [CrossRef]
- Zou, L.; Kang, F.Y.; Zheng, Y.P.; Shen, W.C. Modified natural flake graphite with high cycle performance as anode material in lithium ion batteries. Electrochim. Acta 2009, 54, 3930–3934. [Google Scholar] [CrossRef]
- Rui, X.; Geng, Y.; Zhuang, M.; Xiao, S.; Sun, X. Emergy-based environmental accounting of graphite anode material production. J. Clean. Prod. 2022, 339, 130705. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Q.; Fang, Z. Effect of graphite on the self-sensing properties of cement and alkali-activated fly ash/slag based composite cementitious materials. J. Build. Eng. 2023, 77, 107493. [Google Scholar] [CrossRef]
- Sheng, K.; Li, D.; Yuan, X. Methyl orange assisted dispersion of graphene oxide in the alkaline environment for improving mechanical properties and fluidity of ordinary portland cement composites. J. Build. Eng. 2021, 43, 103166. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qin, Y.; Liang, S.; Chen, K.; Tian, C.; Wang, J.; Luo, X.; Zhang, L. Graphene/SiO2/n-octadecane nanoencapsulated phase change material with flower like morphology, high thermal conductivity, and suppressed supercooling. Appl. Energy 2019, 250, 98–108. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Miao, W.; Jiang, B.; Su, Y.; He, X.; Ma, M.; Strnadel, B. Mechanical and microstructure development of portland cement modified with micro-encapsulated phase change materials. Constr. Build. Mater. 2021, 304, 124652. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, X.; Miao, X.; Zhang, Y.; Guo, M.; Cheng, F.; Zhang, M. Preparation and characterization of SiO2@n-octadecane capsules with controllable size and structure. Thermochim. Acta 2021, 705, 179037. [Google Scholar] [CrossRef]
- GB175-2007; Common Portland Cement. Standards Press of China: Beijing, China, 2007. (In Chinese)
- da Silva, A.d.S.; Dos Santos, J.H.Z. Stöber method and its nuances over the years. Adv. Colloid Interface Sci. 2023, 314, 102888. [Google Scholar] [CrossRef]
- GB/T17671–2021; Test Method of Cement Mortar Strength (ISO Method). Standards Press of China: Beijing, China, 2021. (In Chinese)
- GB/T 13464-2008; Thermal Analysis Test Methods for Thermal Stability of Materials. Standards Press of China: Beijing, China, 2008. (In Chinese)
- GB/T2419-2005; The Method for Fluidity of Cement Mortar. Standards Press of China: Beijing, China, 2005. (In Chinese)



| Thermal Enthalpy | Initial Melting Temperature | Thermal Conductivity Coefficient | |
|---|---|---|---|
| MPCM | 124.6 J/g | 22.8 °C | 0.48 W/m·K |
| Oxide | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 21.5 | 5.1 | 2.8 | 62.9 | 1.4 | 0.8 | 0.1 | 2.3 | 2.8 |
| Code | Graphite (g) | TEOS (g) |
|---|---|---|
| S1 | 6 | 4 |
| S2 | 5 | 5 |
| S3 | 4 | 6 |
| S4 | 3 | 7 |
| Code | Cement (vol%) | MPCM (vol%) | C@SiO2 (vol%) | b/s | w/b |
|---|---|---|---|---|---|
| PC | 100 | 0 | 0 | 1/3 | 0.5 |
| 0S | 90 | 10 | 0 | ||
| 5S | 85 | 10 | 5 | ||
| 10S | 80 | 10 | 10 | ||
| 15S | 75 | 10 | 15 | ||
| 20S | 70 | 10 | 20 |
| No. | C | Si | O |
|---|---|---|---|
| S1 | 90.01 | 2.61 | 7.38 |
| S2 | 81.78 | 4.88 | 13.34 |
| S3 | 75.97 | 6.57 | 17.46 |
| S4 | 65.88 | 9.16 | 24.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Li, X.; Lv, Y.; Dan, J.; Yan, H.; Shi, X. Preparation and Characterization of Graphite–SiO2 Composites for Thermal Storage Cement-Based Materials. Materials 2024, 17, 2880. https://doi.org/10.3390/ma17122880
He C, Li X, Lv Y, Dan J, Yan H, Shi X. Preparation and Characterization of Graphite–SiO2 Composites for Thermal Storage Cement-Based Materials. Materials. 2024; 17(12):2880. https://doi.org/10.3390/ma17122880
Chicago/Turabian StyleHe, Chenhao, Xiangguo Li, Yang Lv, Jianming Dan, Haitian Yan, and Xiangqin Shi. 2024. "Preparation and Characterization of Graphite–SiO2 Composites for Thermal Storage Cement-Based Materials" Materials 17, no. 12: 2880. https://doi.org/10.3390/ma17122880




