New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys
Abstract
:1. Introduction
2. Methodology and Experiments
- A known weight of powder alloy (~1 g per sample) was placed in the reaction chamber of the magnetic suspension balance. The system was sealed and evacuated to a vacuum of <5 Pa.
- The alloy was then activated by exposure to a low hydrogen pressure of ~100 kPa at room temperature for 1 h to reduce the oxides on the surfaces of the powder particles. To remove the absorbed hydrogen, the sample was then heated to 350 °C in a vacuum of ~2 Pa.
- Following activation, the first hydrogen absorption measurement was performed by reducing the sample temperature to 200 °C and by filling the reaction chamber with hydrogen to a pressure of 2 MPa. The increase in the sample’s weight due to hydrogen absorption was recorded for 1 h.
- After the absorption measurement, the reaction chamber was evacuated and the sample was heated to 370 °C, causing the desorption of hydrogen. The weight loss during this process was recorded.
- A second absorption cycle was conducted under the same conditions as the first.
3. Results and Discussion
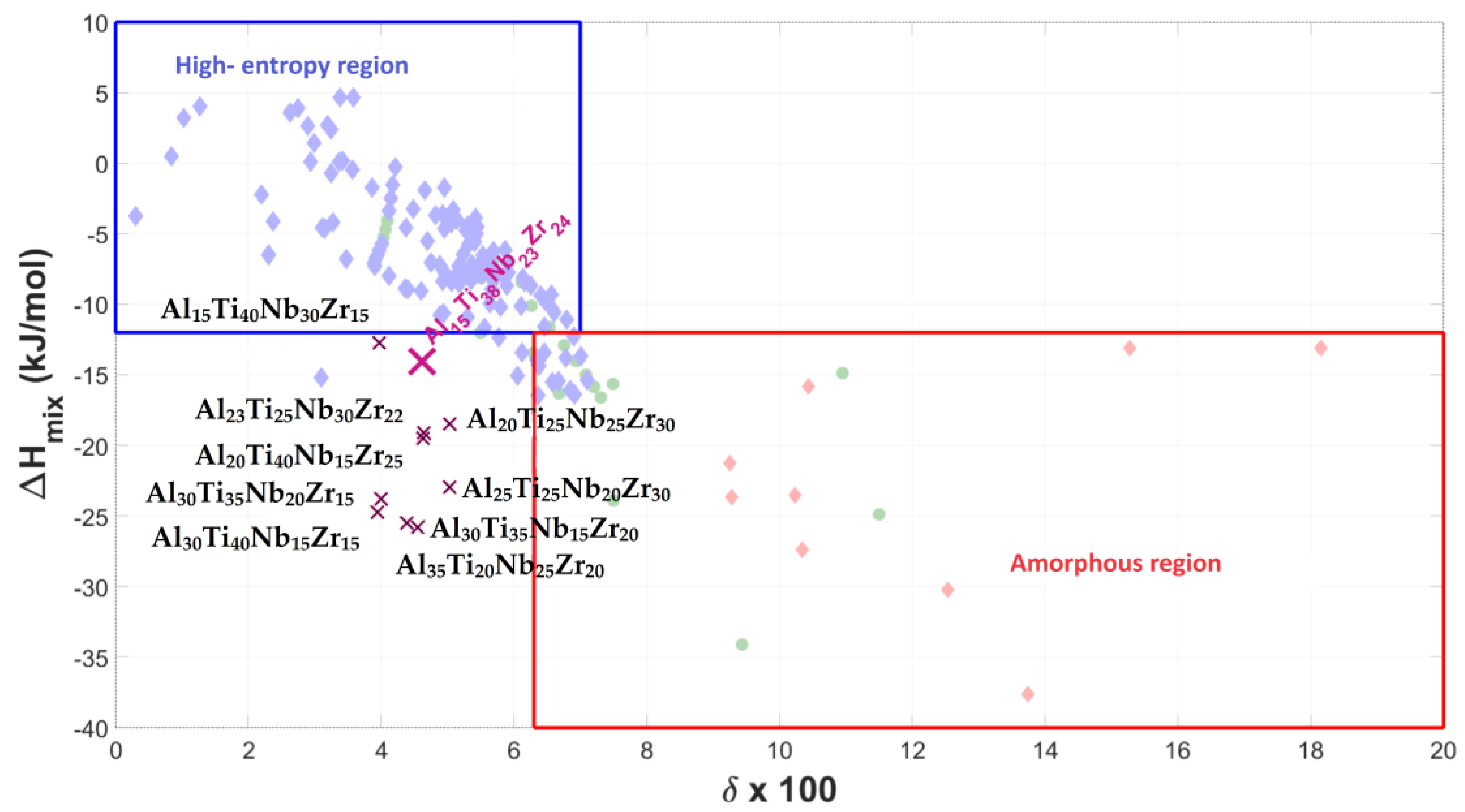
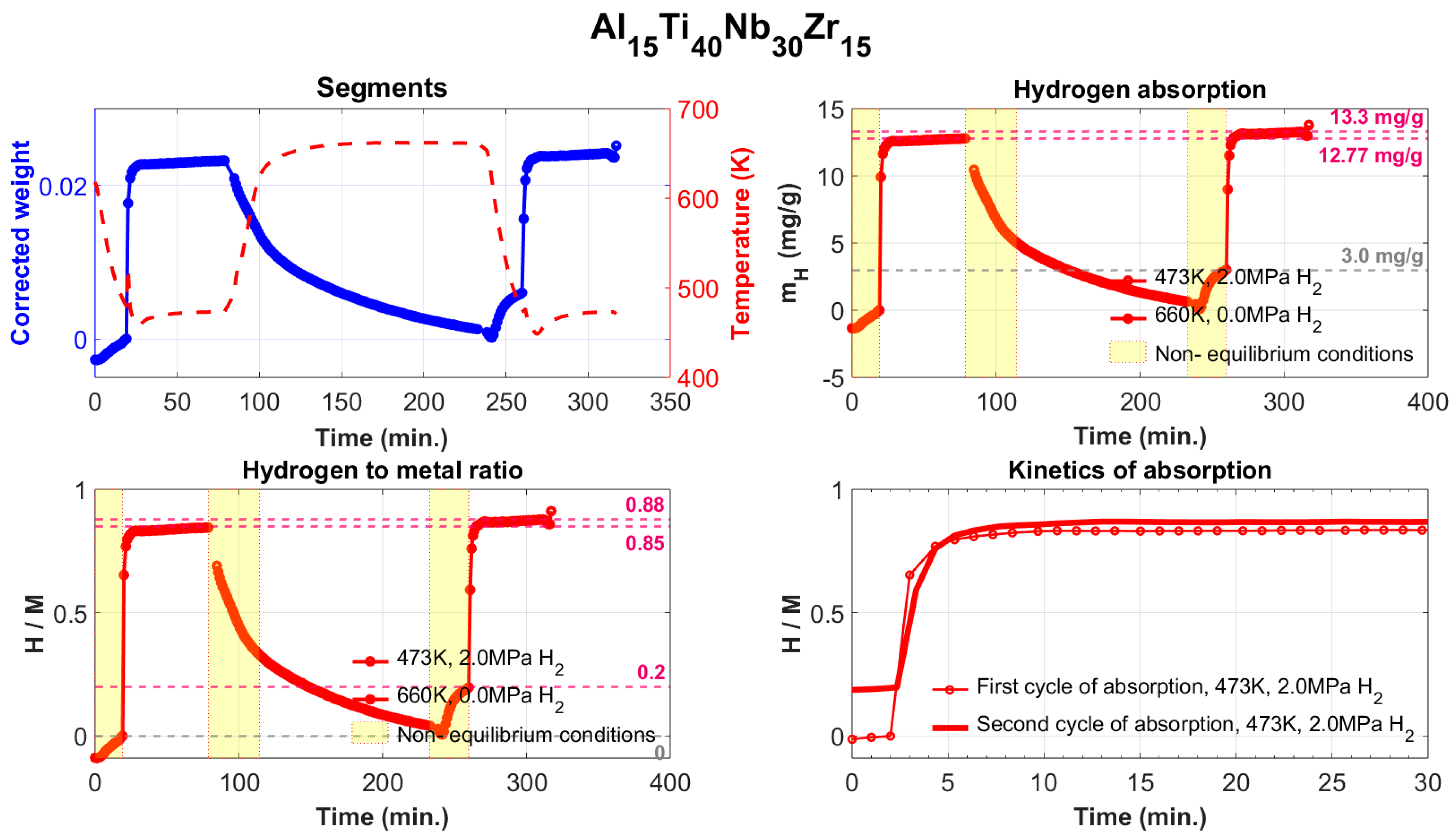
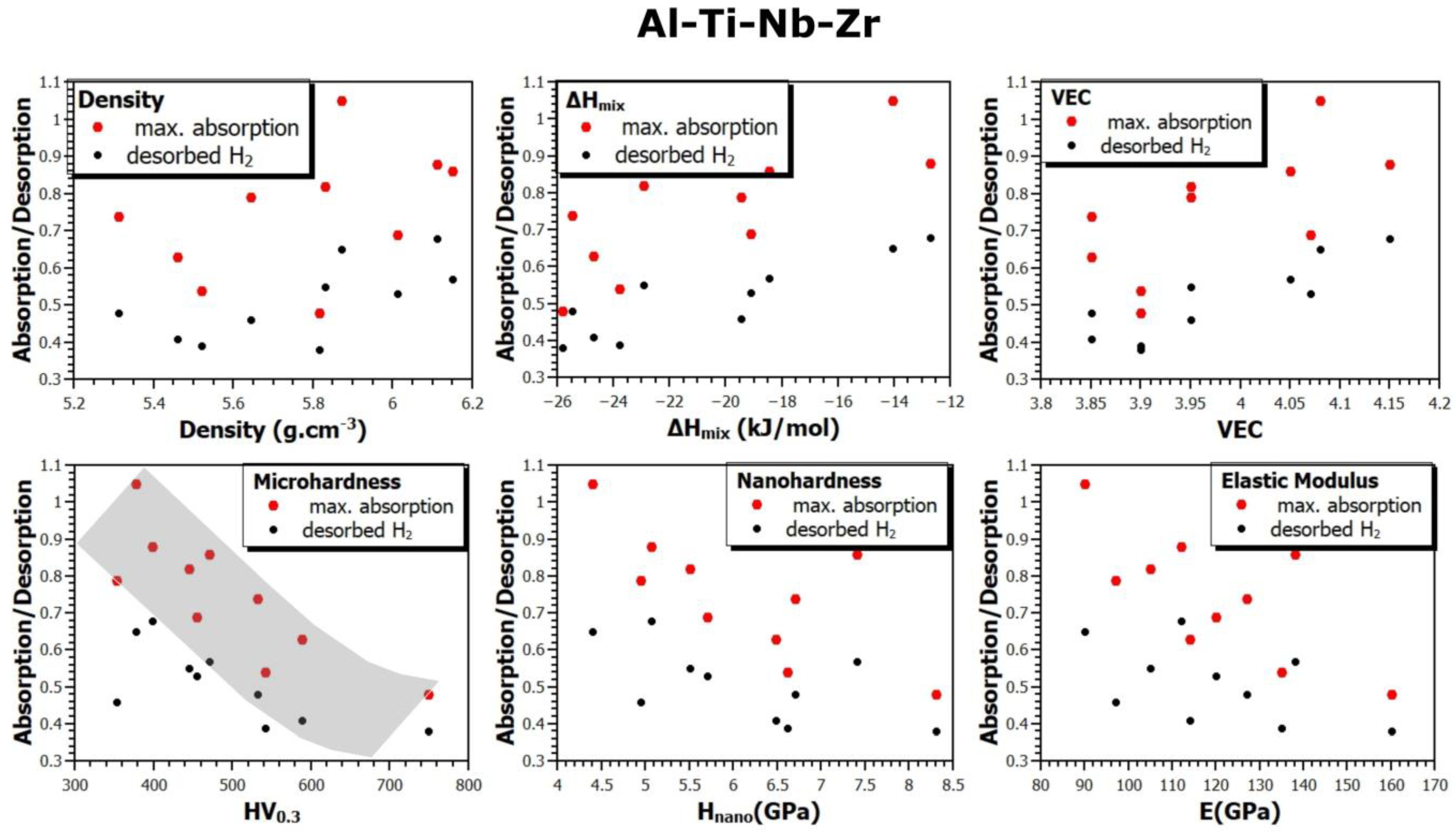
- The Al15Ti38Nb23Zr24 alloy is the most hydrogen-absorbing alloy in the Al-Ti-Nb-Zr system, with a maximum absorption of 1.61 wt.% (H/M = 1.05). However, after annealing at 370 °C in a vacuum, it still retains up to 0.62 wt.% (H/M = 1.05) of chemically bounded hydrogen, meaning that the amount of reversibly released hydrogen is only 0.99 wt.% (H/M = 0.65). From this perspective, the Al15Ti40Nb30Zr15 alloy is more advantageous, with a maximum reversible release of 1.03 wt.% of hydrogen (H/M = 0.68).
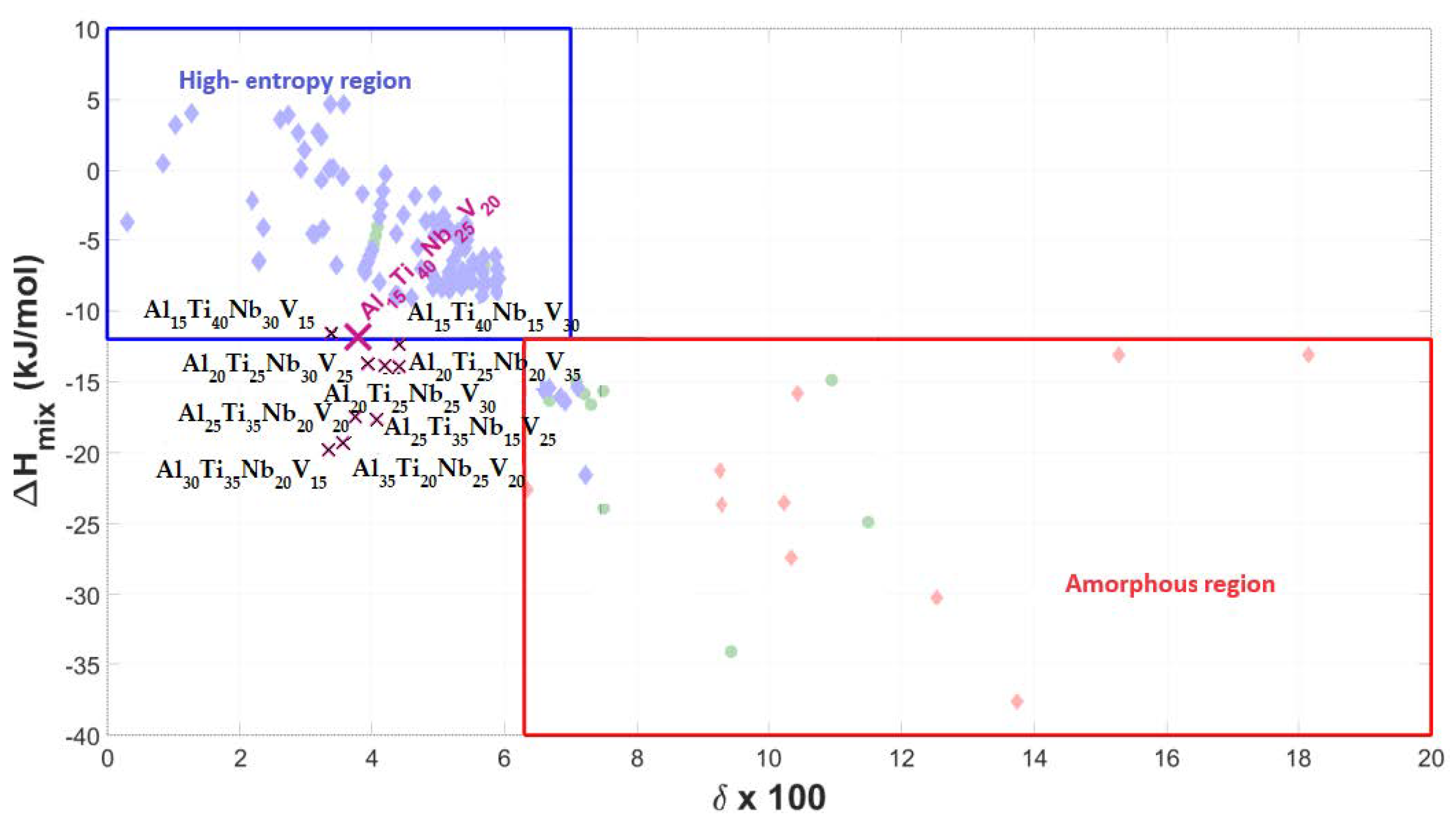
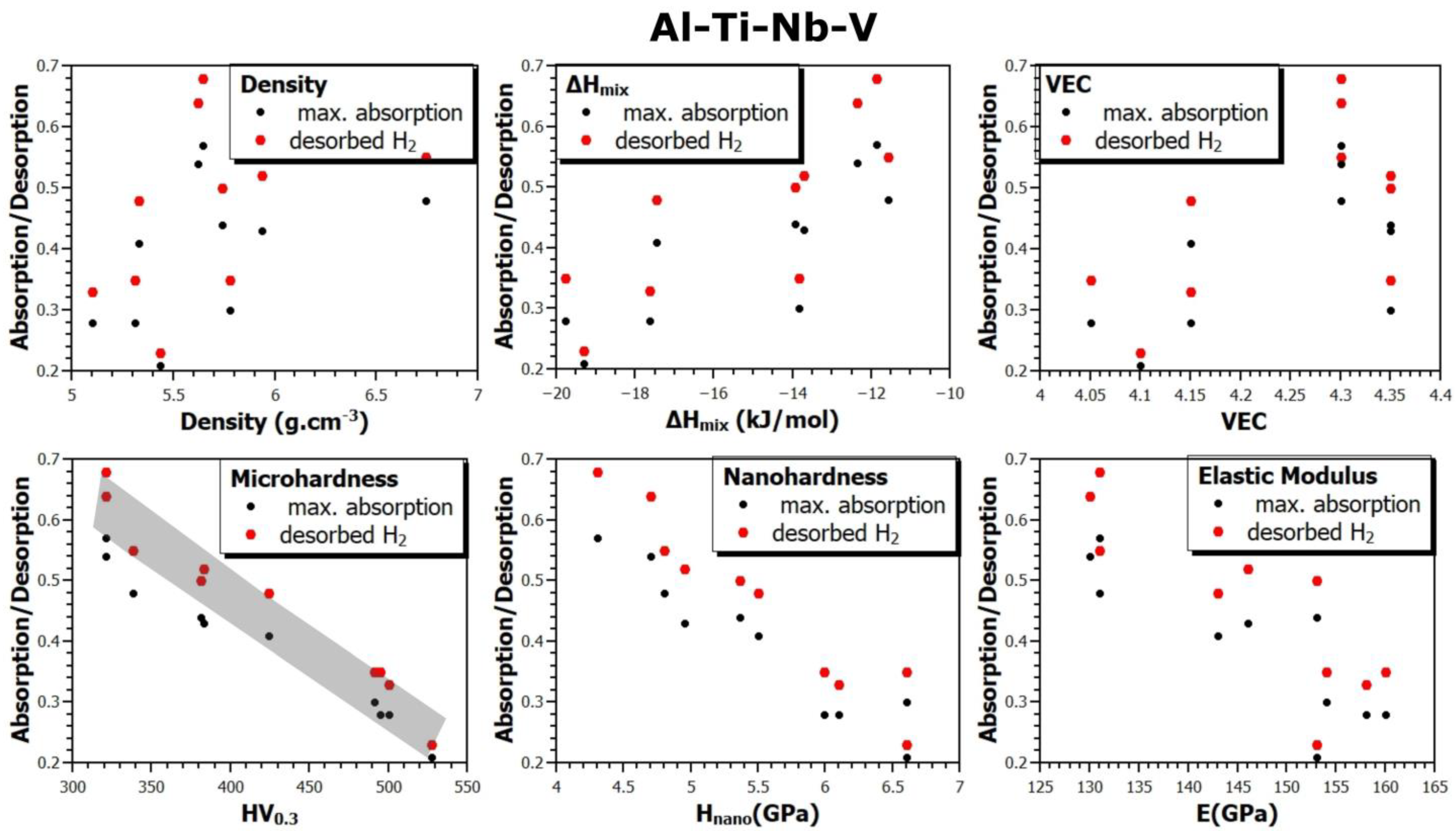
- In the case of the Al-Ti-Nb-V system, the Al15Ti40Nb25V20 alloy exhibits a maximum capacity of reversibly storable hydrogen of 1.02 wt.% (H/M = 0.57).
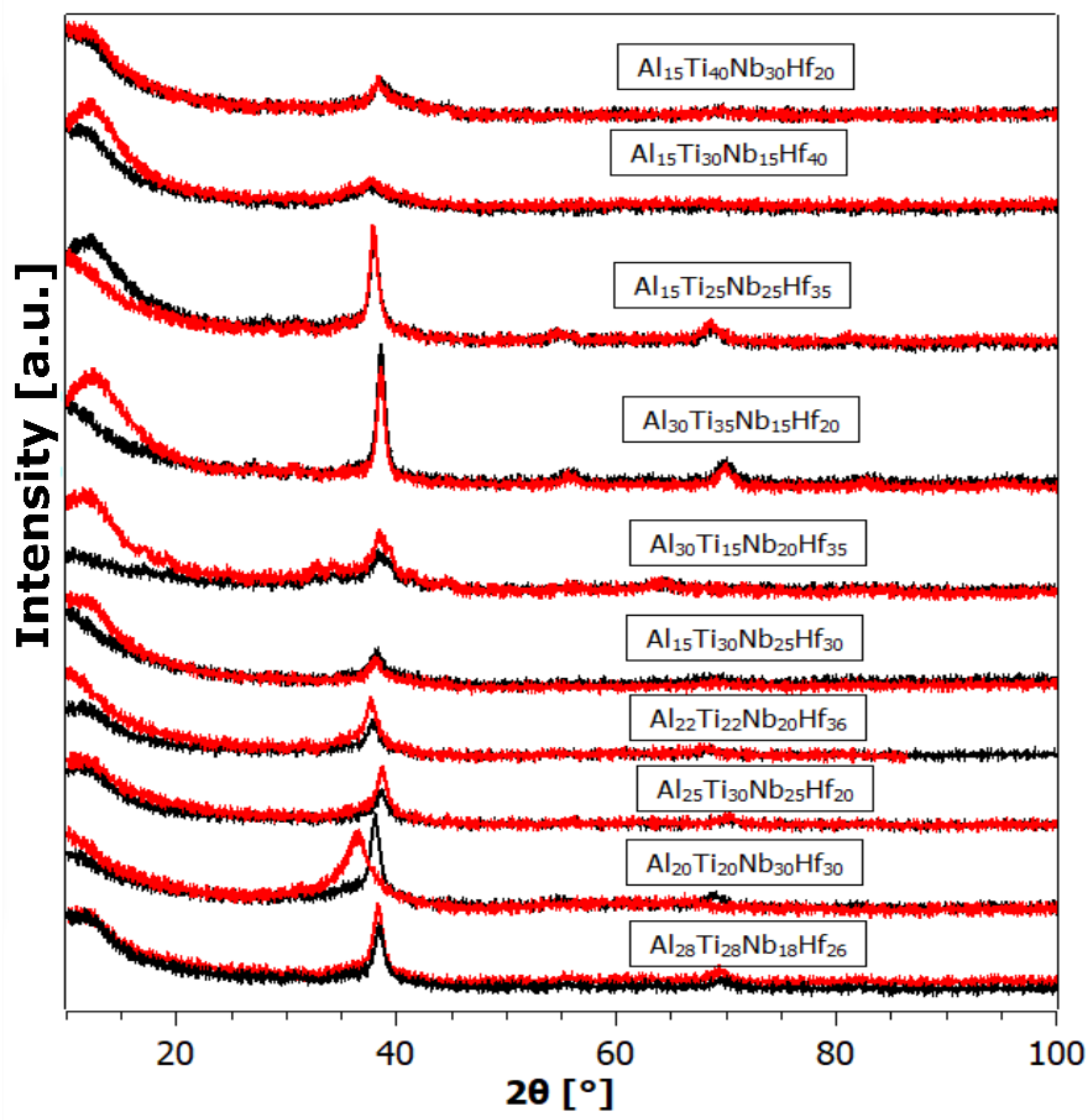
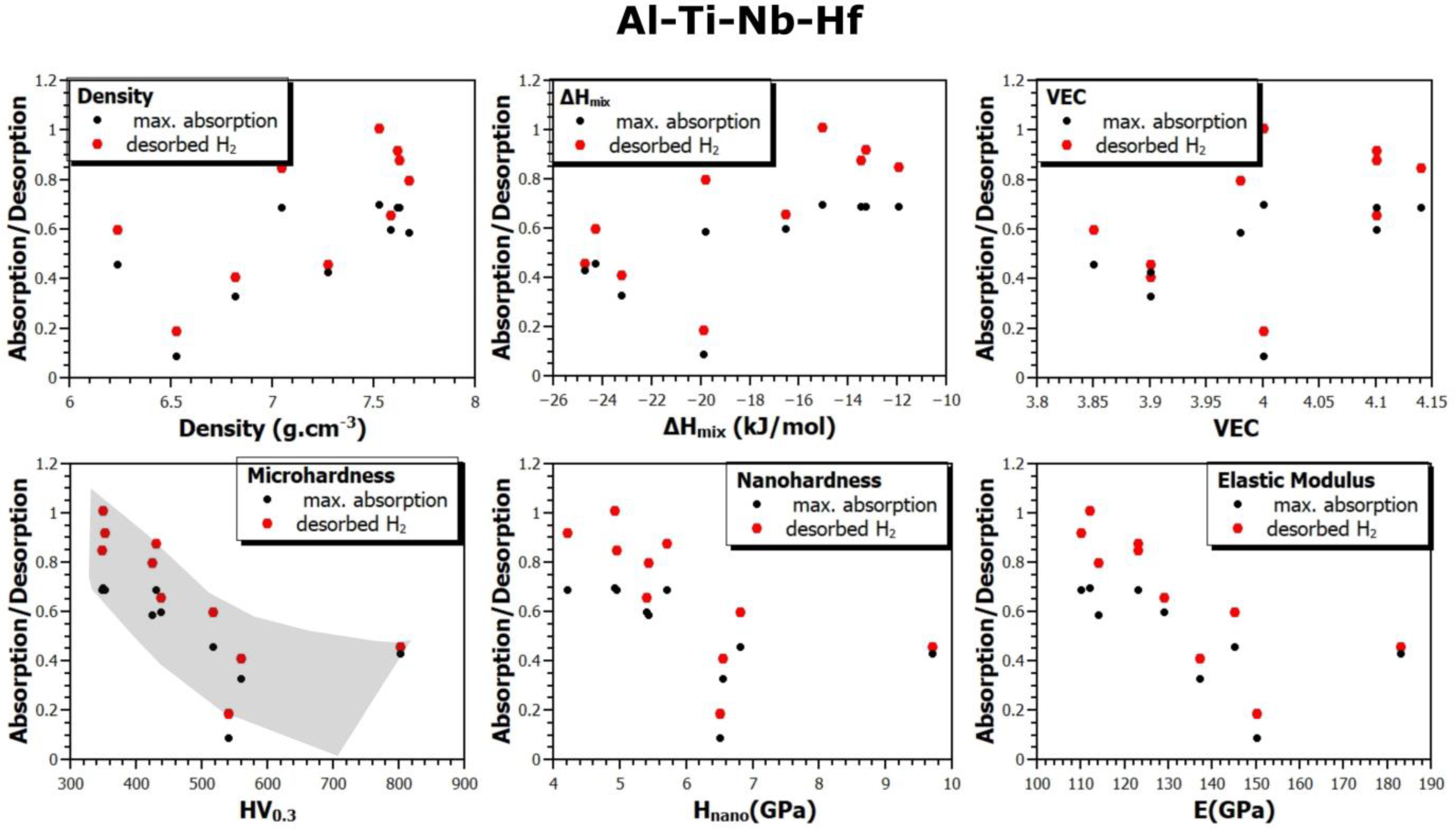
- In the Al-Ti-Nb-Hf system, the best is the Al15Ti40Nb30Hf20 alloy, which has a maximum capacity of reversibly storable hydrogen of 0.82 wt.% (H/M = 0.69).
- Considering the densities of the Al15Ti40Nb30Zr15, Al15Ti40Nb25V20, and Al15Ti40Nb30Hf20 alloys (6.11 g·cm−3, 5.646 g·cm−3, and 7.041 g·cm−3, respectively), the most efficient alloy appears to be Al15Ti40Nb30Zr15. It is 8.2% heavier than the vanadium alloy, but it has a significantly higher amount of reversibly storable hydrogen—H/M = 0.68, compared to H/M = 0.57 for the Al15Ti40Nb25V20. The best Hf-containing sample has a very similar amount of reversibly storable hydrogen as the Zr-containing sample, but it is 15.2% heavier.
4. Conclusions
- Despite the fact that empirical rules suggested that the alloys would fall outside the stability range of single-phase solid solutions, most of the alloys are single-phase with a simple BCC structure.
- The hardness of the Al-Ti-Nb-X alloys (X = Zr, V, or Hf) ranges from 320 to 800 HV0.3 units.
- The most promising alloy is Al15Ti40Nb30Zr15. It has the highest-reversible hydrogen storage capacity of all the alloys, at 1.03 wt.% of hydrogen, corresponding to and H/M = 0.68. This is 40% lower than the reversible hydrogen storage capacity of LaNi5, but Al15Ti40Nb30Zr15 is 23% lighter and does not contain rare earth elements.
- Since this work summarizes the results for a relatively large number of alloys produced in the same way, it was possible to verify the correlation between the material, its thermodynamic characteristics, and the sorption properties of these alloys. Among all the verified dependencies, the most pronounced trend shows the influence of hardness (micro and nano) on the absorption or desorption of hydrogen into or from the alloys. With the increasing hardness of the alloys, the amount of absorbed or desorbed hydrogen decreases. This observation is fundamentally understandable. Hardness is a macroscopical property of the strength of interatomic bonds, so it can be assumed that the stronger the bonds, the more difficult it is for hydrogen to penetrate into the volume of the alloy and create a hydride phase there. To our knowledge, this phenomenon has not been reported before, and this article provides experimental evidence for it.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Energy Strategy. Available online: https://energy.ec.europa.eu/topics/energy-strategy_en (accessed on 1 December 2023).
- European Commission—HyResource. Available online: https://research.csiro.au/hyresource/policy/international/european-commission/ (accessed on 1 December 2023).
- European Commission. The Hydrogen Strategy for a Climate-Neutral Europe. Dk, Vol. 53, No. 9, pp. 1689–1699. 2015. Available online: https://energy.ec.europa.eu/system/files/2020-07/hydrogen_strategy_0.pdf/ (accessed on 10 May 2024).
- Hassanpouryouzband, A.; Wilkinson, M.; Haszeldine, R.S. Hydrogen energy futures—Foraging or farming? Chem. Soc. Rev. 2024, 53, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, G. A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloys Compd. 1999, 293–295, 877–888. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Jiang, Y.; Feng, L.; Sun, Y.; Wang, Y. Recent progress in thermodynamic and kinetics modification of magnesium hydride hydrogen storage materials. Mater. Rep. Energy 2024, 4, 100252. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.; Akiba, E.; Albert, R.; Antonov, V.; Ares, J.; Baricco, M.; Bourgeois, N.; Buckley, C.; von Colbe, J.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.; Yartys, V.; Maehlen, J.; Bulychev, B.; Antonov, V.; Tarasov, B.; Gabis, I. Aluminum hydride as a hydrogen and energy storage material: Past, present and future. J. Alloys Compd. 2011, 509 (Suppl. S2), S517–S528. [Google Scholar] [CrossRef]
- Advances in Hydrogen Production, Storage and Distribution—Google Knihy. Available online: https://www.sciencedirect.com/book/9780857097682/advances-in-hydrogen-production-storage-and-distribution (accessed on 22 May 2024).
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Filho, F.D.C.G.; Ritchie, R.O.; Meyers, M.A.; Monteiro, S.N. Cantor-derived medium-entropy alloys: Bridging the gap between traditional metallic and high-entropy alloys. J. Mater. Res. Technol. 2022, 17, 1868–1895. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Schweidler, S.; Botros, M.; Fu, T.; Hahn, H.; Brezesinski, T.; Breitung, B. High-entropy energy materials: Challenges and new opportunities. Energy Environ. Sci. 2021, 14, 2883–2905. [Google Scholar] [CrossRef]
- Tian, J.; Tang, K.; Wu, Y.; Cao, T.; Pang, J.; Jiang, F. Effects of Al alloying on microstructure and mechanical properties of VCoNi medium entropy alloy. Mater. Sci. Eng. A 2021, 811, 141054. [Google Scholar] [CrossRef]
- Schneider, M.; Laplanche, G. Effects of temperature on mechanical properties and deformation mechanisms of the equiatomic CrFeNi medium-entropy alloy. Acta Mater. 2021, 204, 116470. [Google Scholar] [CrossRef]
- Pan, Z.; Luo, H.; Zhao, Q.; Cheng, H.; Wei, Y.; Wang, X.; Zhang, B.; Li, X. Tailoring microstructure and corrosion behavior of CoNiVAlx medium entropy alloys via Al addition. Corros. Sci. 2022, 207, 110570. [Google Scholar] [CrossRef]
- Fu, A.; Liu, B.; Lu, W.; Liu, B.; Li, J.; Fang, Q.; Li, Z.; Liu, Y. A novel supersaturated medium entropy alloy with superior tensile properties and corrosion resistance. Scr. Mater. 2020, 186, 381–386. [Google Scholar] [CrossRef]
- Deng, H.W.; Xie, Z.; Wang, M.; Chen, Y.; Liu, R.; Yang, J.; Zhang, T.; Wang, X.; Fang, Q.; Liu, C.; et al. A nanocrystalline AlCoCuNi medium-entropy alloy with high thermal stability via entropy and boundary engineering. Mater. Sci. Eng. A 2020, 774, 138925. [Google Scholar] [CrossRef]
- Zhao, Q.; Luo, H.; Pan, Z.; Wang, X.; Li, X. Effect of hydrogen charging on the corrosion behavior of a body-centered cubic FeCrV medium entropy alloy. Corros. Sci. 2023, 223, 111468. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sørby, M.H.; Hauback, B.C. Hydrogen storage in high-entropy alloys with varying degree of local lattice strain. Int. J. Hydrogen Energy 2019, 44, 29140–29149. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sørby, M.H.; Sahlberg, M.; Hauback, B.C. Counting electrons—A new approach to tailor the hydrogen sorption properties of high-entropy alloys. Acta Mater. 2019, 175, 121–129. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.; Strozi, R.; Bedoch, A.; Figueroa, S.; Ishikawa, T.; Botta, W. Hydrogen-induced phase transition of MgZrTiFe0.5Co0.5Ni0.5 high entropy alloy. Int. J. Hydrogen Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Montero, J.; Zlotea, C.; Ek, G.; Crivello, J.-C.; Laversenne, L.; Sahlberg, M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules 2019, 24, 2799. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Subramaniam, A. Vacuum Arc Melting Unit. Iitc, Vol. Department, No. i, p. 1. 2016. Available online: http://home.iitk.ac.in/~anandh/lab/Arc_Melting.pdf (accessed on 13 March 2023).
- TESCAN SEM Solutions for Materials Science|TESCAN. Available online: https://www.tescan.com/product-portfolio/sem/sem-for-materials-science/ (accessed on 30 March 2021).
- Keysight Nano Indenter G200—Keysight News Image Library. Available online: https://about.keysight.com/en/newsroom/images/G200/ (accessed on 7 March 2023).
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High—Entropy Alloys, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Modern Inorganic Chemistry: Jolly, William L: Free Download, Borrow, and Streaming: Internet Archive. Available online: https://archive.org/details/trent_0116300649799 (accessed on 24 January 2024).
- Jia, Y.; Zhang, L.; Li, P.; Ma, X.; Xu, L.; Wu, S.; Jia, Y.; Wang, G. Microstructure and Mechanical Properties of Nb-Ti-V-Zr Refractory Medium-Entropy Alloys. Front. Mater. 2020, 7, 172. [Google Scholar] [CrossRef]
- Zhang, Y.; Bu, Z.; Yao, T.; Yang, L.; Li, W.; Li, J. Novel BCC Ti-Al-Nb-Zr medium-entropy alloys with ultrahigh specific strength and ductility. J. Alloys Compd. 2023, 936, 168290. [Google Scholar] [CrossRef]
- Cieślak, G.; Dąbrowa, J.; Jawańska, M.; Parzuchowska, A.; Oleszak, D. Microstructure and Mechanical Properties of the Ductile Al–Ti–Mo–Nb–V Refractory High Entropy Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2022, 53, 653–662. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Wang, P.; Sun, S.-H.; Nakano, T. Design and development of (Ti, Zr, Hf)-Al based medium entropy alloys and high entropy alloys. Mater. Chem. Phys. 2022, 276, 125409. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Suhane, A. Mechanically alloyed high entropy alloys: Existing challenges and opportunities. J. Mater. Res. Technol. 2022, 17, 2431–2456. [Google Scholar] [CrossRef]
- Šittner, P.; Heller, L.; Pilch, J.; Curfs, C.; Alonso, T.; Favier, D. Young’s modulus of austenite and martensite phases in superelastic NiTi wires. J. Mater. Eng. Perform. 2014, 23, 2303–2314. [Google Scholar] [CrossRef]
- MODUL PRUŽNOSTI—Fyzikální Kabinet FyzKAB. Available online: http://kabinet.fyzika.net/studium/tabulky/modul-pruznosti.php (accessed on 30 May 2023).
- Quan, M.; Yan, Z.; Zou, C. Research on the microstructures and mechanical properties of novel Cu-rich CuxNiTiCr (x = 2, 3, 4, 5) medium-entropy alloys. Mater. Charact. 2024, 212, 113961. [Google Scholar] [CrossRef]
- Li, N.; Feng, S.; Sun, S.; Wei, R. A novel low-cost medium entropy alloys with excellent mechanical properties via multiple strengthening mechanisms. Intermetallics 2024, 170, 108329. [Google Scholar] [CrossRef]
- Azeem, W.; Shahzad, M.K.; Wong, Y.H.; Tahir, M.B. Ab-initio calculations for the study of the hydrogen storage properties of CsXH3 (X= Co, Zn) perovskite-type hydrides. Int. J. Hydrogen Energy 2024, 50, 305–313. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, S.; Lu, H.; Wu, J.; Yan, K.; Cheng, H.; Liu, J. Stability of LaNi5-xCox alloys cycled in hydrogen—Part 1 evolution in gaseous hydrogen storage performance. Int. J. Hydrogen Energy 2019, 44, 15159–15172. [Google Scholar] [CrossRef]



| Alloy | EDX [at.%] | Density [g·cm−3] | Microhardness HV0.3 | Nanohardness [GPa] | Modulus of Elasticity [GPa] |
|---|---|---|---|---|---|
| Al30Ti35Nb15Zr20 | Al30Ti34Nb14Zr22 | 5.312 ± 0.009 | 532 ± 93 | 6.7 ± 0.7 | 127 ± 10 |
| Al23Ti25Nb30Zr22 | Al23Ti27Nb28Zr22 | 6.011 ± 0.006 | 454 ± 44 | 5.7 ± 0.2 | 120 ± 3.7 |
| Al30Ti35Nb20Zr15 | Al31Ti36Nb20Zr13 | 5.52 ± 0.14 | 542 ± 21 | 6.61 ± 0.02 | 135 ± 5 |
| Al25Ti25Nb20Zr30 | Al24Ti26Nb21Zr30 | 5.83 ± 0.02 | 444 ± 7 | 5.5 ± 0.12 | 105 ± 2 |
| Al15Ti38Nb23Zr24 | Al16Ti38Nb23Zr23 | 5.87 ± 0.012 | 377 ± 9 | 4.4 ± 0.12 | 90 ± 3 |
| Al35Ti20Nb25Zr20 | Al33Ti23Nb24Zr20 | 5.815 ± 0.008 | 748 ± 31 | 8.3 ± 0.77 | 160 ± 8 |
| Al30Ti40Nb15Zr15 | Al28Ti38Nb16Zr18 | 5.46 ± 0.017 | 588 ± 19 | 6.48 ± 0.06 | 114 ± 2 |
| Al20Ti25Nb25Zr30 | Al21Ti26Nb26Zr27 | 6.15 ± 0.019 | 470 ± 141 | 7.4 ± 0.11 | 138 ± 5 |
| Al20Ti40Nb15Zr25 | Al19Ti41Nb15Zr25 | 5.643 ± 0.004 | 353 ± 11 | 4.94 ± 0.08 | 97 ± 4 |
| Al15Ti40Nb30Zr15 | Al14Ti39Nb29Zr18 | 6.11 ± 0.02 | 398 ± 21 | 5.07 ± 0.12 | 112 ± 3 |
| Alloy | EDX [at.%] | Density [g·cm−3] | Microhardness HV0.3 | Nanohardness [GPa] | Modulus of Elasticity [GPa] |
|---|---|---|---|---|---|
| Al25Ti35Nb15V25 | Al26Ti36Nb13V25 | 5.101 ± 0.001 | 500 ± 10 | 6.1 ± 0.12 | 158 ± 5 |
| Al20Ti25Nb30V25 | Al18Ti26Nb30V27 | 5.934 ± 0.007 | 383 ± 42 | 4.95 ± 0.06 | 146 ± 3 |
| Al30Ti35Nb20V15 | Al27Ti35Nb22V16 | 5.31 ± 0.013 | 495 ± 14 | 5.99 ± 0.09 | 160 ± 3 |
| Al20Ti25Nb20V35 | Al18Ti24Nb22V35 | 5.74 ± 0.012 | 381 ± 34 | 5.36 ± 0.07 | 153 ± 3 |
| Al15Ti40Nb25V20 | Al16Ti38Nb23V22 | 5.646 ± 0.006 | 321 ± 8 | 4.3 ± 0.18 | 131 ± 3 |
| Al35Ti20Nb25V20 | Al34Ti19Nb25V22 | 5.432 ± 0.009 | 527 ± 14 | 6.6 ± 0.16 | 153 ± 5 |
| Al20Ti25Nb25V30 | Al18Ti25Nb27V30 | 5.78 ± 0.01 | 491 ± 21 | 6.6 ± 0.13 | 154 ± 2 |
| Al25Ti35Nb20V20 | Al25Ti36Nb19V20 | 5.330 ± 0.007 | 424 ± 15 | 5.5 ± 0.11 | 143 ± 3 |
| Al15Ti40Nb15V30 | Al13Ti40Nb15V31 | 5.620 ± 0.009 | 321 ± 7 | 4.7 ± 0.1 | 130 ± 2 |
| Al15Ti40Nb30V15 | Al14Ti43Nb29 V15 | 6.74 ± 0.012 | 338 ± 18 | 4.8 ± 0.09 | 131 ± 3 |
| Alloy | EDX [at.%] | Density [g·cm−3] | Microhardness HV0.3 | Nanohardness [GPa] | Modulus of Elasticity [GPa] |
|---|---|---|---|---|---|
| Al28Ti28Nb18Hf26 | Al28Ti28Nb18Hf26 | 6.813 ± 0.007 | 559 ± 15 | 6.54 ± 0.09 | 137 ± 6 |
| Al20Ti20Nb30Hf30 | Al21Ti21Nb30Hf29 | 7.58 ± 0.01 | 437 ± 7 | 5.4 ± 0.19 | 129 ± 1 |
| Al25Ti30Nb25Hf20 | Al26Ti31Nb24Hf19 | 6.522 ± 0.008 | 540 ± 41 | 6.5 ± 0.17 | 150 ± 3 |
| Al22Ti22Nb20Hf36 | Al22Ti22Nb20Hf36 | 7.67 ± 0.02 | 424 ± 45 | 5.42 ± 0.04 | 114 ± 4 |
| Al15Ti30Nb25Hf30 | Al18Ti32Nb23Hf28 | 7.612 ± 0.007 | 351 ± 5 | 4.2 ± 0.26 | 110 ± 4 |
| Al30Ti15Nb20Hf35 | Al31Ti16Nb21Hf32 | 7.27 ± 0.012 | 801 ± 57 | 9.7 ± 1.7 | 183 ± 23 |
| Al30Ti35Nb15Hf20 | Al30Ti33Nb17Hf20 | 6.234 ± 0.008 | 516 ± 8 | 6.8 ± 0.1 | 145 ± 4 |
| Al15Ti25Nb25Hf35 | Al15Ti25Nb27Hf34 | 7.622 ± 0.006 | 430 ± 9 | 5.7 ± 0.9 | 123 ± 14 |
| Al15Ti30Nb15Hf40 | Al17Ti31Nb15Hf37 | 7.523 ± 0.018 | 349 ± 6 | 4.91 ± 0.08 | 112 ± 3 |
| Al15Ti40Nb30Hf20 | Al14Ti36Nb32Hf19 | 7.041 ± 0.008 | 347 ± 5 | 4.95 ± 0.09 | 123 ± 2 |
| Alloy | ΔHmix [kJ/mol] | δ × 100 | ΔSmix | VEC | Absorption H [wt.%]/[H/M] | Residual H [wt.%]/[H/M] | Desorption H [wt.%]/[H/M] | Cycle Efficiency [%] |
|---|---|---|---|---|---|---|---|---|
| Al30Ti35Nb15Zr20 | −25.50 | 4.39 | 11.10 | 3.85 | 1.28/0.74 | 0.46/0.26 | 0.82/0.48 | 64.86 |
| Al23Ti25Nb30Zr22 | −19.11 | 4.64 | 11.46 | 4.07 | 1.06/0.69 | 0.25/0.16 | 0.81/0.53 | 76.91 |
| Al30Ti35Nb20Zr15 | −23.80 | 3.99 | 11.10 | 3.90 | 0.95/0.54 | 0.27/0.15 | 0.71/0.39 | 72.22 |
| Al25Ti25Nb20Zr30 | −22.94 | 5.03 | 11.44 | 3.95 | 1.23/0.82 | 0.41/0.27 | 0.82/0.55 | 67.07 |
| Al15Ti38Nb23Zr24 | −14.08 | 4.61 | 11.08 | 4.08 | 1.61/1.05 | 0.62/0.40 | 0.99/0.65 | 61.90 |
| Al35Ti20Nb25Zr20 | −25.82 | 4.54 | 11.29 | 3.90 | 0.79/0.48 | 0.18/0.10 | 0.61/0.38 | 79.16 |
| Al30Ti40Nb15Zr15 | −24.72 | 3.95 | 10.78 | 3.85 | 1.10/0.63 | 0.37/0.22 | 0.73/0.41 | 65.08 |
| Al20Ti25Nb25Zr30 | −18.46 | 5.04 | 11.44 | 4.05 | 1.28/0.86 | 0.43/0.29 | 0.85/0.57 | 66.28 |
| Al20Ti40Nb15Zr25 | −19.48 | 4.62 | 10.97 | 3.95 | 1.28/0.79 | 0.54/0.33 | 0.74/0.46 | 58.23 |
| Al15Ti40Nb30Zr15 | −12.72 | 3.96 | 10.78 | 4.15 | 1.33/0.88 | 0.30/0.20 | 1.03/0.68 | 77.27 |
| Alloy | ΔHmix [kJ/mol] | δ × 100 | ΔSmix | VEC | Absorption H [wt.%]/H/M | Residual H [wt.%]/H/M | Desorption H [wt.%]/ H/M | Cycle Efficiency [%] |
|---|---|---|---|---|---|---|---|---|
| Al25Ti35Nb15V25 | −17.63 | 4.08 | 11.18 | 4.15 | 0.68/0.33 | 0.1/0.05 | 0.58/0.28 | 84.85 |
| Al20Ti25Nb30V25 | −13.72 | 3.94 | 11.44 | 4.35 | 0.88/0.52 | 0.15/0.09 | 0.73/0.43 | 82.69 |
| Al30Ti35Nb20V15 | −19.78 | 3.35 | 11.10 | 4.05 | 0.67/0.35 | 0.13/0.07 | 0.54/0.28 | 80.00 |
| Al20Ti25Nb20V35 | −13.94 | 4.41 | 11.29 | 4.35 | 0.91/0.50 | 0.12/0.06 | 0.79/0.44 | 88.00 |
| Al15Ti40Nb25V20 | −11.86 | 3.79 | 10.97 | 4.30 | 1.23/0.68 | 0.21/0.11 | 1.02/0.57 | 83.82 |
| Al35Ti20Nb25V20 | −19.30 | 3.57 | 11.28 | 4.10 | 0.44/0.23 | 0.04/0.02 | 0.4/0.21 | 91.3 |
| Al20Ti25Nb25V30 | −13.84 | 4.20 | 11.44 | 4.35 | 0.61/0.35 | 0.09/0.05 | 0.52/0.3 | 85.71 |
| Al25Ti35Nb20V20 | −17.46 | 3.75 | 11.29 | 4.15 | 0.92/0.48 | 0.13/0.07 | 0.79/0.41 | 85.42 |
| Al15Ti40Nb15V30 | −12.36 | 4.40 | 10.78 | 4.30 | 1.2/0.64 | 0.19/0.1 | 1.01/0.54 | 84.38 |
| Al15Ti40Nb30V15 | −11.58 | 3.39 | 10.78 | 4.30 | 0.95/0.55 | 0.13/0.07 | 0.82/0.48 | 27.27 |
| Alloy | ΔHmix [kJ/mol] | δ × 100 | ΔSmix | VEC | Absorption H [wt.%]/H/M | Residual H [wt.%]/H/M | Desorption H [wt.%]/ H/M | Cycle Efficiency [%] |
|---|---|---|---|---|---|---|---|---|
| Al28Ti28Nb18Hf26 | −23.24 | 4.11 | 11.41 | 3.90 | 0.63/0.41 | 0.10/0.08 | 0.53/0.33 | 80.34 |
| Al20Ti20Nb30Hf30 | −16.56 | 4.37 | 11.36 | 4.10 | 0.69/0.66 | 0.06/0.06 | 0.63/0.60 | 90.91 |
| Al25Ti30Nb25Hf20 | −19.90 | 3.81 | 11.44 | 4.00 | 0.24/0.19 | 0.21/0.10 | 0.03/0.09 | 47.37 |
| Al22Ti22Nb20Hf36 | −19.83 | 4.47 | 11.27 | 3.98 | 0.80/0.80 | 0.22/0.21 | 0.58/0.59 | 73.75 |
| Al15Ti30Nb25Hf30 | −13.32 | 4.24 | 11.25 | 4.10 | 1.01/0.92 | 0.25/0.23 | 0.76/0.69 | 75.00 |
| Al30Ti15Nb20Hf35 | −24.74 | 4.55 | 11.10 | 3.90 | 0.50/0.46 | 0.03/0.03 | 0.47/0.43 | 93.48 |
| Al30Ti35Nb15Hf20 | −24.30 | 3.75 | 11.10 | 3.85 | 0.80/0.60 | 0.19/0.14 | 0.61/0.46 | 76.67 |
| Al15Ti25Nb25Hf35 | −13.49 | 4.42 | 11.18 | 4.10 | 0.86/0.88 | 0.19/0.19 | 0.67/0.69 | 78.41 |
| Al15Ti30Nb15Hf40 | −15.06 | 4.38 | 10.78 | 4.00 | 1.02/1.01 | 0.32/0.31 | 0.70/0.70 | 69.30 |
| Al15Ti40Nb30Hf20 | −11.97 | 3.68 | 10.97 | 4.14 | 1.01/0.85 | 0.19/0.16 | 0.82/0.69 | 81.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varcholová, D.; Kušnírová, K.; Oroszová, L.; Möllmer, J.; Lange, M.; Gáborová, K.; Buľko, B.; Demeter, P.; Saksl, K. New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys. Materials 2024, 17, 2897. https://doi.org/10.3390/ma17122897
Varcholová D, Kušnírová K, Oroszová L, Möllmer J, Lange M, Gáborová K, Buľko B, Demeter P, Saksl K. New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys. Materials. 2024; 17(12):2897. https://doi.org/10.3390/ma17122897
Chicago/Turabian StyleVarcholová, Dagmara, Katarína Kušnírová, Lenka Oroszová, Jens Möllmer, Marcus Lange, Katarína Gáborová, Branislav Buľko, Peter Demeter, and Karel Saksl. 2024. "New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys" Materials 17, no. 12: 2897. https://doi.org/10.3390/ma17122897









