Recent Advances in and Challenges with Fe-Based Metallic Glasses for Catalytic Efficiency: Environment and Energy Fields
Abstract
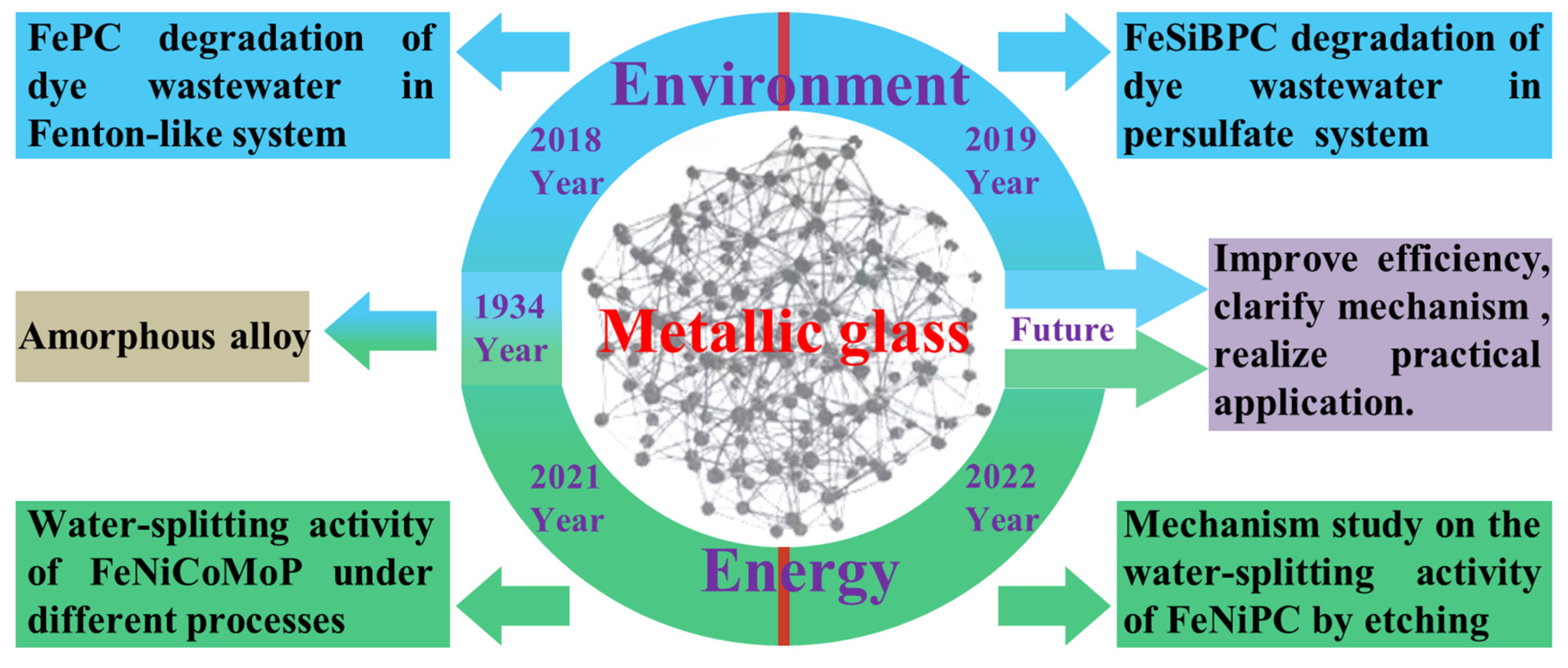
1. Introduction
2. Metallic Glass Preparation Methods
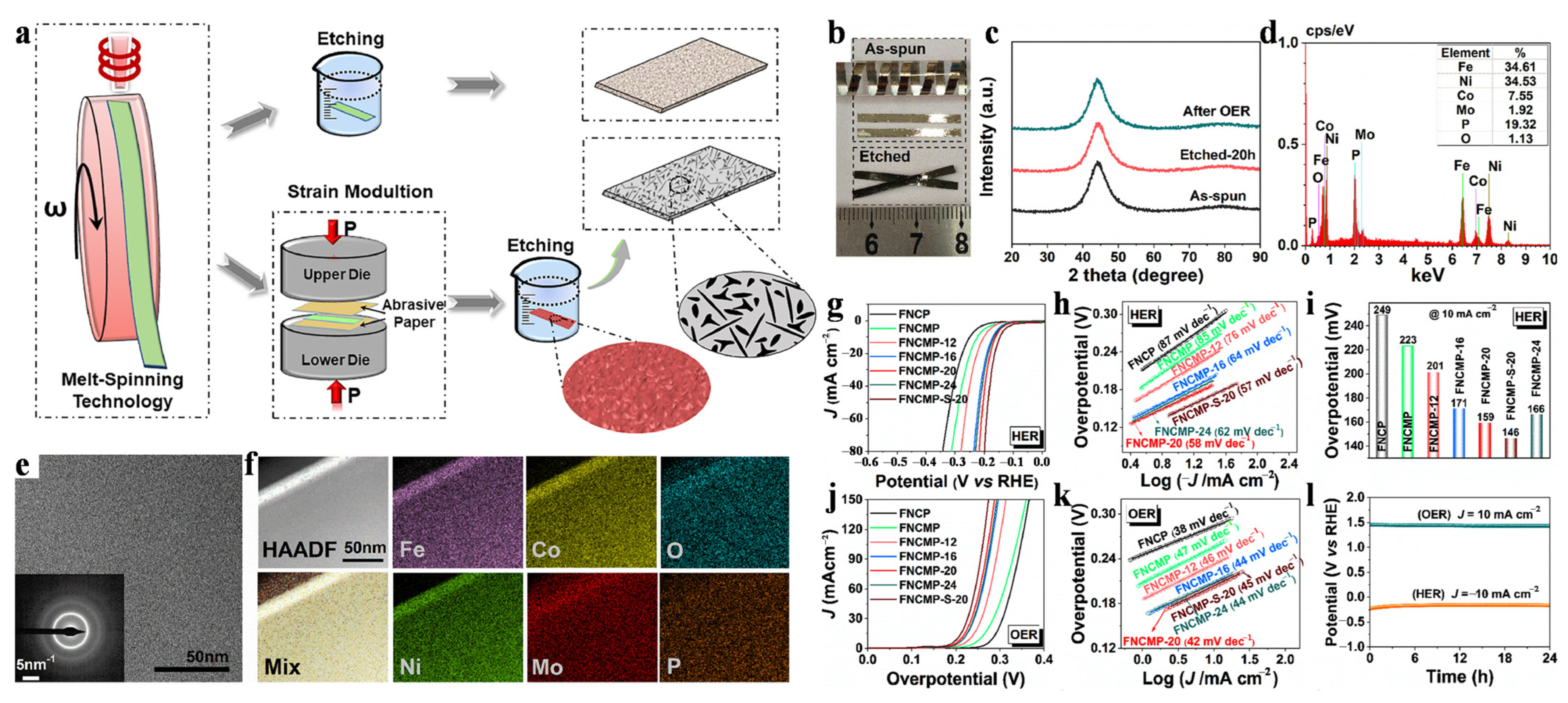
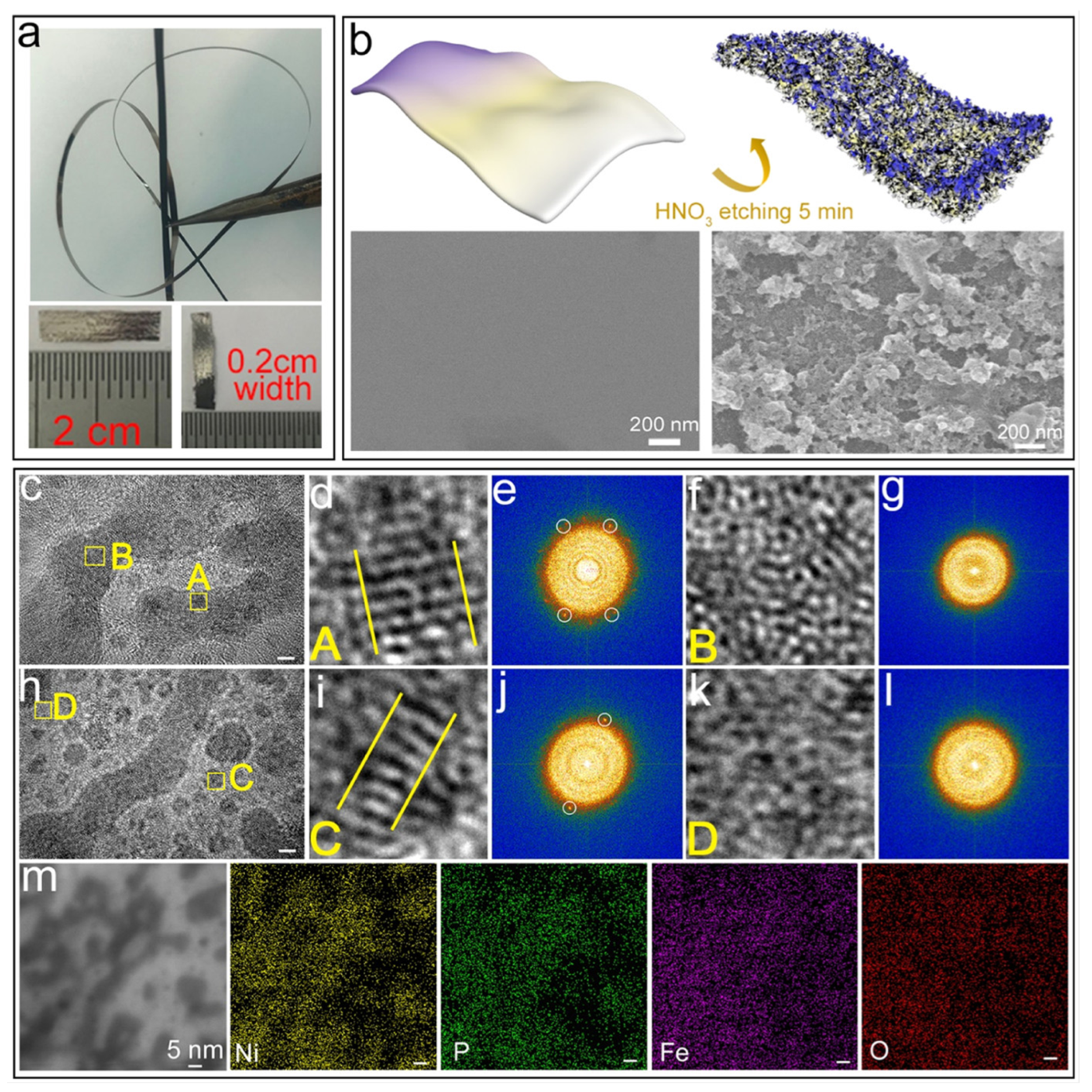
2.1. Melt-Spinning Method
2.2. Ball Milling and Gas Atomization
3. Catalytic Performance Evaluation
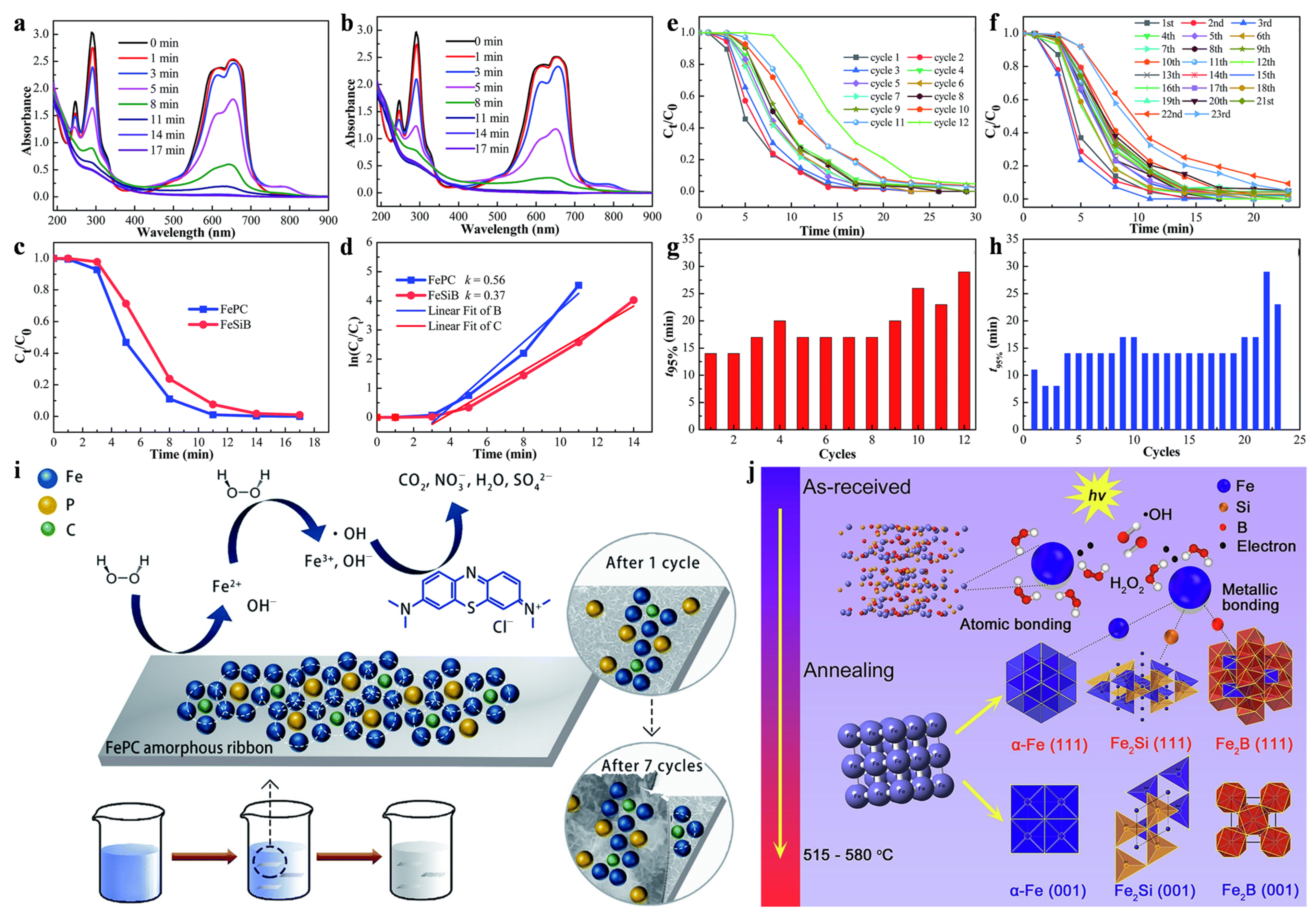
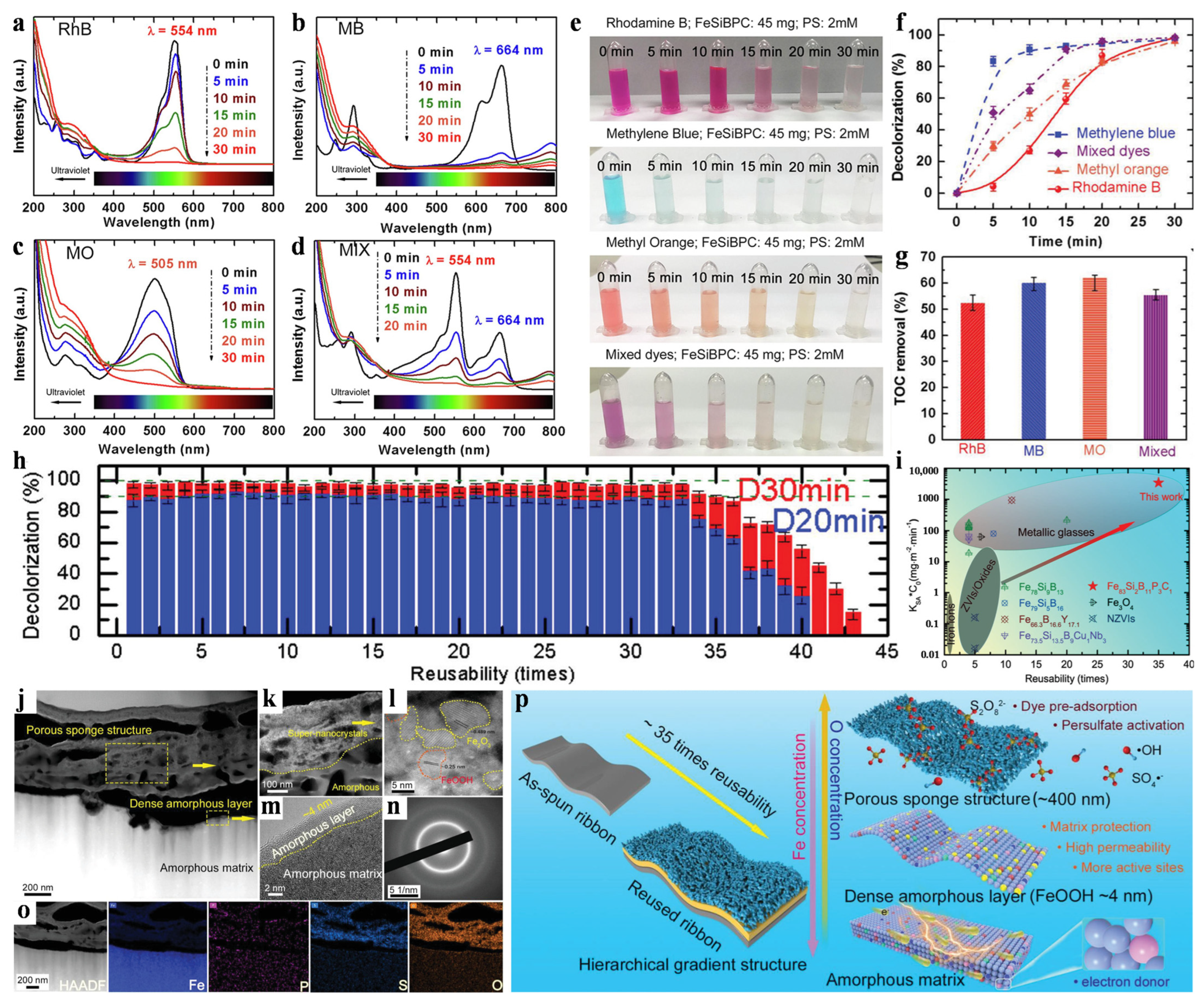
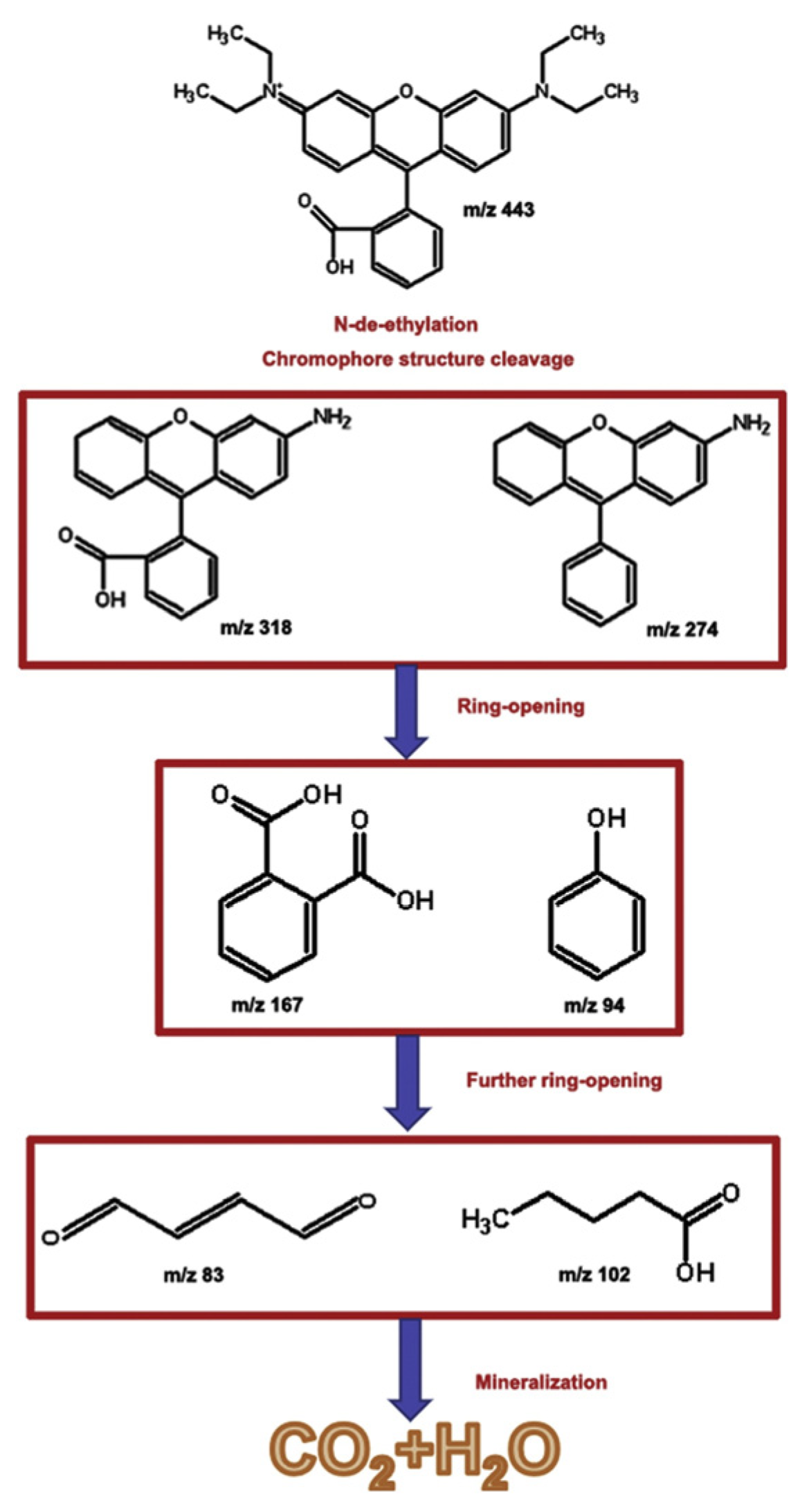
3.1. Environmental Catalysts
3.2. Energy Catalyst
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Armakovic, S.J.; Savanovic, M.M.; Siljegovic, M.V.; Kisic, M.; Scepanovic, M.; Grujic-Brojcin, M.; Simic, N.; Gavanski, L.; Armakovic, S. Self-Cleaning and Charge Transport Properties of Foils Coated with Acrylic Paint Containing TiO2 Nanoparticles. Inorganics 2024, 12, 35. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hyun, S.; Shanmugam, S. Pulse Electrodeposition of a Superhydrophilic and Binder-Free Ni-Fe-P Nanostructure as Highly Active and Durable Electrocatalyst for Both Hydrogen and Oxygen Evolution Reactions. ACS Appl. Mater. Interfaces 2020, 12, 53719–53730. [Google Scholar] [CrossRef] [PubMed]
- El-Refaei, S.M.; Russo, P.A.; Pinna, N. Recent Advances in Multimetal and Doped Transition-Metal Phosphides for the Hydrogen Evolution Reaction at Different pH values. ACS Appl. Mater. Interfaces 2021, 13, 22077–22097. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zou, P.; Yue, Y.; Wang, M.; Fu, W.; Si, S.; Wei, L.; Zhao, X.; Hu, G.; Xin, H.L. One-Pot Synthesis of B/P-Codoped Co-Mo Dual-Nanowafer Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 20024–20033. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Luo, G.; Lu, Y.R.; Chen, C.; Zou, Y.; Dong, C.L.; Li, Y.; Wang, S. Zirconium-Regulation-Induced Bifunctionality in 3D Cobalt-Iron Oxide Nanosheets for Overall Water Splitting. Adv. Mater. 2019, 31, 1901439. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Singh, S.; Ediger, M.D.; de Pablo, J.J. Ultrastable glasses from in silico vapour deposition. Nat. Mater. 2013, 12, 139–144. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, Z.; Guo, L.; Zhang, H.; Zhang, L.; Kim, K.; Li, X.; Wang, W. Enhancing the acid orange dye degradation efficiency of Mg-based glassy alloys with introducing porous structure and zinc oxide. J. Alloys Compd. 2020, 831, 154817. [Google Scholar] [CrossRef]
- Chen, Q.; Pang, J.; Yan, Z.; Hu, Y.; Guo, L.; Zhang, H.; Zhang, L.; Wang, W. MgZn-based amorphous ribbon as a benign decolorizer in methyl blue solution. J. Non-Cryst. Solids 2020, 529, 119802. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.Q.; Li, H.; Yang, H.; Huo, J.; Wang, J.; Chang, C.; Wang, X.; Li, R.W.; Wang, G. Fast decolorization of azo dyes in both alkaline and acidic solutions by Al-based metallic glasses. J. Alloys Compd. 2017, 701, 759–767. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, Z.; Guo, L.; Zhang, H.; Zhang, L.C.; Wang, W. Role of maze like structure and Y2O3 on Al-based amorphous ribbon surface in MO solution degradation. J. Mol. Liq. 2020, 318, 114318. [Google Scholar] [CrossRef]
- Tang, M.; Lai, L.; Ding, D.; Liu, T.; Kang, W.; Guo, N.; Song, B.; Guo, S. Rapid degradation of Direct Blue dye by Co-based amorphous alloy wire. J. Non-Cryst. Solids 2022, 576, 121282. [Google Scholar] [CrossRef]
- Chen, S.; Li, M.; Ji, Q.; Feng, T.; Lan, S.; Yao, K. Effect of the chloride ion on advanced oxidation processes catalyzed by Fe-based metallic glass for wastewater treatment. J. Mater. Sci. Technol. 2022, 117, 49–58. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Z.; Feng, Y.; Liu, H.; Wang, Z.; Zhang, L.; Wang, W. Nanostructured metallic glass contributing to efficient catalytic degradation of dye wastewater. J. Non-Cryst. Solids 2022, 598, 121952. [Google Scholar] [CrossRef]
- Chen, Q.; Di, H.; Qi, Z.; Wang, Z.; Song, Z.; Zhang, L.C.; Guo, L.; Wang, W. Highly efficient catalytic performance and self-renewing behavior of Fe-based glass induced by pulsed laser. J. Mater. Sci. Technol. 2024, 188, 191–201. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Z.; Wang, Z.; Song, Z.; Zhang, L.C.; Guo, L.; Wang, W. Pulsed laser-indued nanoscale phase transition in FePC alloy with enhancing the catalytic activity in dye wastewater degradation. Appl. Surf. Sci. 2024, 659, 159946. [Google Scholar] [CrossRef]
- Zhang, L.C.; Jia, Z.; Lyu, F.; Liang, S.X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q.; Louzguine Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Rapid Degradation of Azo Dye by Fe-Based Metallic Glass Powder. Adv. Funct. Mater. 2012, 22, 2567–2570. [Google Scholar] [CrossRef]
- Longhi, M.; Cova, C.; Pargoletti, E.; Coduri, M.; Santangelo, S.; Patanè, S.; Ditaranto, N.; Cioffi, N.; Facibeni, A.; Scavini, M. Synergistic Effects of Active Sites’ Nature and Hydrophilicity on the Oxygen Reduction Reaction Activity of Pt-Free Catalysts. Nanomaterials 2018, 8, 643. [Google Scholar] [CrossRef]
- Zuliani, A.; Cano, M.; Calsolaro, F.; Santiago, A.R.P.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Berlier, G.; Cravotto, G.; Martina, K.; Luque, R. Improving the electrocatalytic performance of sustainable Co/carbon materials for the oxygen evolution reaction by ultrasound and microwave assisted synthesis. Sustain. Energy Fuels 2021, 5, 720–731. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, Y.Z.; Su, R.; Cao, C.R.; Li, F.; Sun, C.W.; Yang, Y.; Guan, P.F.; Ding, D.W.; Wang, Z.L.; et al. A Highly Efficient and Self-Stabilizing Metallic-Glass Catalyst for Electrochemical Hydrogen Generation. Adv. Mater. 2016, 28, 10293–10297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Li, M.X.; Yu, J.H.; Ge, X.B.; Liu, Y.H.; Wang, W.H. Low-Iridium-Content IrNiTa Metallic Glass Films as Intrinsically Active Catalysts for Hydrogen Evolution Reaction. Adv. Mater. 2019, 32, 1906384. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, X.; Wu, R.; Wang, J.; Li, Z.; Chan, K.C.; Wang, H.; Wu, Y.; Lu, Z. Flexible Honeycombed Nanoporous/Glassy Hybrid for Efficient Electrocatalytic Hydrogen Generation. Adv. Mater. 2019, 31, 1904989. [Google Scholar] [CrossRef]
- Khan, I.; Qurashi, A.; Berdiyorov, G.; Iqbal, N.; Fuji, K.; Yamani, Z.H. Single-step strategy for the fabrication of GaON/ZnO nanoarchitectured photoanode their experimental and computational photoelectrochemical water splitting. Nano Energy 2018, 44, 23–33. [Google Scholar] [CrossRef]
- Tang, J.W.; Durrant, J.R.; Klug, D.R. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes, Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef]
- Jia, Z.; Zhao, Y.; Wang, Q.; Lyu, F.; Tian, X.; Liang, S.X.; Zhang, L.C.; Luan, J.; Wang, Q.; Sun, L.; et al. Nanoscale Heterogeneities of Non-Noble Iron-Based Metallic Glasses toward Efficient Water Oxidation at Industrial-Level Current Densities. ACS Appl. Mater. Interfaces 2022, 14, 10288–10297. [Google Scholar] [CrossRef]
- Wang, W.H. The nature and properties of amorphous substances. Prog. Phys. 2013, 33, 177–351. [Google Scholar]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Kramer, J. Produced the first amorphous metals through vapor deposition. Ann. Phys. 1934, 37, 19–21. [Google Scholar]
- Brenner, A.; Couch, D.E.; Williams, E.K. Electrodeposition of alloys of phosphorus with nickel or cobalt. J. Res. Natl. Bur. Stand. 1950, 44, 109. [Google Scholar] [CrossRef]
- Klement, W.; Willens, R.; Duwez, P. Non-crystalline structure in solidified gold-silicon alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Zha, Q.S.J. Glass and Amorphous Materials; Science Press: Beijing, China, 2001. [Google Scholar]
- Wang, W.H. Roles of minor additions in formation and properties of bulk metallic glasses. Prog. Mater. Sci. 2007, 52, 540–596. [Google Scholar] [CrossRef]
- Hu, Y.C.; Li, F.X.; Li, M.Z.; Bai, H.Y.; Wang, W.H. Five-fold symmetry as indicator of dynamic arrest in metallic glass-forming liquids. Nat. Commun. 2015, 6, 8310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012, 57, 487–656. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super plastic bulk metallic glasses at room temperature. Science 2007, 315, 1385–1388. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, L.; Di, H.; Qi, Z.; Wang, Z.; Song, Z.; Zhang, L.; Hu, L.; Wang, W. Nanoscale Oxygenous Heterogeneity in FePC Glass for Highly Efficient and Reusable Catalytic Performance. Adv. Sci. 2023, 10, 2304045. [Google Scholar] [CrossRef]
- Pei, C.; Chen, S.; Zhao, T.; Li, M.; Cui, Z.; Sun, B.; Hu, S.; Lan, S.; Hahn, H.; Feng, T. Nanostructured Metallic Glass in a Highly Upgraded Energy State Contributing to Efficient Catalytic Performance. Adv. Mater. 2022, 34, 2200850. [Google Scholar] [CrossRef]
- Jia, Z.; Duan, X.; Qin, P.; Zhang, W.; Wang, W.; Yang, C.; Sun, H.; Wang, S.; Zhang, L.C. Disordered Atomic Packing Structure of Metallic Glass: Toward Ultrafast Hydroxyl Radicals Production Rate and Strong Electron Transfer Ability in Catalytic Performance. Adv. Funct. Mater. 2017, 27, 1702258. [Google Scholar] [CrossRef]
- Zhang, L.C.; Shen, Z.Q.; Xu, J. Glass formation in a (Ti, Zr, Hf)–(Cu, Ni, Ag)–Al high-order alloy system by mechanical alloying. J. Mater. Res. 2002, 18, 2141–2149. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Chu, W.; Zhao, X.; Hu, L. Evolution path of metallic glasses under extensive cryogenic thermal cycling: Rejuvenation or relaxation? Mater. Sci. Eng. A 2022, 850, 143551. [Google Scholar] [CrossRef]
- Zhang, L.C.; Calin, M.; Branzei, M.; Schultz, L.; Eckert, J. Phase stability and consolidation of glassy/nanostructured Al85Ni9Nd4Co2 alloys. J. Mater. Res. 2011, 22, 1145–1155. [Google Scholar] [CrossRef]
- Trigunayat, G.; Chadha, G. Progress in the study of polytypism in crystals (I). Phys. Status Solidi A 1971, 4, 9–42. [Google Scholar] [CrossRef]
- Jia, Z.; Duan, X.G.; Zhang, W.C.; Wang, W.M.; Sun, H.Q.; Wang, S.B.; Zhang, L.C. Ultra-sustainable Fe78Si9B13 metallic glass as a catalyst for activation of persulfate on methylene blue degradation under UV-Vis light. Sci. Rep. 2016, 6, 38520. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, Q.; Sun, L.; Wang, Q.; Zhang, L.C.; Wu, G.; Luan, J.H.; Jiao, Z.B.; Wang, A.; Liang, S.X.; et al. Attractive In Situ Self-Reconstructed Hierarchical Gradient Structure of Metallic Glass for High Efficiency and Remarkable Stability in Catalytic Performance. Adv. Funct. Mater. 2019, 29, 1807857. [Google Scholar] [CrossRef]
- Parray, R.A.; Ravichandran, K. Structural, thermal, electrical and magnetic properties of Fe2CrAl FulHeusler alloy prepared by ball milling. Physica B 2023, 653, 414665. [Google Scholar] [CrossRef]
- Akmal, M.; Malik, A.; Jeong, W.; Ryu, H.J. Incorporating microstructural and mechanical heterogeneity to Ti–Zr–Nb alloy by partial high-energy ball milling. Mater. Chem. Phys. 2024, 315, 129037. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Li, X.F.; Wang, W.M.; Lin, H.C.; Zhang, L.C. Ultrafast activation efficiency of three peroxides by Fe78Si9B13 metallic glass under photo-enhanced catalytic oxidation: A comparative study. Appl. Catal. B 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, W.C.; Wang, W.M.; Habibi, D.; Zhang, L.C. Amorphous Fe78Si9B13 alloy: An efficient and reusable photo-enhanced Fenton-like catalyst in degradation of cibacron brilliant red 3B-A dye under UV–vis light. Appl. Catal. B 2016, 192, 46–56. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Pei, Y.; Zhou, G.; Luan, N.; Zong, B.; Qiao, M.; Tao, F.F. Synthesis and catalysis of chemically reduced metal-metalloid amorphous alloys. Chem. Soc. Rev. 2012, 41, 8140–8162. [Google Scholar] [CrossRef] [PubMed]
- Chemelewski, W.D.; Lee, H.C.; Lin, J.F.; Bard, A.J.; Mullins, C.B. Amorphous FeOOH Oxygen Evolution Reaction Catalyst for Photoelectrochemical Water Splitting. J. Am. Chem. Soc. 2014, 136, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hou, H.; Fujii, A.; Hosomi, M.; Li, F. Degradation of 1,4-dioxane in water with heat- and Fe2+-activated persulfate oxidation. Environ. Sci. Pollut. Res. Int. 2014, 21, 7457–7465. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J.; Zhou, Q. Heat-activated persulfate oxidation of atrazine: Implications for remediation of groundwater contaminated by herbicides. Chem. Eng. J. 2015, 263, 45–54. [Google Scholar] [CrossRef]
- Pang, Y.; Kong, L.; Chen, D.; Yuvaraja, G.; Mehmood, S. Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B. J. Hazard. Mater. 2020, 384, 121447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhu, L.; Yang, Z.; Wang, Y. Morphological-modulated FeNi-based amorphous alloys as efficient alkaline water splitting electrocatalysts. Electrochim. Acta 2021, 389, 138756. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Liu, X.; Chen, S.Q.; Yao, K.F. Insight into the high reactivity of commercial Fe–Si–B amorphous zero-valent iron in degrading azo dye solutions. RSC Adv. 2015, 5, 34032–34039. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Liu, Y.J.; Zhang, W.C.; Wang, W.M.; Lu, J.; Zhang, L.C. Compelling Rejuvenated Catalytic Performance in Metallic Glasses. Adv. Mater. 2018, 30, 1802764. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Lin, P.; Cui, Z.; Chu, C.; Shen, B. Investigation of FePC amorphous alloys with self-renewing behaviour for highly efficient decolorization of methylene blue. J. Mater. Chem. A 2018, 6, 10686–10699. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H.; Hu, Z. Rapid reductive degradation of azo dyes by a unique structure of amorphous alloys. Chin. Sci. Bull. 2011, 56, 3988–3992. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H.; Hu, Z. Rapid decolorization of Acid Orange II aqueous solution by amorphous zero-valent iron. J. Environ. Sci. 2012, 24, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, J.L.; Zha, M.Q.; Shek, C.H. Synthesis of an Fe rich amorphous structure with a catalytic effect to rapidly decolorize Azo dye at room temperature. ACS Appl. Mater. Interfaces 2014, 6, 5500–5505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Z.; Zhang, H.; Hu, Z. On the decolorization property of Fe–Mo–Si–B alloys with different structures. J. Non-Cryst. Solids 2012, 358, 61–64. [Google Scholar] [CrossRef]
- Chen, S.Q.; Li, M.; Ji, Q.; Chen, X.; Lan, S.; Feng, T.; Yao, K.F. Functional 3D nanoporous Fe-based alloy from metallic glass for high-efficiency water splitting and wastewater treatment. J. Non-Cryst. Solids 2021, 571, 121070. [Google Scholar] [CrossRef]
- Wang, S.L.; Kim, D.H.; Yi, S. Electrocatalytic properties of Fe-based bulk metallic glasses for hydrogen evolution reaction. Korean J. Chem. Eng. 2011, 28, 1672–1676. [Google Scholar] [CrossRef]
- Zhang, F.; Shan, W.; Hu, Q.; Jiang, W.; Li, D.; Zhang, B. The Effect of Mo Addition on Electrocatalytic Activity and Stability of Fe-Co-P-C Metallic Glasses for Hydrogen Evolution. J. Electrochem. Soc. 2021, 168, 076510. [Google Scholar] [CrossRef]
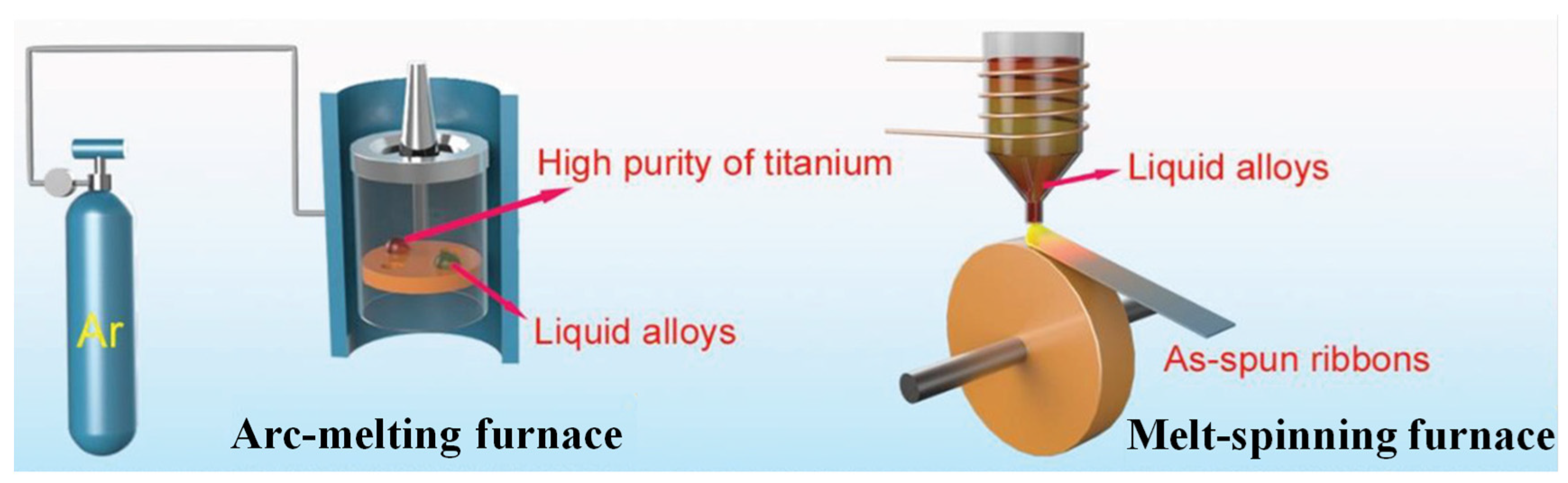
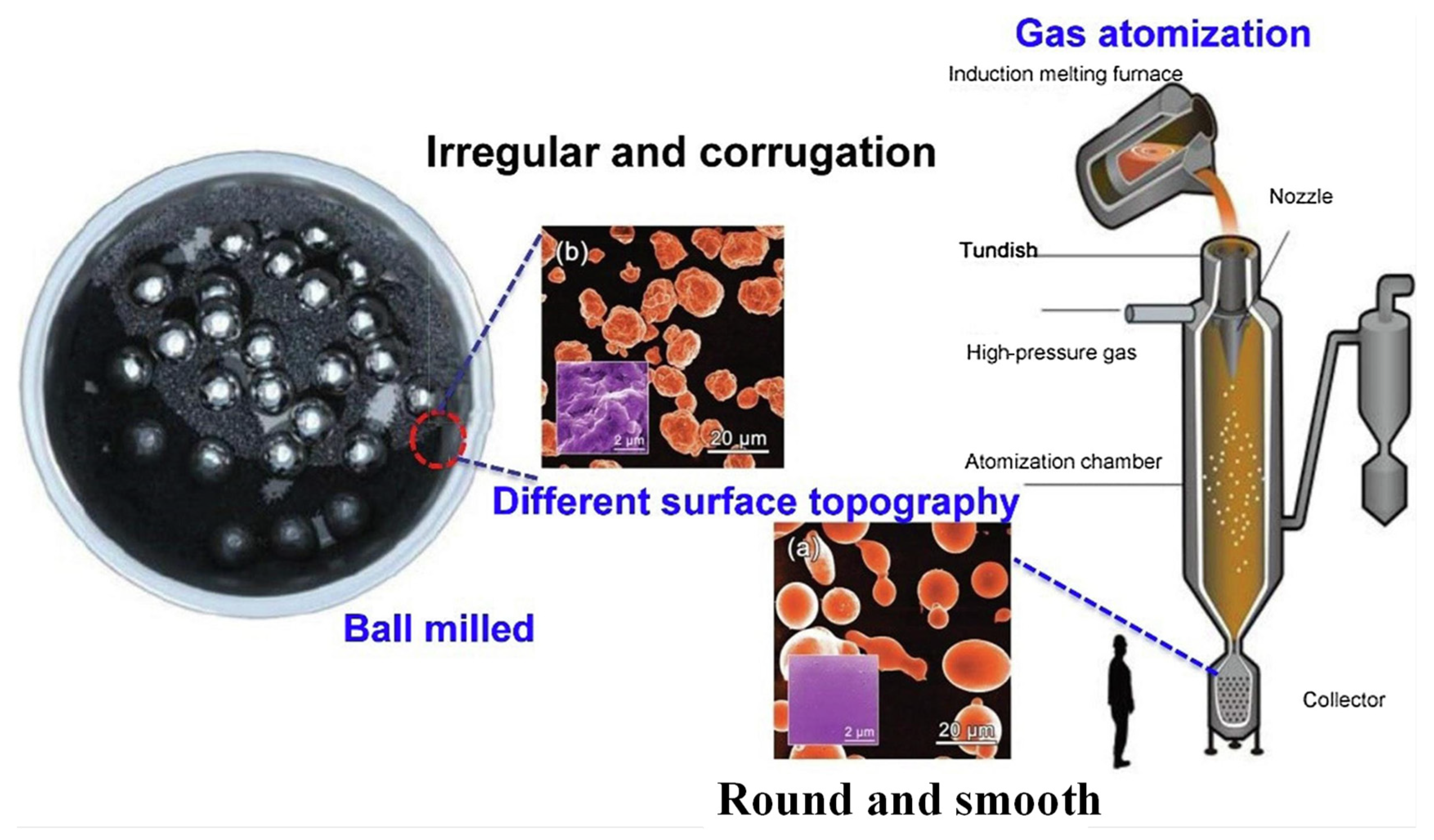


| Method | Mechanism | Morphology | Specific Surface Area | Preparation Cycle | Refs. |
|---|---|---|---|---|---|
| Melt spinning | Rapid cooling | Smooth ribbon | Low | Fast | [39,43] |
| Ball milling | Mechanical crushing | Rough powder | High | Slow | [20,44] |
| Gas atomization | Rapid cooling | Smooth powder | Medium | Fast | [19,20] |
| Metallic Glass (Environment) | Organic Pollutant | Initial Concentration (mg·L−1) | k (min−1) | Ref. |
|---|---|---|---|---|
| Fe78Si9B13 | Methyl blue | 20 | 0.381 | [50] |
| Fe78Si9B13 | Blliant red 3B-A | 50 | 0.654 | [52] |
| Fe78Si9B13 | Methyl blue | 20 | 0.356 | [41] |
| Fe78Si9B13 | Brilliant Yellow 3G-P | 20 | 0.466 | [41] |
| Fe78Si9B13 | Brilliant red 3B-A | 20 | 0.372 | [41] |
| Fe78Si9B13 | Malachite green | 20 | 0.519 | [41] |
| Fe78Si9B13 | Malachite green | 20 | 0.057 | [41] |
| Fe78Si9B13 | Methylene blue | 20 | 0.64 | [46] |
| Fe78Si13B9 | Direct blue 6 | 200 | 0.115 | [60] |
| Fe78Si13B9 | Orange II | 100 | 0.238 | [60] |
| Fe78Si13B9 | Methyl orange | 25 | 0.103 | [60] |
| Fe78Si9B13 | Methylene blue | 20 | 0.302 | [61] |
| Fe78Si9B13 | Reactive red 195 | 100 | 0.495 | [17] |
| Fe78Si9B13 | Methylene blue | 100 | 0.37 | [62] |
| Fe80P13C7 | Methylene blue | 100 | 0.56 | [62] |
| Fe78(Si,B)22 | Orange II | 100 | 0.125 | [63] |
| Fe78Si8B14 | Acid orange II | 200 | 0.174 | [64] |
| Fe79B16Si5 | Orange G | 100 | 0.004 | [65] |
| Fe66.3B16.6Y17.1 | Orange G | 100 | 0.047 | [65] |
| Fe75P15C10 | Reactive red 195 | 100 | 0.307 | [39] |
| (Fe0.99Mo0.01)78Si9B13 | Acid orange II | 100 | 0.168 | [66] |
| Fe73.5Si13.5B9Cu1Nb3 | Methyl orange | 20 | 0.152 | [50] |
| Fe73.5Si13.5B9Cu1Nb3 | Methyl blue | 20 | 0.201 | [50] |
| Fe73.5Si13.5B9Cu1Nb3 | Methylene blue | 20 | 0.119 | [61] |
| Fe83Si2B11P3C1 | Rhodamine B | 20 | 0.09 | [47] |
| Fe83Si2B11P3C1 | Rhodamine B | 20 | 0.36 | [47] |
| Fe83Si2B11P3C1 | Rhodamine B | 20 | 0.165 | [47] |
| Metallic Glass (Energy) | Electrolyte Solution | Overpotential (mV) | Tafel Slope (mV dec−1) | Ref. |
| Fe35Ni35Co8Mo2P20 (HER) | 1 M NaOH | 159 | 58 | [59] |
| Fe35Ni35Co8Mo2P20 (OER) | 1 M NaOH | 213 | 42 | [59] |
| Fe50Ni30P13C7 (OER) | 1 M KOH | 327 | 33.2 | [28] |
| Fe50Ni30P13C7 (HER) | 1 M KOH | 113 | 40.6 | [28] |
| (Fe73.5Si13.5B9Nb3Cu1)91.5Ni8.5 (OER) | 1 M KOH | 395 | 100 | [67] |
| Fe73C3Si7.3B8.5P5.7Mo2.5 (HER) | 1 M KOH | 476 | 115 | [68] |
| Fe59.5C3Si7.3B8.5P5.7Mo2.5Co13.5 (HER) | 1 M KOH | 462 | 110 | [68] |
| Fe40Co35Mo5P13C7 (HER) | 0.5 M H2SO4 | 90 | 42 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Qi, Z.; Wang, Z.; Song, Z.; Wang, W. Recent Advances in and Challenges with Fe-Based Metallic Glasses for Catalytic Efficiency: Environment and Energy Fields. Materials 2024, 17, 2922. https://doi.org/10.3390/ma17122922
Chen Q, Qi Z, Wang Z, Song Z, Wang W. Recent Advances in and Challenges with Fe-Based Metallic Glasses for Catalytic Efficiency: Environment and Energy Fields. Materials. 2024; 17(12):2922. https://doi.org/10.3390/ma17122922
Chicago/Turabian StyleChen, Qi, Zhigang Qi, Zhaoxuan Wang, Ziqi Song, and Weimin Wang. 2024. "Recent Advances in and Challenges with Fe-Based Metallic Glasses for Catalytic Efficiency: Environment and Energy Fields" Materials 17, no. 12: 2922. https://doi.org/10.3390/ma17122922
APA StyleChen, Q., Qi, Z., Wang, Z., Song, Z., & Wang, W. (2024). Recent Advances in and Challenges with Fe-Based Metallic Glasses for Catalytic Efficiency: Environment and Energy Fields. Materials, 17(12), 2922. https://doi.org/10.3390/ma17122922







