Incorporation of Anions into Anodic Alumina—A New Track in Cr(VI) Anodizing Substitution?
Abstract
:1. Introduction
2. Common Cr(VI)-Free Anodizing Alternatives
| Type of Material | Bath Composition | Anodizing Conditions | Corrosion Performance (Quantitative) | Remarks | Reference | ||
|---|---|---|---|---|---|---|---|
| Ecorr/mV | Jcorr/A.cm−2 | Other | |||||
| AA 2024 T3 | 0.46 M sulfuric acid and 0.1 M ammonium pentaborate with 80 g/L (0.53 M) or 150 g/L (1 M) of tartaric acid | 14 V vs. SCE (3-electrode cell), RT 1 | - | - | - | It was revealed that tartaric acid decreases the dissolution rate of the anodic oxide in the acidic environment. | [12] |
| AA 2099 | 0.4 M H2SO4 | 14 V, 37 °C, 1500 s | - | - | Since 0.53 M of tartaric acid NSST 2 shows significant improvement in corrosion performance. | Study of the impact of tartaric acid concentration in the anodizing bath on corrosion performance (EIS 3, NSST). | [13] |
| 0.4 M H2SO4 +0.1 M C4H4O6 | |||||||
| 0.4 M H2SO4 +0.3 M C4H4O6 | |||||||
| 0.4 M H2SO4 +0.53 M C4H4O6 | |||||||
| 0.4 M H2SO4 +0.7 M C4H4O6 | |||||||
| 0.4 M H2SO4 +0.9 M C4H4O6 | |||||||
| AA 2024 clad with AA 1050 | 40 g/L H2SO4 | 10 V, 37 °C, 20 min | - | - | - | The higher the electrolyte temperature, the better the adhesion of the film to a certain extent. | [14] |
| 40 g/L H2SO4 + 80 g/L C4H4O6 | 14 V, 45 °C, 20 min | - | - | - | |||
| 40 g/L H2SO4 + 150 g/L C4H4O6 | 20 V, 55 °C, 20 min | - | - | - | |||
| AA 2024 T3 | 44 g/L H2SO4 + 87 g/L C4H6O6 | 11 V, 37 °C, 20 min | −0.721 | 3.32 × 10−8 | - | The impact of the anodizing voltage on corrosion performance was studied; corrosion potentials are given vs. Ag|AgCl. | [15] |
| 13 V, 37 °C, 20 min | −0.672 | 4.85 × 10−8 | - | ||||
| 15 V, 37 °C, 20 min | −0.637 | 2.91 × 10−9 | - | ||||
| 17 V, 37 °C, 20 min | −0.556 | 2.99 × 10−9 | - | ||||
| 19 V, 37 °C, 20 min | −0.311 | 1.52 × 10−8 | - | ||||
| 21 V, 37 °C, 20 min | −0.636 | 1.79 × 10−6 | - | ||||
| 46 g/L CrO3 | 40 V, 40 °C, 40 min | −0.555 | 1.26 × 10−7 | - | |||
| AA 2024 T3 | 0.46 M H2SO4 + 0.533 M C4H4O6 | 14 V, 37 °C, 1500 s | - | - | - | Films with similar thickness (ca. 3 µm) but different pore morphologies were obtained; 7 V films have better performance in immersion tests, while 14 V has better performance in EIS and NSSTs. | [16] |
| 7 V, 37 °C, 3200–3300 s | - | - | - | ||||
| AA 2024 T3 AA 7075 T6 AA 7175 T6 | 40 g/L H2SO4 + 80 g/L C4H4O6 | 14 V, 37 °C, 25 or 45 min | - | - | - | Wetting angle and EIS study of samples anodized for different times; a semi-industrial 200 L bath with samples as big as 10 per 12 cm2 was used. | [18] |
| 7 V, 37 °C, 3200–3300 s | - | - | - | ||||
| AA 2024 T3 | 0.4 M H2SO4 + 0.53 M C4H4O6 | 5 mA/cm2 or 18 V, 308 K (35 °C), 1800 s | - | - | - | Rutherford backscattering spectroscopy (RBS) has proven that Cu from the alloy is incorporated into the anodic oxide. | [20] |
| AA 2024 T4 | 55 g/L H2SO4 + 88 g/L C4H6O6 | 14 V, 37 °C, 23 min | - | - | - | A comparative study of AA 2024 T4 TSA after various sealing is reported. Authors used sealing (1) in boiling DI water for 30 min (HWS); (2) in 4 g/L NiF2 at 25 °C for 30 min, followed by in DI water at 60 °C for 30 min; (3) in 50 g/L K2Cr2O7 (potassium dichromate) at 90–95 °C, pH = 6.7 (measured in 25 °C) for 30 min; and (4) in potassium permanganate, lithium nitrate and sodium molybdate at 70 °C for 30 min. | [21] |
| 99.999% Al | 0.41 M H2SO4 + 0.53 M C4H6O6 | 25 V, 5–7 °C, 20 h (1st step), 1 h (2nd step) | - | 2.63 × 10−7; after sealing jcorr was below detection limit | 1.20 × 105 | Two-step anodizing for corrosion protection was investigated; pores were sealed using boiling water (60 min). | [22] |
| Type of Material | Bath Composition | Anodizing Conditions | Corrosion Performance (Quantitative) | Remarks | Reference | ||
|---|---|---|---|---|---|---|---|
| Ecorr/mV | Jcorr/A.cm−2 | Other | |||||
| AA 6061 | 199.04 g/L H2SO4 | 1.0 A/cm2, 5 °C, 30 min | 0.629 | 2.22 × 10−8 | - | Anodizing was followed by HWS (95 °C, 30 min). | [24] |
| 7.2 A/cm2, 5 °C, 30 min | −0.566 | 5.93 × 10−9 | Epit = −0.297 mV | ||||
| 5 mL/L of H2SO4 + 210.12 g/L of citric acid | 1.0 A/cm2, 5 °C, 30 min | −0.343 | 1.7 × 10−8 | - | |||
| 7.2 A/cm2, 5 °C, 30 min | −0.693 | 2.62 × 10−6 | Epit = −0.693 mV | ||||
| AA 2024 T3 | 1.5 M H2SO4 | 15 V, 25 °C, | - | - | - | EIS study of CSA, TSA and TSAA covered with Ce- and Li-rich hybrid sol–gel coatings was reported. Active corrosion protection was proven. | [25] |
| 1.5 M H2SO4 + 0.1 citric acid | 15 V, 25 °C, | - | - | - | |||
| 1.5 M H2SO4 + 0.25 M citric acid | 15 V, 25 °C, | - | - | - | |||
| 1.5 M H2SO4 + 0.50 M citric acid | 15 V, 25 °C, | - | - | - | |||
| AA 7150 | 45 g/L of H2SO4 + 8 g/L of citric acid | 15 V, 25 °C, 20 min | −0.9827 | 8.86 × 10−8 | - | Various amounts of Ti sol was added to the samples; the greater the amount of sol, the greater the corrosion potential and the lower the corrosion current density. | [26] |
| 45 g/L of H2SO4 + 8 g/L of citric acid + 25 mL/L Ti sol | −0.7652 | 3.36 × 10−8 | - | ||||
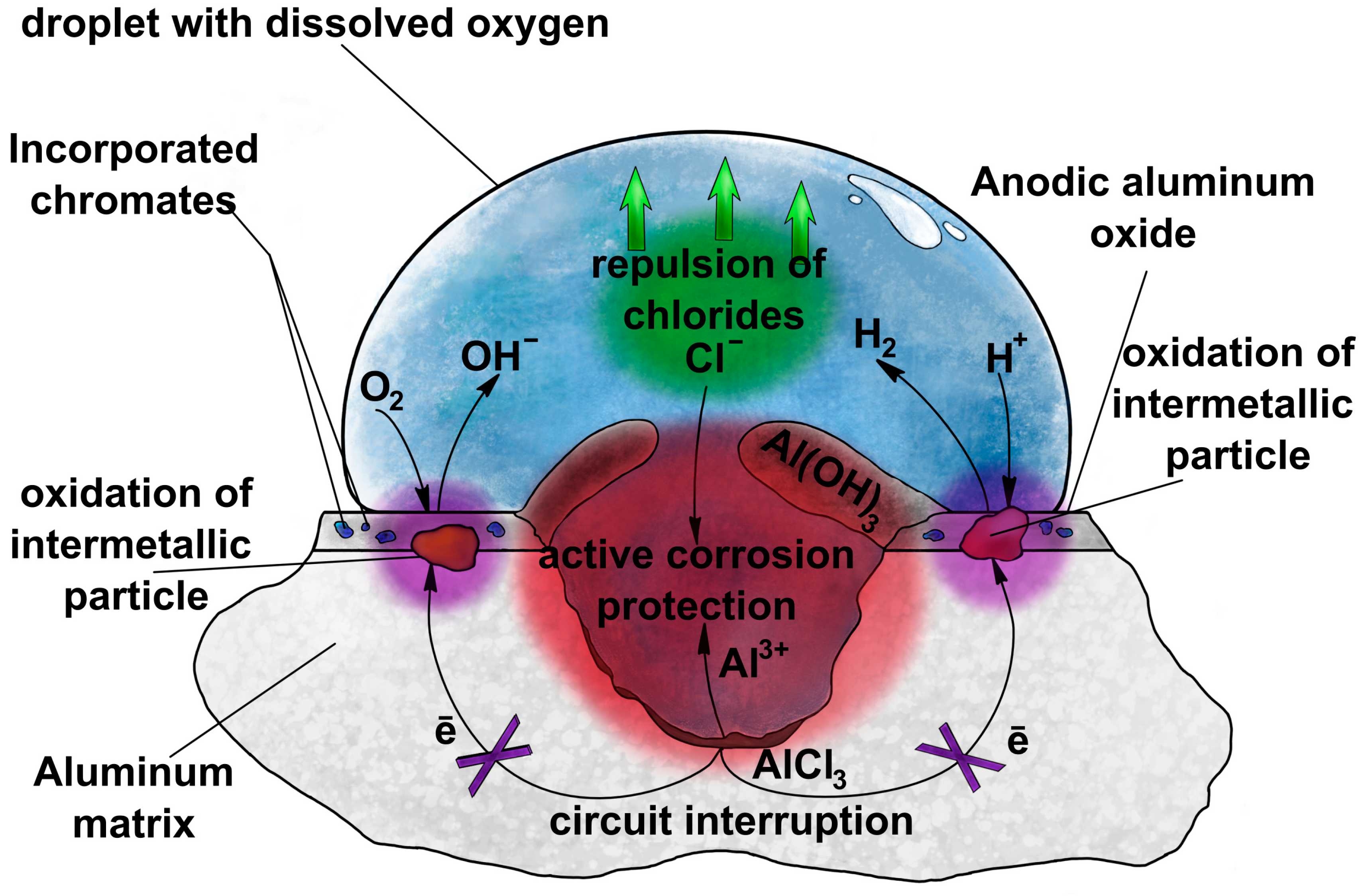
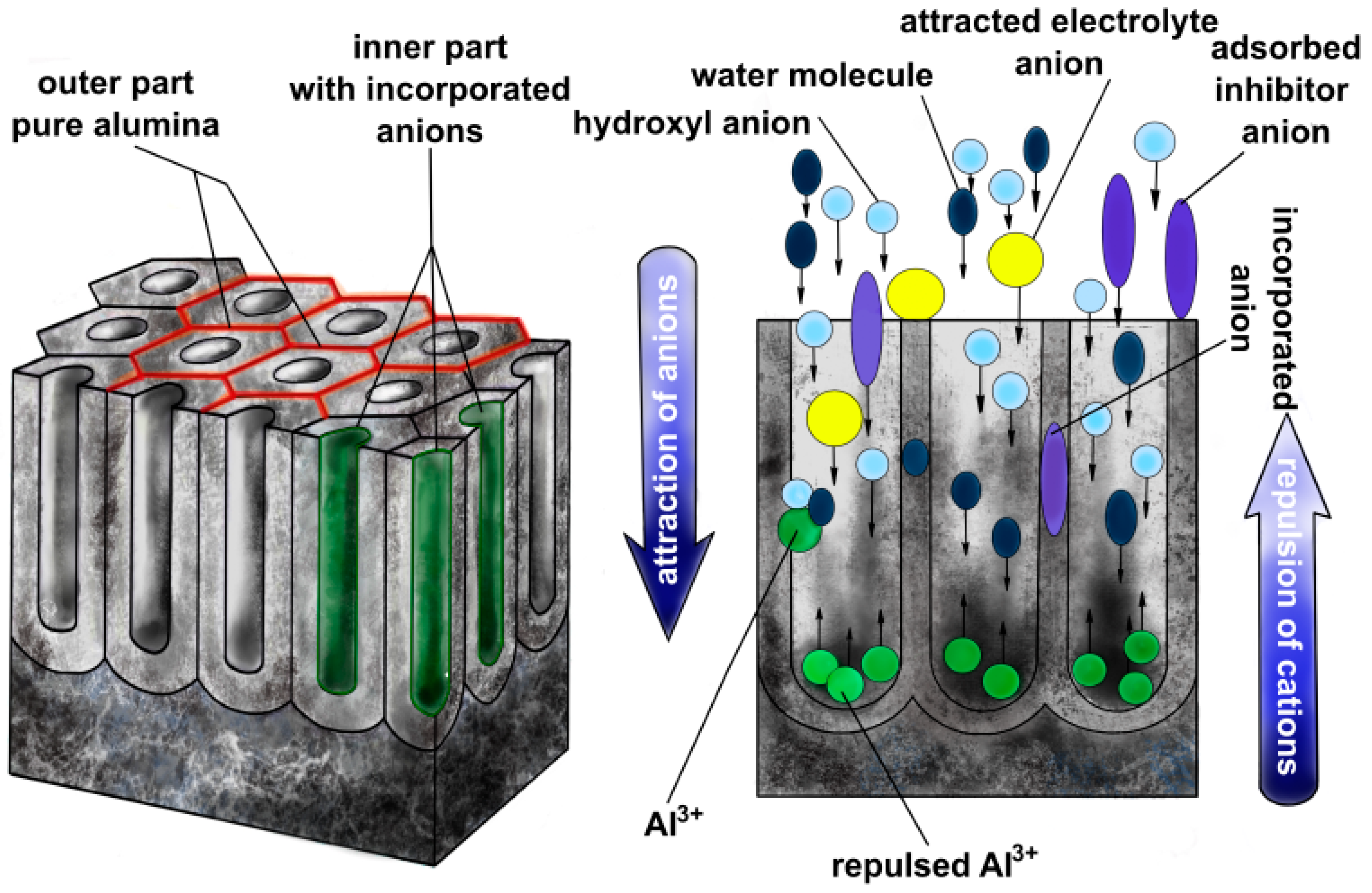
3. Incorporation of Anions into Anodic Alumina
4. Incorporation of Corrosion Inhibitors into Anodic Alumina
| Type of Material | Bath Composition | Anodizing Conditions | Corrosion Performance (Quantitative) | Remarks | Reference | ||
|---|---|---|---|---|---|---|---|
| Ecorr/mV | Jcorr/A.cm−2 | Other | |||||
| AA 2024 T3 | 17 wt.% H2SO4 | 20 V, 30 min, RT | −0.633 | 2.23 × 10−6 | Rb = 4295 Ω Cb = 37.9 µF | - | [75] |
| 17 wt.% H2SO4 + 50 mM KMnO4 | −0.555 | 4.11 × 10−7 | Rb = 9027 Ω Cb = 20 µF | 0.46 at. % (EDS) of Mn | |||
| 17 wt.% H2SO4 + 100 mM KMnO4 | −0.543 | 1.34 × 10−7 | Rb = 27,200 Ω Cb = 8.88 µF | 0.82 at. % (EDS) of Mn | |||
| 17 wt.% H2SO4 + 50 mM NH4H2PO4 | −0.550 | 5.8 × 10−8 | Rb = 27,900 Ω Cb = 4.71 µF | 2.61 at. % (EDS) of P | |||
| 17 wt.% H2SO4 + 100 mM NH4H2PO4 | −0.587 | 2.5 × 10−8 | Rb = 116,000 Ω Cb = 2.28 µF | 4.25 at. % (EDS) of P | |||
| AA 2024 T3 | 17 wt.% H2SO4 | 20 V, 30 min, RT | −0.633 | 2.23 × 10−6 | Rb = 4295 Ω Cb = 37.9 µF | The impact of potassium permanganate additive to the sulfuric acid was investigated; however, no chemical composition examinations of the anodic film were shown. Nevertheless, a strong inhibition effect was noticed. | [76] |
| 17 wt.% H2SO4 + 0.01 M KMnO4 | −0.578 | 1.23 × 10−6 | Rb = 5526 Ω Cb = 34 µF | ||||
| 17 wt.% H2SO4 + 0.05 M KMnO4 | −0.555 | 4.11 × 10−7 | Rb = 9027 Ω Cb = 20 µF | ||||
| 17 wt.% H2SO4 + 0.10 M KMnO4 | −0.543 | 1.34 × 10−7 | Rb = 27,200 Ω Cb = 8.88 µF | ||||
| 17 wt.% H2SO4 + 0.25 M KMnO4 | −0.531 | 2.6 × 10−8 | Rb = 148,000 Ω Cb = 2.94 µF | ||||
| AA 2024 T3 | 17 wt.% H2SO4 | 1 mA/cm2, 20 min, 20 °C | After anodizing, samples were immersed in 5 wt.% NaCl (35 °C) for 1, 2, 10, 24, and 48 h and EIS spectra were recorded. The capacitance of the barrier layer (cb) increased from 0.6 (1 h) to 10.2 µF/cm2 (48 h). After hot water sealing, cb ranged from 0.5 (1 h) to 3.9 µF/cm2 (48 h). | [77] | |||
| 17 wt.% H2SO4 + 0.1 M KMnO4 | The capacitance of the barrier layer grew from 0.5 (1 h) to 7.8 µF/cm2 (48 h). After hot water sealing, cb ranged from 0.2 (1 h) to 2.4 µF/cm2 (48 h). | ||||||
| AA 2024 | 150 g/L H2SO4 | 15–20 V, 20 °C, | Epit =−400 mV vs. Ag|AgCl, Rpo = 4100 Ω·cm2, cb = 0.46 µF/cm2. | HWS for 30 min; GD OES confirmed the presence of Mn in anodic alumina. | [81] | ||
| 150 g/L H2SO4 + 0.01 M KMnO4 | Epit = −90 mV vs. Ag|AgCl, Rpo = 16,000 Ω·cm2, cb = 0.19 µF/cm2. | ||||||
| Type of Material | Bath Composition | Anodizing Conditions | Corrosion Performance (Quantitative) | Remarks | Reference | |
|---|---|---|---|---|---|---|
| AA 2024 T3 | 17 wt.% H2SO4 | 1 mA/cm2, 20 min, 20 °C | After anodizing, samples were immersed in 5 wt.% NaCl (35 °C) for 1, 2, 10, 24, and 48 h and EIS spectra were recorded. The capacitance of the barrier layer (cb) increased from 0.6 (1 h) to 10.2 µF/cm2 (48 h). After hot water sealing, cb ranged from 0.5 (1 h) to 3.9 µF/cm2 (48 h). | [77] | ||
| 17 wt.% H2SO4 + 0.1 M Na2MoO4 | The capacitance of the barrier layer grew from 0.6 (1 h) to 9.8 µF/cm2 (48 h). After hot water sealing, cb ranged from 0.5 (1 h) to 3.4 µF/cm2 (48 h). | |||||
| AA 2024 T3 | 150 g/L H2SO4 + 0.1 M Na2MoO4 | 1.0 or 1.5 mA/cm2, 20 min, 20 °C | When Mo-containing electrolyte was used, greater voltage was recorded and thinner oxide was formed. | [80] | ||
| AA 2024 | 150 g/L H2SO4 | 15–20 V, 20 °C, | Epit = −400 mV vs. Ag|AgCl, Rpo = 4100 Ω·cm2, cb = 0.46 µF/cm2. | HWS for 30 min; GD OES confirmed the presence of Mo in anodic alumina. | [81] | |
| 150 g/L H2SO4 + 0.01 M Na2MoO4 | Epit = −250 mV vs. Ag|AgCl, Rpo = 8500 Ω·cm2, cb = 0.28 µF/cm2. | |||||
| AA 2024 T3 | 150 g/L H2SO4 | 1.0 mA/cm2, 20 min, 20 °C | Epit = −400 mV vs. Ag|AgCl, Rb = 3.5 × 107 Ω·cm2, cb = 1.01 µF/cm2. | HWS for 30 min. | [82] | |
| 150 g/L H2SO4 + 0.01 M Na2MoO4 | - | |||||
| 150 g/L H2SO4 + 0.1 M Na2MoO4 | Epit = −300 mV vs. Ag|AgCl, Rb = 4 × 107 Ω·cm2, cb = 0.86 µF/cm2. | |||||
| 150 g/L H2SO4 + 0.5 M Na2MoO4 | Epit = 0 mV vs. Ag|AgCl, Rb = 8 × 107 Ω·cm2, cb = 0.54 µF/cm2. | |||||
| AA 2024 T3 | 1.5 M H2SO4 | 1.0 mA/cm2, 20 min, 20 °C | Cb = 0.62 µF/cm2 | Atomic Absorption Spectroscopy (AAS) results have shown that anodizing with 0.1 M and 0.5 M Na2MoO4 provides 0.16 µg/cm2 and 0.96 µg/cm2 of Mo into anodic alumina. XPS and GDOES also confirmed the presence of Mo. | [83] | |
| 1.5 M H2SO4 + 0.1 M Na2MoO4 | Cb = 0.60 µF/cm2 | |||||
| 1.5 M H2SO4 + 0.5 M Na2MoO4 | Cb = 0.58 µF/cm2 | |||||
| AA 2024 T3 | 50 g/L Na2B4O7·10H2O | 40 V, 70 °C, 60 min, pH = 10 (NaOH) | The maximum amount of Mo was incorporated when the alloy was anodized in an electrolyte containing 0.3 M Na2MoO4 (EDS). The highest values of pitting potentials were for samples anodized in electrolytes containing 0.3 and 0.4 M Na2MoO4 | [84] | ||
| 50 g/L Na2B4O7·10H2O + 0.1 M Na2MoO4 | ||||||
| 50 g/L Na2B4O7·10H2O + 0.2 M Na2MoO4 | ||||||
| 50 g/L Na2B4O7·10H2O + 0.3 M Na2MoO4 | ||||||
| 50 g/L Na2B4O7·10H2O + 0.4 M Na2MoO4 | ||||||
| 50 g/L Na2B4O7·10H2O + 0.5 M Na2MoO4 | ||||||
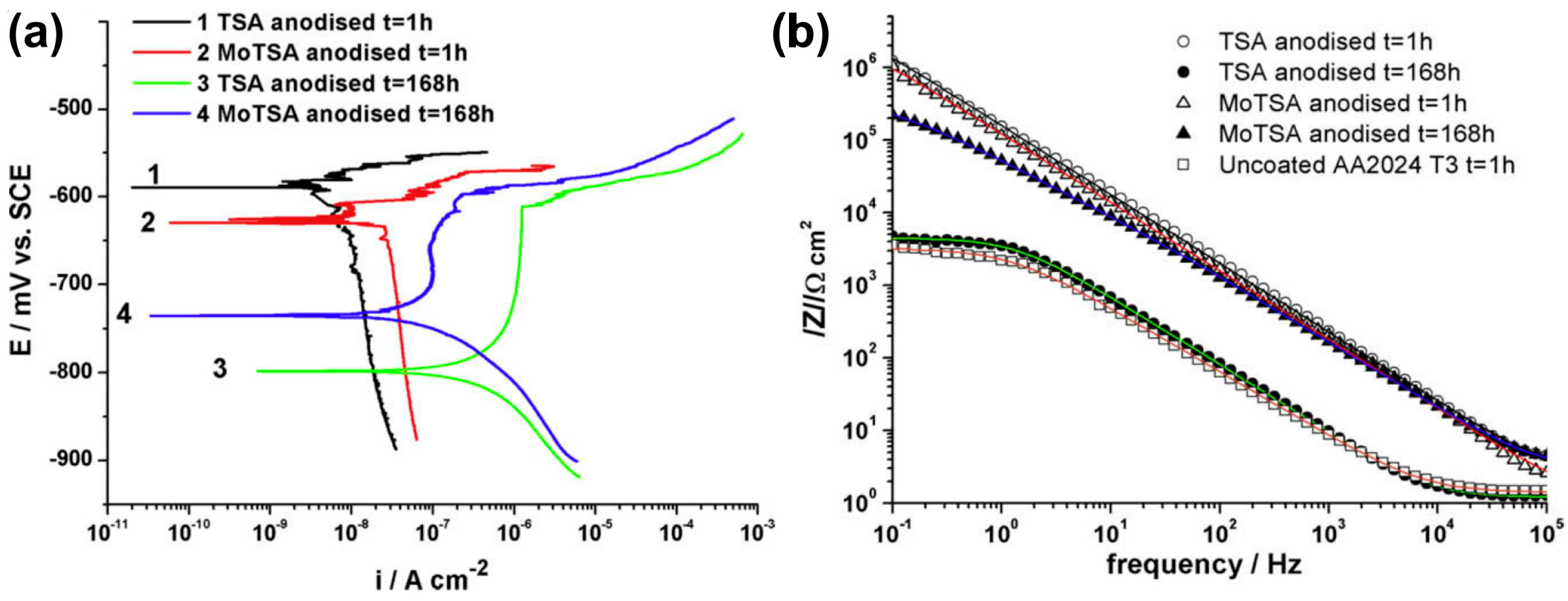
| AA 2024 T3 | 0.53 M C4H6O6 + 0.46 M H2SO4 | 14 V, 37 °C, 20 min | 1 h immersion in 3 wt.% NaCl: Ecorr = −594 mV vs. SCE, jcorr = 8 nA/cm2, Epitt = −569 mV vs. SCE, cb = 0.66 µF/cm2 168 h immersion in 3 wt.% NaCl: Ecorr = −798 mV vs. SCE jcorr = 1590 nA/cm2, Epitt = −611 mV vs. SCE, cb = 30.3 µF/cm2 | RBS and GDOES confirmed the presence of incorporated sulfates. | [85] | |
| 0.53 M C4H6O6 + 0.46 M H2SO4 + 0.25 M Na2MoO4 | 1 h immersion in 3 wt.% NaCl: Ecorr = −627 mV vs. SCE, jcorr = 24 nA/cm2, Epitt = −601 mV vs. SCE, cb = 0.95 µF/cm2 168 h immersion in 3 wt.% NaCl: Ecorr = −735 mV vs. SCE, jcorr = 132 nA/cm2, Epitt = −598 mV vs. SCE, cb = 3.87 µF/cm2 | RBS and GDOES confirmed the presence of Mo species: up to 0.15 at.% of Mo (RBS). | ||||
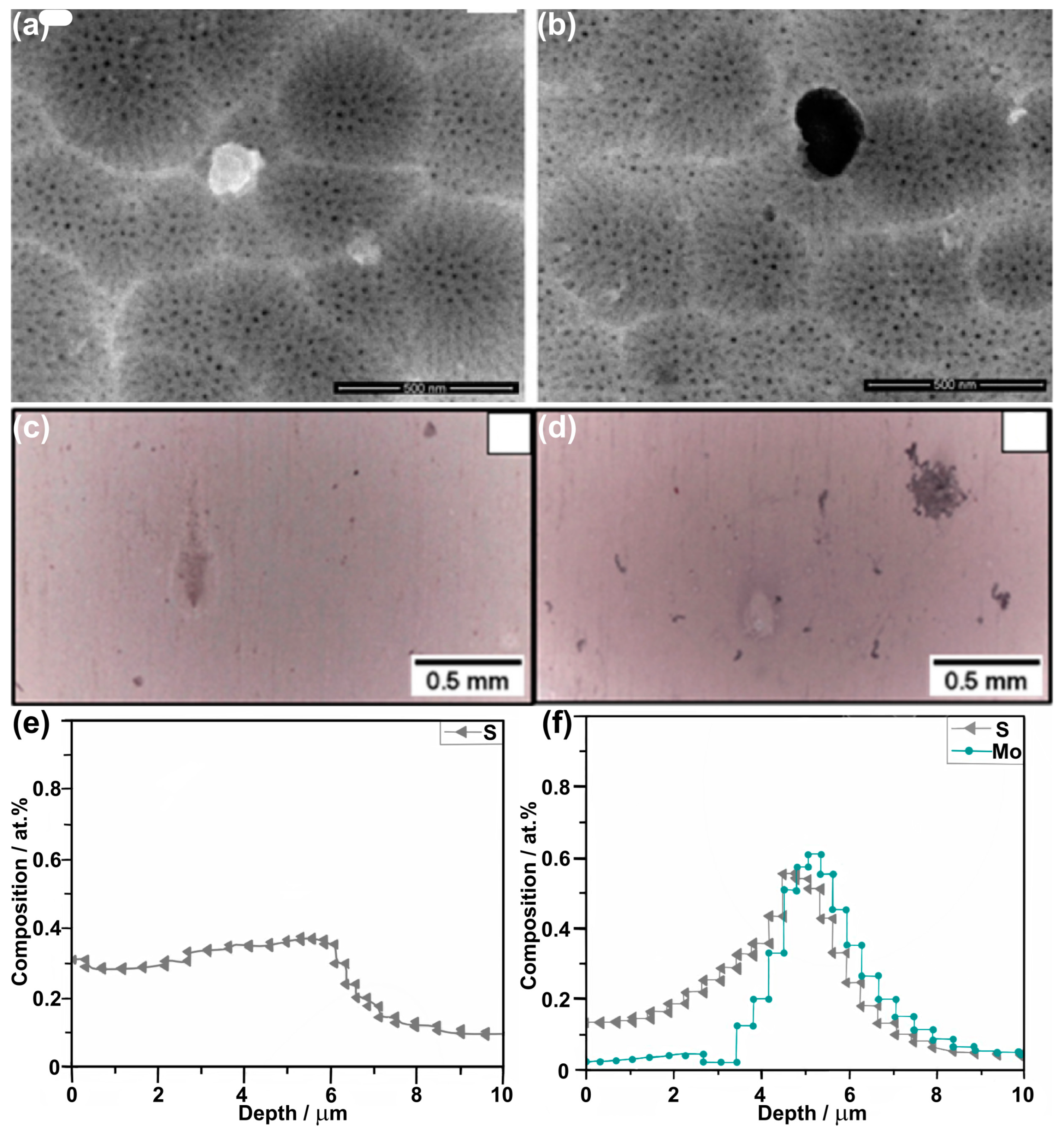
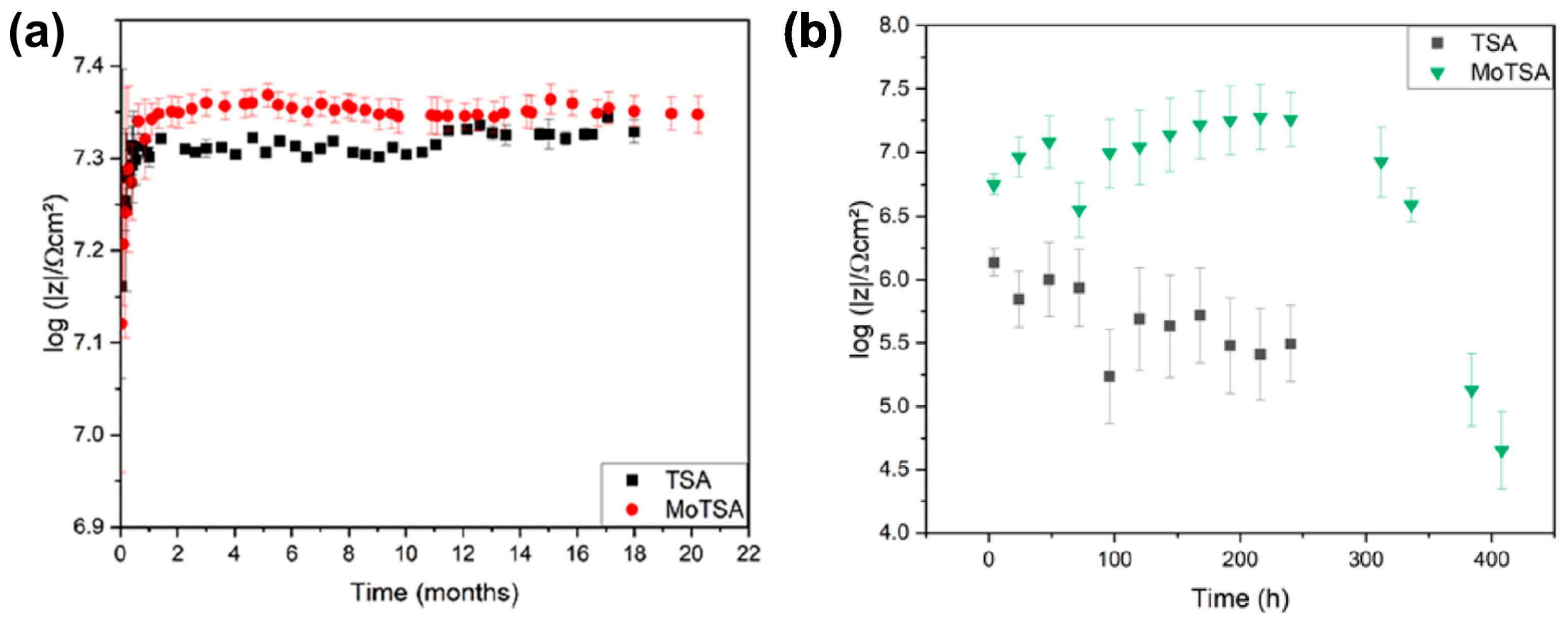
| AA 2024 T3 | (40 g/L 1 H2SO4 (0.47 M) + 80 g/L C4H6O6 (0.53 M) | 14 V, 37 °C, 20 min | EIS measurements were conducted in naturally aerated 0.5 M NaCl and acidified 0.1 M NaCl (pH = 4.0); XPS, RBS, and STEM-HAADF confirmed incorporation of the Mo species, up to 0.6 at% of Mo by RBS. | [86] | ||
| (40 g/L 1 H2SO4 (0.47 M) + 80 g/L C4H6O6 (0.53 M) + 0.1 M Na2MoO4 | ||||||
| AA 5052 | 70 wt% H4P2O7 | 15 °C, 30 V, 0 min | Ecorr = −0.729 V vs. Ag|AgCl, jcorr = 27.69 µA/cm2 | Corrosion research was conducted in 3.5 wt.% NaCl, but at 70 C, to simulate the exploitation conditions; XPS and EDS confirmed the incorporation of Mo species into anodic alumina (up to 0.48 wt.%), as well as the incorporation of pyrophosphates (up to 2.49 wt.%). | [87] | |
| 15 °C, 30 V, 20 min | Ecorr = −0.715 V vs. Ag|AgCl, jcorr = 20.76 µA/cm2 | |||||
| 15 °C, 30 V, 40 min | Ecorr = −0.722 V vs. Ag|AgCl, jcorr = 21.47 µA/cm2 | |||||
| 15 °C, 30 V, 60 min | Ecorr = −0.729 V vs. Ag|AgCl, jcorr = 22.14 µA/cm2 | |||||
| 15 °C, 30 V, 20 min | Ecorr = −0.715 V vs. Ag|AgCl, jcorr = 20.76 µA/cm2 | |||||
| 15 °C, 40 V, 20 min | Ecorr = −0.702 V vs. Ag|AgCl, jcorr = 19.23 µA/cm2 | |||||
| 15 °C, 50 V, 20 min | Ecorr = −0.687 V vs. Ag|AgCl, jcorr = 17.66 µA/cm2 | |||||
| 15 °C, 60 V, 20 min | Ecorr = −0.668 V vs. Ag|AgCl, jcorr = 14.32 µA/cm2 | |||||
| 15 °C, 30 V, 20 min | Ecorr = −0.715 V vs. Ag|AgCl, jcorr = 20.76 µA/cm2 CPEb = 1.36 µF/cm2, Rb = 3165 Ω·cm2 | Barrier layer thickening with Mo amount increase; BL = 6.5 nm. | ||||
| 70 wt% H4P2O7 + 0.01 M Na2MoO4 | 15 °C, 30 V, 20 min | Ecorr = −0.642 V vs. Ag|AgCl, jcorr = 17.55 µA/cm2, CPEb = 1.17 µF/cm2, Rb = 3590 Ω·cm2 | BL = 7.6 nm (0.13 wt.% of Mo; EDS) | |||
| 70 wt% H4P2O7 + 0.1 M Na2MoO4 | 15 °C, 30 V, 30 min | Ecorr = −0.528 V vs. Ag|AgCl, jcorr = 10.1 µA/cm2 CPEb = 0.68 µF/cm2, Rb = 4548 Ω·cm2 | BL = 13.0 nm (0.33 wt.% of Mo; EDS) | |||
| 70 wt% H4P2O7 + 0.5 M Na2MoO4 | 15 °C, 30 V, 40 min | Ecorr = −0.264 V vs. Ag|AgCl, jcorr = 5.67 µA/cm2 CPEb = 0.58 µF/cm2, Rb = 6252 Ω·cm2 | BL = 15.3 nm (0.48 wt.% of Mo; EDS); 0 h immersion in 3.5 wt.% NaCl, 70 °C | |||
| Ecorr = −0.345 V vs. Ag|AgCl, jcorr = 8.86 µA/cm2 CPEb = 1.54 µF/cm2, Rb = 5689 Ω·cm2 | 24 h immersion in 3.5 wt.% NaCl, 70 °C | |||||
| Ecorr = −0.428 V vs. Ag|AgCl, jcorr = 12.23 µA/cm2 CPEb = 2.68 µF/cm2, Rb = 4985 Ω·cm2 | 72 h immersion in 3.5 wt.% NaCl, 70 °C | |||||
| Ecorr = −0.462 V vs. Ag|AgCl, jcorr = 13.29 µA/cm2 CPEb = 2.93 µF/cm2, Rb = 4608 Ω·cm2 | 120 h immersion in 3.5 wt.% NaCl, 70 °C | |||||
| Ecorr = −0.488 V vs. Ag|AgCl, jcorr = 13.84 µA/cm2 CPEb = 3.24 µF/cm2, Rb = 4257 Ω·cm2 | 168 h immersion in 3.5 wt.% NaCl, 70 °C | |||||
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrahami, S.T.; de Kok, J.M.M.; Terryn, H.; Mol, J.M.C. Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: A review. Front. Chem. Sci. Eng. 2017, 11, 465–482. [Google Scholar] [CrossRef]
- United States Department of Labor OSHA. Toxic and Hazardous Substances in Occupational Exposure to Hexavalent Chromium. Regulatory Standard 29 CFR 1910.1026 and 29 CFR 1926.1126 Federal Register Number; United States Department of Labor OSHA: Washington, DC, USA, 2006; Volume 71, pp. 10099–10385. [Google Scholar]
- Regulation (EC). No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC 2006. Off. J. 1999, L396, 1–849. [Google Scholar]
- Koop, R.; Moji, Y. Boric/Sulfuric Acid Anodize-Alternative to Chromic Acid Anodize; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1992; p. 920944. [Google Scholar] [CrossRef]
- Thompson, G.E.; Zhang, L.; Smith, C.J.E.; Skeldon, P. Boric/Sulfuric Acid Anodizing of Aluminum Alloys 2024 and 7075: Film Growth and Corrosion Resistance. Corrosion 1999, 55, 1052–1061. [Google Scholar] [CrossRef]
- Zhang, L.; Thompson, G.E.; Curioni, M.; Skeldon, P. Anodizing of Aluminum in Sulfuric Acid/Boric Acid Mixed Electrolyte. J. Electrochem. Soc. 2013, 160, C179–C184. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Zuo, Y.; Xiong, J. The bonding strength and corrosion resistance of aluminum alloy by anodizing treatment in a phosphoric acid modified boric acid/sulfuric acid bath. Surf. Coat. Technol. 2008, 202, 3149–3156. [Google Scholar] [CrossRef]
- Domingues, L.; Fernandes, J.C.S.; Da Cunha Belo, M.; Ferreira, M.G.S.; Guerra-Rosa, L. Anodising of Al 2024-T3 in a modified sulphuric acid/boric acid bath for aeronautical applications. Corr. Sci. 2003, 45, 149–160. [Google Scholar] [CrossRef]
- Ding, Z.; Smith, B.A.; Hebert, R.R.; Zhang, W.; Jaworowski, M.R. Morphology perspective on chromic acid anodizing replacement by thin film sulfuric acid anodizing. Surf. Coat. Technol. 2018, 350, 31–39. [Google Scholar] [CrossRef]
- Ding, Z. Mechanistic study of thin film sulfuric acid anodizing rate difference between Al2024 T3 and Al6061 T6. Surf. Coat. Technol. 2019, 357, 280–288. [Google Scholar] [CrossRef]
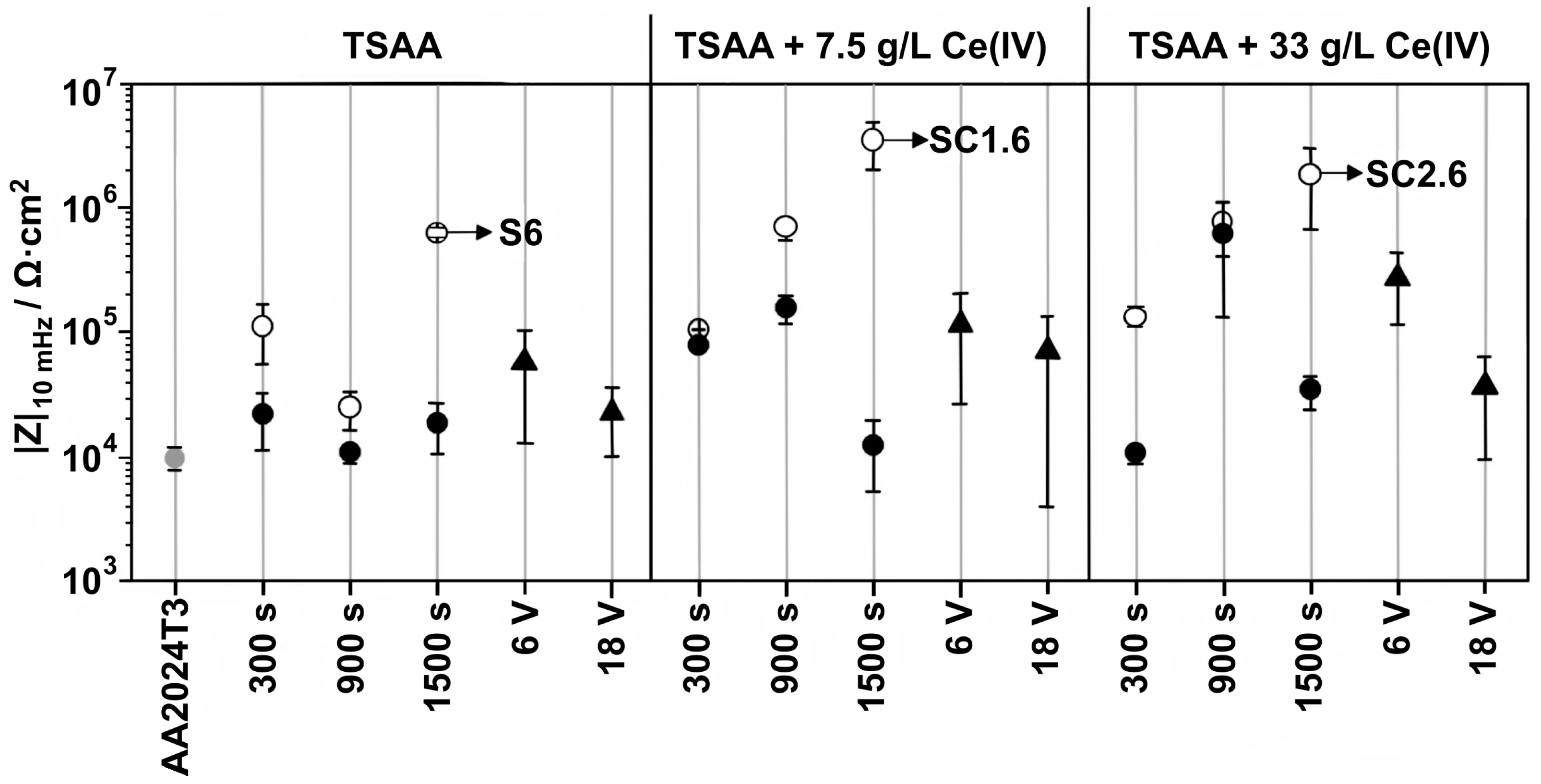
- Del Olmo, R.; Mohedano, M.; Visser, P.; Rodriguez, A.; Matykina, E.; Arrabal, R. Effect of cerium (IV) on thin sulfuric acid anodizing of 2024-T3 alloy. J. Mater. Res. Technol. 2021, 15, 3240–3245. [Google Scholar] [CrossRef]
- Curioni, M.; Skeldon, P.; Koroleva, E.; Thompson, G.E.; Ferguson, J. Role of Tartaric Acid on the Anodizing and Corrosion Behavior of AA 2024 T3 Aluminum Alloy. J. Electrochem. Soc. 2009, 156, C147–C153. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Liu, W. Effects of tartaric acid on the structure and corrosion resistance for anodizing films of aerospace aluminium alloys. Mater. Res. Innov. 2024, 28, 1–7. [Google Scholar] [CrossRef]
- Martinez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. Adhesion properties of tartaric sulfuric acid anodic films assessed by a fast and quantitative peel tape adhesion test. Int. J. Adhes. Adhes. 2022, 116, 103156. [Google Scholar] [CrossRef]
- Setianto, M.H.; Korda, A.A. Characterization of Tartaric-Sulphuric Acid Anodized 2024-T3 Aluminium Alloys with Anodizing Potential Variation. J. Phys. Conf. Ser. 2019, 1204, 012039. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. Corrosion Testing of Anodized Aerospace Alloys: Comparison Between Immersion and Salt Spray Testing using Electrochemical Impedance Spectroscopy. J. Electroch. Soc. 2020, 167, 041505. [Google Scholar] [CrossRef]
- Sulka, G.D.; Parkoła, K. Anodising potential influence on well-ordered nanostructures formed by anodisation of aluminium in sulphuric acid. Thin Solid Films 2006, 515, 338–345. [Google Scholar] [CrossRef]
- Raffin, F.; Echouard, J.; Volovitch, P. Influence of the Anodizing Time on the Microstructure and Immersion Stability of Tartaric-Sulfuric Acid Anodized Aluminum Alloys. Metals 2023, 13, 993. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Bojar, Z. Synthesis of anodic aluminum oxide (AAO) at relatively high temperatures. Study of the influence of anodization conditions on the alumina structural features. Surf. Coat. Technol. 2011, 206, 265–272. [Google Scholar] [CrossRef]
- Iglesias-Rubianes, L.; Garcia-Vergara, S.J.; Skeldon, P.; Thompson, G.E.; Ferguson, J.; Beneke, M. Cyclic oxidation processes during anodizing of Al–Cu alloys. Electrochim. Acta 2007, 52, 7148–7157. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; He, C.; Lu, M.; Sun, L. Studies on the sealing processes of corrosion resistant coatings formed on 2024 aluminium alloy with tartaric-sulfuric anodizing. Surf. Coat. Technol. 2019, 360, 369–375. [Google Scholar] [CrossRef]
- González-Rovira, L.; González-Souto, L.; Astola, P.J.; Bravo-Benítez, C.; Botana, F.J. Assessment of the corrosion resistance of self-ordered anodic aluminum oxide (AAO) obtained in tartaric-sulfuric acid (TSA). Surf. Coat. Technol. 2020, 399, 126131. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Del Olmo, R.; Tiringer, U.; Milosev, I.; Visser, P.; Arrabal, R.; Matykina, E.; Mol, J.M.C. Hybrid sol-gel coatings applied on anodized AA2024-T3 for active corrosion protection. Surf. Coat. Technol. 2021, 419, 127251. [Google Scholar] [CrossRef]
- Ji, M.; Li, W.; Liu, H.; Zhu, L.; Chen, H.; Li, W. Effect of titanium sol on sulfuric-citric acids anodizing of 7150 aluminum alloy. Surf. Interfaces 2020, 19, 100479. [Google Scholar] [CrossRef]
- Machado, T.V.; Dick, P.A.; Knörnschild, G.H.; Dick, L.F.P. The effect of different carboxylic acids on the sulfuric acid anodizing of AA2024. Surf. Coat. Technol. 2020, 383, 125283. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kawahara, K.; Kikuchi, T.; Suzuki, R.O.; Natsui, S. Corrosion-Resistant Porous Alumina Formed via Anodizing Aluminum in Etidronic Acid and Its Pore-Sealing Behavior in Boiling Water. J. Electrochem. Soc. 2019, 166, C261–C269. [Google Scholar] [CrossRef]
- Yoganandan, G.; Balaraju, J.N.; Manikandanath, N.T.; Ezhilselvi, V.; Srivastava, M.; Nagacharan, K.V.; Anilchandra, A.R.; Manjunatha, C.M. Surface and Electrochemical Characteristics of Novel Chromate-Free Mn-V Oxyanion Sealed Tartaric–Sulfuric Acid Anodized Coating. J. Mater. Eng. Perf. 2018, 27, 6175–6188. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Fabrication of Anodic Nanoporous Alumina via Acetylenedicarboxylic Acid Anodizing. ECS Electrochem. Lett. 2014, 3, C25–C28. [Google Scholar] [CrossRef]
- Akiya, S.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Nanostructural characterization of large-scale porous alumina fabricated via anodizing in arsenic acid solution. Appl. Surf. Sci. 2017, 403, 652–661. [Google Scholar] [CrossRef]
- Norek, M.; Łażewski, M. Manufacturing of highly ordered porous anodic alumina with conical pore shape and tunable interpore distance in the range of 550 nm to 650 nm. Mater. Sci. 2017, 35, 511–518. [Google Scholar] [CrossRef]
- Norek, M.; Włodarski, M. Morphological and chemical characterization of highly ordered conical-pore anodic alumina prepared by multistep citric acid anodizing and chemical etching process. J. Porous. Mater. 2018, 25, 45–53. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nakajima, D.; Kawashima, J.; Natsui, S.; Suzuki, R.O. Fabrication of anodic porous alumina via anodizing in cyclicoxocarbon acids. Appl. Surf. Sci. 2014, 313, 276–285. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Growth behavior of anodic oxide formed by aluminum anodizing in glutaric and its derivative acid electrolytes. Appl. Surf. Sci. 2014, 321, 364–370. [Google Scholar] [CrossRef]
- Zajączkowska, L.; Norek, M. Peculiarities of Aluminum Anodization in AHAs-Based Electrolytes: Case Study of the Anodization in Glycolic Acid Solution. Materials 2021, 14, 5362. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, L.; Siemaszko, D.; Norek, M. Towards Self-Organized Anodization of Aluminum in Malic Acid Solutions—New Aspects of Anodization in the Organic Acid. Materials 2020, 13, 3899. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Yamamoto, T.; Suzuki, R.O. Growth behavior of anodic porous alumina formed in malic acid solution. Appl. Surf. Sci. 2013, 284, 907–913. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Jagminas, A.; Hemeltjen, S.; Goedel, W.A. Photoluminescent behavior of heat-treated porous alumina films formed in malonic acid. Appl. Surf. Sci. 2010, 256, 2013–2017. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Jagminas, A.; Hemeltjen, S.; Goedel, W.A. Behavior of acid species during heat treatment and re-anodizing of porous alumina films formed in malonic acid. J. Solid State Electrochem. 2009, 13, 1873–1880. [Google Scholar] [CrossRef]
- Poznyak, A.; Knörnschild, G.; Karoza, A.; Norek, M.; Pligovka, A. Peculiar Porous Aluminum Oxide Films Produced via Electrochemical Anodizing in Malonic Acid Solution with Arsenazo-I Additive. Materials 2021, 14, 5118. [Google Scholar] [CrossRef]
- Akiya, S.; Kikuchi, T.; Natsui, S.; Sakaguchi, N.; Suzuki, R.O. Self-ordered porous alumina fabricated via phosphonic acid anodizing. Electrochim. Acta 2016, 190, 471–479. [Google Scholar] [CrossRef]
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Self-Ordered Aluminum Anodizing in Phosphonoacetic Acid and Its Structural Coloration. ECS Solid State Lett. 2015, 4, P55–P58. [Google Scholar] [CrossRef]
- Kikuchi, T.; Onoda, F.; Iwai, M.; Suzuki, R.O. Influence of sub-10 nm anodic alumina nanowire morphology formed by two-step anodizing aluminum on water wettability and slipping behavior. Appl. Surf. Sci. 2021, 546, 149090. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Advancing and receding contact angle investigations for highly sticky and slippery aluminum surfaces fabricated from nanostructured anodic oxide. RSC Adv. 2018, 8, 37315. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, D.; Kikuchi, T.; Yoshioka, T.; Matsushima, H.; Ueda, M.; Suzuki, R.O.; Natsui, S. A Superhydrophilic Aluminum Surface with Fast Water Evaporation Based on Anodic Alumina Bundle Structures via Anodizing in Pyrophosphoric Acid. Materials 2019, 12, 3497. [Google Scholar] [CrossRef] [PubMed]
- Akiya, S.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Optimum Exploration for the Self-Ordering of Anodic Porous Alumina Formed via Selenic Acid Anodizing. J. Electrochem. Soc. 2015, 162, E244–E250. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Self-Ordering Behavior of Anodic Porous Alumina via Selenic Acid Anodizing. Electrochim. Acta 2014, 137, 728–735. [Google Scholar] [CrossRef]
- Nishinaga, O.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Rapid fabrication of self-ordered porous alumina with 10-/sub-10-nm-scale nanostructures by selenic acid anodizing. Sci. Rep. 2013, 3, 2748. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Yamamoto, T.; Natsui, S.; Suzuki, R.O. Fabrication of Anodic Porous Alumina by Squaric Acid Anodizing. Electrochim. Acta 2014, 123, 14–22. [Google Scholar] [CrossRef]
- Ispas, A.; Bund, A.; Vrublevsky, I. Effects of a magnetic field on growth of porous alumina films on aluminum. Electrochim. Acta 2010, 55, 4180–4187. [Google Scholar] [CrossRef]
- Dorsey, G.A. The characterization of anodic aluminas. I. Composition of films from acidelectrolytes. J. Electrochem. Soc. 1966, 113, 169–172. [Google Scholar] [CrossRef]
- Dorsey, G.A. The Characterization of Anodic Aluminas: II. Effect of Anodizing Temperature on Coatings from Aliphatic Dicarboxylic Electrolytes. J. Electrochem. Soc. 1966, 113, 172–176. [Google Scholar] [CrossRef]
- Davenoport, A.J.; Isaacs, H.S. Glancing Angle studies of oxide films. Corr. Sci. 1990, 31, 105–110. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Baba, N. Nature of the carboxylate species incorporated in anodic alumina films formed in oxalic acid solution. Thin Solid Films 1983, 101, 329–338. [Google Scholar] [CrossRef]
- Le Coz, F.; Arurault, L.; Datas, L. Chemical analysis of a single basic cell porous anodic aluminium oxide templates. Mater. Charact. 2010, 61, 282–288. [Google Scholar] [CrossRef]
- Parkhutik, V.P.; Abella, J.M.; Makushok, Y.E.; Monteri, I.; Martinez Duart, J.M.; Shershulskii, V.I. Study of aluminium in sulphuric and chromic acid solutions—I kinetics of growth and composition of oxides. Electrochim. Acta 1990, 35, 955–960. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Chernyakova, K.V.; Ispas, A.; Bund, A.; Zavadski, S. Optical properties of thin anodic alumina membranes formed in a solution of tartaric acid. This Solid Films 2014, 556, 230–235. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Cantelli, L.; Santos, J.S.; Silva, T.F.; Tabacniks, M.H.; Delgado-Silva, A.O.; Trivinho-Strixino, F. Unveiling the origin of photoluminescence in nanoporous anodic alumina (NAA) obtained by constant current regime. J. Lumin. 2019, 207, 63–69. [Google Scholar] [CrossRef]
- Cantelli, L.; Santos, J.S.; Trivinho-Strixino, F. The effect of anodization temperature on optical properties of nanoporous anodic alumina (NAA) films. J. Electroanal. Chem. 2016, 780, 386–390. [Google Scholar] [CrossRef]
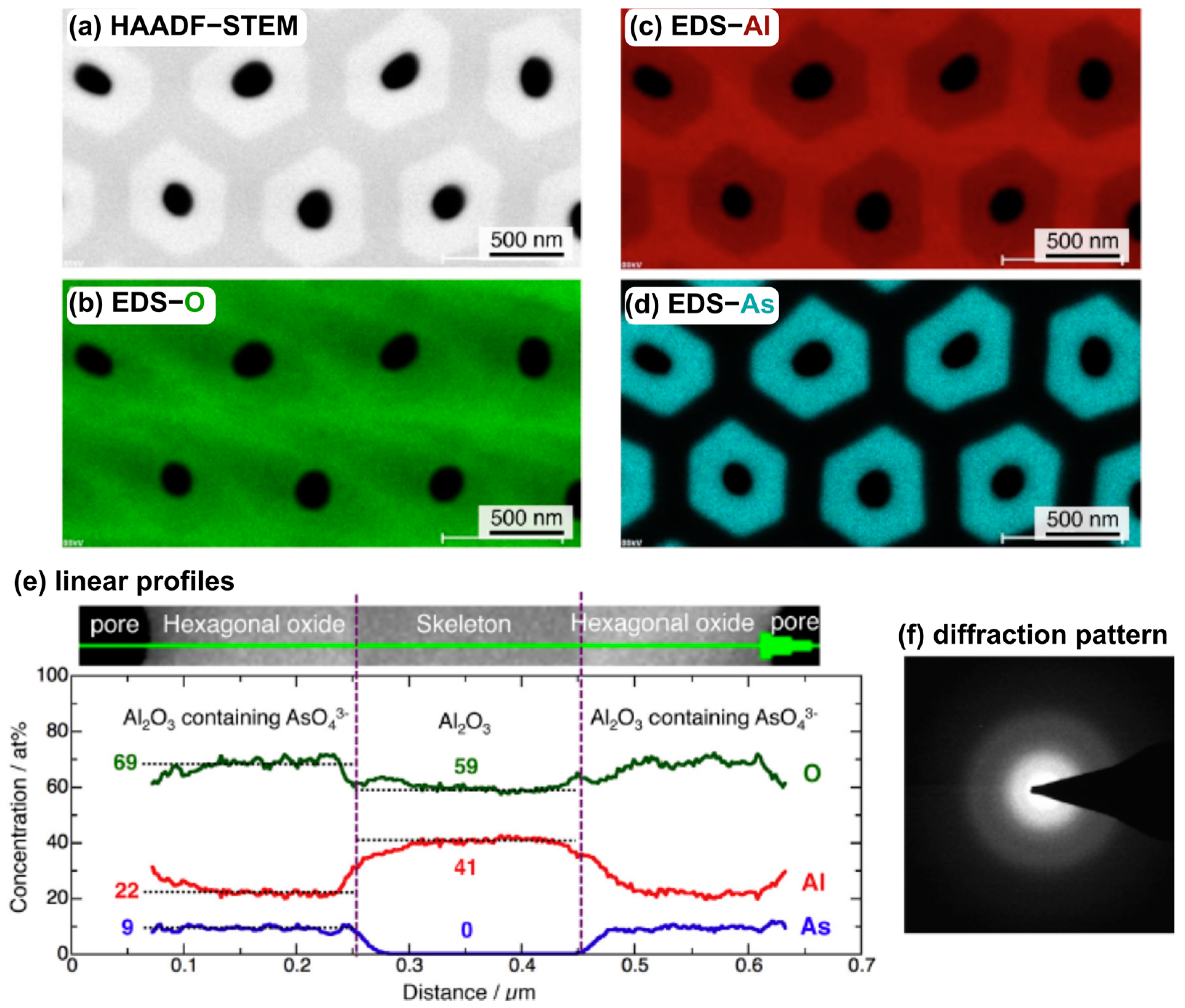
- Stępniowski, W.J.; Norek, M.; Michalska-Domańska, M.; Bombalska, A.; Nowak-Stępniowska, A.; Kwaśny, M.; Bojar, Z. Fabrication of anodic aluminum oxide with incorporated chromate ions. Appl. Surf. Sci. 2012, 259, 324–330. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yamashita, M.; Iwai, M.; Suzuki, R.O. Self-Ordering of Porous Anodic Alumina Fabricated by Anodizing in Chromic Acid at High Temperature. J. Electrochem. Soc. 2021, 168, 093501. [Google Scholar] [CrossRef]
- Sato, Y.; Asoh, H.; Ono, S. Effects of Electrolyte Species and Their Combination on Film Structures and Dielectric Properties of Crystalline Anodic Alumina Films Formed by Two-Step Anodization. Mater. Trans. 2013, 54, 1993–1999. [Google Scholar] [CrossRef]
- Mínguez-Bacho, I.; Rodríguez-López, S.; Climent-Font, A.; Fichou, D.; Vázquez, M.; Hernández-Vélez, M. Variation of the refractive index by means of sulfate anion incorporation into nanoporous anodic aluminum oxide films. Micropor. Mesopor. Mater. 2016, 225, 192–197. [Google Scholar] [CrossRef]
- Choi, J.; Luo, Y.; Wehrspohn, R.B.; Hillebrand, R.; Schilling, J.; Gösele, U. Perfect two-dimensional porous alumina photonic crystals with duplex oxide layers. J. Appl. Phys. 2003, 94, 4757–4762. [Google Scholar] [CrossRef]
- Han, H.; Park, S.-J.; Jang, J.S.; Ryu, H.; Kim, K.J.; Baik, S.; Lee, W. In Situ Determination of the Pore Opening Point during Wet-Chemical Etching of the Barrier Layer of Porous Anodic Aluminum Oxide: Nonuniform Impurity Distribution in Anodic Oxide. ACS Appl. Mater. Interfaces 2013, 5, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Bikulcius, G.; Suchodolskis, A.; Selskiene, A.; Matijosius, T. Formation of a black anodic coating on alloy AA5052 by using anodizing in a tri-acid environmentally friendly electrolyte. Mater. Chem. Phys. 2024, 316, 129124. [Google Scholar]
- Stępniowski, W.J.; Norek, M.; Michalska-Domańska, M.; Nowak-Stępniowska, A.; Bombalska, A.; Włodarski, M.; Bojar, Z. Incorporation of copper chelate ions into anodic alumina walls. Mater. Lett. 2013, 106, 242–245. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Norek, M.; Budner, B.; Michalska-Domańska, M.; Nowak-Stępniowska, A.; Bombalska, A.; Kaliszewski, M.; Mostek, A.; Thorat, S.; Salerno, M.; et al. In-situ electrochemical doping of nanoporous anodic aluminum oxide with indigo carmine organic dye. Thin Solid Films 2016, 598, 60–64. [Google Scholar] [CrossRef]
- Chernyakova, K.; Jasulaitiene, V.; Naujokaitis, A.; Karpicz, R.; Matulaitiene, I.; Klimas, V.; Jagminas, A. Aluminum Anodizing in an Aqueous Solution of Formic Acid with Ammonium Heptamolybdate Additive. J. Electrochem. Soc. 2023, 170, 013501. [Google Scholar] [CrossRef]
- Chernyakova, K.; Klimas, V.; Karpicz, R.; Naujokaitis, A.; Jagminas, A. Effect of Oxalic Acid Additives on Aluminum Anodizing in Formic Acid Containing Ammonium Heptamolybdate. J. Electrochem. Soc. 2023, 170, 103511. [Google Scholar] [CrossRef]
- Selegård, L.; Poot, T.; Eriksson, P.; Palisaitis, J.; Persson, P.O.Å.; Hu, Z.; Uvdal, K. In-situ growth of cerium nanoparticles for chrome-free, corrosion resistant anodic coatings. Surf. Coat. Technol. 2021, 410, 126958. [Google Scholar] [CrossRef]
- Ramirez, O.M.P.; Queiroz, F.M.; Tunes, M.A.; Antunes, R.A.; Rodrigues, C.L.; Lanzutti, A.; Pogatscher, S.; Olivier, M.G.; De Melo, H.G. Tartaric-sulphuric acid anodized clad AA2024-T3 post-treated in Cecontaining solutions at different temperatures: Corrosion behaviour and Ce ions distribution. Appl. Surf. Sci. 2020, 534, 14763. [Google Scholar] [CrossRef]
- Mohammadi, M.; Yazdani, A.; Bahrololoom, M.E.; Alfantazi, A. Corrosion behavior of 2024 aluminum alloy anodized in presence of permanganate and phosphate ions. J. Coat. Technol. Res. 2013, 10, 219–229. [Google Scholar] [CrossRef]
- Mohammadi, M.; Yazdani, A.; Mohammadi, F.; Alfantazi, A. Corrosion behavior of 2024 aluminum alloy anodized in sulfuric acid containing inorganic inhibitor. In Light Metals 2013. The Minerals, Metals & Materials Series; Sadler, B.A., Ed.; Springer: Cham, Switzerland, 2013. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Normand, B.; Pagetti, J. EIS characterisation of anodic films formed on 2024 aluminium alloy, in sulphuric acid containing molybdate or permanganate species. Corr. Sci. 2005, 47, 937–951. [Google Scholar] [CrossRef]
- Kwolek, P. Corrosion behaviour of 7075 aluminium alloy in acidic solution. RSC Adv. 2020, 10, 26078. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garrity, O.; Frankel, G.S. Corrosion Inhibition of Aluminum Alloy 2024-T3 by Sodium Molybdate. J. Electrochem. Soc. 2014, 161, C95–C106. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Pagetti, J.; Normand, B. An electrochemical approach to the anodic oxidation of Al 2024 alloy in sulfuric acid containing inhibitors. Surf. Coat. Technol. 2002, 161, 267–274. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Ricq, L.; Pagetti, J. Electrochemical characterisation of anodic oxidation films formed in presence of corrosion inhibitors. Appl. Surf. Sci. 2001, 183, 1–9. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Pagetti, J.; Ricq, L. Molybdate/sulfuric acid anodising of 2024-aluminium alloy: Influence of inhibitor concentration on film growth and on corrosion resistance. Surf. Coat. Technol. 2003, 17, 87–95. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Pagetti, J.; Linget, S. Influence of molybdate species added to sulphuric acid on composition and morphology of the anodic layers formed on 2024 aluminium alloy. Thin Solid Films 2005, 483, 197–204. [Google Scholar] [CrossRef]
- Moutarlier, V.; Pelletier, S.; Lallemand, F.; Gigandet, M.P.; Mekhalif, Z. Characterisation of the anodic layers formed on 2024 aluminium alloy, in tetraborate electrolyte containing molybdate ions. Appl. Surf. Sci. 2005, 252, 1739–1746. [Google Scholar] [CrossRef]
- García-Rubio, M.; Ocón, P.; Climent-Font, A.; Smith, R.W.; Curioni, M.; Thompson, G.E.; Skeldon, P.; Lavía, A.; García, I. Influence of molybdate species on the tartaric acid/sulphuric acid anodic films grown on AA2024 T3 aerospace alloy. Corr. Sci. 2009, 51, 2034–2042. [Google Scholar] [CrossRef]
- de Almeida, T.F.; Prada Ramirez, O.M.; Lanzutti, A.; Rodrigues, C.L.; Brabetz, M.; Kremmer, T.M.; Hammer, P.; de Melo, H.G. Addition of molybdate ions to the anodizing bath to improve the corrosion resistance of clad 2024-T3 alloy anodized in tartaric-sulfuric acid. Surf. Coat. Technol. 2024, 482, 130682. [Google Scholar] [CrossRef]
- Lv, J.; Chen, Z.-L.; Tang, J.; Chen, L.; Xie, W.-J.; Sun, M.-X.; Huang, X.-J. Study on the superhydrophilic modification and enhanced corrosion resistance method of aluminum alloy distillation desalination tubes. Surf. Coat. Technol. 2022, 446, 128770. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, K.; Stępniowski, W.J. Incorporation of Anions into Anodic Alumina—A New Track in Cr(VI) Anodizing Substitution? Materials 2024, 17, 2938. https://doi.org/10.3390/ma17122938
Tomczyk K, Stępniowski WJ. Incorporation of Anions into Anodic Alumina—A New Track in Cr(VI) Anodizing Substitution? Materials. 2024; 17(12):2938. https://doi.org/10.3390/ma17122938
Chicago/Turabian StyleTomczyk, Katarzyna, and Wojciech J. Stępniowski. 2024. "Incorporation of Anions into Anodic Alumina—A New Track in Cr(VI) Anodizing Substitution?" Materials 17, no. 12: 2938. https://doi.org/10.3390/ma17122938





