The Effect of Implantoplasty on the Fatigue Behavior and Corrosion Resistance in Titanium Dental Implants
Abstract
1. Introduction
2. Materials and Methods
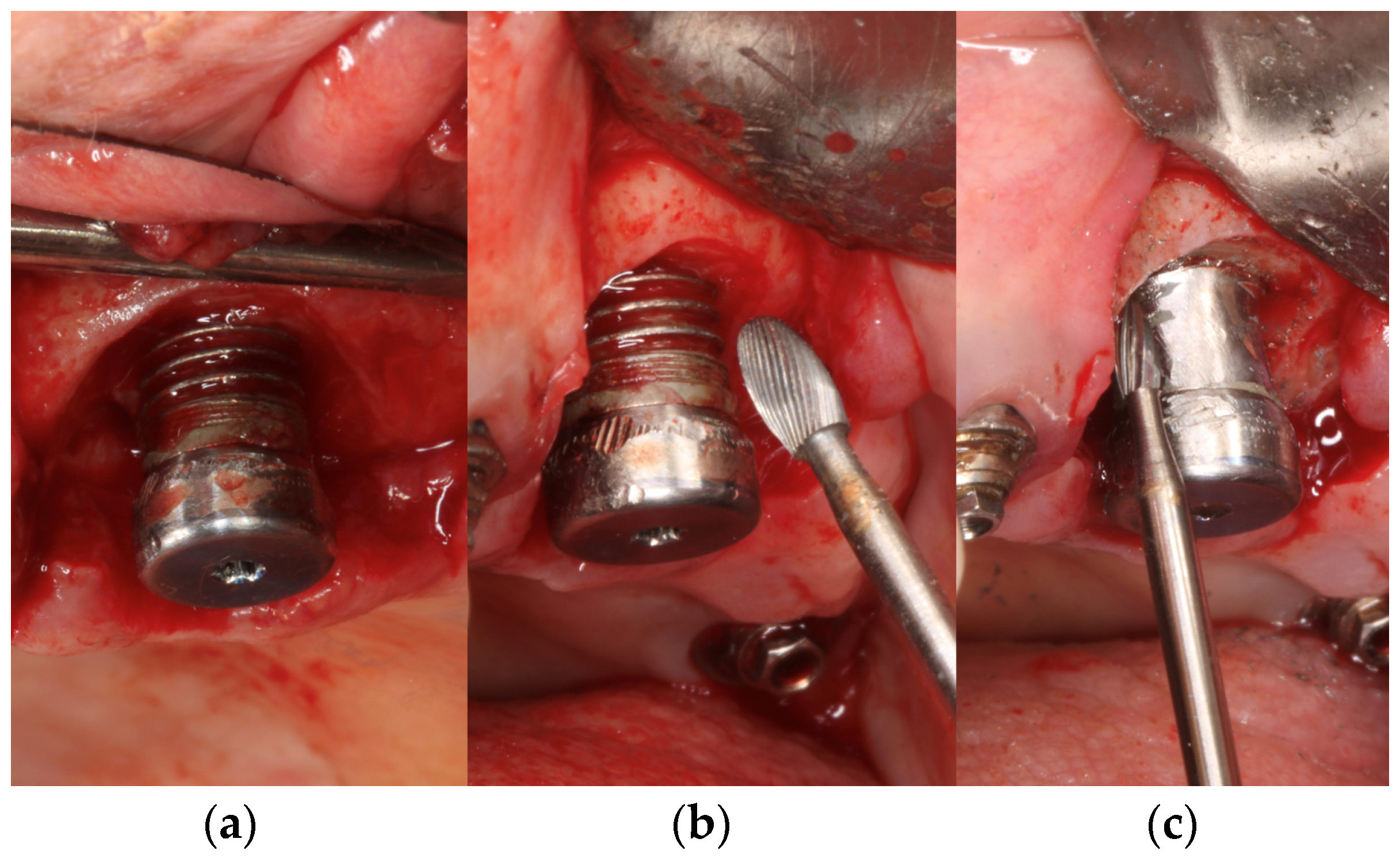
2.1. Sample Preparation and Collection
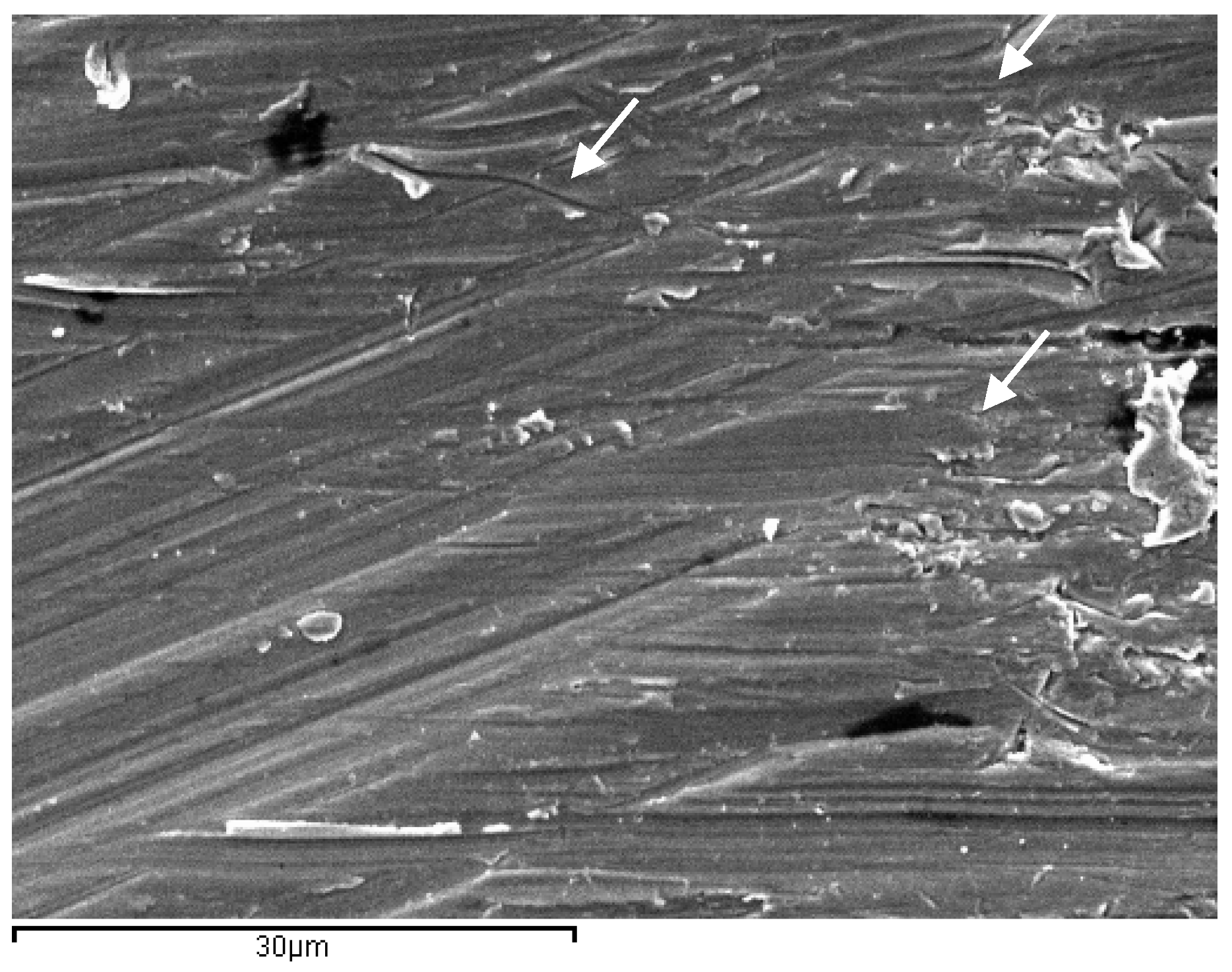
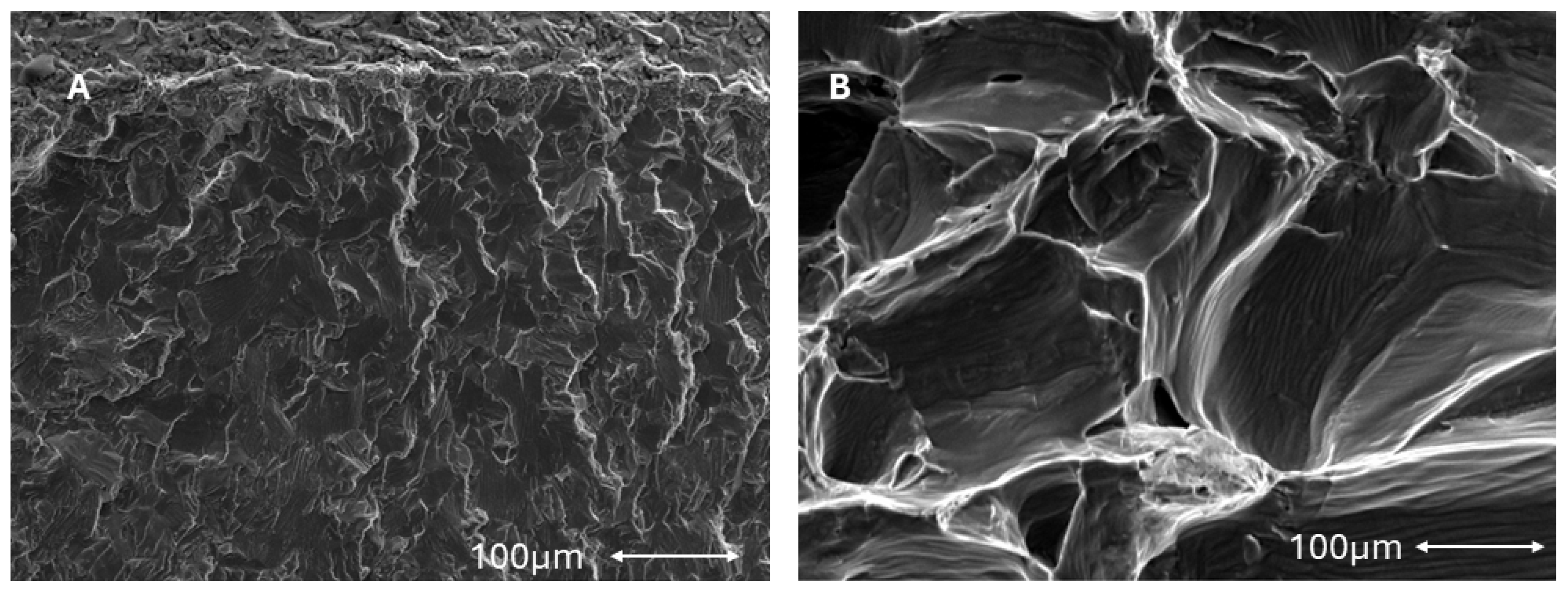
2.2. Scanning Electron Microscopy
2.3. Mechanical Characterization
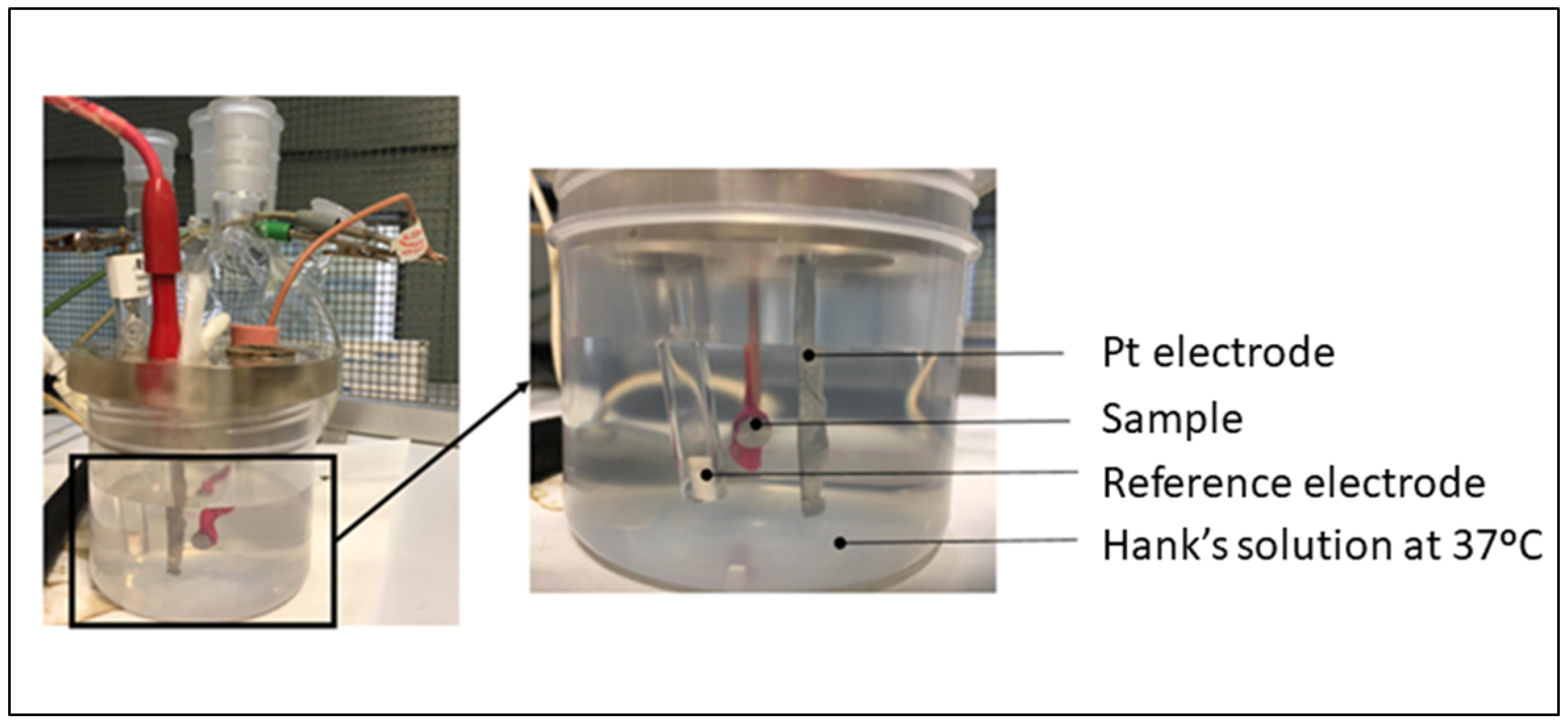
2.4. Corrosion Resistance
- icorr (μA/cm2)/corrosion current density.
- Ecorr (mV)/Corrosion potential: value at which the current density changes from cathodic to anodic.
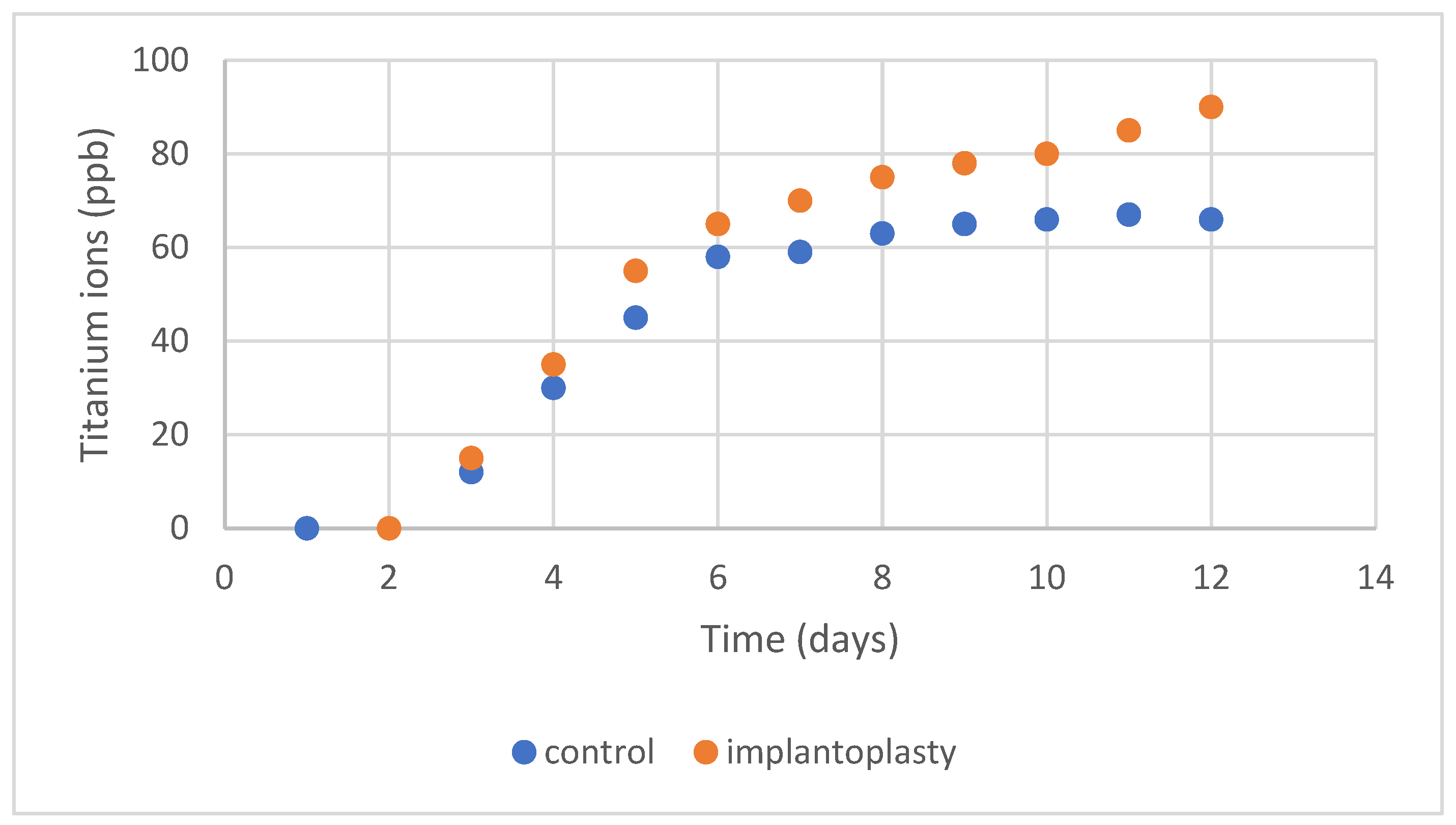
2.5. Ion Release
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), 246–266. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 68–81. [Google Scholar] [CrossRef]
- Albouy, J.P.; Abrahamsson, I.; Persson, L.G.; Berglundh, T. Spontaneous progression of peri-implantitis at different types of implants. An experimental study in dogs. I: Clinical and radiographic observations. Clin. Oral Implant. Res. 2008, 19, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Albouy, J.P.; Abrahamsson, I.; Persson, L.G.; Berglundh, T. Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: Histological observations. Clin. Oral Implant. Res. 2009, 20, 366–371. [Google Scholar] [CrossRef]
- Albouy, J.P.; Abrahamsson, I.; Persson, L.G.; Berglundh, T. Implant surface characteristics influence the outcome of treatment of peri-implantitis: An experimental study in dogs. J. Clin. Periodontol. 2011, 38, 58–64. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I.; Claffey, N. How do implant surface characteristics influence peri-implant disease? J. Clin. Periodontol. 2011, 38 (Suppl. S11), 214–222. [Google Scholar] [CrossRef] [PubMed]
- Almohandes, A.; Carcuac, O.; Abrahamsson, I.; Lund, H.; Berglundh, T. Re-osseointegration following reconstructive surgical therapy of experimental peri-implantitis. A pre-clinical in vivo study. Clin. Oral Implant. Res. 2019, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Azzola, F.; Ionescu, A.C.; Ottobelli, M.; Cavalli, N.; Brambilla, E.; Corbella, S.; Francetti, L. Biofilm formation on dental implant surface treated by implantoplasty: An in-situ study. Dent. J. 2020, 8, 40. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T.; Heitz-Mayfield, L.J.; Pjetursson, B.E.; Salvi, G.E.; Sanz, M. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int. J. Oral Maxillofac. Implants 2004, 19, 150–154. [Google Scholar]
- Parma-Benfenati, S.; Roncati, M.; Tinti, C. Treatment of peri-implantitis: Surgical therapeutic approaches based on peri-implantitis defects. Int. J. Periodontics Restor. Dent. 2013, 33, 627–633. [Google Scholar] [CrossRef]
- Rimondini, L.; Cicognani Simoncini, F.; Carrassi, A. Micro-morphometric assessment of titanium plasma-sprayed coating removal using burs for the treatment of peri-implant disease. Clin. Oral Implant. Res. 2000, 11, 129–138. [Google Scholar] [PubMed]
- Lozada, J.L.; James, R.A.; Boskovic, M.; Cordova, C.; Emanuelli, S. Surgical repair of peri-implant defects. J. Oral Implantol. 1990, 16, 42–46. [Google Scholar] [PubMed]
- Ramel, C.F.; Lussi, A.; Ozcan, M.; Jung, R.E.; Hammerle, C.H.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implant. Res. 2016, 27, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Costa-Berenguer, X.; Garcia-Garcia, M.; Sanchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellon, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implant. Res. 2018, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, C.; Gil, J.; Jantz, E.; Al Sakka, Y.; Padial-Molina, M.; Suárez-López del Amo, F. Characteristics of Particles and Debris Released after Implantoplasty: A Comparative Study. Materials 2022, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellon, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosión behavior of Ti6Al4V particles obtained by Implatoplasty. An in vivo study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Verket, A. Implantoplasty-provoking or reducing inflammation?—A systematic scoping review. Acta Odontol. Scand. 2022, 80, 105–116. [Google Scholar] [CrossRef]
- Romeo, E.; Ghisolfi, M.; Murgolo, N.; Chiapasco, M.; Lops, D.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: Clinical outcome. Clin. Oral Implant. Res. 2005, 16, 9–18. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2022, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Daugela, P.; Faria de Almeida, R.; Saulacic, N. Surgical Non-Regenerative Treatments for Peri-Implantitis: A Systematic Review. J. Oral Maxillofac. Res. 2016, 7, e14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravidà, A.; Siqueira, R.; Saleh, I.; Saleh, M.H.A.; Giannobile, A.; Wang, H.L. Lack of Clinical Benefit of Implantoplasty to Improve Implant Survival Rate. J. Dent. Res. 2020, 99, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; AramburúJúnior, J.S.; Dedavid, B.A.; Shibli, J.A. Analysis of implant strength after implantoplasty in three implant-abutment connection designs: An in vitro study. Int. J. Oral Maxillofac. Implants 2016, 31, e65–e70. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Bosch, B.M.; Díez-Tercero, L.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Evaluation of the inflammatory and osteogenic response induced by titanium particles released during implantoplasty of dental implants. Sci Rep. 2022, 12, 15790. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Schunemann, W.V.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Ingham, E.; Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Manero, J.M.; Gil, F.J.; Padrós, E.; Planell, J.A. Applications of environmental scanning electron microscopy (ESEM) in biomaterials field. Microsc. Res. Tech. 2003, 61, 469–480. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Fos-Parra, I.; Cabanillas-Balsera, D.; Gil, J.; Ortiz-Garcia, I.; Giner, M.; Bocio-Núñez, J.; Montoya-García, M.J.; Jiménez-Guerra, A. Osteoblastic Cell Behavior and Gene Expression Related to Bone Metabolism on Different Titanium Surfaces. Int. J. Mol. Sci. 2023, 24, 3523. [Google Scholar] [CrossRef]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the Titanium Implant Surface Treatment on the Surface Roughness and Chemical Composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef]
- Pazos, L.; Corengia, P.; Svoboda, H. Effect of surface treatments on the fatigue life of titanium for biomedical applications. J. Mech. Behav. Biomed. Mater. 2010, 3, 416–424. [Google Scholar] [CrossRef]
- Gil, F.J.; Espinar, E.; Llamas, J.M.; Sevilla, P. Fatigue life of bioactive titanium dental implants treated by means of Grit Blasting and Thermo-Chemical treatment. Clin. Implant Dent. Relat. Res. 2014, 16, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Shemtov-Yona, K.; Rittel, D.; Levin, L.; Machtei, E.E. Effect of dental implant diameter on fatigue performance. Part I: Mechanical behavior. Clin. Implant Dent. Relat. Res. 2014, 16, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Shemtov-Yona, K.; Rittel, D.; Machtei, E.E.; Levin, L. Effect of Dental Implant Diameter on Fatigue Performance. Part II: Failure Analysis. Clin. Implant Dent. Relat. Res. 2012, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- ASTM G31-90; Standard Practice for Laboratory Immersion Corrosion Testing Metals. 1992 Annual Book of ASTM Standards, Vol. 03.02; ASTM: Philadelphia, PA, USA, 1992; pp. 102–109.
- ASTM G5; Potentiodynamic Anodic Polarization Measurements. 1992 Annual Book of ASTM Standards, Vol. 03.02; ASTM: Philadelphia, PA, USA, 1992; pp. 188–189.
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. 1999 Annual Book of ASTM Standards, Vol. 03.02; ASTM: Philadelphia, PA, USA, 1992; pp. 231–235.
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron–Nickel, or Cobalt-Based Alloys. 1992 Annual Book of ASTM Standards, Vol. 03.02; ASTM: Philadelphia, PA, USA, 1992; pp. 231–235.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. Internation Standard Organization: Geneva, Switzerland, 2021; pp. 45–54.
- Kim, T.I.; Han, J.H.; Lee, I.S.; Lee, K.H.; Shin, M.C.; Choi, B.B. New Ti alloys for biomaterials: A study of mechanical and corrosion properties and cytotoxicity. Bio-Med. Mater. 1997, 7, 253–263. [Google Scholar]
- Schmalz, G.; Garhammer, P. Biological interactions of dental cast alloys with oral tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. (Eds.) Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In vivo severe corrosion and hydrogen embrittlement of retrieved modular body titanium alloy hip-implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Monsalve-Guil, L.; Jiménez-Guerra, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pequeroles, M.; Perez, R.A.; Gil, F.J. Importance of the roughness and residual stresses of dental implants on fatigue and osseointegration be-havior. In vivo study in rabbits. J. Oral Implantol. 2016, 42, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Flichy-Fernández, A.; Punset, M.; Jiménez-Guerra, A.; Manero, J.M.; Gil, J. Fracture and Fatigue of Titanium Narrow Dental Implants: New Trends in Order to Improve the Mechanical Response. Materials 2019, 12, 3728. [Google Scholar] [CrossRef]
- Colling, E.W. The Physical Metallurgy of Titanium Alloys. In American Society for Metals; ASM International: Metals Park, OH, USA, 1984. [Google Scholar]
- Manero, J.M.; Gil, F.J.; Planell, J.A. Deformation mechanisms of Ti-6Al-4V alloy with a martensitic microstructure subjected to oligocyclic fatigue. Acta Materialia. 2014, 48, 3353–3359. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 524–532. [Google Scholar] [CrossRef]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef]
- Guillem-Marti, J.; Cinca, N.; Punset, M.; García Cano, I.; Gil, F.J.; Guilemany, J.M.; Dosta, S. Porous titanium-hydroxyapatite composite coating obtained on titanium by cold gas spray with high bond strength for biomedical applications. Colloids Surf. B Biointerfaces 2019, 180, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Maia, P.; Rios-Santos, J.V.; Herrero-Climent, M.; Rios-Carrasco, B.; Aparicio, C.; Gil, J. Influence of Titanium Surface Residual Stresses on Osteoblastic Response and Bacteria Colonization. Materials 2024, 17, 1626. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A.; Padrós, A.; Aparicio, C. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar]
- Hoyos-Nogués, M.; Buxadera-Palomero, J.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. All-in-one trifunctional strategy: A cell adhesive, bacteriostatic and bactericidal coating for titanium implants. Colloids Surf. B Biointerfaces 2018, 169, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, M.; Brizuela-Velasco, A.; Gil, J.; Cerrolaza, M.; Montalvillo, E.; Fernández-Hernández, S.; Robles, D. Hybrid surface implants: Influence of residual stress on mechanical behavior, evaluated by finite element analysis and validation by fatigue tests. Dent. Mater. 2024, 40, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gutelmacher, E.; Eliezer, D. Hydrogen cracking in titanium-based alloys. J. Alloys Compd. 2005, 404, 621–625. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M. The effect of temperature on the nucleation of corrosion pits on titanium in Ringer’s physiological solution. Biomaterials 2005, 26, 245–256. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef]
- Ferguson, A.B.; Laing, P.G.; Hodge, E.S. The ionization of metal implants in living tissues. J. Bone Jt. Surg. 1960, 42, 77–90. [Google Scholar] [CrossRef]
- Ferguson, A.B.; Akahoshi, Y.; Laing, P.G.; Hodge, E.S. Characteristics of trace ion release from embedded metal implants in the rabbit. J. Bone Jt. Surg. 1962, 44, 317–336. [Google Scholar] [CrossRef]
- Meachim, G.; Williams, D.F. Changes in nonosseous tissue adjacent to titanium implants. J. Biomed. Mater. Res. 1973, 7, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Ichinose, S.; Kimijima, Y.; Mimura, M. Investigation of titanium leak to bone tissue surrounding dental titanium implant: Electron microscopic findings and analysis by electron diffraction. Med. Electron. Microsc. 2000, 33, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Englezos, E.; Cosyn, J.; Koole, S.; Jacquet, W.; De Bruyn, H. Resective Treatment of Peri-implantitis: Clinical and Radiographic Outcomes After 2 Years. Int. J. Periodontics Restor. Dent. 2018, 38, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Regidor, E.; Ortiz-Vigón, A.; Derks, J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 340–356. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin. Oral Implant. Res. 2014, 25, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Schwarz, F. Principles of Combined Surgical Therapy for the Management of Peri-Implantitis. Clin. Adv. Periodontics 2022, 12, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Amerio, E.; Cha, J.K.; Kotsakis, G.; Pons, R.; Renvert, S.; Sanz-Martin, I.; Schwarz, F.; Sculean, A.; Stavropoulos, A.; et al. Strategies for implant surface decontamination in peri-implantitis therapy. Int. J. Oral Implantol. 2022, 15, 213–248. [Google Scholar]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 305–315. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant. Dent. 2015, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Hentenaar, D.F.; De Waal, Y.C.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer, H.J.; Raghoebar, G.M. Erythritol airpolishing in the non-surgical treatment of peri-implantitis: A randomized controlled trial. Clin. Oral Implant. Res. 2021, 32, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontology 2000 2019, 81, 29–40. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef]






| Chemical Product | Composition (mM) |
|---|---|
| K2HPO4 | 0.44 |
| KCl | 5.4 |
| CaCl2 | 1.3 |
| Na2HPO4 | 0.25 |
| NaCl | 137 |
| NaHCO3 | 4.2 |
| MgSO4 | 1.0 |
| C6H12O6 | 5.5 |
| Samples | Ecorr (mV) | icorr (μA/cm2) | Rp (Ω/cm2) | Corrosion Rate (mm/year) |
|---|---|---|---|---|
| Control | −302 ± 22 | 0.041 ± 0.006 | ||
| Implantoplasty | −345 ± 38 * | 0.050 ± 0.0085 * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, D.; de Tapia, B.; Pons, R.; Aparicio, C.; Guerra, F.; Messias, A.; Gil, J. The Effect of Implantoplasty on the Fatigue Behavior and Corrosion Resistance in Titanium Dental Implants. Materials 2024, 17, 2944. https://doi.org/10.3390/ma17122944
Fonseca D, de Tapia B, Pons R, Aparicio C, Guerra F, Messias A, Gil J. The Effect of Implantoplasty on the Fatigue Behavior and Corrosion Resistance in Titanium Dental Implants. Materials. 2024; 17(12):2944. https://doi.org/10.3390/ma17122944
Chicago/Turabian StyleFonseca, Darcio, Beatriz de Tapia, Ramon Pons, Conrado Aparicio, Fernando Guerra, Ana Messias, and Javier Gil. 2024. "The Effect of Implantoplasty on the Fatigue Behavior and Corrosion Resistance in Titanium Dental Implants" Materials 17, no. 12: 2944. https://doi.org/10.3390/ma17122944
APA StyleFonseca, D., de Tapia, B., Pons, R., Aparicio, C., Guerra, F., Messias, A., & Gil, J. (2024). The Effect of Implantoplasty on the Fatigue Behavior and Corrosion Resistance in Titanium Dental Implants. Materials, 17(12), 2944. https://doi.org/10.3390/ma17122944








