Study of Coating Growth Direction of 6061 Aluminum Alloy in Soft Spark Discharge of Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Plasma Electrolytic Oxidation
2.3. Methods for the Study of Coating Properties
3. Results
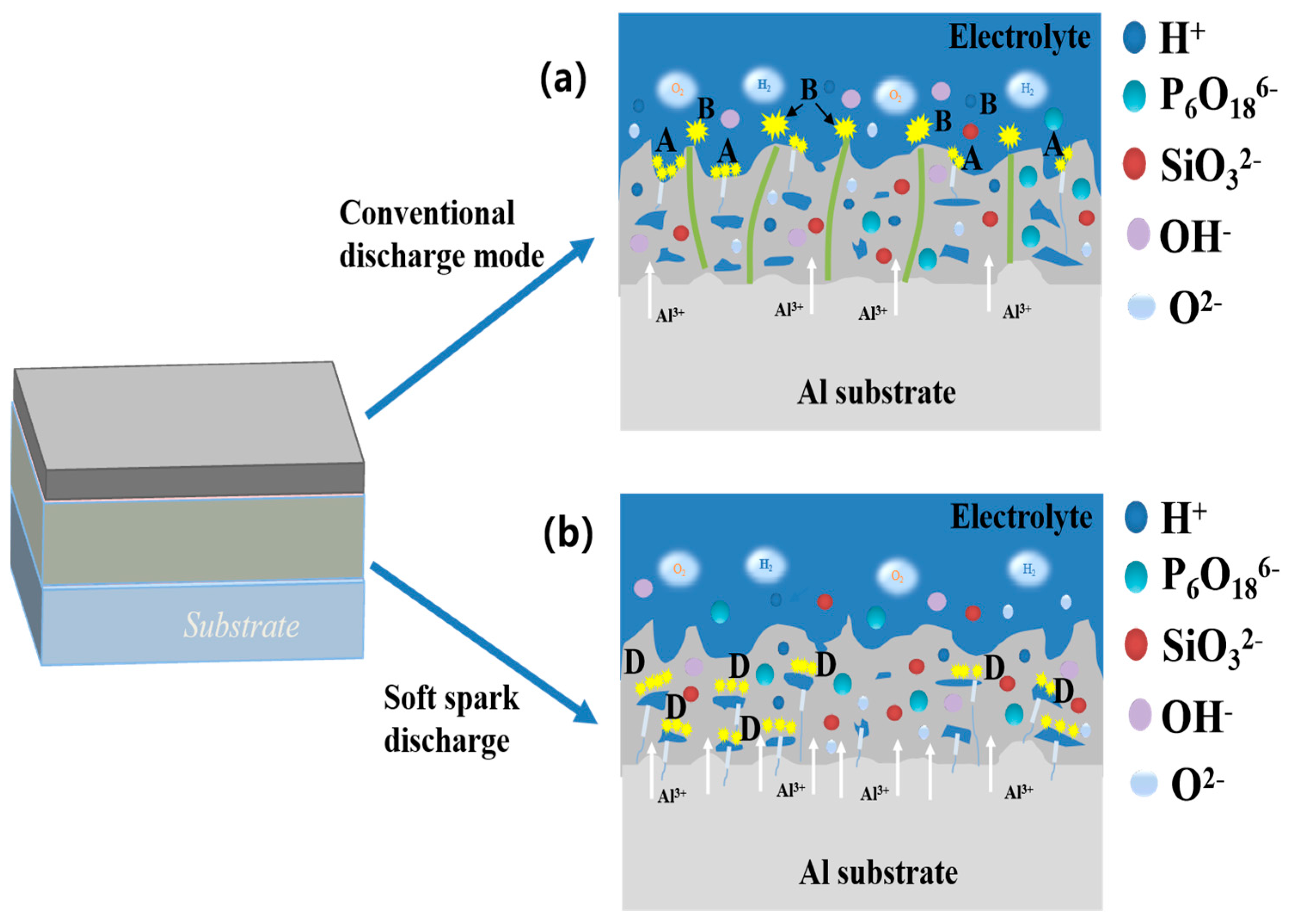
3.1. Voltage-versus-Time Curves and Spark Morphology Evolution during the PEO Process
3.2. Effect of Soft Spark Discharge on Phase Composition
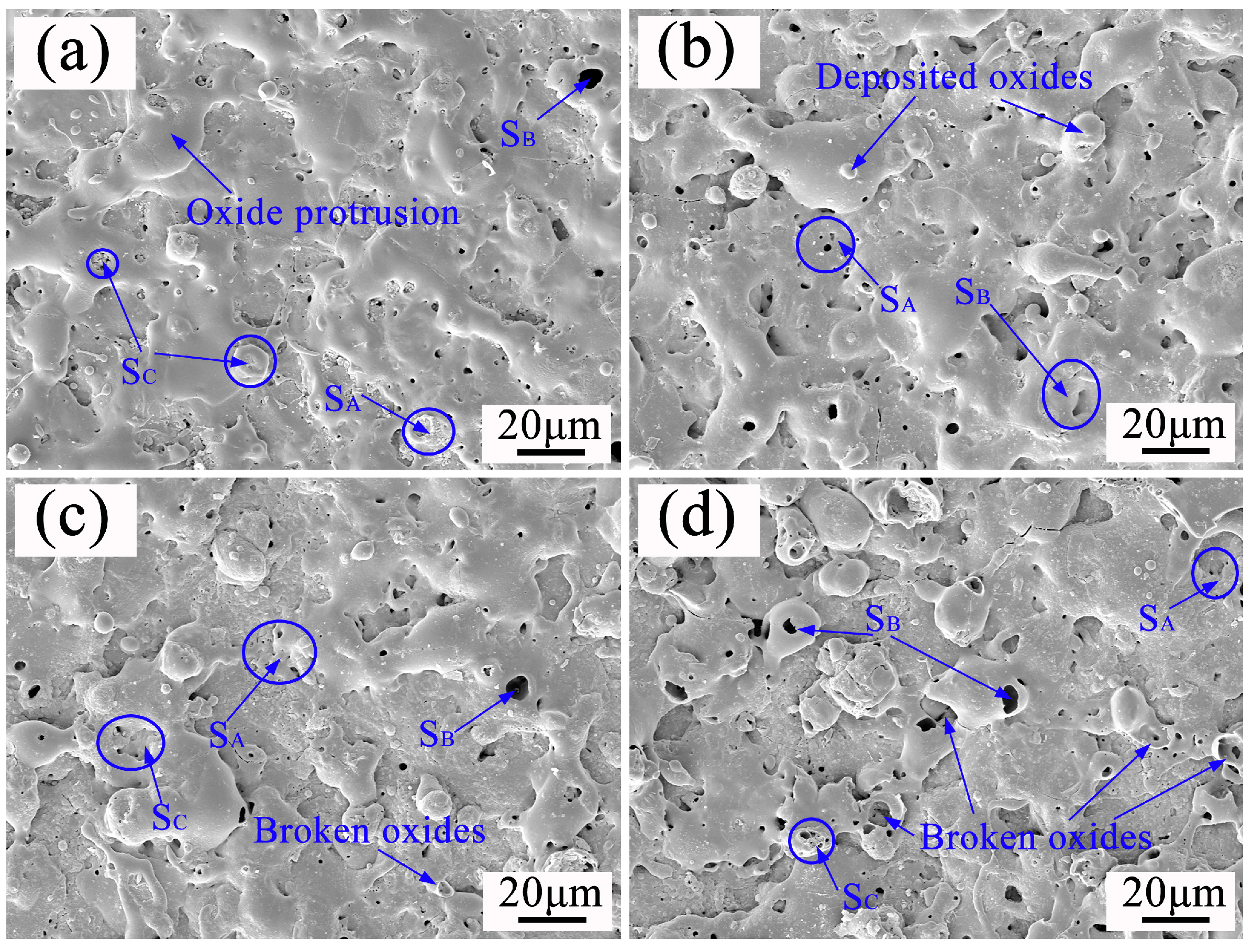
3.3. Effect of Soft Spark Discharge on the Microscopic Morphology of Ceramic Layers
3.3.1. Effect of Soft Spark Discharge on the Surface Morphology of Ceramic Layers
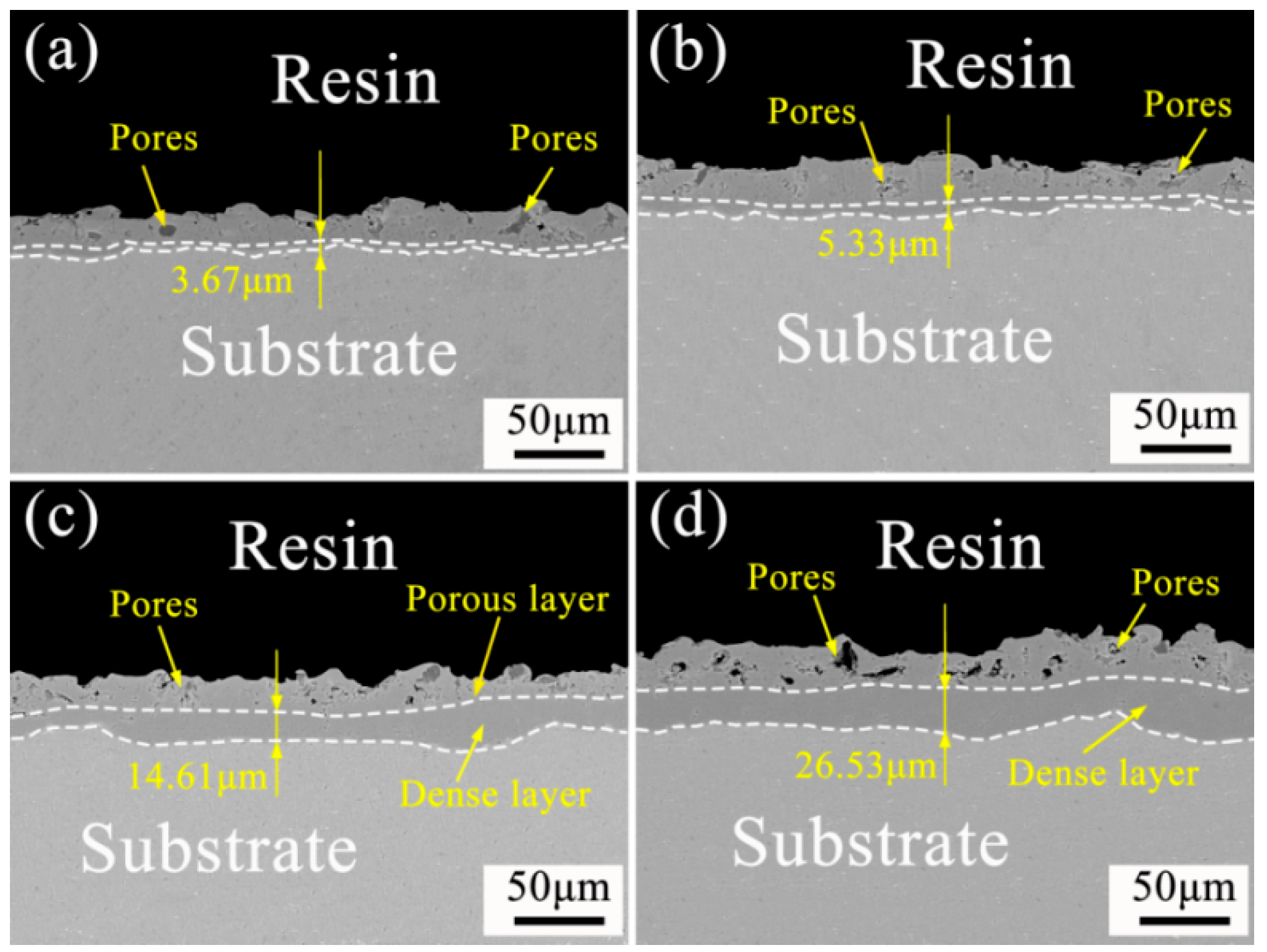
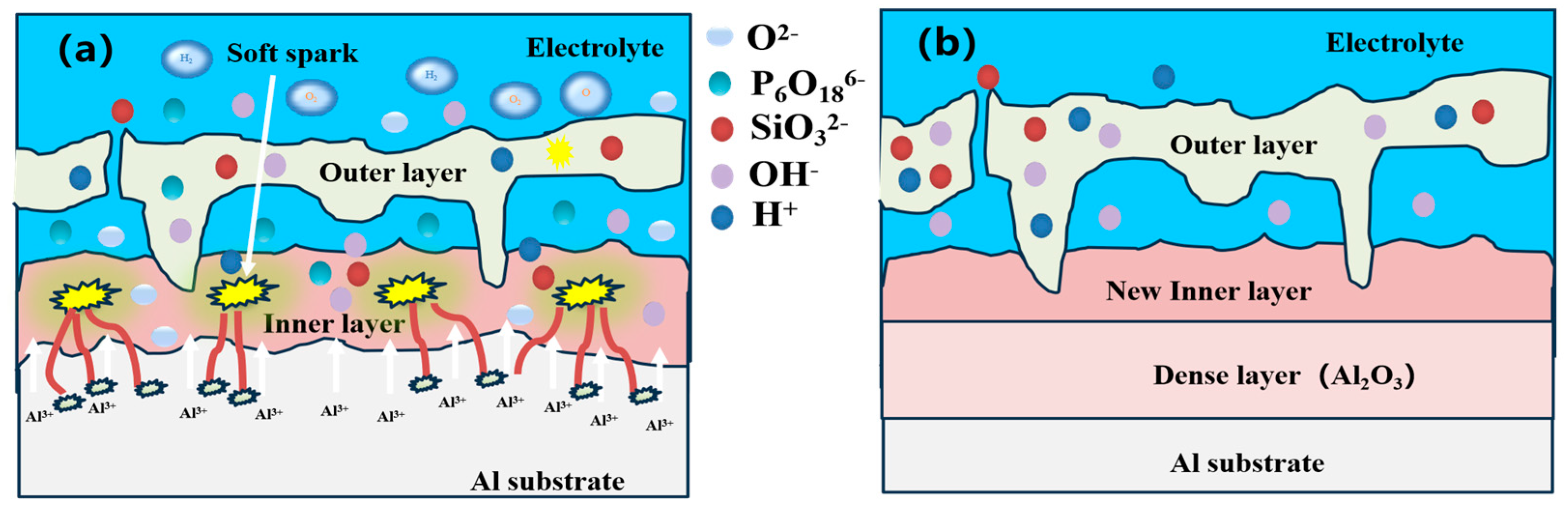
3.3.2. Effect of Soft Spark Discharge on the Cross-Sectional Morphology of Ceramic Layers
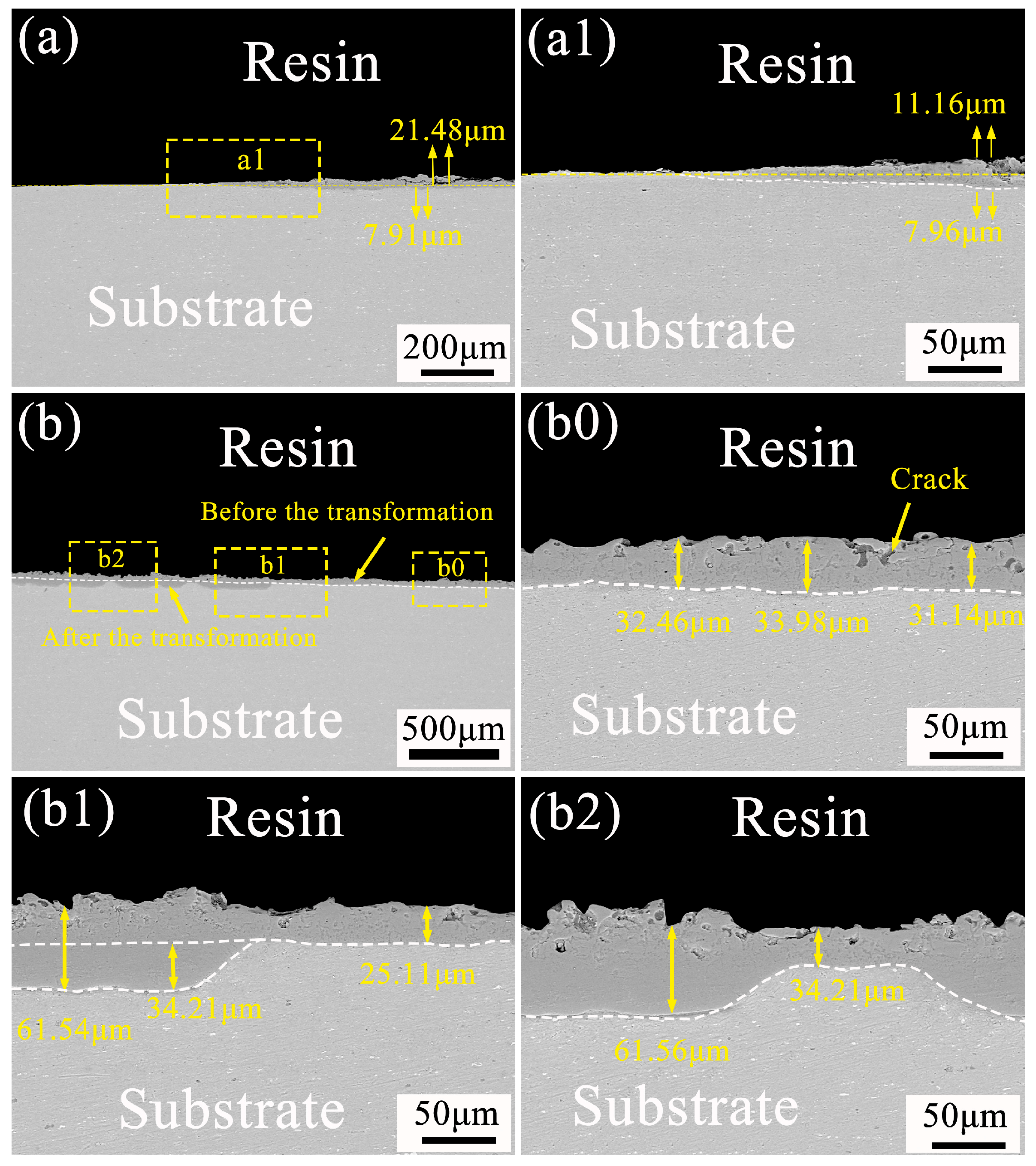
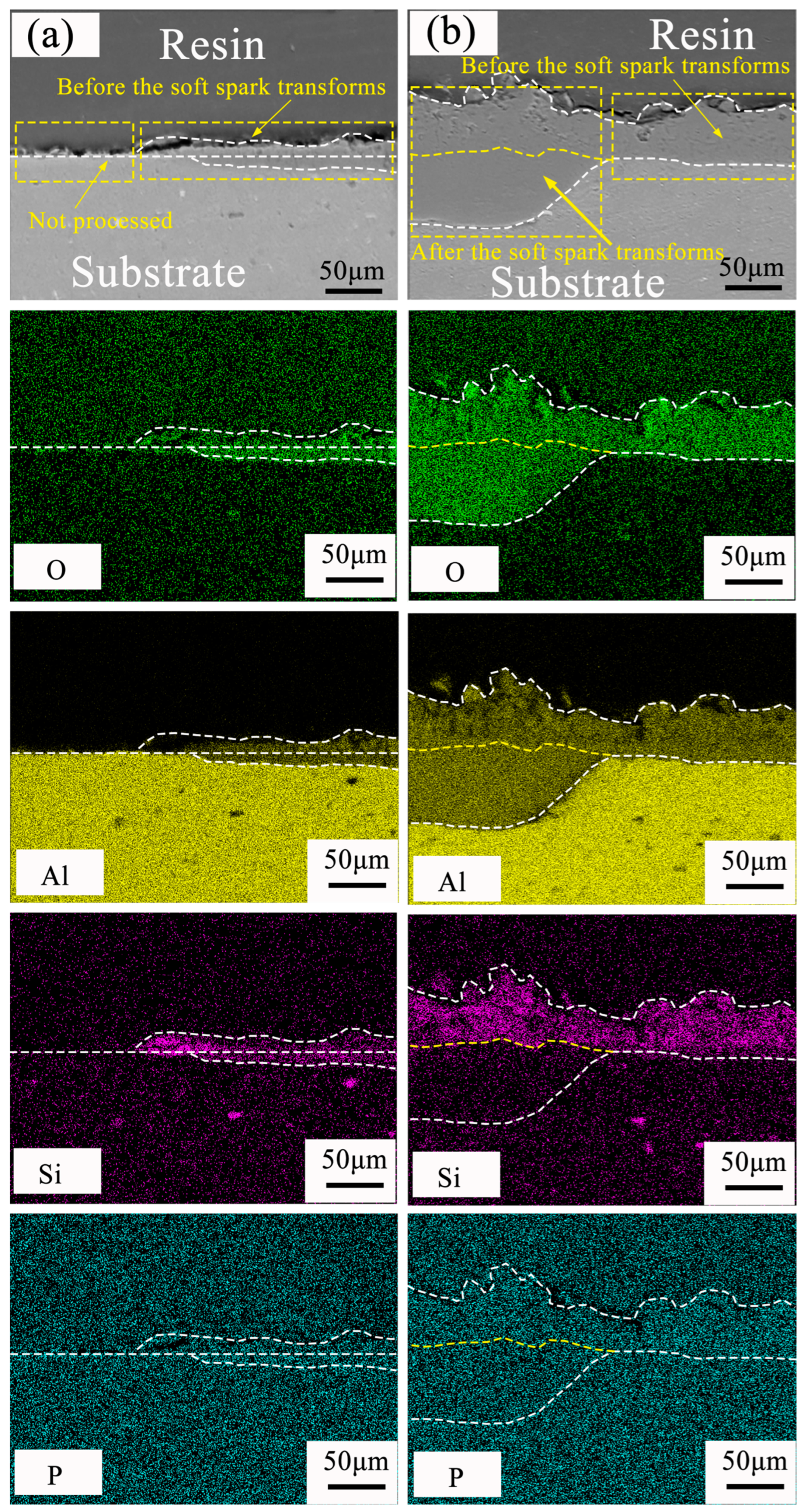
3.4. Direction of Ceramic Layer Growth before and after Soft Spark Discharge
4. Discussion
5. Conclusions
- Relative to the substrate, the growth direction of the PEO ceramic layer before the soft spark discharge transition is dominated by outward growth. The growth direction of the PEO ceramic layer after the soft spark discharge transition is dominated by inward growth relative to that before the soft spark discharge transition.
- After soft spark discharge, the Si element is distributed in the outer layer, which is due to the fact that SiO32−, on account of its large size, cannot enter the interface between the substrate and the inner layer of the ceramic layer to participate in the film formation through the D-type discharge channel.
- The relative phase content of α-Al2O3 in the coating increased by 30.2% from 0.487 to 0.634 after 10 min of trans-spark discharge compared to that before the soft spark discharge transformation, while the relative phase content of α-Al2O3 in the coating decreased by 55.6% from 0.487 to 0.313 after 20 min of trans-spark discharge compared to that before the soft spark discharge transformation.
- The film formation rate was 0.014 μm/s before the soft spark discharge transition and 0.026 μm/s after the soft spark discharge transition, and, comparing the two, the film formation rate was improved by 85.14% under soft spark discharge.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troughton, S.C.; Nominé, A.; Dean, J.; Clyne, T.W. Effect of individual discharge cascades on the microstructure of plasma electrolytic oxidation coatings. Appl. Surf. Sci. 2016, 389, 260–269. [Google Scholar] [CrossRef]
- Hussain, T.; Kaseem, M.; Ko, Y.G. Hard acid–hard base interactions responsible for densification of alumina layer for superior electrochemical performance. Corros. Sci. 2020, 170, 108663. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Qian, C.; Chen, B.; Li, H.; Shi, R.; Yang, Z.; Zhang, N.; Song, S.; Liu, C.; Yang, B. Growth Behavior and Wear Resistance of PEO Coatings in Bipolar Mode. Trans. Indian Inst. Met. 2023, 76, 2263–2271. [Google Scholar] [CrossRef]
- Tsai, D.-S.; Chou, C.-C. Influences of Growth Species and Inclusions on the Current–Voltage Behavior of Plasma Electrolytic Oxidation: A Review. Coatings 2021, 11, 270. [Google Scholar] [CrossRef]
- Melhem, A.; Henrion, G.; Czerwiec, T.; Briançon, J.L.; Duchanoy, T.; Brochard, F.; Belmonte, T. Changes induced by process parameters in oxide layers grown by the PEO process on Al alloys. Surf. Coat. Technol. 2011, 205, S133–S136. [Google Scholar] [CrossRef]
- Bahador, R.; Hosseinabadi, N.; Yaghtin, A. Effect of Power Duty Cycle on Plasma Electrolytic Oxidation of A356-Nb2O5 Metal Matrix Composites. J. Mater. Eng. Perform. 2021, 30, 2586–2604. [Google Scholar] [CrossRef]
- Song, S.; Chen, B.; Li, H.; Shi, R.; Liu, C.; Yang, B.; de la Fuente, G.F. Growth behavior and insulation property of the oxide layer during micro-arc oxidation of aluminium in “soft” regime condition. J. Mater. Sci. 2023, 58, 7136–7148. [Google Scholar] [CrossRef]
- Martin, J.; Akoda, K.; Ntomprougkidis, V.; Ferry, O.; Maizeray, A.; Bastien, A.; Brenot, P.; Ezo’o, G.; Henrion, G. Duplex surface treatment of metallic alloys combining cold-spray and plasma electrolytic oxidation technologies. Surf. Coat. Technol. 2020, 392, 125756. [Google Scholar] [CrossRef]
- Martin, J.; Leone, P.; Nominé, A.; Veys-Renaux, D.; Henrion, G.; Belmonte, T. Influence of electrolyte ageing on the Plasma Electrolytic Oxidation of aluminium. Surf. Coat. Technol. 2015, 269, 36–46. [Google Scholar] [CrossRef]
- Martin, J.; Nominé, A.; Brochard, F.; Briançon, J.L.; Noël, C.; Belmonte, T.; Czerwiec, T.; Henrion, G. Delay in micro-discharges appearance during PEO of Al: Evidence of a mechanism of charge accumulation at the electrolyte/oxide interface. Appl. Surf. Sci. 2017, 410, 29–41. [Google Scholar] [CrossRef]
- Martin, J.; Nominé, A.; Ntomprougkidis, V.; Migot, S.; Bruyère, S.; Soldera, F.; Belmonte, T.; Henrion, G. Formation of a metastable nanostructured mullite during Plasma Electrolytic Oxidation of aluminium in “soft” regime condition. Mater. Des. 2019, 180, 107977. [Google Scholar] [CrossRef]
- Tjiang, F.; Ye, L.-W.; Huang, Y.-J.; Chou, C.-C.; Tsai, D.-S. Effect of processing parameters on soft regime behavior of plasma electrolytic oxidation of magnesium. Ceram. Int. 2017, 43, S567–S572. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, T.; Cheng, Y. A Systematic Study of the Role of Cathodic Polarization and New Findings on the Soft Sparking Phenomenon from Plasma Electrolytic Oxidation of an Al-Cu-Li Alloy. J. Electrochem. Soc. 2022, 169, 071505. [Google Scholar] [CrossRef]
- Tsai, D.-S.; Chou, C.-C. Review of the Soft Sparking Issues in Plasma Electrolytic Oxidation. Metals 2018, 8, 105. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Q.; Zhu, Z.; Tu, W.; Cheng, Y.; Skeldon, P. Potential and morphological transitions during bipolar plasma electrolytic oxidation of tantalum in silicate electrolyte. Ceram. Int. 2020, 46, 13385–13396. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft plasma electrolysis with complex ions for optimizing electrochemical performance. Sci. Rep. 2017, 7, 44458. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, S.; Hazmatulhaq, F.; Sheng, Y.; Suhartono, T.; Oh, J.M.; Nashrah, N.; Kang, J.H.; Ko, Y.G. Effect of Ultrasonic Frequency on Structure and Corrosion Properties of Coating Formed on Magnesium Alloy via Plasma Electrolytic Oxidation. Materials 2023, 16, 5424. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Ma, R.; Du, A.; Zhao, X.; Fan, Y. The Effects of the Pre-Anodized Film Thickness on Growth Mechanism of Plasma Electrolytic Oxidation Coatings on the 1060 Al Substrate. Materials 2023, 16, 5922. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Tang, Q.; Ni, P.; Li, X.; Shen, J. Growth pattern of soft-spark micro-arc oxide coating on titanium alloy in silicon anion electrolyte. Surf. Coat. Technol. 2023, 473, 130030. [Google Scholar] [CrossRef]
- He, X.; Feng, T.; Cheng, Y.; Hu, P.; Le, Z.; Liu, Z.; Cheng, Y. Fast formation of a black inner α-Al2O3 layer doped with CuO on Al–Cu–Li alloy by soft sparking PEO process. J. Am. Ceram. Soc. 2023, 106, 7019–7042. [Google Scholar] [CrossRef]
- Xiang, M.; Li, T.; Zhao, Y.; Chen, M. The Influence of Negative Voltage on Corrosion Behavior of Ceramic Coatings Prepared by MAO Treatment on Steel. Coatings 2022, 12, 710. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, M.; You, C.; Li, W.; Tie, D.; Liu, H. Effect of α-Al2O3 additive on the microstructure and properties of MAO coatings prepared on low carbon steel. J. Mater. Res. Technol. 2020, 9, 3875–3884. [Google Scholar] [CrossRef]
- Li, D.; Shi, R.; Liu, C.; Tu, C.; Li, H.; Miao, X.; Chen, D. Effects of H3PO4 content on the properties of plasma electrolytic fluorination coatings on AZ31 Mg alloy in ethylene glycol electrolyte. Ceram. Int. 2023, 49, 29055–29063. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, W.; Liu, H.; Liu, L.; Wang, F.; Qiao, Z. Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges. Materials 2022, 15, 4635. [Google Scholar] [CrossRef] [PubMed]
- Vrublevsky, I.; Chernyakova, K.; Bund, A.; Ispas, A.; Schmidt, U. Effect of anodizing voltage on the sorption of water molecules on porous alumina. Appl. Surf. Sci. 2012, 258, 5394–5398. [Google Scholar] [CrossRef]
- Ji, R.; Liu, L.; Yin, H.; Li, W.; Sun, J.; Zhu, P.; Cao, C. Synthesis of corundum rich coating on 7055 aluminum alloy by micro-arc oxidation and its corrosion resistant property in a saline medium. Surf. Coat. Technol. 2024, 476, 130182. [Google Scholar] [CrossRef]
- Curran, J.A.; Kalkancı, H.; Magurova, Y.; Clyne, T.W. Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications. Surf. Coat. Technol. 2007, 201, 8683–8687. [Google Scholar] [CrossRef]
- Rogov, A.B.; Huang, Y.; Shore, D.; Matthews, A.; Yerokhin, A. Toward rational design of ceramic coatings generated on valve metals by plasma electrolytic oxidation: The role of cathodic polarisation. Ceram. Int. 2021, 47, 34137–34158. [Google Scholar] [CrossRef]
- Rogov, A.B.; Yerokhin, A.; Matthews, A. The Role of Cathodic Current in Plasma Electrolytic Oxidation of Aluminum: Phenomenological Concepts of the “Soft Sparking” Mode. Langmuir 2017, 33, 11059–11069. [Google Scholar] [CrossRef] [PubMed]
- Jovović, J.; Stojadinović, S.; Šišović, N.M.; Konjević, N. Spectroscopic study of plasma during electrolytic oxidation of magnesium- and aluminium-alloy. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 1928–1937. [Google Scholar] [CrossRef]
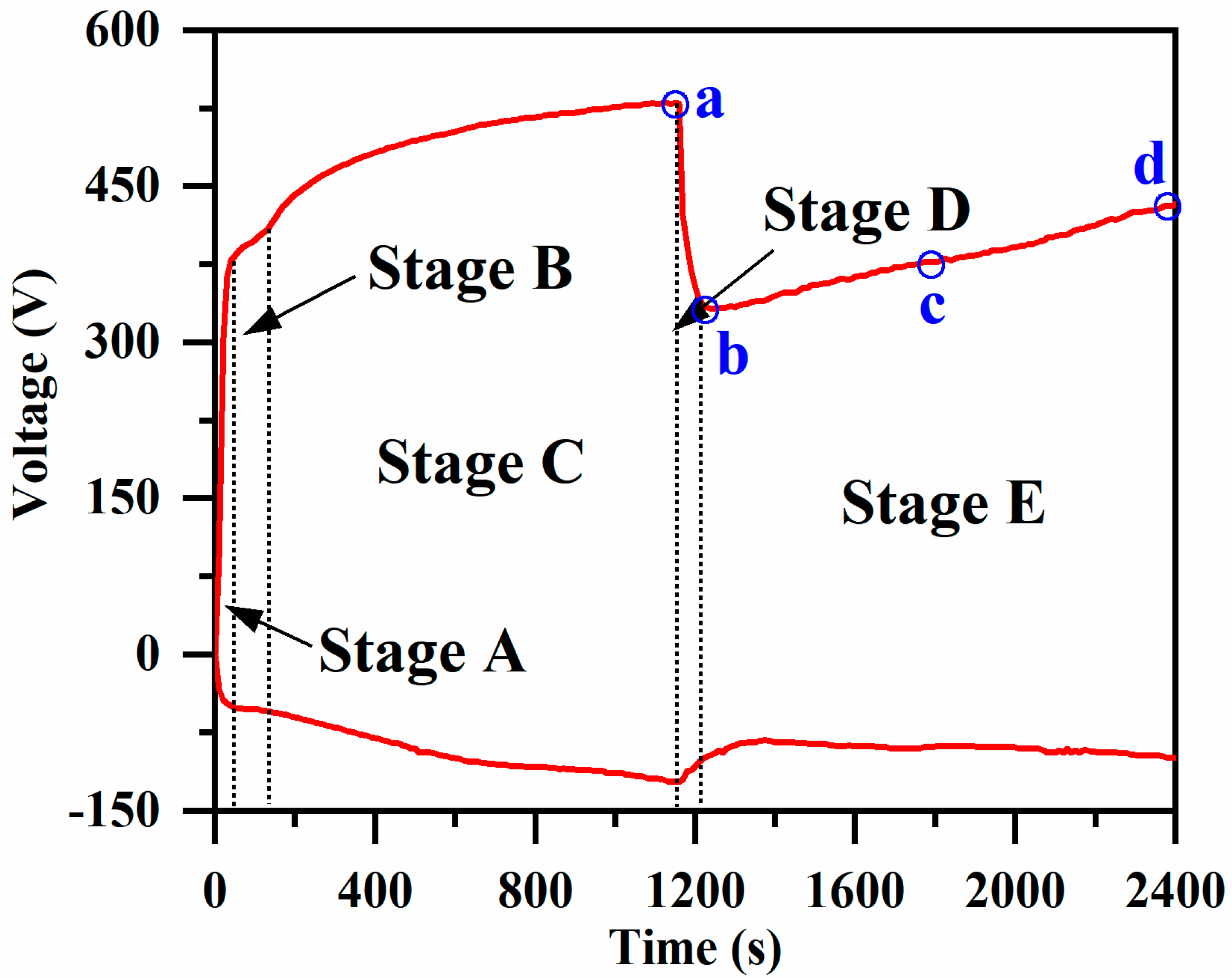
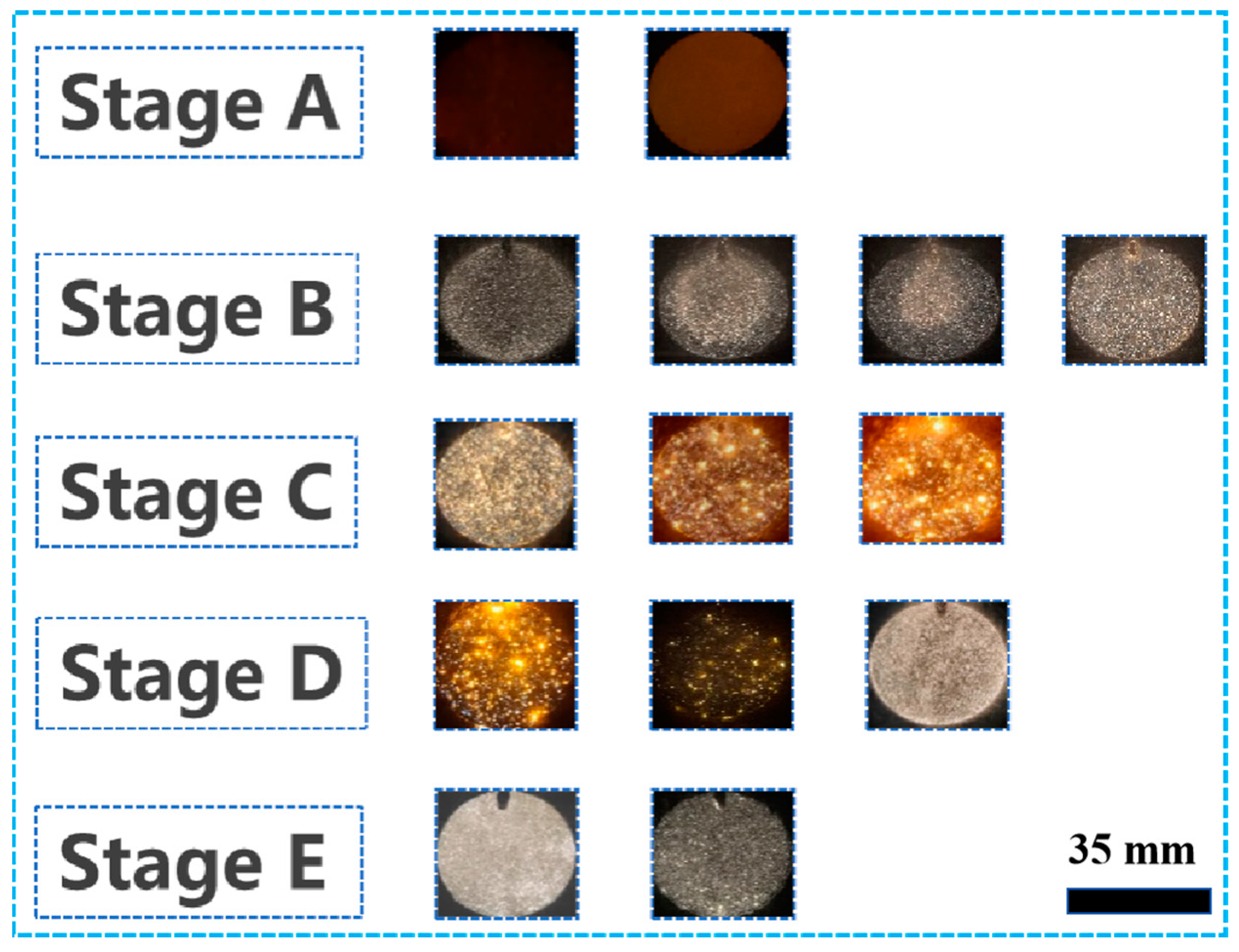
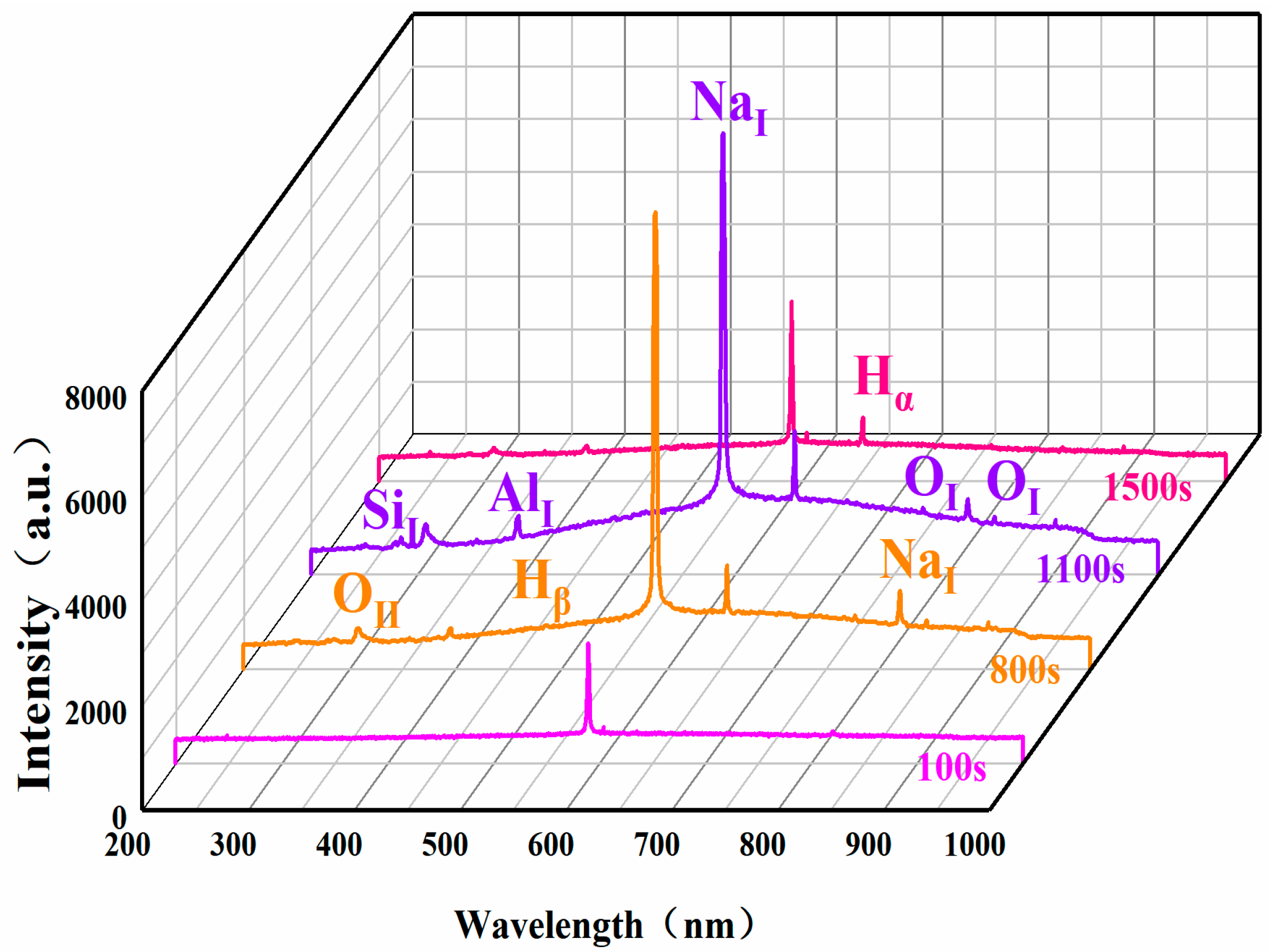
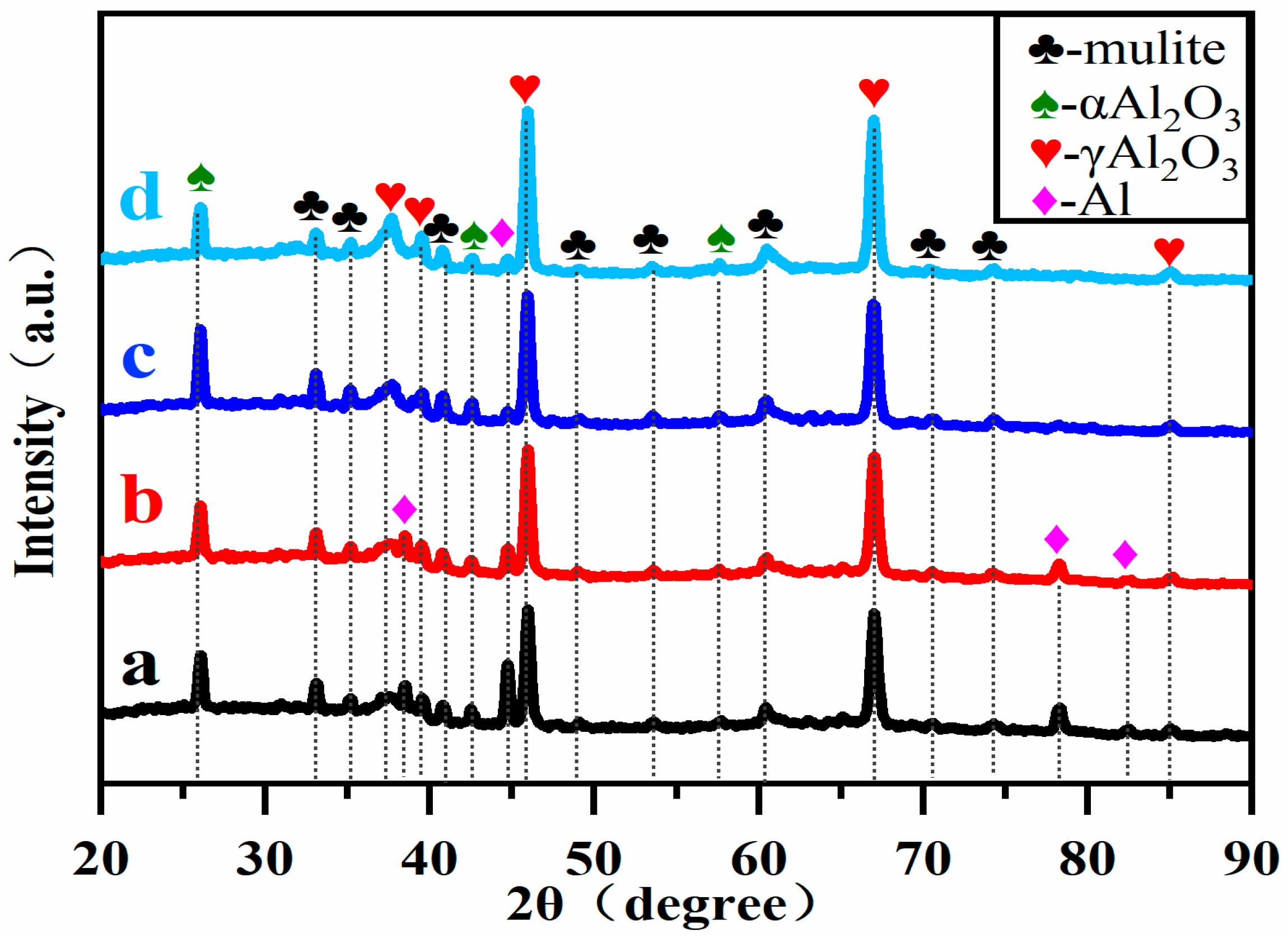


| Specimen Number | a | b | c | d |
|---|---|---|---|---|
| Frequency (Hz) | 500 | |||
| Duty cycle (%) | +20%, −20% | |||
| Current density (A/dm2) | +6, −7.5 | |||
| Processing time (s) | 1160 | 1200 | 1800 | 2400 |
| Specimen Number | Al2O3 (wt%) | Mullite (wt%) | Al (wt%) | Iα/Iγ |
|---|---|---|---|---|
| a | 60.5 | 33.1 | 6.4 | 0.487 |
| b | 63.8 | 32.3 | 3.9 | 0.408 |
| c | 64.7 | 34.2 | 1.1 | 0.634 |
| d | 70.6 | 28.4 | 1.0 | 0.313 |
| Specimen Number | Thickness of Dense Layer (μm) | Outer Layer Thickness (μm) | Coating Thickness (μm) |
|---|---|---|---|
| a | 3.76 | 20.95 | 24.67 |
| b | 5.33 | 22.88 | 28.21 |
| c | 14.61 | 25.62 | 40.23 |
| d | 26.53 | 32.63 | 59.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yang, Y.; Liu, C.; Chen, B.; Chen, X.; Wang, H.; Tong, R.; Zhou, S. Study of Coating Growth Direction of 6061 Aluminum Alloy in Soft Spark Discharge of Plasma Electrolytic Oxidation. Materials 2024, 17, 2947. https://doi.org/10.3390/ma17122947
Wang W, Yang Y, Liu C, Chen B, Chen X, Wang H, Tong R, Zhou S. Study of Coating Growth Direction of 6061 Aluminum Alloy in Soft Spark Discharge of Plasma Electrolytic Oxidation. Materials. 2024; 17(12):2947. https://doi.org/10.3390/ma17122947
Chicago/Turabian StyleWang, Wenqiang, Yifeng Yang, Cancan Liu, Bo Chen, Xuanyu Chen, Hao Wang, Rui Tong, and Shiquan Zhou. 2024. "Study of Coating Growth Direction of 6061 Aluminum Alloy in Soft Spark Discharge of Plasma Electrolytic Oxidation" Materials 17, no. 12: 2947. https://doi.org/10.3390/ma17122947





