Tribocorrosion Behavior of NiCoCrMoCu Alloys Containing Different Carbides in Acidic Media at Various Applied Loads and Sliding Speeds
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Phase Identification
2.3. Mechanical Behavior
2.4. Dry Sliding Wear and Tribocorrosion Tests
2.5. Characterization of the Microstructure
3. Results and Discussion
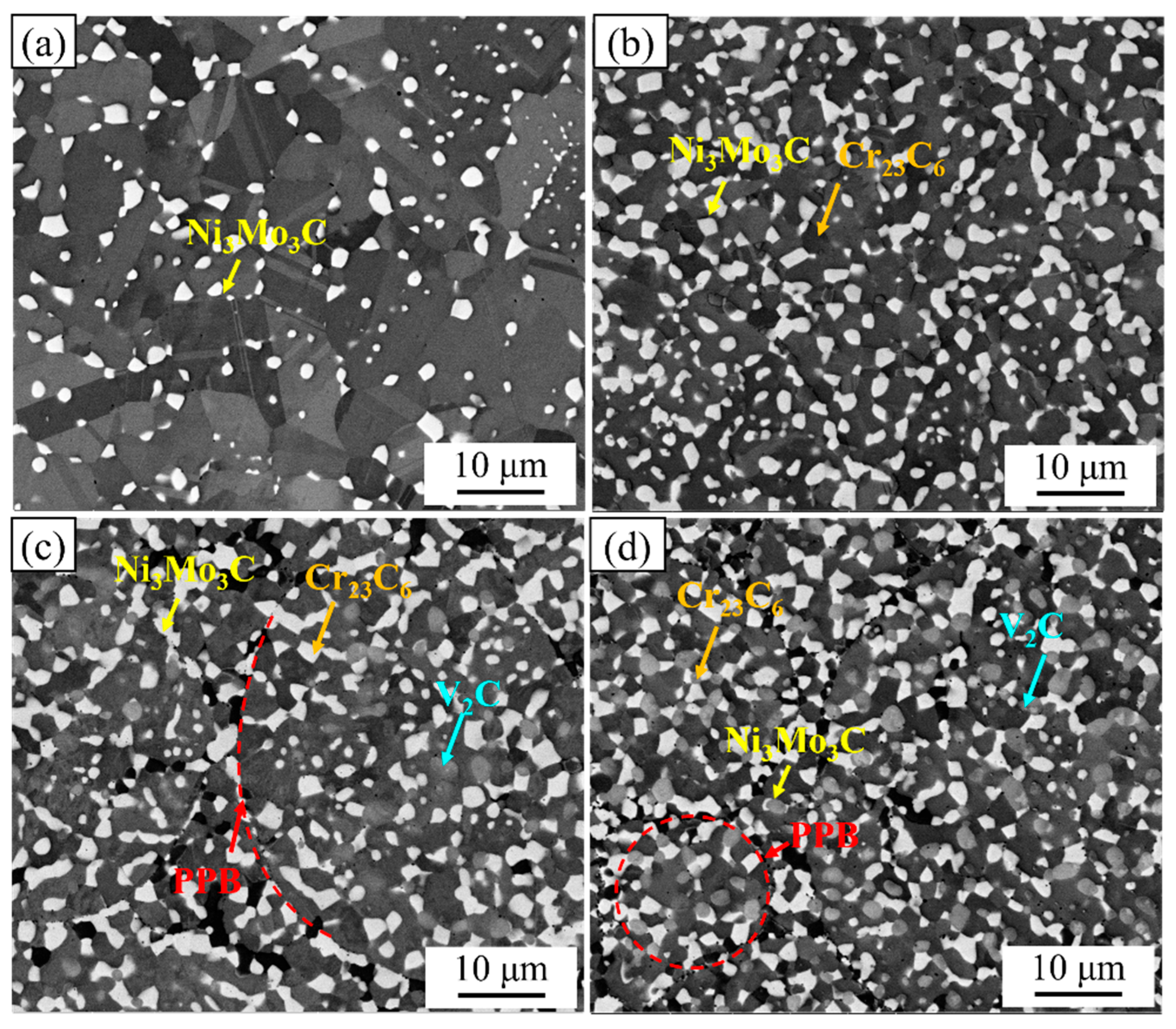
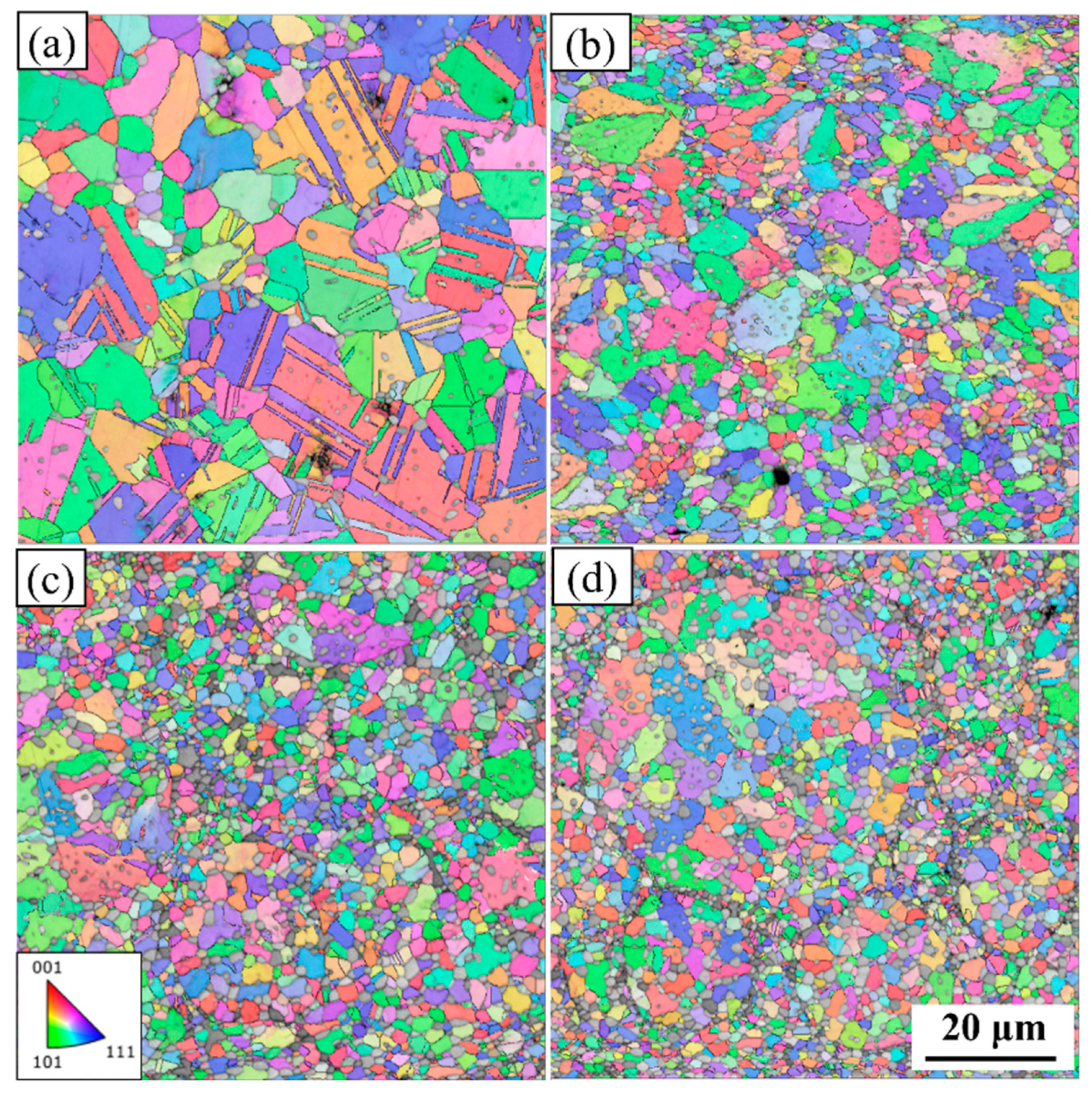
3.1. Microstructure of the NiCoCrMoCu-xC Alloy
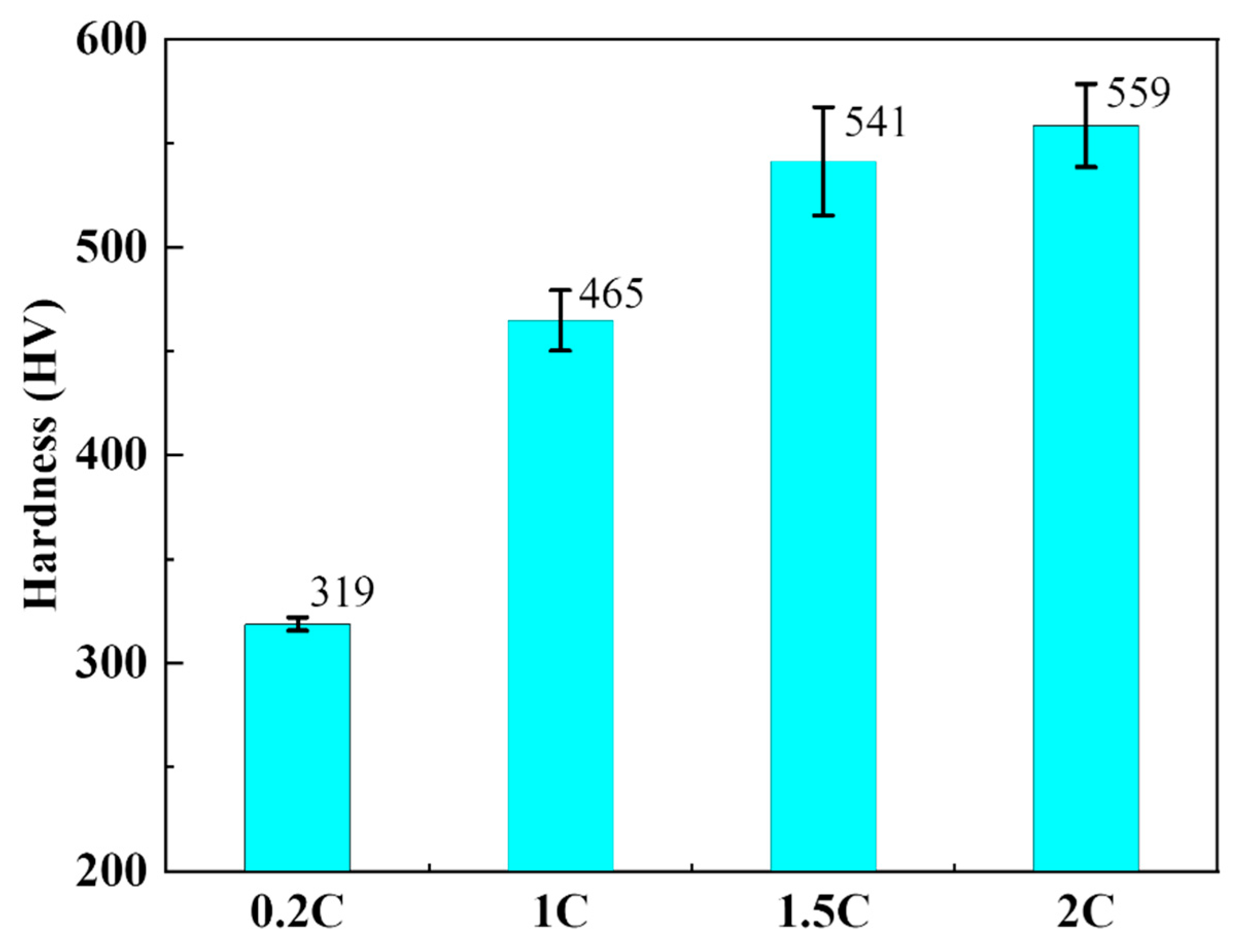
3.2. Mechanical Behavior of the NiCoCrMoCu-xC Alloys
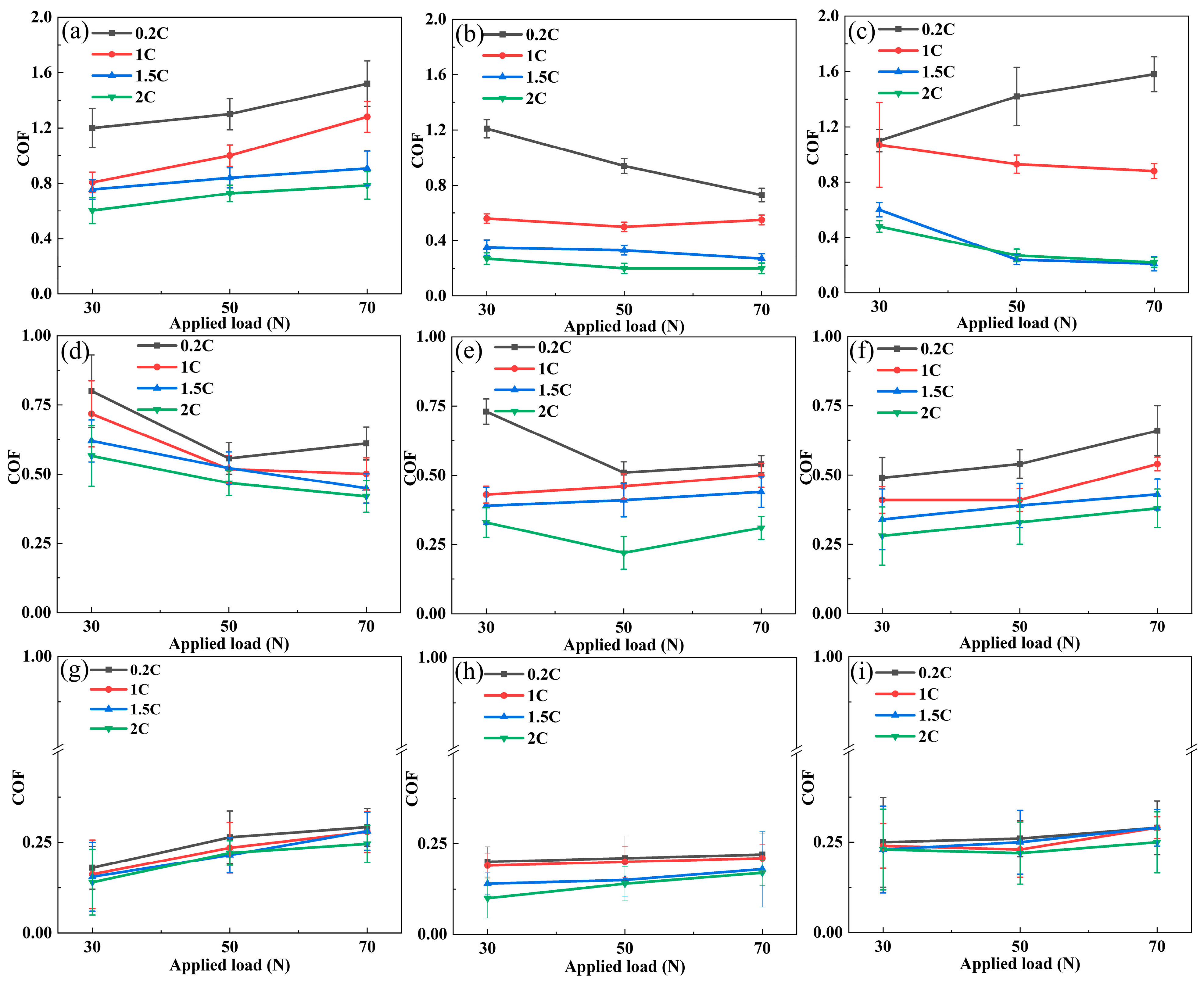
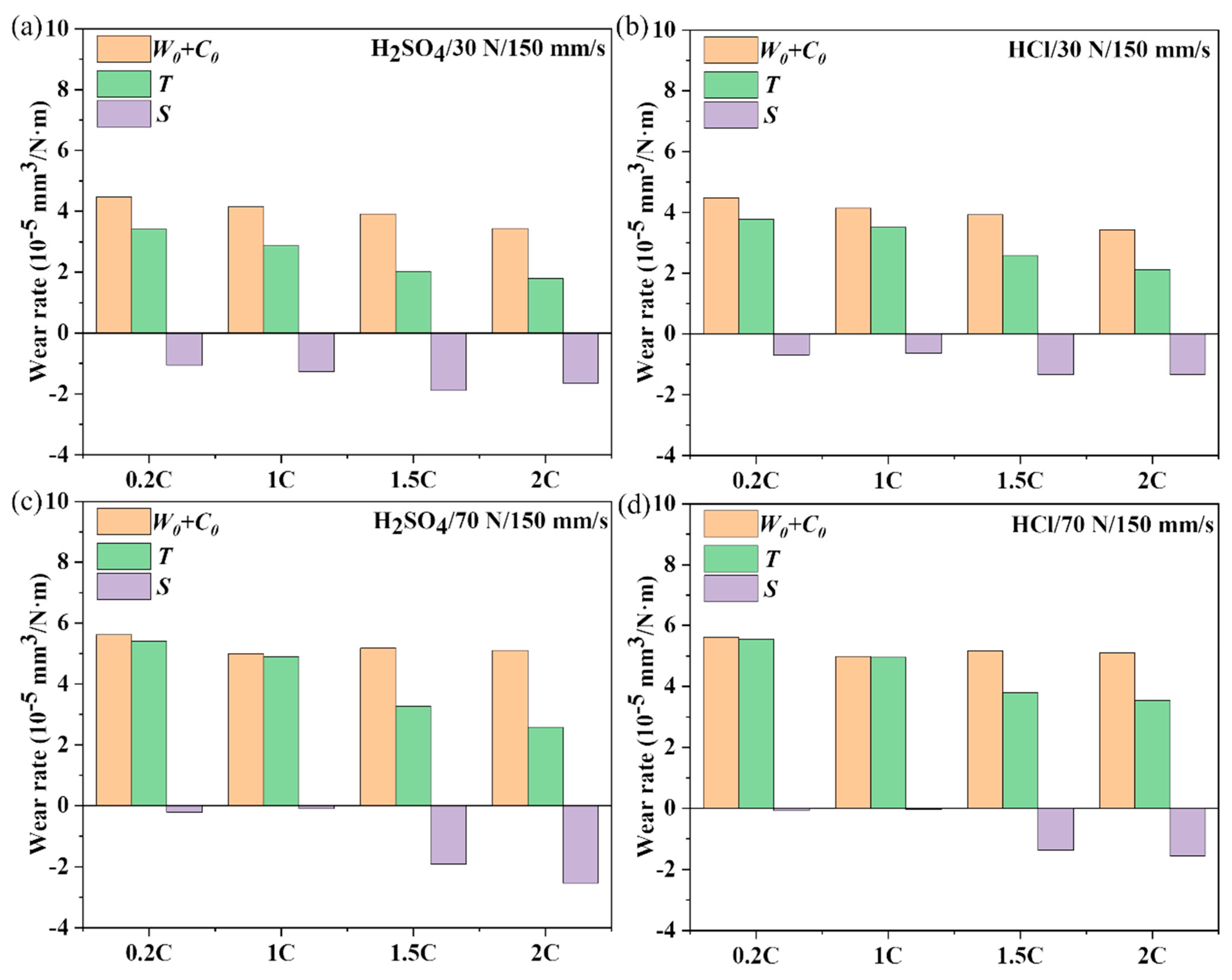
3.3. Friction Coefficient and Wear Rate of the NiCoCrMoCu-xC Alloys in Various Conditions
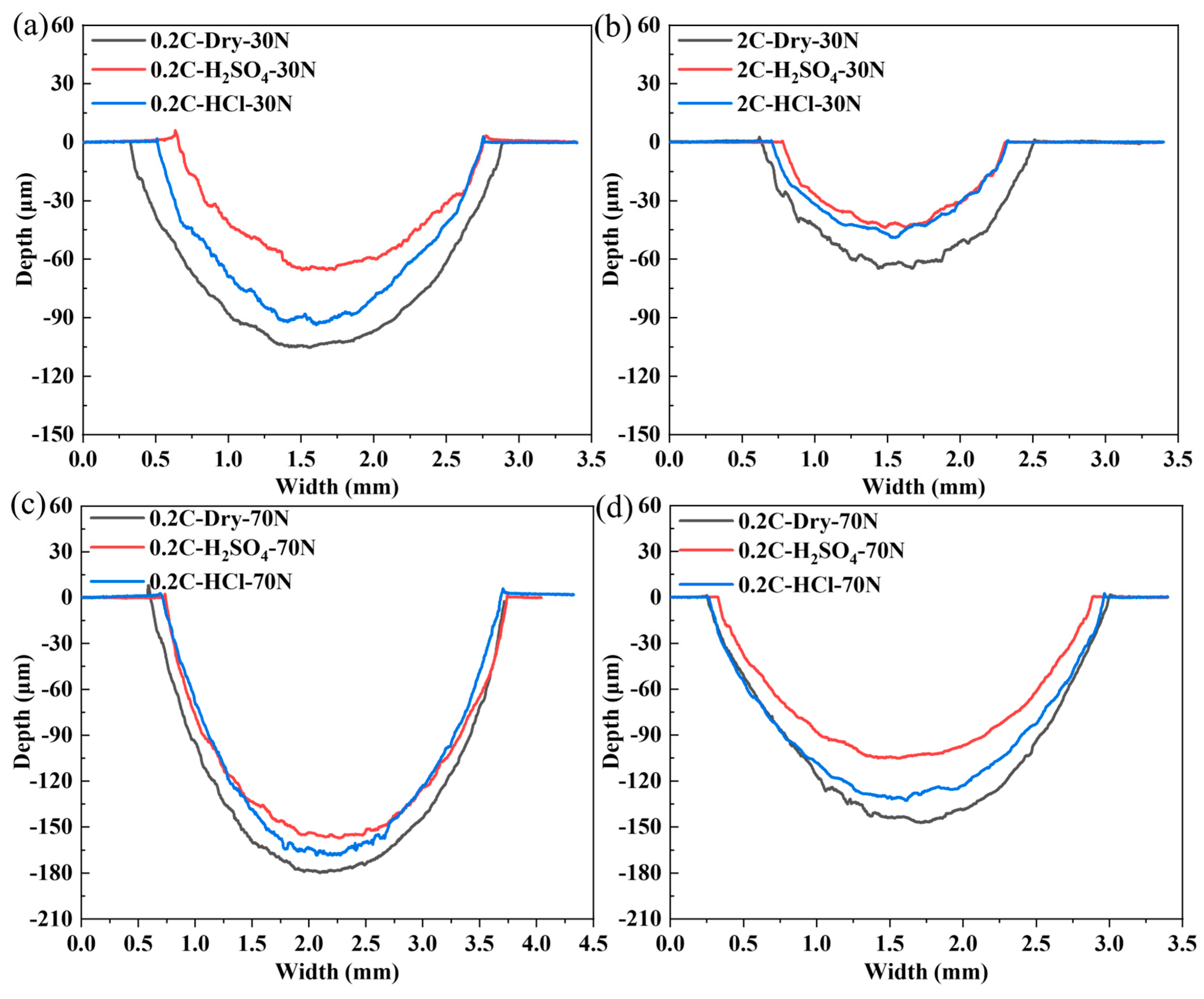
3.4. Dry Sliding and Tribocorrosion Behavior of the NiCoCrMoCu-xC Alloys under Various Loads
3.5. Wear and Tribocorrosion Behavior of the NiCoCrMoCu-xC Alloys under Various Sliding Speeds
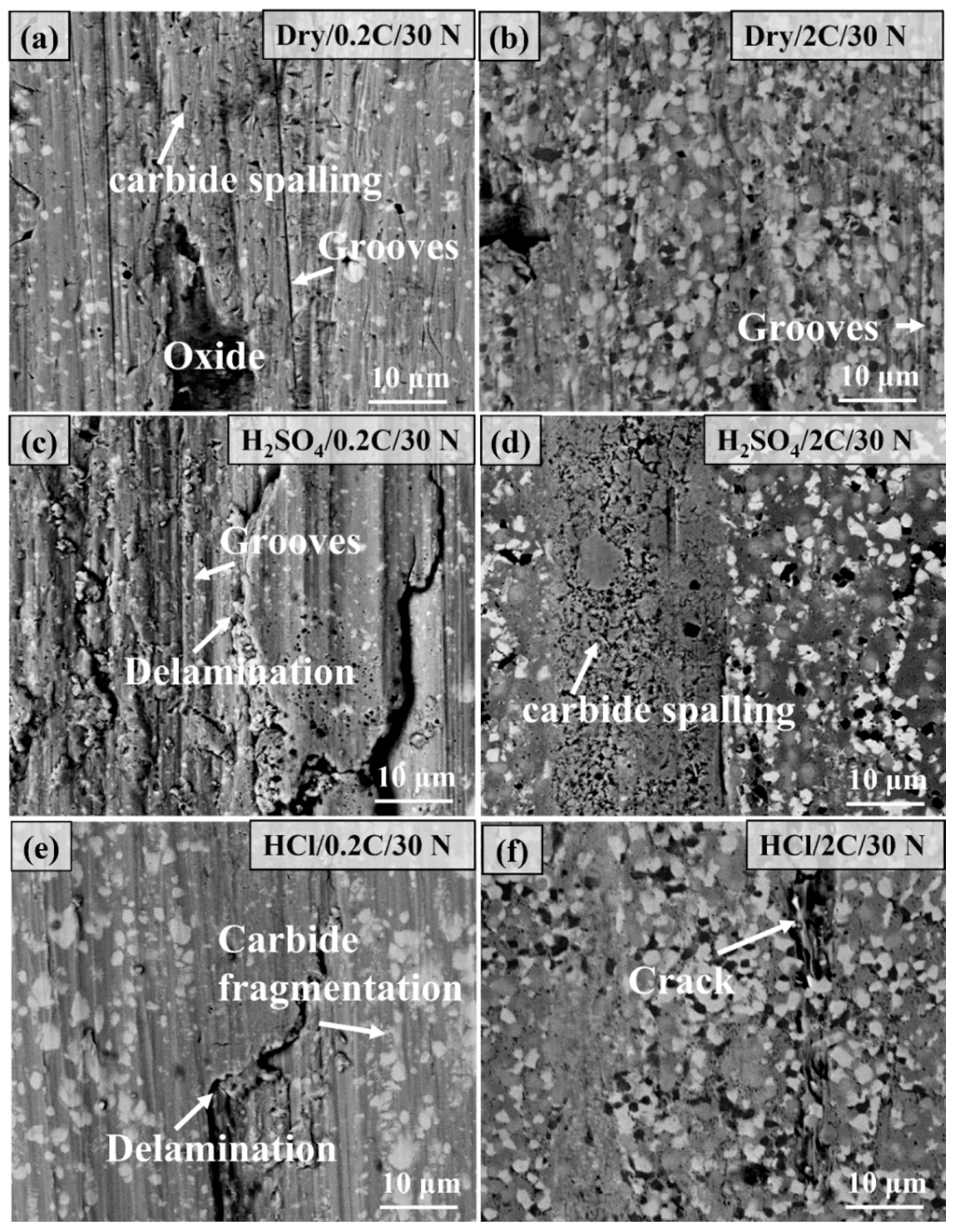
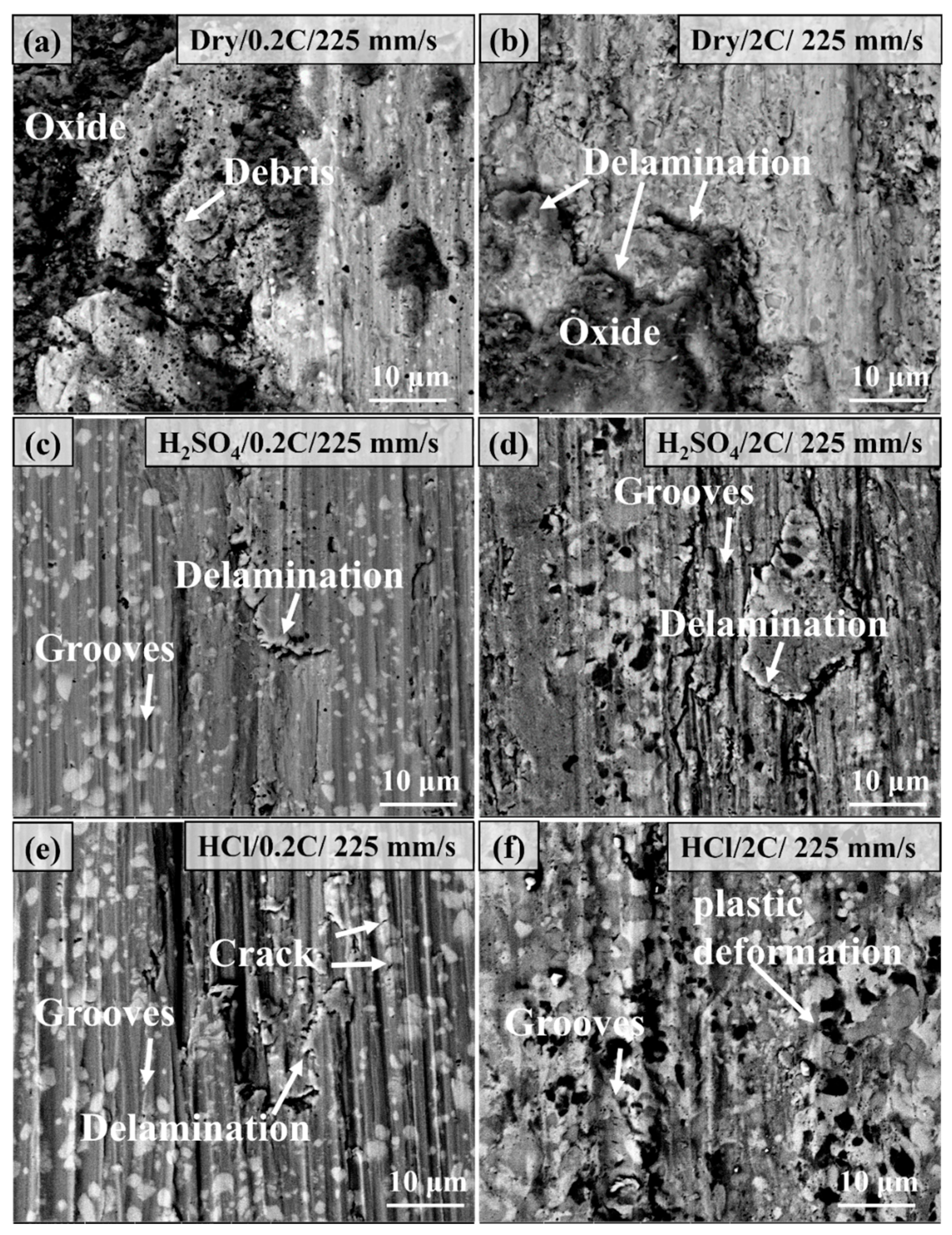
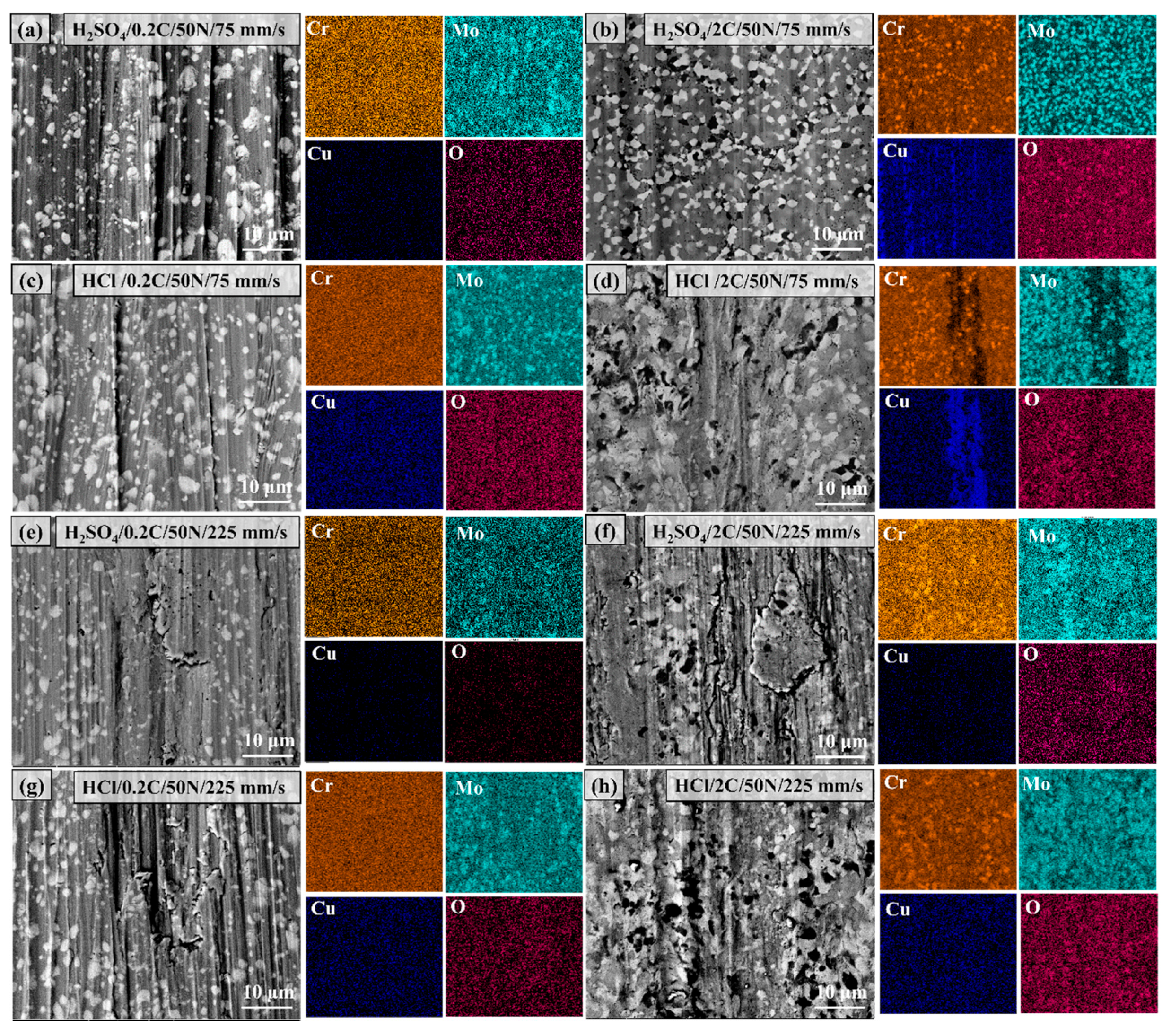
3.6. Worn Surface Characterization of the 0.2C and 2C Alloys in Various Conditions
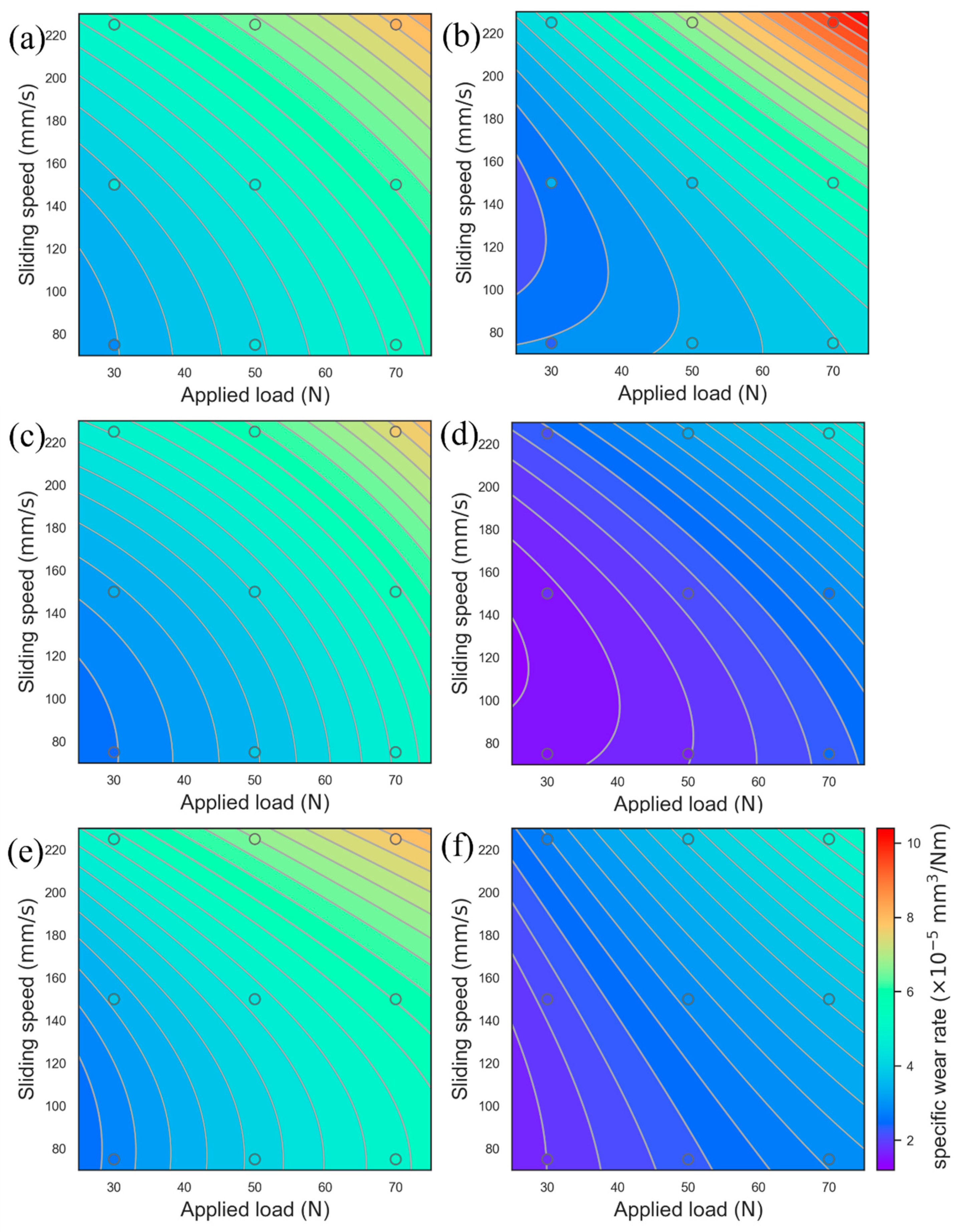
3.7. Optimization of the COF and Wear Rate Using the Response Surface Model
4. Conclusions
- The carbide content, Vickers hardness, and yield strength in the NiCoCrMoCu-xC alloy increase with the C content, whereas the alloy grain size is refined with the increase of carbide content.
- Compared with dry sliding wear, the NiCoCrMoCu-xC alloys exhibit a lower wear rate in the tribocorrosion process due to the lubrication effect of the solution, regardless of wear parameters. The lubrication effect can be promoted by the enrichment of Cu elements and the Cr2O3 on the surface. In addition, the H2SO4 solution has a better lubricating effect than HCl because of its higher viscosity.
- A general antagonistic effect was observed in the tribocorrosion of the NiCoCrMoCu-xC alloy. As the load and sliding rate increased, the antagonistic effect of the 0.2C and 1C alloys decreased because the destruction of the integrity of the wear surface leads to the disappearance of the Cu-rich zone and Cr2O3.
- The COF and wear rate under different loads and sliding rates were analyzed using the response surface analysis method. It was found that the COF mainly showed dependence on the sliding rate, while the wear rate showed dependence on load and the sliding rate.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ω | wear rate(10−5 mm3/N·m) |
| V | wear volume loss (mm3) |
| L | the sliding distance (m) |
| P | the applied load (N) |
| T | wear rate caused by tribocorrosion (10−5 mm3/N·m) |
| W0 | wear rate caused by dry sliding wear (10−5 mm3/N·m) |
| C0 | wear rate caused by immersion corrosion (10−5 mm3/N·m) |
| S | wear rate caused by the synergistic effect of corrosion and wear (10−5 mm3/N·m) |
| Hmin | the minimum hydrodynamic film thickness (μm) |
| α | constant |
| η | the liquid viscosity (Pa·s) |
| U | sliding speed (m/s) |
| R | equivalent radius of curvature(mm) |
| the equivalent elastic modulus of the wear system (GPa) | |
| k | the ellipticity of the contact domain |
References
- Guo, S.; Xu, D.; Jiang, G.; Kuang, W. Corrosion behavior and mechanism of Ni-based alloys Hastelloy C2000 and Inconel 740 in chloride-containing supercritical water oxidation. J. Alloys Compd. 2022, 907, 164452. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Y.; Zhong, X.; Wang, Z.; Oyama, K.; Ogota, S.; Watanabe, Y.; Shoji, T. Quantitatively evaluating the contribution of intergranular carbides, Cr-depleted zone, and grain boundary to intergranular stress corrosion cracking of Alloy 600 in a simulated boiling water reactors environment with high oxygen concentrations. Acta Mater. 2024, 269, 119794. [Google Scholar] [CrossRef]
- Dai, H.; Shi, S.; Tang, J.; Guo, C.; Ning, Z.; Chen, X. Revealing the effect of heat treatment on stress corrosion cracking behavior of Monel 400 alloy in hydrofluoric acid vapor environment. Corros. Sci. 2023, 215, 111046. [Google Scholar] [CrossRef]
- Bai, G.; Li, J.; Hu, R.; Zhang, T.; Kou, H.; Fu, H. Effect of thermal exposure on the stability of carbides in Ni–Cr–W based superalloy. Mater. Sci. Eng. A 2011, 528, 2339–2344. [Google Scholar] [CrossRef]
- Acharya, M.; Fuchs, G. The effect of stress on the microstructural stability of CMSX-10 single crystal Ni-base superalloys. Scr. Mater. 2006, 54, 61–64. [Google Scholar] [CrossRef]
- Teng, J.; Gong, X.; Yang, B.; Yu, S.; Lai, R.; Liu, J.; Li, Y. High temperature oxidation behavior of a novel Ni-Cr-W-Al-Ti superalloy. Corros. Sci. 2022, 198, 110141. [Google Scholar] [CrossRef]
- Teng, J.; Gong, X.; Yang, B.; Yu, S.; Liu, J.; Li, Y. Influence of Ti addition on oxidation behavior of Ni-Cr-W-based superalloys. Corros. Sci. 2021, 193, 109882. [Google Scholar] [CrossRef]
- Ye, X.; Yang, B.; Nie, Y.; Yu, S.; Li, Y. Influence of Nb addition on the oxidation behavior of novel Ni-base superalloy. Corros. Sci. 2021, 185, 109436. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhu, M.; Guo, Y.; Qin, H.; Tian, Q. Precipitation characteristics of γ’ precipitates of the GH4742 nickel-based superalloy at a slow cooling rate. J. Alloys Compd. 2023, 941, 169013. [Google Scholar] [CrossRef]
- Ecob, R.; Ricks, R.; Porter, A. The measurement of precipitate/matrix lattice mismatch in nickel-base superalloys. Scr. Met. 1982, 16, 1085–1090. [Google Scholar] [CrossRef]
- Meher, S.; Carroll, L.; Pollock, T.; Carroll, M. Solute partitioning in multi-component γ/γ′ Co–Ni-base superalloys with near-zero lattice misfit. Scr. Mater. 2016, 113, 185–189. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Harada, H.; Koizumi, Y. The effect of lattice misfit on the dislocation motion in superalloys during high-temperature low-stress creep. Acta Mater. 2005, 53, 4623–4633. [Google Scholar] [CrossRef]
- Kumar, B.K.A.; Ananthaprasad, M.G.; GopalaKrishna, K. A Review on Mechanical and Tribological Behaviors of Nickel Matrix Composites. Indian J. Sci. Technol. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Zhou, D.; Qiu, F.; Wang, H.; Jiang, Q. Manufacture of Nano-Sized Particle-Reinforced Metal Matrix Composites: A Review. Acta Met. Sin. (Engl. Lett.) 2014, 27, 798–805. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Contribution of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites. Mater. Sci. Eng. A 2008, 483–484, 148–152. [Google Scholar] [CrossRef]
- Proville, L.; Bakó, B. Dislocation depinning from ordered nanophases in a model fcc crystal: From cutting mechanism to Orowan looping. Acta Mater. 2010, 58, 5565–5571. [Google Scholar] [CrossRef]
- Cai, X.; Shen, M.; Cao, B.; Liu, M.; Xu, Y. Microstructural characterization of chromium carbide-reinforced iron matrix composites prepared by combination of casting and heat treatment. Vacuum 2023, 216, 112477. [Google Scholar] [CrossRef]
- Xing, L.; Hao, S.; Huang, H.; Yao, L.; Ding, A.; Zhang, M.; Zhang, Y.; Zhao, X.; Wang, J.; Xu, R.; et al. Ni-base superalloys enhanced by in-situ MC carbides precipitant on TiC nanoparticles. Mater. Today Commun. 2024, 38, 108262. [Google Scholar] [CrossRef]
- Antonov, S.; Chen, W.; Huo, J.; Feng, Q.; Isheim, D.; Seidman, D.N.; Sun, E.; Tin, S. MC Carbide Characterization in High Refractory Content Powder-Processed Ni-Based Superalloys. Met. Mater. Trans. A 2018, 49, 2340–2351. [Google Scholar] [CrossRef]
- Ye, W.; Xie, M.; Huang, Z.; Wang, H.; Zhou, Q.; Wang, L.; Chen, B.; Wang, H.; Liu, W. Microstructure and tribological properties of in-situ carbide/CoCrFeNiMn high entropy alloy composites synthesized by flake powder metallurgy. Tribol. Int. 2023, 181, 108295. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, W.; Yi, G.; Wang, B.; Feng, X.; Shi, P.; Hua, M. High temperature and room temperature tribological behaviors of in-situ carbides reinforced Ni-based composites by reactive sintering Ni and Ti2AlC precursor. Wear 2022, 488–489, 204165. [Google Scholar] [CrossRef]
- Handoko, W.; Anurag, A.; Pahlevani, F.; Hossain, R.; Privat, K.; Sahajwalla, V. Effect of selective-precipitations process on the corrosion resistance and hardness of dual-phase high-carbon steel. Sci. Rep. 2019, 9, 15631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yamanaka, K.; Bian, H.; Chiba, A. Corrosion-resistant carbide-reinforced martensitic steel by Cu modification. NPJ Mater. Degrad. 2019, 3, 30. [Google Scholar] [CrossRef]
- Cao, S.; Mischler, S. Modeling tribocorrosion of passive metals—A review. Curr. Opin. Solid State Mater. Sci. 2018, 22, 127–141. [Google Scholar] [CrossRef]
- Mischler, S.; Debaud, S.; Landolt, D. Wear-Accelerated Corrosion of Passive Metals in Tribocorrosion Systems. J. Electrochem. Soc. 1998, 145, 750–758. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Cao, S.; Maldonado, S.G.; Mischler, S. Tribocorrosion of passive metals in the mixed lubrication regime: Theoretical model and application to metal-on-metal artificial hip joints. Wear 2015, 324–325, 55–63. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Wang, J.; Yan, F. Antagonistic effect of electrochemical corrosion on the mechanical wear of Monel 400 alloy in seawater. Corros. Sci. 2022, 198, 110120. [Google Scholar] [CrossRef]
- Gao, R.; Liu, E.; Zhang, Y.; Zhu, L.; Zeng, Z. Tribocorrosion Behavior of SAF 2205 Duplex Stainless Steel in Artificial Seawater. J. Mater. Eng. Perform. 2019, 28, 414–422. [Google Scholar] [CrossRef]
- Sun, Y.; Rana, V. Tribocorrosion behaviour of AISI 304 stainless steel in 0.5M NaCl solution. Mater. Chem. Phys. 2011, 129, 138–147. [Google Scholar] [CrossRef]
- Namus, R.; Nutter, J.; Qi, J.; Rainforth, W. Sliding speed influence on the tribo-corrosion behaviour of Ti6Al4V alloy in simulated body fluid. Tribol. Int. 2021, 160, 107023. [Google Scholar] [CrossRef]
- Yang, B.; Shi, C.; Li, Y.; Lei, Q.; Nie, Y. Effect of Cu on the corrosion resistance and electrochemical response of a Ni-Co-Cr-Mo alloy in acidic chloride solution. J. Mater. Res. 2018, 33, 3801–3808. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Wang, F.; Zhang, C.; Koizumi, Y.; Chiba, A. Influence of Mo concentration on corrosion resistance to HF acid solution of Ni–Co–Cr–Mo alloys with and without Cu. Corros. Sci. 2015, 99, 185–193. [Google Scholar] [CrossRef]
- Yang, B.; Li, J.; Gong, X.; Nie, Y.; Li, Y. Effects of Cu addition on the corrosion behavior of NiCoCrMo alloys in neutral chloride solution. RSC Adv. 2017, 7, 40779–40790. [Google Scholar] [CrossRef]
- Arjunraj, R.; Sengottaiyan, M.; Venkateshan, T.; Chandramohan, V.; Santhosh, V.; Gunasekar, C.; Kavinkumar, D. Study on the tribological characteristics of AA5010/fly ash/ boron carbide metal matrix composites using response surface methodology. Mater. Today Proc. 2023, S2214785323053269. [Google Scholar] [CrossRef]
- Srinivasan, R.; Pridhar, T.; Kirubakaran, R.; Ramesh, A. Prediction of wear strength of squeeze cast aluminium hybrid metal matrix composites using response surface methodology. Mater. Today Proc. 2020, 27, 1806–1811. [Google Scholar] [CrossRef]
- ASTM-E963-95; Standard Practice for Electrolytic Extraction of Phases from Ni and Ni-Fe Base Superalloys Using a Hydrochloric-Methanol Electrolyte. ASTM International: West Conshohocken, PA, USA, 2010.
- Landolt, D.; Mischler, S.; Stemp, M. Electrochemical methods in tribocorrosion: A critical appraisal. Electrochim. Acta 2001, 46, 3913–3929. [Google Scholar] [CrossRef]
- Ponthiaux, P.; Wenger, F.; Drees, D.; Celis, J. Electrochemical techniques for studying tribocorrosion processes. Wear 2004, 256, 459–468. [Google Scholar] [CrossRef]
- Li, C.; Teng, J.; Yang, B.; Ye, X.; Li, Y. Effect of Carbon Content on Wear Behavior of Ni-Co-Cr-Mo-Cu Alloy. J. Mater. Eng. Perform. 2023, 1–15. [Google Scholar] [CrossRef]
- Yao, Z.; Hou, J.; Chen, Y.; Xu, W.; Jiang, H.; Dong, J. Effect of micron-sized particles on the crack growth behavior of a Ni-based powder metallurgy superalloy. Mater. Sci. Eng. A 2022, 860, 144242. [Google Scholar] [CrossRef]
- Du, B.; Yang, J.; Cui, C.; Sun, X. Effects of grain refinement on the microstructure and tensile behavior of K417G superalloy. Mater. Sci. Eng. A 2015, 623, 59–67. [Google Scholar] [CrossRef]
- Bian, H.; Xu, X.; Li, Y.; Koizumi, Y.; Wang, Z.; Chen, M.; Yamanaka, K.; Chiba, A. Regulating the coarsening of the γ′ phase in superalloys. NPG Asia Mater. 2015, 7, e212. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, L.; Huang, Z.; Wan, T.; Zhu, M.; Ren, F. Achieving low wear in a μ-phase reinforced high-entropy alloy and associated subsurface microstructure evolution. Wear 2021, 474–475, 203755. [Google Scholar] [CrossRef]
- Zhao, G.; Zang, X.; Jing, Y.; Lü, N.; Wu, J. Role of carbides on hot deformation behavior and dynamic recrystallization of hard-deformed superalloy U720Li. Mater. Sci. Eng. A 2021, 815, 141293. [Google Scholar] [CrossRef]
- Hamrock, B.J.; Dowson, D. Elastohydrodynamic Lubrication of Elliptical Contacts for Materials of Low Elastic Modulus I—Fully Flooded Conjunction. J. Lubr. Technol. 1978, 100, 236–245. [Google Scholar] [CrossRef]
- Bright, N.F.H.; Hutchison, H.; Smith, D. The viscosity and density of sulphuric acid and oleum. J. Chem. Technol. Biotechnol. 1946, 65, 385–388. [Google Scholar] [CrossRef]
- Nishikata, E.; Ishii, T.; Ohta, T. Viscosities of aqueous hydrochloric acid solutions, and densities and viscosities of aqueous hydroiodic acid solutions. J. Chem. Eng. Data 1981, 26, 254–256. [Google Scholar] [CrossRef]
- Feng, M.; Wu, Q.; Xue, J.; Luo, Z.; Wang, Z.; An, X.; Liao, X.; Li, J.; Jin, S.; Sha, G. High-pressure-torsion-induced segregation, precipitation and grain refinement of Al-(Si, Mg and Cu) binary alloys. J. Mater. Sci. Technol. 2024, 199, S1005030224003311. [Google Scholar] [CrossRef]
- Yamanaka, K.; Mori, M.; Yoshida, K.; Onuki, Y.; Sato, S.; Chiba, A. Surface evolution and corrosion behaviour of Cu-doped carbide-reinforced martensitic steels in a sulfuric acid solution. NPJ Mater. Degrad. 2021, 5, 43. [Google Scholar] [CrossRef]
- Webb, W.W.; Norton, J.T.; Wagner, C. Oxidation Studies in Metal-Carbon Systems. J. Electrochem. Soc. 1956, 103, 112–117. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. The role of microstructure in the high temperature oxidation mechanism of Cr3C2–NiCr composite coatings. Corros. Sci. 2009, 51, 1172–1180. [Google Scholar] [CrossRef]
- Yang, C.-M.; Liu, X.-B.; Liu, Y.-F.; Zhu, Z.-X.; Meng, Y.; Zhou, H.-B.; Zhang, S.-H. Effect of Cu-doping on tribological properties of laser-cladded FeCoCrNiCux high-entropy alloy coatings. Tribol. Int. 2023, 188, 108868. [Google Scholar] [CrossRef]
- Stott, F. The role of oxidation in the wear of alloys. Tribol. Int. 1998, 31, 61–71. [Google Scholar] [CrossRef]
- García-León, R.; Martínez-Trinidad, J.; Zepeda-Bautista, R.; Campos-Silva, I.; Guevara-Morales, A.; Martínez-Londoño, J.; Barbosa-Saldaña, J. Dry sliding wear test on borided AISI 316L stainless steel under ball-on-flat configuration: A statistical analysis. Tribol. Int. 2021, 157, 106885. [Google Scholar] [CrossRef]
- García-León, R.A.; Martínez-Trinidad, J.; Campos-Silva, I.; Figueroa-López, U.; Guevara-Morales, A. Development of tribological maps on borided AISI 316L stainless steel under ball-on-flat wet sliding conditions. Tribol. Int. 2021, 163, 107161. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Espallargas, N. Tribocorrosion mechanisms in sliding contacts. In Tribocorrosion of Passive Metals and Coatings; Woodhead Publishing: Cambridge, UK, 2011; pp. 118–152. [Google Scholar]
- Guadalupe, S.; Cao, S.; Cantoni, M.; Chitty, W.-J.; Falcand, C.; Mischler, S. Applicability of a recently proposed tribocorrosion model to CoCr alloys with different carbides content. Wear 2017, 376–377, 203–211. [Google Scholar] [CrossRef]








| Nominations | Ni | Co | Cr | Mo | Cu | W | V | Si | Ti | Mn | C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.2C | Bal. | 30 | 17 | 16 | 2 | 4 | — | — | — | — | 0.2 |
| 1C | Bal. | 20 | 20 | 16 | 1.5 | 2 | 2 | 0.25 | 0.25 | 0.5 | 1 |
| 1.5C | Bal. | 20 | 20 | 16 | 1.5 | 3 | 3 | 0.375 | 0.375 | 0.5 | 1.5 |
| 2C | Bal. | 20 | 20 | 16 | 1.5 | 4 | 4 | 0.5 | 0.5 | 0.5 | 2 |
| Alloys | Ni3Mo3C | Cr23C6 | V2C | Area Fraction, % | |
|---|---|---|---|---|---|
| 0.2C | Phase fraction, % | 9.38 | — | — | 9.5 |
| Size, μm | 1.03 | — | — | — | |
| 1C | Phase fraction, % | 25.0 | 10.0 | — | 35 |
| Size, μm | 1.39 | 1.77 | — | — | |
| 1.5C | Phase fraction, % | 23.5 | 5.8 | 11.8 | 41.1 |
| Size, μm | 1.42 | 0.67 | 0.7 | — | |
| 2C | Phase fraction, % | 17.3 | 6.1 | 17.8 | 41.2 |
| Size, μm | 1.08 | 0.63 | 0.73 | — |
| Alloys | Yield Strength (MPa) | Ultimate Compressive Strength (MPa) | Maximum Contact Stress (MPa) | ||
|---|---|---|---|---|---|
| 30 N | 50 N | 70 N | |||
| 0.2C | 698 | 2287 | 2077 | 2463 | 2755 |
| 1C | 978 | 1873 | 2114 | 2507 | 2804 |
| 1.5C | 1166 | 2007 | 2141 | 2538 | 2839 |
| 2C | 1335 | 2088 | 2163 | 2565 | 2870 |
| Roughness/μm | 0.2C-Dry | 0.2C-H2SO4 | 0.2C-HCl | 2C-Dry | 2C-H2SO4 | 2C-HCl |
|---|---|---|---|---|---|---|
| 30 N/75 mm/s | 0.228 | 0.109 | 0.138 | 0.144 | 0.074 | 0.166 |
| 70 N/75 mm/s | 0.282 | 0.045 | 0.176 | 0.150 | 0.062 | 0.119 |
| 75 mm/s/50 N | 0.175 | 0.085 | 0.136 | 0.267 | 0.031 | 0.042 |
| 225 mm/s/50 N | 0.185 | 0.127 | 0.142 | 0.256 | 0.14 | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zeng, Z.; Teng, J.; Yang, B.; Li, Y. Tribocorrosion Behavior of NiCoCrMoCu Alloys Containing Different Carbides in Acidic Media at Various Applied Loads and Sliding Speeds. Materials 2024, 17, 2971. https://doi.org/10.3390/ma17122971
Li C, Zeng Z, Teng J, Yang B, Li Y. Tribocorrosion Behavior of NiCoCrMoCu Alloys Containing Different Carbides in Acidic Media at Various Applied Loads and Sliding Speeds. Materials. 2024; 17(12):2971. https://doi.org/10.3390/ma17122971
Chicago/Turabian StyleLi, Chao, Ziming Zeng, Jianwei Teng, Biaobiao Yang, and Yunping Li. 2024. "Tribocorrosion Behavior of NiCoCrMoCu Alloys Containing Different Carbides in Acidic Media at Various Applied Loads and Sliding Speeds" Materials 17, no. 12: 2971. https://doi.org/10.3390/ma17122971





