Synergistic Effect of Blended Precursors and Silica Fume on Strength and High Temperature Resistance of Geopolymer
Abstract
:1. Introduction
2. Experimental Program
2.1. Raw Materials
2.2. Mix Design and Preparation
2.3. Testing Methods
2.3.1. Flowability Test
2.3.2. Volume and Mass Loss Test
2.3.3. Compressive Strength Test
2.3.4. High Temperature Test
2.3.5. X-ray Diffraction Test
2.3.6. Thermogravimetric Test
2.3.7. Mercury Intrusion Porosimetry Test
2.3.8. Scanning Electron Microscopy Test
3. Results and Discussion
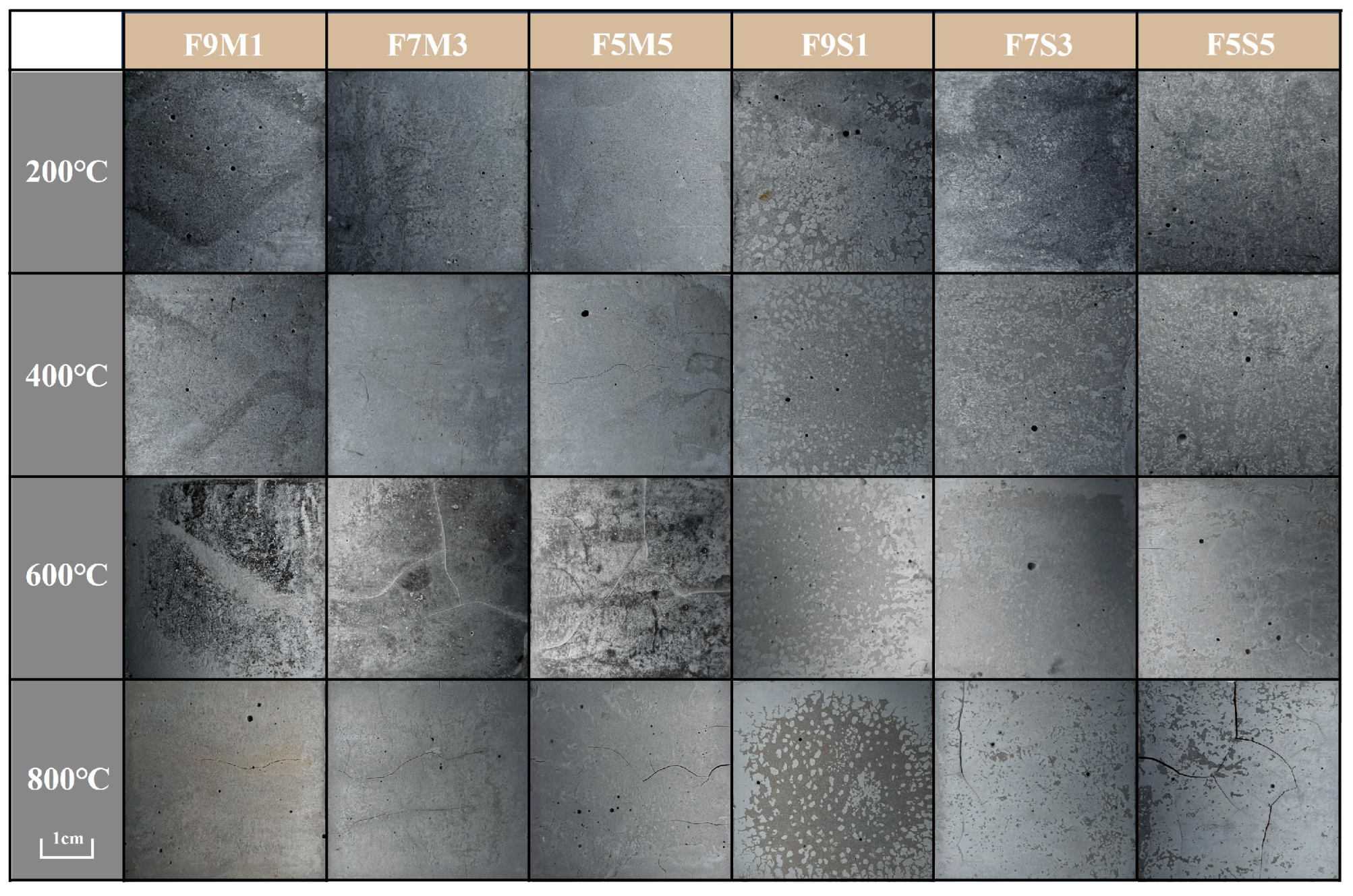
3.1. Macroscopic Morphology and Failure Mode
3.2. Volume Shrinkage and Mass Loss
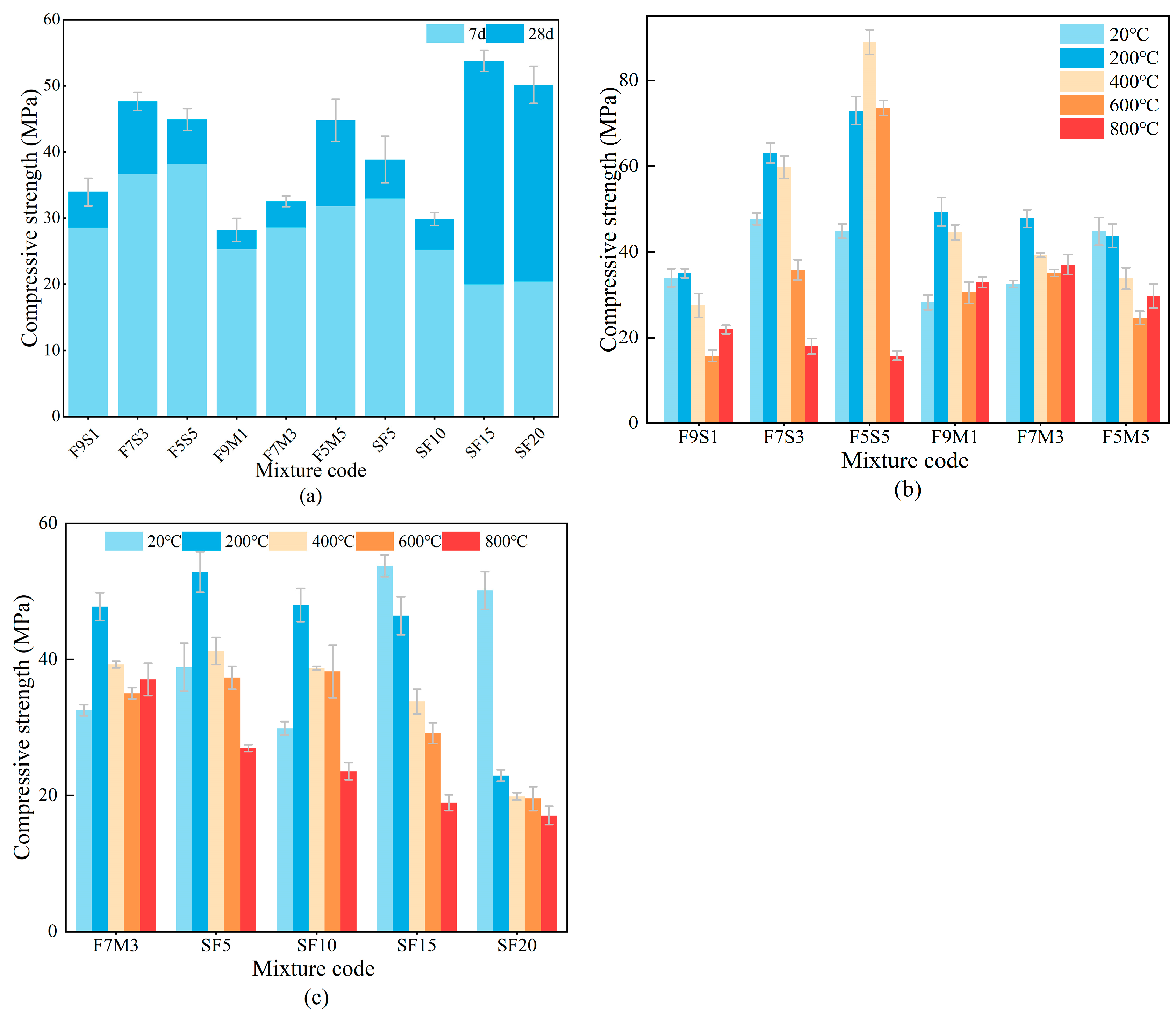
3.3. Compressive Strength
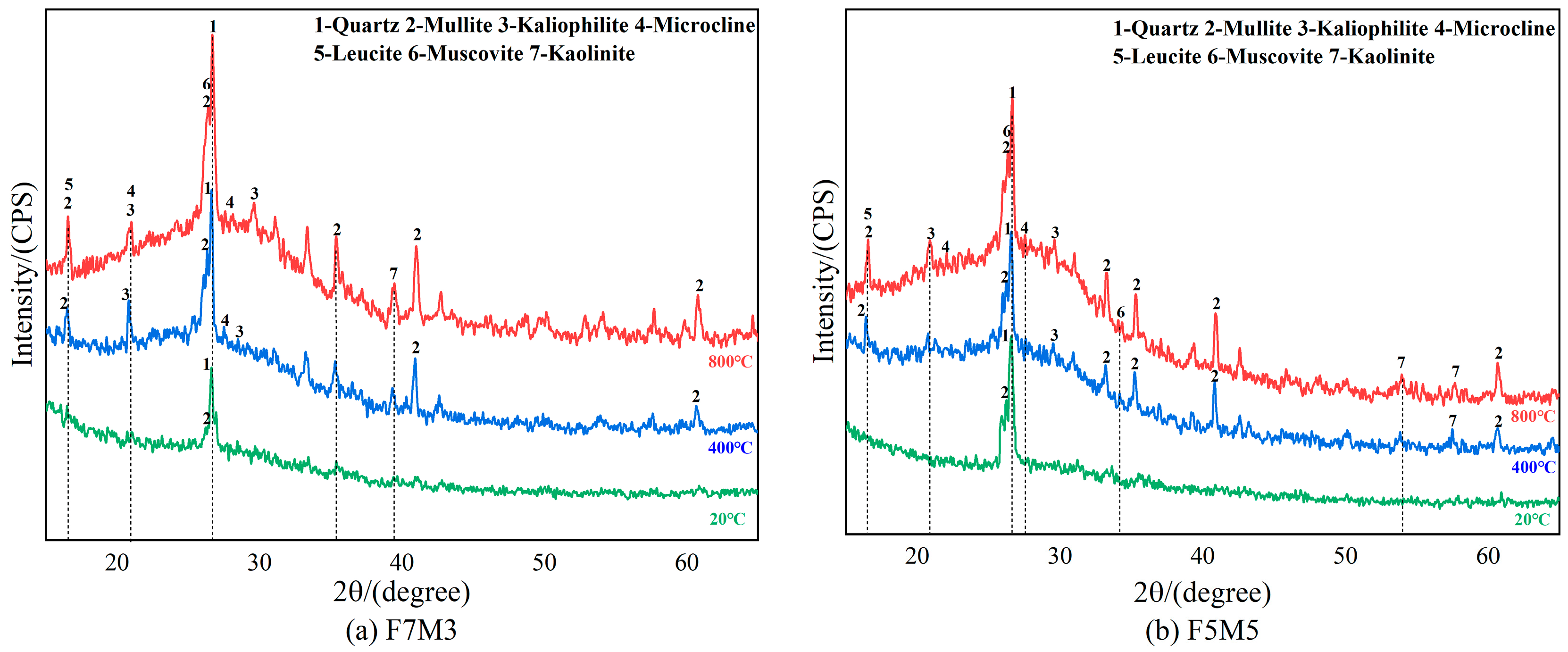
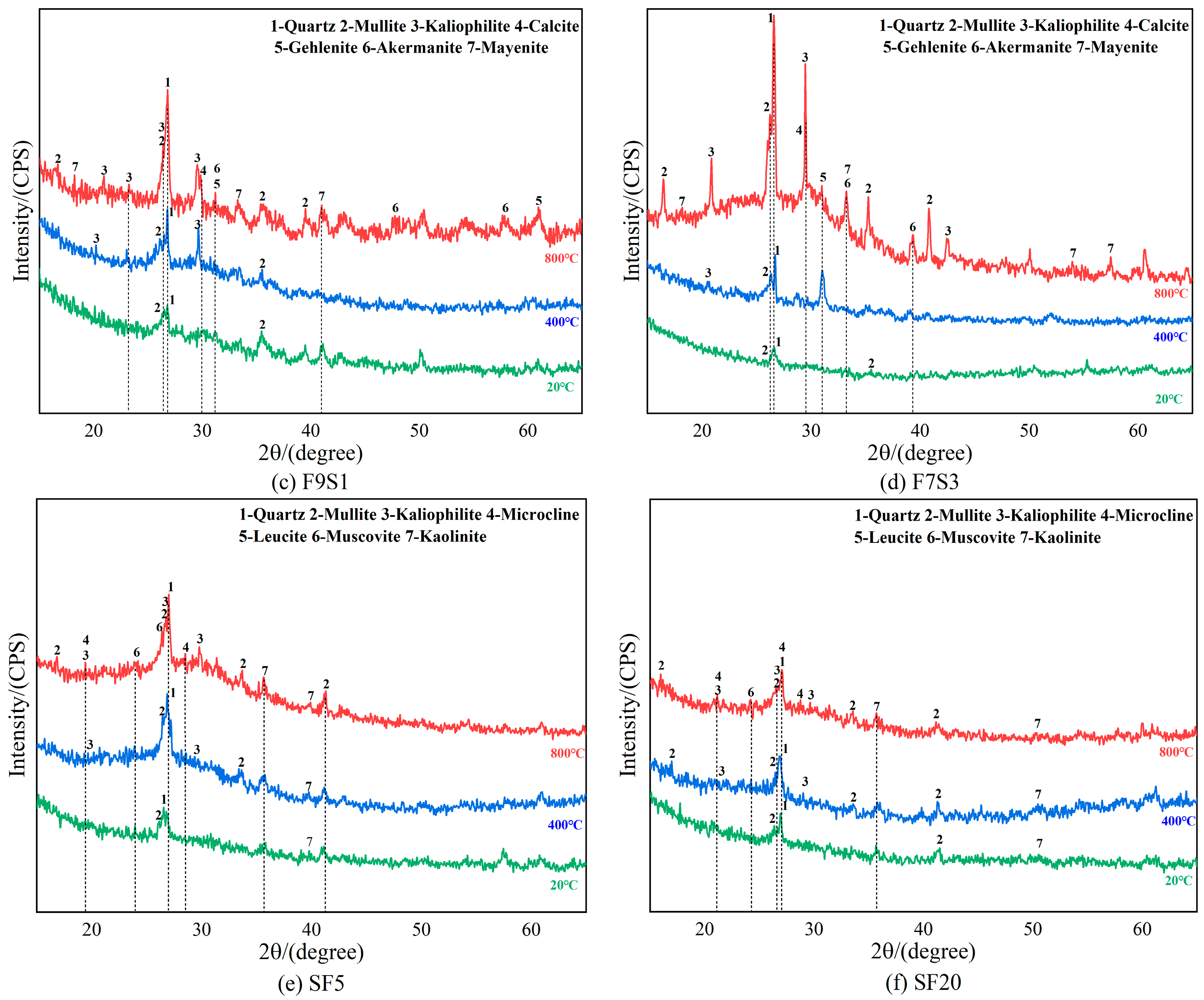
3.4. X-ray Diffraction Analysis
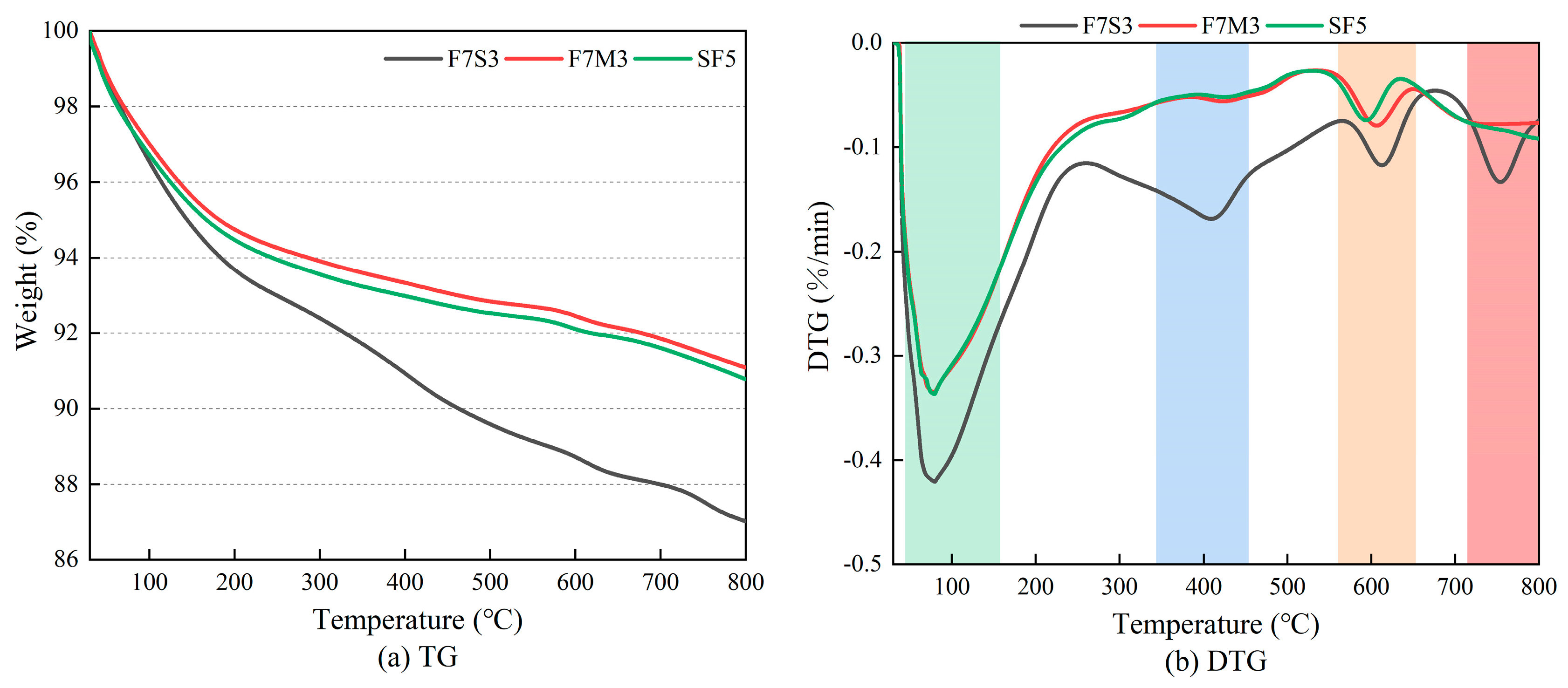
3.5. Thermal Analysis
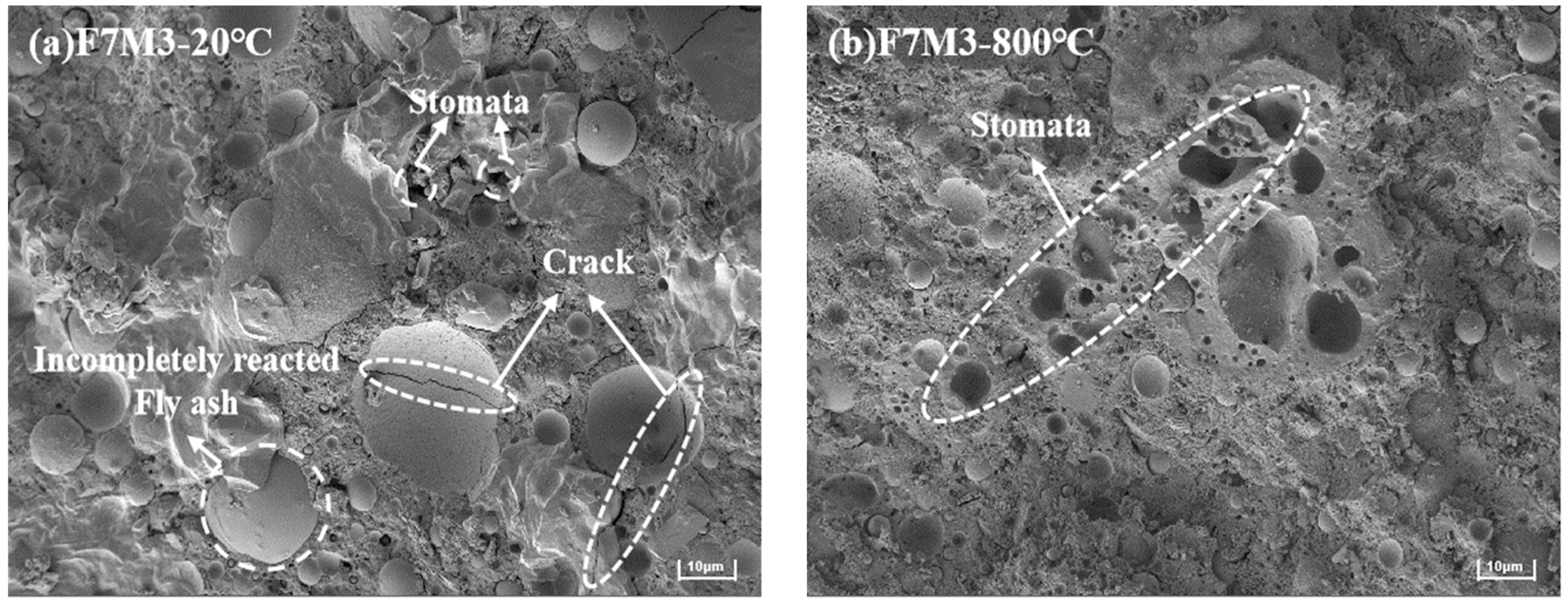
3.6. SEM Analysis
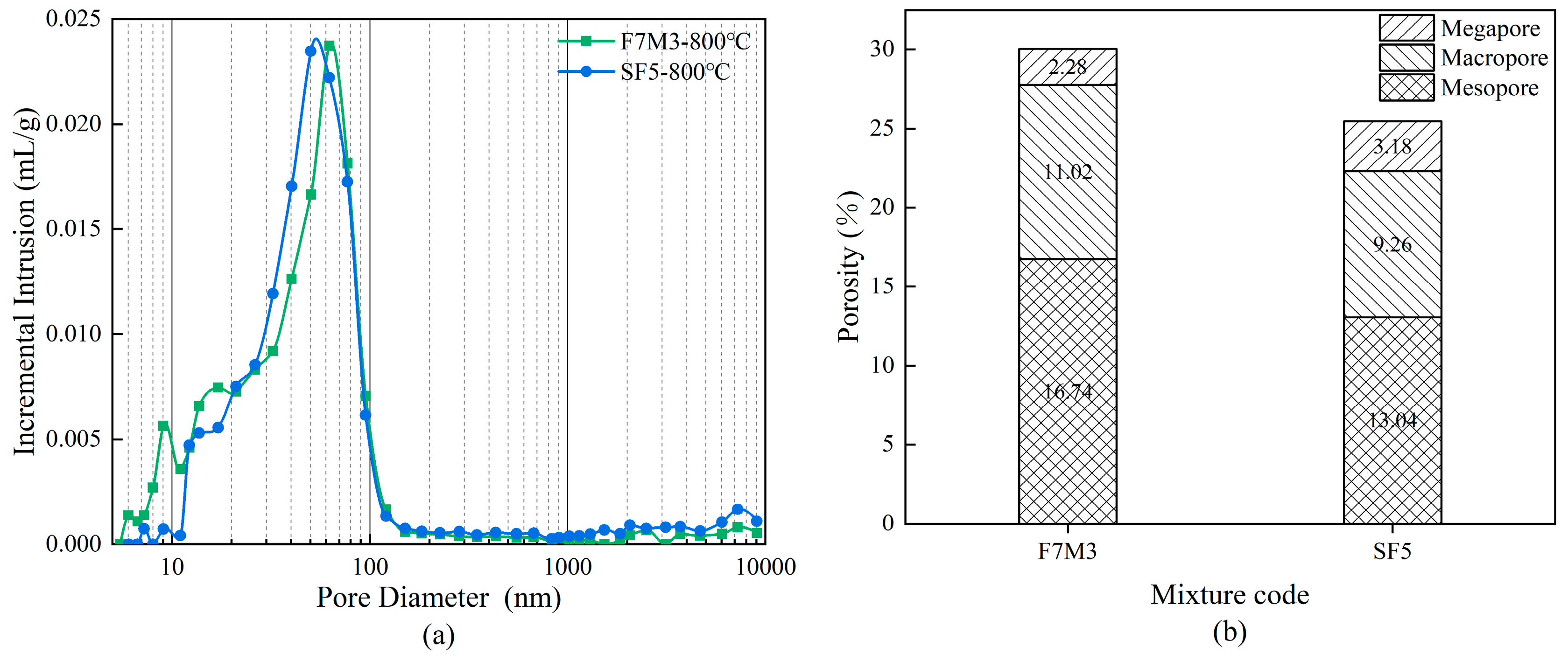
3.7. Pore Structure Analysis
4. Conclusions
- The development of blended precursor geopolymers by partially substituting fly ash with slag significantly benefits dense structures, 28 d compressive strength, and residual strength below moderate temperature exposure. However, the creation of the M-(C)-A-S-H hybrid gel due to higher calcium contents adversely reduces the thermal stability leading to enlarged volume shrinkage and mass loss at high temperatures. The residual compressive strength dramatically declines after being exposed to high temperatures such as 800 °C due to the generation of expansive akermanite and gehlenite to result in porous microstructure.
- The blended precursor geopolymers prepared from fly ash and metakaolin have favorable mechanical properties and excellent high-temperature resistance. The large amount of reactive Al2O3 in metakaolin promotes the formation of silica-aluminate gels and enhances the microstructure and compressive strength of the geopolymers. Large gels generate kaliophilite by viscous sintering to compensate for the strength loss at high temperatures.
- An appropriate silica fume content in the blended precursor geopolymer contributed to reduced porosity and enhanced compressive strength and mitigates pore collapse at high temperatures. However, excessive silica fume content reduced the residual compressive strength of the blended precursor geopolymers at high temperatures due to the generation of alkali aluminosilicate gels and ceramic-like phases being hindered. In addition, the presence of more free water increases the mass loss and volume shrinkage of geopolymers which include silica fume.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Man-made rock geosynthesis and the resulting development of very early high strength cement. J. Mater. Educ. 1994, 16, 91–139. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Effect of curing condition on the mechanical properties of fly ash-based geopolymer concrete. SN Appl. Sci. 2019, 1, 1694. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Ge, X.; Hu, X.; Shi, C. The effect of different types of class F fly ashes on the mechanical properties of geopolymers cured at ambient environment. Cem. Concr. Compos. 2022, 130, 104528. [Google Scholar] [CrossRef]
- Brough, A.R.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium silicate-based alkali-activated slag mortars Part II. The retarding effect of additions of sodium chloride or malic acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Kathirvel, P.; Sreekumaran, S. Sustainable development of ultra high performance concrete using geopolymer technology. J. Build. Eng. 2021, 39, 102267. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Bellum, R.R.; Muniraj, K.; Indukuri, C.S.R.; Madduru, S.R.C. Investigation on Performance Enhancement of Fly ash-GGBFS Based Graphene Geopolymer Concrete. J. Build. Eng. 2020, 32, 101659. [Google Scholar] [CrossRef]
- Pradhan, P.; Dwibedy, S.; Pradhan, M.; Panda, S.; Panigrahi, S.K. Durability characteristics of geopolymer concrete—Progress and perspectives. J. Build. Eng. 2022, 59, 105100. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Borstel, C.D.; Van Riessen, A. The effect of pre-treatment on the thermal performance of fly ash geopolymers. Thermochim. Acta 2013, 573, 130–137. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Van Deventer, J.S.J. Thermal evolution of metakaolin geopolymers: Part 1—Physical evolution. J. Non-Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Gluth, G.J.G.; Pistol, K. In-situ thermo-mechanical testing of fly ash geopolymer concretes made with quartz and expanded clay aggregates. Cem. Concr. Res. 2016, 80, 33–43. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Luo, Y.; Klima, K.M.; Brouwers, H.J.H.; Yu, Q. Effects of ladle slag on Class F fly ash geopolymer: Reaction mechanism and high temperature behavior. Cem. Concr. Compos. 2022, 129, 104468. [Google Scholar] [CrossRef]
- Cai, R.; Ye, H. Clinkerless ultra-high strength concrete based on alkali-activated slag at high temperatures. Cem. Concr. Res. 2021, 145, 106465. [Google Scholar] [CrossRef]
- Rivera, O.G.; Long, W.R.; Weiss, C.A., Jr.; Moser, R.D.; Williams, B.A.; Torres-Cancel, K.; Gore, E.R.; Allison, P.G. Effect of elevated temperature on alkali-activated geopolymeric binders compared to portland cement-based binders. Cem. Concr. Res. 2016, 90, 43–51. [Google Scholar] [CrossRef]
- Çelikten, S.; Sarıdemir, M.; Özgür Deneme, İ. Mechanical and microstructural properties of alkali-activated slag and slag + fly ash mortars exposed to high temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Qiu, G.H.; Kodur, V.; Yuan, Z.S. Spalling behavior of metakaolin-fly ash based geopolymer concrete under elevated temperature exposure. Cem. Concr. Compos. 2020, 106, 103483. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Comparative performance of alkali activated slag/metakaolin cement pastes exposed to high temperatures. Cem. Concr. Compos. 2017, 84, 157–166. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Lahoti, M.; Yang, E.-H. Influence of Mix Design Parameters on Geopolymer Mechanical Properties and Microstructure. In Ceramic Engineering and Science Proceedings; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 21–33. [Google Scholar]
- Tayeh, B.A.; Akeed, M.H.; Qaidi, S.; Bakar, B.H.A. Influence of microsilica and polypropylene fibers on the fresh and mechanical properties of ultra-high performance geopolymer concrete (UHP-GPC). Case Stud. Constr. Mater. 2022, 17, e01367. [Google Scholar] [CrossRef]
- Bai, W.; Lu, X.; Guan, J.; Yuan, C. Experimental study on uniaxial compression mechanical properties of recycled concrete with silica fume considering the effect of curing age. Constr. Build. Mater. 2022, 350, 128758. [Google Scholar] [CrossRef]
- Okoye, F.N.; Durgaprasad, J.; Singh, N.B. Effect of silica fume on the mechanical properties of fly ash based-geopolymer concrete. Ceram. Int. 2016, 42, 3000–3006. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Paisitsrisawat, P.; Rattanasak, U. Strength and resistance to sulfate and sulfuric acid of ground fluidized bed combustion fly ash–silica fume alkali-activated composite. Adv. Powder Technol. 2014, 25, 1087–1093. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.-H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Tu, W.; Zhang, M. Behaviour of alkali-activated concrete at elevated temperatures: A critical review. Cem. Concr. Compos. 2023, 138, 104961. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Temuujin, J.; Van Riessen, A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Liu, H.; Jing, W.; Qin, L.; Duan, P.; Zhang, Z.; Guo, R.; Li, W. Thermal stability of geopolymer modified by different silicon source materials prepared from solid wastes. Constr. Build. Mater. 2022, 315, 125709. [Google Scholar] [CrossRef]
- Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q. Thermal and fire resistance of Class F fly ash based geopolymers—A review. Constr. Build. Mater. 2022, 323, 126529. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, R.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A laboratory investigation of steel to fly ash-based geopolymer paste bonding behavior after exposure to elevated temperatures. Constr. Build. Mater. 2020, 254, 119267. [Google Scholar] [CrossRef]
- Khater, H.M.; Gharieb, M. Synergetic effect of nano-silica fume for enhancing physico-mechanical properties and thermal behavior of MK-geopolymer composites. Constr. Build. Mater. 2022, 350, 128879. [Google Scholar] [CrossRef]
- Li, B.; Gao, A.; Li, Y.; Xiao, H.; Chen, N.; Xia, D.; Wang, S.; Li, C. Effect of silica fume content on the mechanical strengths, compressive stress–strain behavior and microstructures of geopolymeric recycled aggregate concrete. Constr. Build. Mater. 2023, 384, 131417. [Google Scholar] [CrossRef]
- Cheng, Y.; Cong, P.; Zhao, Q.; Hao, H.; Mei, L.; Zhang, A.; Han, Z.; Hu, M. Study on the effectiveness of silica fume-derived activator as a substitute for water glass in fly ash-based geopolymer. J. Build. Eng. 2022, 51, 104228. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y.; Ge, W.; Zhang, A. Mechanical, microstructure and reaction process of calcium carbide slag-waste red brick powder based alkali-activated materials (CWAAMs). J. Clean. Prod. 2022, 331, 129845. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Optimization of brick waste-based geopolymer binders at ambient temperature and pre-targeted chemical parameters. J. Clean. Prod. 2020, 268, 122285. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Riessen, A.V.; Walls, P. Thermal Character of Geopolymers Synthesized from Class F Fly Ash Containing High Concentrations of Iron and α-Quartz. Int. J. Appl. Ceram. Tec. 2010, 7, 81–88. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Vickers, L.; Van Riessen, A. Performance of fibre reinforced, low density metakaolin geopolymers under simulated fire conditions. Appl. Clay Sci. 2013, 73, 71–77. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Rovnaník, P.; Bayer, P.; Rovnaníková, P. Characterization of alkali activated slag paste after exposure to high temperatures. Constr. Build. Mater. 2013, 47, 1479–1487. [Google Scholar] [CrossRef]
- Rashad, A.; Bai, Y.; Basheer, P.; Collier, N.; Milestone, N. Chemical and mechanical stability of sodium sulfate activated slag after exposure to elevated temperature. Cem. Concr. Res. 2012, 42, 333–343. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, P.; Zhang, Z. Application of silica-rich biomass ash solid waste in geopolymer preparation: A review. Constr. Build. Mater. 2022, 356, 129142. [Google Scholar] [CrossRef]
- Luo, Y.; Li, S.; Klima, K.; Brouwers, H.; Yu, Q. Degradation mechanism of hybrid fly ash/slag based geopolymers exposed to elevated temperatures. Cem. Concr. Res. 2022, 151, 106649. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal stability and microstructure of metakaolin-based geopolymer blended with rice husk ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- Li, P.P.; Brouwers, H.J.H.; Chen, W.; Yu, Q. Optimization and characterization of high-volume limestone powder in sustainable ultra-high performance concrete. Constr. Build. Mater. 2020, 242, 118112. [Google Scholar] [CrossRef]






| Oxide (%) | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | K2O | TiO2 | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|
| FA | 60.91 | 31.09 | 3.29 | 0.38 | 0.3 | 1.29 | 0.92 | 1.82 | 4.66 |
| MK | 52.09 | 46.03 | 0.10 | 0.12 | 0.26 | 0.11 | 0.95 | 0.34 | 1.10 |
| GGBS | 30.16 | 19.60 | 35.32 | 9.31 | 1.99 | 0.35 | 0.48 | 2.79 | 4.84 |
| SF | 97.51 | 0.16 | 0.38 | 0.88 | - | 0.29 | - | 0.78 | 3.59 |
| Sample Code | FA (wt.%) | MK (wt.%) | Slag (wt.%) | SF (wt.%) | K2O% a (wt.%) | w/b | Si/Al Ratio | Flowability (mm) |
|---|---|---|---|---|---|---|---|---|
| F9M1 | 90% | 10% | 0% | 0% | 9.2% | 0.18 | 3.44 | 235 |
| F7M3 | 70% | 30% | 0% | 0% | 11.6% | 0.27 | 3.14 | 225 |
| F5M5 | 50% | 50% | 0% | 0% | 14.0% | 0.34 | 2.88 | 215 |
| F9S1 | 90% | 0% | 10% | 0% | 7.7% | 0.205 | 3.56 | 225 |
| F7S3 | 70% | 0% | 30% | 0% | 7.1% | 0.23 | 3.46 | 227 |
| F5S5 | 50% | 0% | 50% | 0% | 6.5% | 0.245 | 3.33 | 232 |
| F7M3SF5 | 66.5% | 28.5% | 0% | 5% | 11.6% | 0.27 | 3.41 | - |
| F7M3SF10 | 63% | 27% | 0% | 10% | 11.6% | 0.27 | 3.71 | - |
| F7M3SF15 | 59.5% | 25.5% | 0% | 15% | 11.6% | 0.27 | 4.04 | - |
| F7M3SF20 | 56% | 24% | 0% | 20% | 11.6% | 0.27 | 4.42 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, B.; Li, Y.; Li, P. Synergistic Effect of Blended Precursors and Silica Fume on Strength and High Temperature Resistance of Geopolymer. Materials 2024, 17, 2975. https://doi.org/10.3390/ma17122975
Cao B, Li Y, Li P. Synergistic Effect of Blended Precursors and Silica Fume on Strength and High Temperature Resistance of Geopolymer. Materials. 2024; 17(12):2975. https://doi.org/10.3390/ma17122975
Chicago/Turabian StyleCao, Bosong, Yi Li, and Peipeng Li. 2024. "Synergistic Effect of Blended Precursors and Silica Fume on Strength and High Temperature Resistance of Geopolymer" Materials 17, no. 12: 2975. https://doi.org/10.3390/ma17122975





