ZrB2–Copper–Graphite Composite for Electric Brushes: Positive Effect of ZrB2 Addition on Composite Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials and Samples
2.2. Experimental Procedure
3. Results and Discussion
3.1. Physical Properties of the Composites
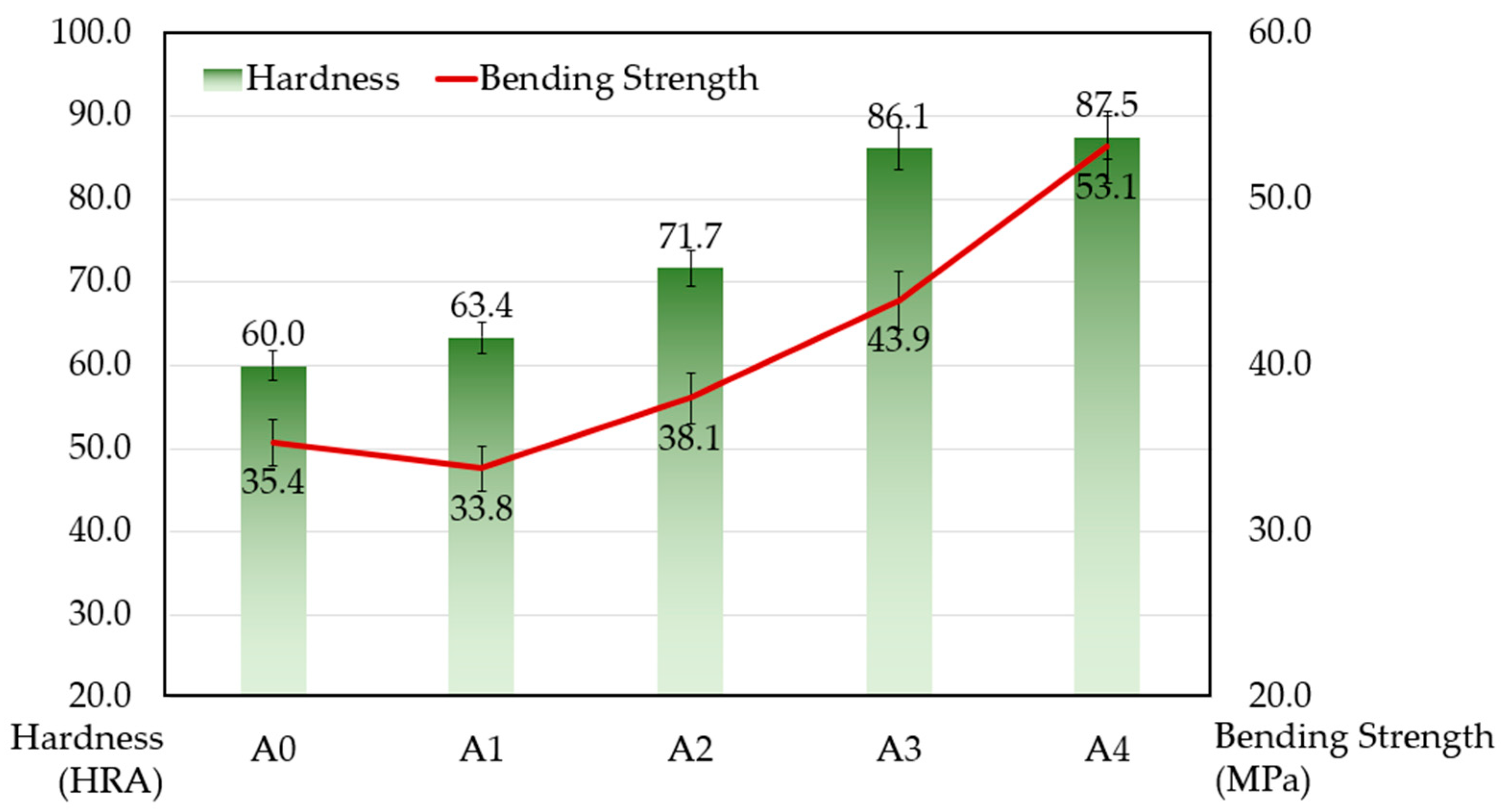
3.2. Mechanical Properties of the Composites
3.3. Wear Resistance of the Composites
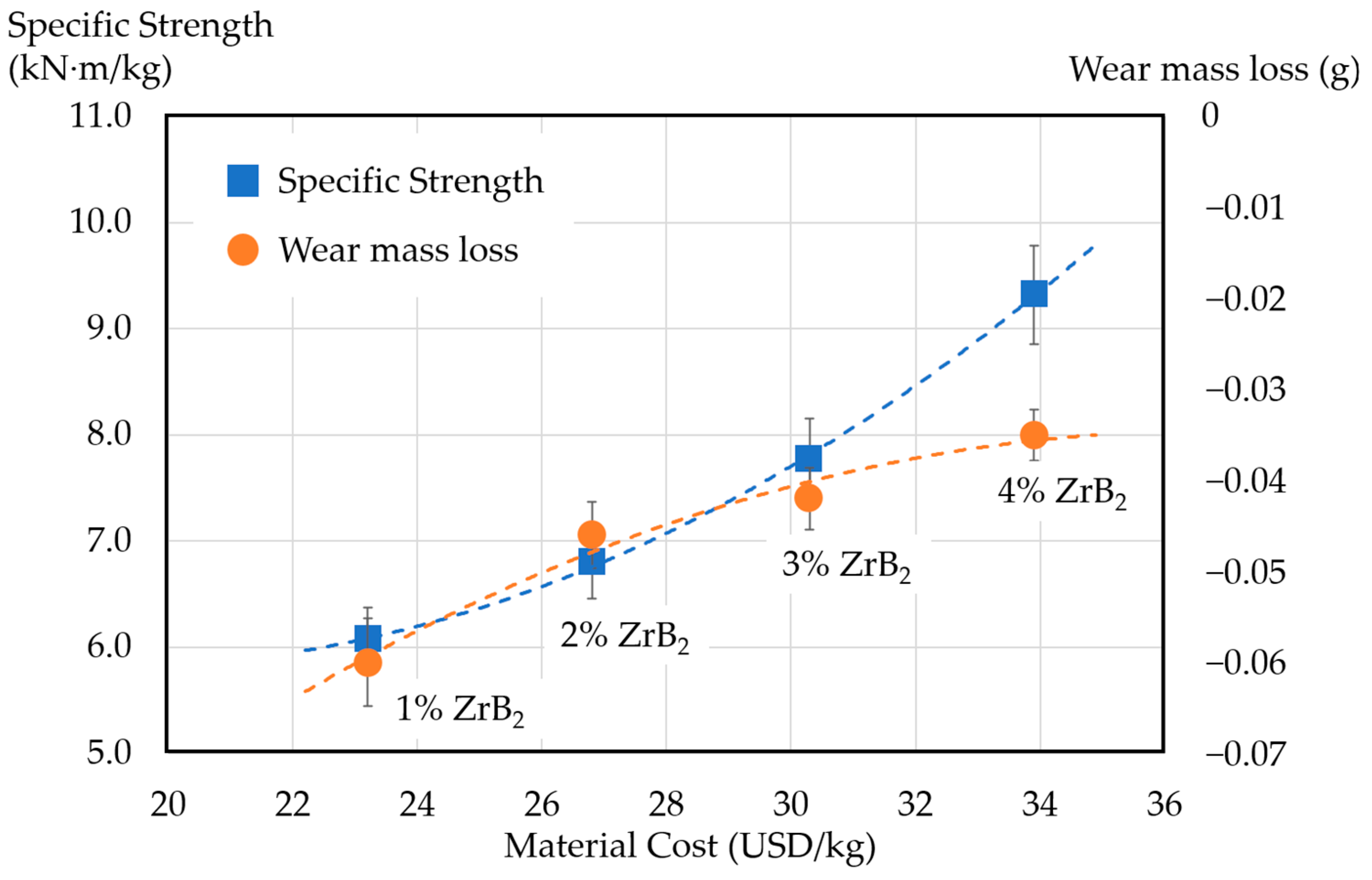
3.4. Performance–Cost Analysis
4. Conclusions
- (1)
- With an increasing content of ZrB2, the density, resistivity, hardness, bending strength, and wear resistance of the composites were enhanced. Compared with samples without ZrB2, samples with a 4% addition of ZrB2 achieved a hardness of 87.5 HRA, which is an improvement of 45.8%, and bending strength reached 53.1 MPa, which is an increase by nearly 50.0%;
- (2)
- Under non-conductive friction, the composite with 1% addition of ZrB2 displays the best wear resistance due to the formation of a lubrication film composed of ZrO2 and graphite. Wear mass loss during the friction test was reduced by 85.2% compared with samples without ZrB2;
- (3)
- Under conductive friction, all samples showed lower wear resistance. The average wear mass loss of the samples was 87.6% higher than that under non-conductive conditions. The composite with a 4% addition of ZrB2 displayed the best wear resistance;
- (4)
- The performance–cost analysis shows that improvements in specific strength and wear resistance are accompanied by increasing material costs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Im, Y.-d.; Matsumoto, R.; Utsunomiya, H. Strength and electrical conductivity of Cu-Al alloy sheets by cryogenic high-speed rolling. Mater. Sci. Eng. A 2021, 799, 139815. [Google Scholar] [CrossRef]
- Zhang, Z.; Ru, Y.; Zuo, T.; Xue, J.; Wu, Y.; Gao, Z.; Liu, Y.; Xiao, L. Achieving High Strength and High Conductivity of Cu-6 wt%Ag Sheets by Controlling the Aging Cooling Rate. Materials 2023, 16, 3632. [Google Scholar] [CrossRef] [PubMed]
- Paweł Strzępek, M.Z. Prospective cold metal working and analysis of deformation susceptibility of CuMg alloys with high magnesium content. Sci. Rep. 2024, 14, 6447. [Google Scholar] [CrossRef] [PubMed]
- Fiantok, T.; Šroba, V.; Koutná, N.; Izai, V.; Roch, T.; Truchlý, M.; Vidiš, M.; Satrapinskyy, L.; Nagy, Š.; Grančič, B.; et al. Structure evolution and mechanical properties of co-sputtered Zr-Al-B2 thin films. J. Vac. Sci. Technol. A 2022, 40, 033414. [Google Scholar] [CrossRef]
- Tengdelius, L.; Broitman, E.; Lu, J.; Eriksson, F.; Birch, J.; Nyberg, T.; Hultman, L.; Ho¨gberg, H. Hard and elastic epitaxial ZrB2 thin films on Al2O3(0001) substrates deposited by magnetron sputtering from a ZrB2 compound target. Acta Mater. 2016, 111, 166–172. [Google Scholar] [CrossRef]
- Prasad, L.; Kumar, N.; Yadav, A.; Kumar, A.; Kumar, V.; Winczek, J. In Situ Formation of ZrB2 and Its Influence on Wear and Mechanical Properties of ADC12 Alloy Mixed Matrix Composites. Materials 2021, 14, 2141. [Google Scholar] [CrossRef]
- Dinaharan, I.; Murugan, N.; Parameswaran, S. Influence of in situ formed ZrB2 particles on microstructure and mechanical properties of AA6061 metal matrix composites. Mater. Sci. Eng. 2011, 528, 5733–5740. [Google Scholar] [CrossRef]
- Fiantok, T.; Truchlý, M.; Šroba, V.; Roch, T.; Izai, V.; Vidiš, M.; Haršáni, M.; Satrapinskyy, L.; Mikula, M. First Approach to ZrB2 Thin Films Alloyed with Silver Prepared by Magnetron Co-Sputtering. Coatings 2023, 13, 663. [Google Scholar] [CrossRef]
- Ding, X.; Guo, Z.; Li, X.; Li, Z.; Li, X. Plasma Oscillatory Pressure Sintering of Mo-9Si-8B Alloy with ZrB2 Addition. Materials 2022, 15, 2387. [Google Scholar] [CrossRef]
- Guan, C.; Chen, G.; Kai, X.; Gao, X.; Huang, L.; Cao, R.; Qian, W.; Zhao, Y. Transformation of dislocation strengthening mechanisms induced by graphene nanoplates and ZrB2 nanoparticle in nanolaminated Al matrix composites. Mater. Charact. 2023, 199, 112773. [Google Scholar] [CrossRef]
- Berner, A.; Mundim, K.C.; Ellis, D.E.; Dorfman, S.; Fuks, D.; Evenhaim, R. Microstructure of Cu-C interface in Cu-based metal matrix composite. Sens. Actuators A Phys. 1999, 74, 86–90. [Google Scholar] [CrossRef]
- García-Márquez, J.M.; Antón, N.; Jimenez, A.; Madrid, M.; Martinez, M.A.; Bas, J.A. Viability study and mechanical characterisation of copper-graphite electrical contacts produced by adhesive joining. J. Mater. Process. Technol. 2003, 143–144, 290–293. [Google Scholar] [CrossRef]
- Bident, A.; Delange, F.; Labrugere, C.; Debiemme-Chouvy, C.; Lu, Y.; Silvain, J.-F. Fabrication and characterization of copper and copper alloys reinforced with graphene. J. Compos. Mater. 2024, 58, 109–117. [Google Scholar] [CrossRef]
- Kato, H.; Takama, M.; Iwai, Y. Wear and mechanical properties of sintered copper-tin composites containing graphite or molybdenum disulfide. Wear 2003, 255, 573–578. [Google Scholar] [CrossRef]
- Moustafa, S.F.; El-Badry, S.A.; Sanad, A.M. Friction and wear of copper-graphite composites made with Cu-coated and uncoated graphite powders. Wear 2002, 253, 699–710. [Google Scholar] [CrossRef]
- Hu, Z.L.; Chen, Z.H.; Xia, J.T.; Ding, G.Y. Properties of electric brushes made with Cu-coated graphite composites and with copper powders. Trans. Nonferrous Met. Soc. China 2007, 17, 1060–1064. [Google Scholar]
- Yakut, R.; Ortakaya, R. Investigation of the Effect of Additional Zirconium Diboride (ZrB2) in Spherical Graphite Cast Iron on Mechanical Properties. Coatings 2023, 13, 1385. [Google Scholar] [CrossRef]
- Sulima, I.; Boczkal, G. Processing and Properties of ZrB2-Copper Matrix Composites Produced by Ball Milling and Spark Plasma Sintering. Materials 2023, 16, 7455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhan, Z.; Lv, X.; Cao, H. Microstructure and Properties of ZrB2-SiC Reinforced Copper Matrix Composite Coatings Prepared by Laser Cladding. Materials 2022, 15, 6777. [Google Scholar] [CrossRef] [PubMed]
- JB/T 8133.13-2013; Test Method for Physical-Chemical Properties of Electrical Carbon Product. Part 13: Mechanical Strength of Carbon Plie. Harbin Institute of Carbon: Harbin, China, 2013.
- JB/T 8155-2017; Test Methods for the Measurement of the Operational Characteristics of Brushes for Electrical Machines. National Energy Administration: Beijing, China, 2017.
- Guo, Z.; Wang, C.; Ding, X.; Li, X.; Li, B. Microstructure and properties of Mo-Si-B alloy fabricated via novel technology: Plasma Oscillatory Pressure Sintering. J. Alloys Compd. 2021, 887, 161274. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, J.; Li, Q.Y.; Hou, C.K. Nano Zirconia reinforced Cu-Matrix Composites. Heat Treat. Met. 2006, 31, 40–42. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Zhang, Z.; Li, W. Fabrication, interfacial characteristics and strengthening mechanisms of ZrB2 microparticles reinforced Cu composites prepared by hot-pressed sintering. J. Alloys Compd. 2018, 748, 546–552. [Google Scholar] [CrossRef]
- Chen, S.Y.; Liu, Y.J.; Liu, C.S.; Sun, G.F. Nano-Al2O3 particles reinforced Cu-based self-lubricating composites. Acta Mater. Compos. Sin. 2009, 26, 109–115. [Google Scholar] [CrossRef]
- Fan, X.; Huang, X.; Liu, Q.; Ding, H.; Wang, H.; Hao, C. The microstructures and properties of in-situ ZrB2 reinforced Cu matrix composites. Results Phys. 2019, 14, 102494. [Google Scholar] [CrossRef]











| Sample Number | Copper | Graphite | ZrB2 |
|---|---|---|---|
| A0 | 88 | 12 | 0 |
| A1 | 88 | 11 | 1 |
| A2 | 88 | 10 | 2 |
| A3 | 88 | 9 | 3 |
| A4 | 88 | 8 | 4 |
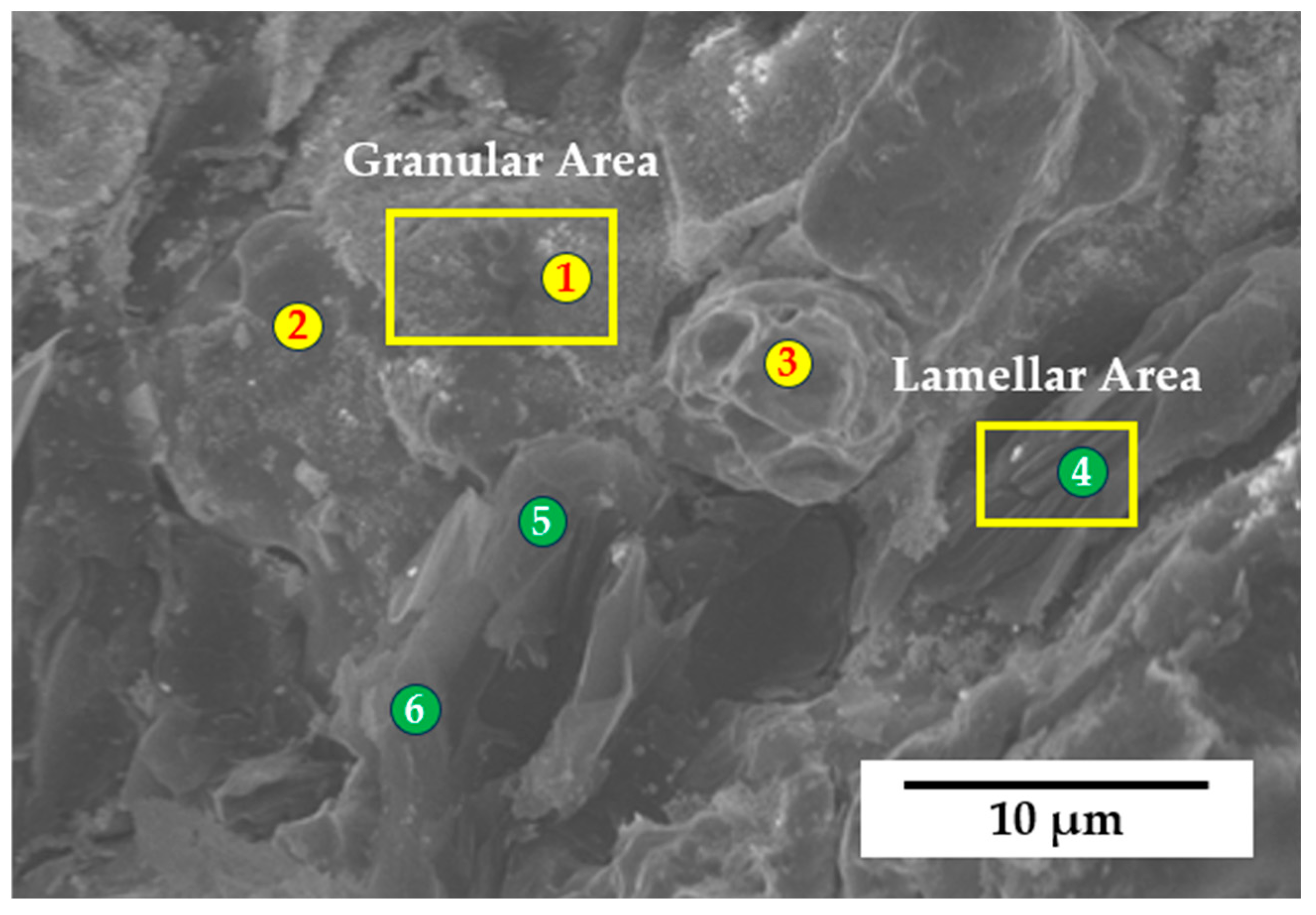
| Area | Position | Cu (wt.%) | Zr (wt.%) | O (wt.%) | C (wt.%) | B (wt.%) |
|---|---|---|---|---|---|---|
| Granular | 1 | 70.7 | 10.2 | 4.3 | 12.5 | 2.3 |
| 2 | 81.2 | 8.9 | 2.1 | 6.7 | 1.1 | |
| 3 | 84.5 | 7.6 | 4.4 | 1.9 | 1.6 | |
| Lamellar | 4 | 17.2 | 9.7 | 1.2 | 68.9 | 3.0 |
| 5 | 16.1 | 5.8 | 6.7 | 70.5 | 0.9 | |
| 6 | 17.8 | 7.2 | 6.3 | 67.4 | 1.3 |
| Sample Number | Copper | Graphite | ZrB2 | Cost USD/kg |
|---|---|---|---|---|
| A0 | 88% | 12% | 0 | 19.6 |
| A1 | 88% | 11% | 1% | 23.2 |
| A2 | 88% | 10% | 2% | 26.8 |
| A3 | 88% | 9% | 3% | 30.3 |
| A4 | 88% | 8% | 4% | 33.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Ding, F.; Wang, S.; Zhu, C. ZrB2–Copper–Graphite Composite for Electric Brushes: Positive Effect of ZrB2 Addition on Composite Properties. Materials 2024, 17, 2980. https://doi.org/10.3390/ma17122980
Feng Y, Ding F, Wang S, Zhu C. ZrB2–Copper–Graphite Composite for Electric Brushes: Positive Effect of ZrB2 Addition on Composite Properties. Materials. 2024; 17(12):2980. https://doi.org/10.3390/ma17122980
Chicago/Turabian StyleFeng, Yuqiang, Feng Ding, Shuxin Wang, and Chengnan Zhu. 2024. "ZrB2–Copper–Graphite Composite for Electric Brushes: Positive Effect of ZrB2 Addition on Composite Properties" Materials 17, no. 12: 2980. https://doi.org/10.3390/ma17122980






