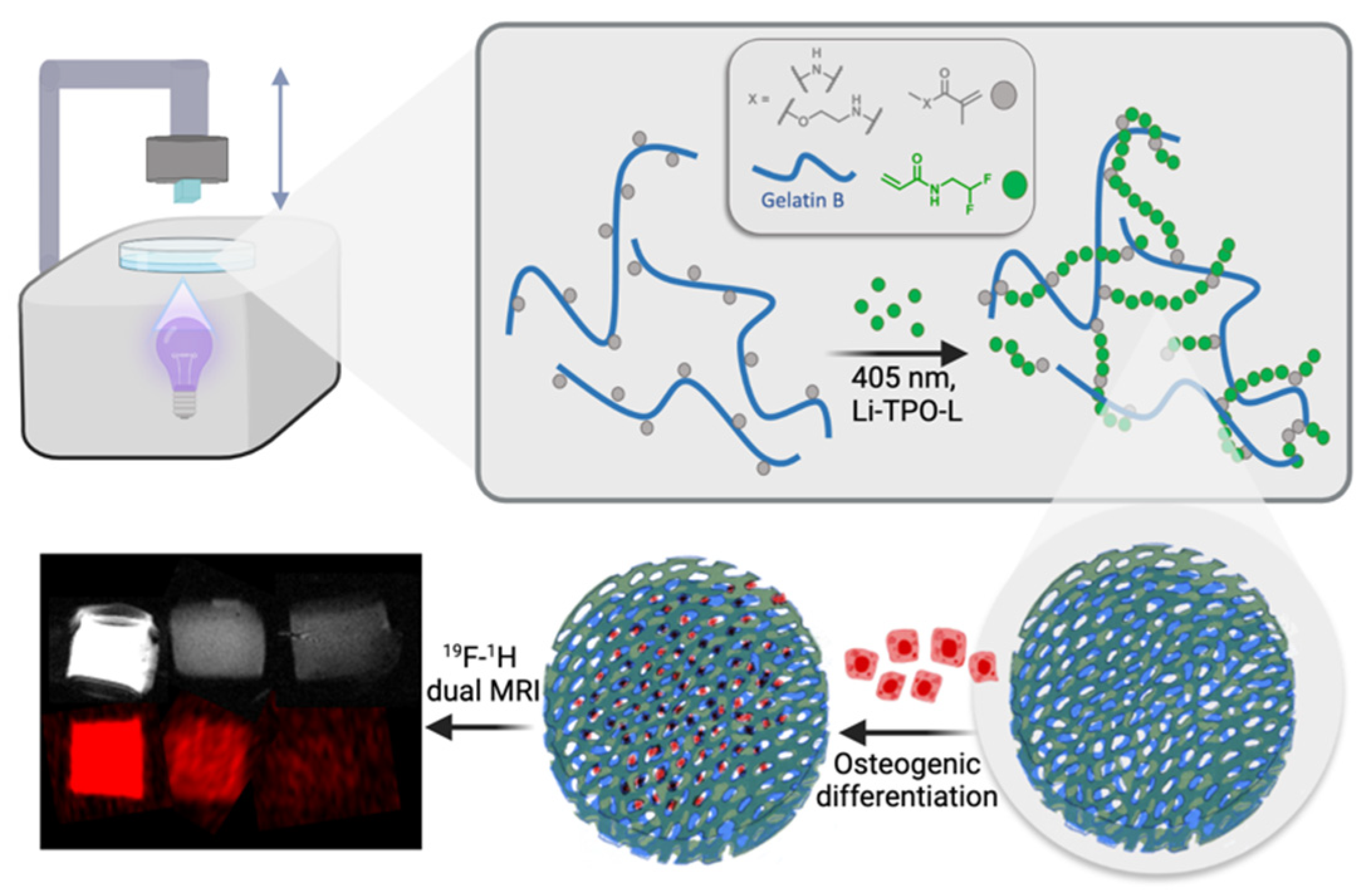
Digital Light Processing of 19F MRI-Traceable Gelatin-Based Biomaterial Inks towards Bone Tissue Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gelatin-Methacryloyl-Aminoethyl-Methacrylate (Gel-MA-AEMA)
2.3. Synthesis of Lithium Phenyl(2,4,6-Trimethylbenzoyl)phosphinate (Li-TPO-L)
2.4. Evaluation of Photo-Crosslinkability of the Gel-MA-AEMA-F-Based Hydrogel Precursor via In Situ Photo-Rheology
2.5. Mechanical Evaluation of the Gel-MA-AEMA-F Hydrogels via Oscillatory Rheology
2.6. Biomaterial Ink Development
2.6.1. Creation of a Computer-Aided Design (CAD)
2.6.2. In Situ Rheological Evaluation of the Crosslinking Kinetics of Different Biomaterial Ink Formulations
2.6.3. DLP Processing of the Gel-MA-AEMA-F Biomaterial Ink
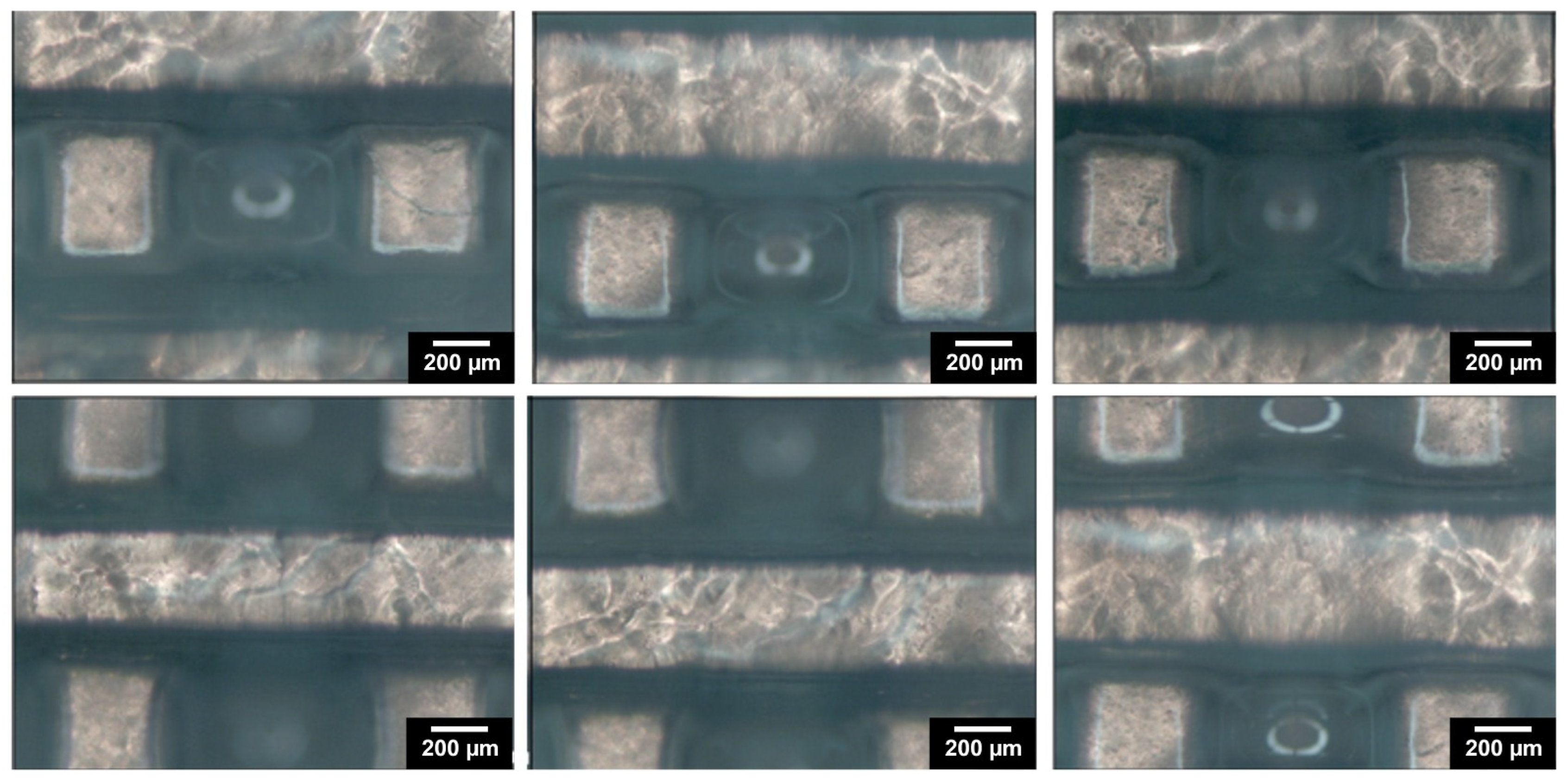
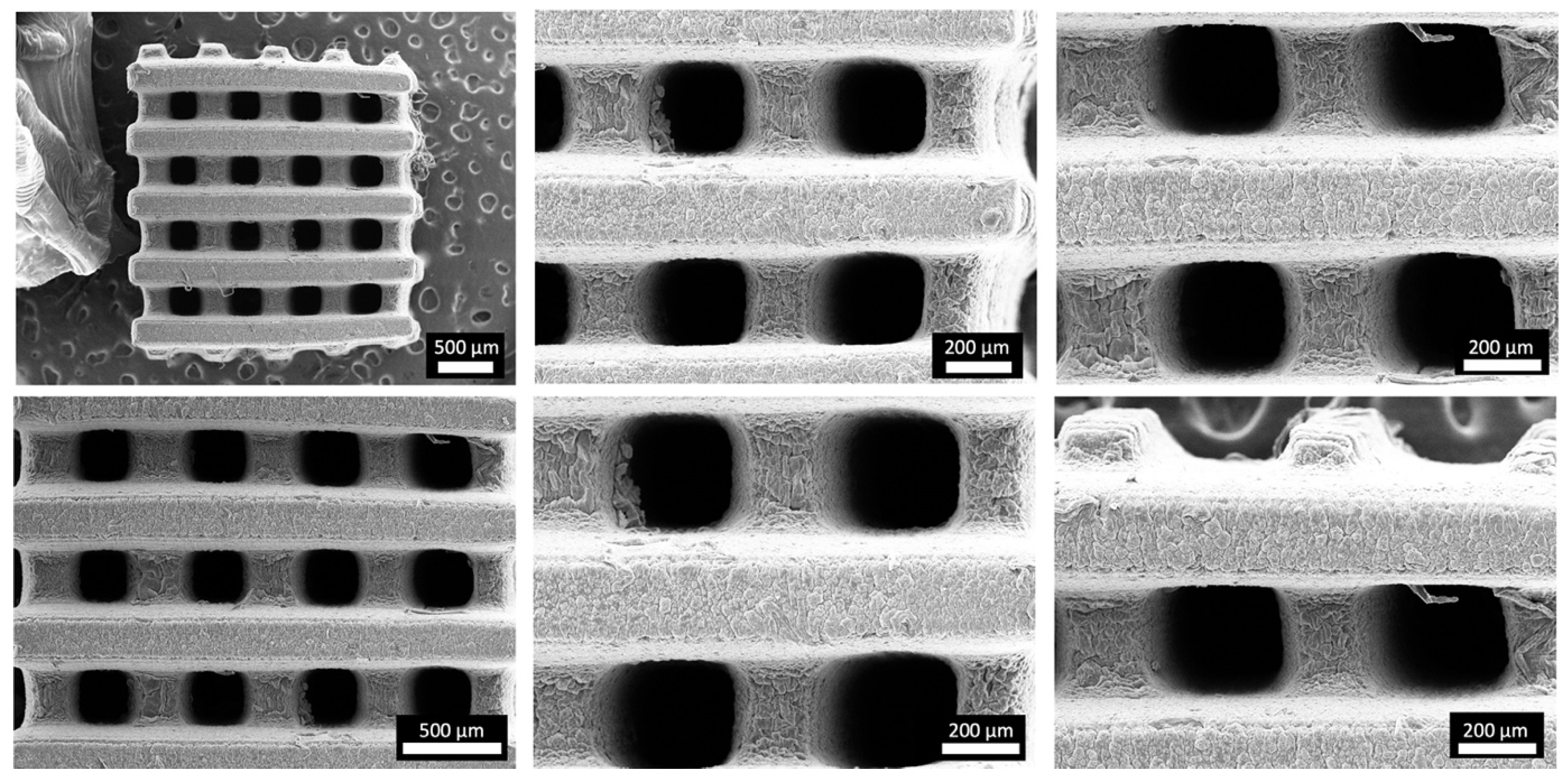
2.7. Morphological Evaluation of the 3D Hydrogel Constructs
2.8. High-Resolution Magic Angle Spinning (HR-MAS) 1H-NMR Spectroscopy
2.9. Determination of Swelling Ratio (SR) and Gel Fraction (GF)
2.10. Elemental Analysis of the DLP-Processed Gel-MA-AEMA-F Scaffolds
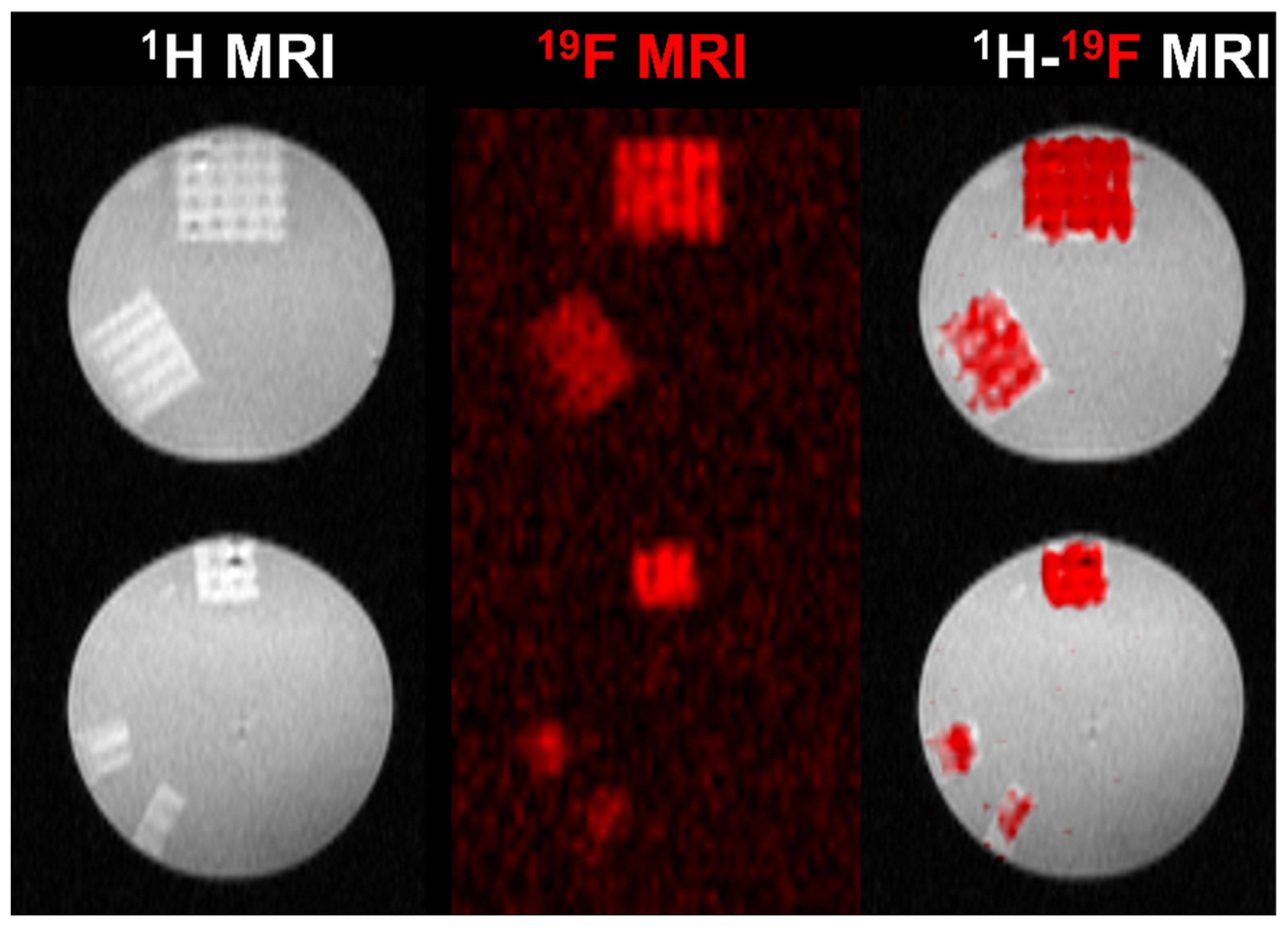
2.11. Acquisition of 19F Magnetic Resonance Spectroscopy (19F MRS) and Imaging (19F MRI)
2.12. Biological Evaluation of the Gel-MA-AEMA-F Hydrogel Scaffolds
2.12.1. Cell Culture Conditions
2.12.2. Evaluation of Biocompatibility of the Gel-MA-AEMA-F Hydrogel Constructs via Live/Dead Staining
2.12.3. Evaluation of ASC Osteogenic Differentiation Capacity via a Ca2+-Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Development of Hydrogel Precursors
3.2. Physico-Chemical Evaluation of the Gel-MA-AEMA-F Hydrogels
3.3. DLP Processability of the Biomaterial Ink towards the Fabrication of a Microporous 3D Architecture
3.4. Physico-Chemical Evaluation of the 3D Hydrogel Constructs
3.4.1. Evaluation of the Composition and Crosslinking Efficiency of the 3D-Processed Hydrogel Precursors
3.4.2. Swelling Ratio and Gel Fraction of 3D-Processed Hydrogel Precursors
3.4.3. 19F MRI Detectability of 3D Hydrogel Precursors
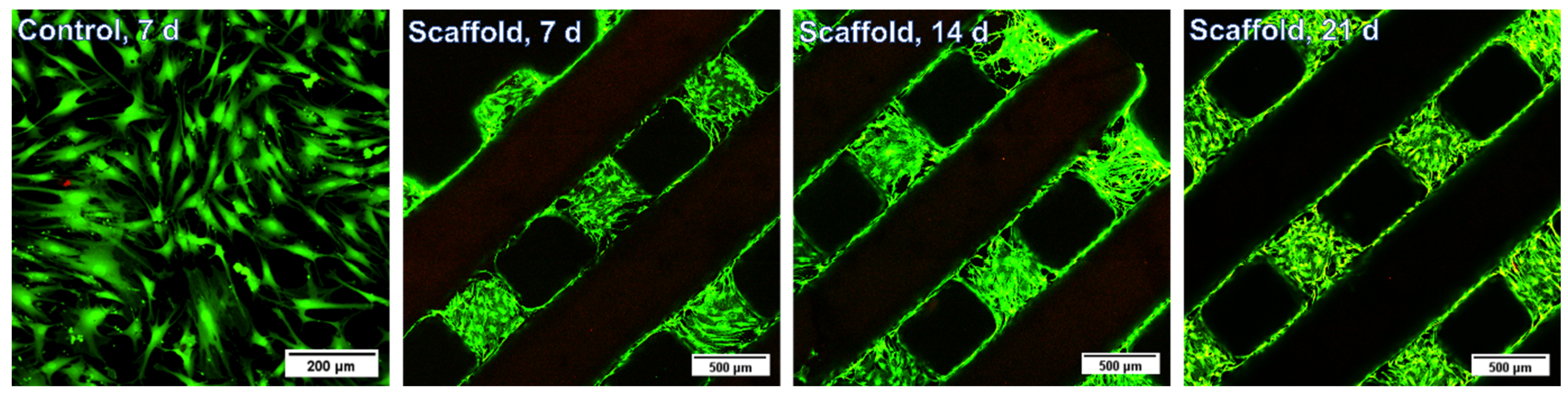
3.5. Biological Evaluation of the 3D Gel-MA-AEMA-F Hydrogel Constructs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen-vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials: Applications for Nanomedicine; Books on Demand: Norderstedt, Germany, 2011; pp. 17–52. [Google Scholar]
- Kolouchova, K.; Herynek, V.; Groborz, O.; Karela, J.; Van Damme, L.; Kucka, J.; Šafanda, A.; Bui, Q.H.; Sefc, L.; Van Vlierberghe, S. 19F MRI In Vivo Monitoring of Gelatin-Based Hydrogels: 3D Scaffolds with Tunable Biodegradation toward Regenerative Medicine. Chem. Mater. 2024, 36, 4417–4425. [Google Scholar] [CrossRef]
- Kolouchova, K.; Groborz, O.; Herynek, V.; Petrov, O.V.; Lang, J.; Dunlop, D.; Parmentier, L.; Szabó, A.; Schaubroeck, D.; Adriaensens, P.; et al. Cell-Interactive Gelatin-Based 19F MRI Tracers: An In Vitro Proof-of-Concept Study. Chem. Mater. 2023, 36, 183–196. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Tytgat, L.; Dobos, A.; Ottevaere, H.; Van Erps, J.; Thienpont, H.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. (Photo-)Crosslinkable Gelatin Derivatives for Biofabrication Applications. Acta Biomater. 2019, 97, 46–73. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Liska, R.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. Cross-Linkable Gelatins with Superior Mechanical Properties Through Carboxylic Acid Modification: Increasing the Two-Photon Polymerization Potential. Biomacromolecules 2017, 18, 3260–3272. [Google Scholar] [CrossRef]
- Dobos, A.; Van Hoorick, J.; Steiger, W.; Gruber, P.; Markovic, M.; Andriotis, O.G.; Rohatschek, A.; Dubruel, P.; Thurner, P.J.; Van Vlierberghe, S.; et al. Thiol–Gelatin–Norbornene Bioink for Laser-Based High-Definition Bioprinting. Adv. Healthc. Mater. 2020, 9, 1900752. [Google Scholar] [CrossRef]
- Szabó, A.; Pasquariello, R.; Costa, P.F.; Pavlovic, R.; Geurs, I.; Dewettinck, K.; Vervaet, C.; Brevini, T.A.L.; Gandolfi, F.; Van Vlierberghe, S. Light-Based 3D Printing of Gelatin-Based Biomaterial Inks to Create a Physiologically Relevant In Vitro Fish Intestinal Model. Macromol. Biosci. 2023, 23, 2300016. [Google Scholar] [CrossRef]
- van Hoorick, J.; Dobos, A.; Markovic, M.; Gheysens, T.; van Damme, L.; Gruber, P.; Tytgat, L.; van Erps, J.; Thienpont, H.; Dubruel, P.; et al. Thiol-Norbornene Gelatin Hydrogels: Influence of Thiolated Crosslinker on Network Properties and High Definition 3D Printing. Biofabrication 2020, 13, 015017. [Google Scholar] [CrossRef]
- Fratzl, P.; Barth, F.G. Biomaterial Systems for Mechanosensing and Actuation. Nature 2009, 462, 442–448. [Google Scholar] [CrossRef]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix Elasticity, Cytoskeletal Forces and Physics of the Nucleus: How Deeply Do Cells “feel” Outside and In? J. Cell Sci. 2010, 123, 297–308. [Google Scholar] [CrossRef]
- Appel, A.A.; Anastasio, M.A.; Larson, J.C.; Brey, E.M. Imaging Challenges in Biomaterials and Tissue Engineering. Biomaterials 2013, 34, 6615–6630. [Google Scholar] [CrossRef]
- Nam, S.Y.; Ricles, L.M.; Suggs, L.J.; Emelianov, S.Y. Imaging Strategies for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2015, 21, 88–102. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Hyun, H.; Ashitate, Y.; Park, G.; Robichaud, K.; Lunsford, E.; Lee, S.J.; Khang, G.; Choi, H.S. Near-Infrared Fluorescence Imaging for Noninvasive Trafficking of Scaffold Degradation. Sci. Rep. 2013, 3, 1198. [Google Scholar] [CrossRef]
- Gudur, M.S.R.; Rao, R.R.; Peterson, A.W.; Caldwell, D.J.; Stegemann, J.P.; Deng, C.X. Noninvasive Quantification of In Vitro Osteoblastic Differentiation in 3D Engineered Tissue Constructs Using Spectral Ultrasound Imaging. PLoS ONE 2014, 9, e85749. [Google Scholar] [CrossRef]
- Hetterich, H.; Willner, M.; Fill, S.; Herzen, J.; Bamberg, F.; Hipp, A.; Schüller, U.; Adam-Neumair, S.; Wirth, S.; Reiser, M.; et al. Phase-Contrast CT: Qualitative and Quantitative Evaluation of Atherosclerotic Carotid Artery Plaque. Radiology 2014, 271, 870–878. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, Y.-H.; Kao, C.-Y.; Lu, C.-H.; Sung, L.-Y.; Yen, T.-C.; Lin, K.-J.; Hu, Y.-C. Augmented Healing of Critical-Size Calvarial Defects by Baculovirus-Engineered MSCs That Persistently Express Growth Factors. Biomaterials 2012, 33, 3682–3692. [Google Scholar] [CrossRef]
- Tondera, C.; Hauser, S.; Krüger-Genge, A.; Jung, F.; Neffe, A.T.; Lendlein, A.; Klopfleisch, R.; Steinbach, J.; Neuber, C.; Pietzsch, J. Gelatin-Based Hydrogel Degradation and Tissue Interaction In Vivo: Insights from Multimodal Preclinical Imaging in Immunocompetent Nude Mice. Theranostics 2016, 6, 2114–2128. [Google Scholar] [CrossRef]
- Patterson, J.; Siew, R.; Herring, S.W.; Lin, A.S.P.; Guldberg, R.; Stayton, P.S. Hyaluronic Acid Hydrogels with Controlled Degradation Properties for Oriented Bone Regeneration. Biomaterials 2010, 31, 6772–6781. [Google Scholar] [CrossRef]
- Colomb, J.; Louie, K.; Massia, S.P.; Bennett, K.M. Self-Degrading, MRI-Detectable Hydrogel Sensors with Picomolar Target Sensitivity. Magn. Reson. Med. 2010, 64, 1792–1799. [Google Scholar] [CrossRef]
- Berdichevski, A.; Shachaf, Y.; Wechsler, R.; Seliktar, D. Protein Composition Alters in Vivo Resorption of PEG-Based Hydrogels as Monitored by Contrast-Enhanced MRI. Biomaterials 2015, 42, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Luan, J.; Wen, Z.; Wang, L.; Liu, Z.; Wu, G.; Zhuo, R. Visualization of in Situ Hydrogels by MRI In Vivo. J. Mater. Chem. B 2016, 4, 1343–1353. [Google Scholar] [CrossRef]
- Sharma, U.; Chang, E.W.; Yun, S.H. Long-Wavelength Optical Coherence Tomography at 1.7 Μm for Enhanced Imaging Depth. Opt. Express 2008, 16, 19712–19723. [Google Scholar] [CrossRef]
- Crosignani, V.; Dvornikov, A.S.; Aguilar, J.S.; Stringari, C.; Edwards, R.A.; Mantulin, W.W.; Gratton, E. Deep Tissue Fluorescence Imaging and In Vivo Biological Applications. J. Biomed. Opt. 2012, 17, 116023. [Google Scholar] [CrossRef]
- Jirak, D.; Galisova, A.; Kolouchova, K.; Babuka, D.; Hruby, M. Fluorine Polymer Probes for Magnetic Resonance Imaging: Quo Vadis? Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 173–185. [Google Scholar] [CrossRef]
- Staal, X.; Koshkina, O.; Srinivas, M. 11—In Vivo 19-Fluorine Magnetic Resonance Imaging. In Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Academic Press: Cambridge, MA, USA, 2019; pp. 397–424. [Google Scholar] [CrossRef]
- Srinivas, M.; Heerschap, A.; Ahrens, E.T.; Figdor, C.G.; Vries, I.J.M.D. 19F MRI for Quantitative in Vivo Cell Tracking. Trends Biotechnol. 2010, 28, 363–370. [Google Scholar] [CrossRef]
- Hockett, F.D.; Wallace, K.D.; Schmieder, A.H.; Caruthers, S.D.; Pham, C.T.N.; Wickline, S.A.; Lanza, G.M. Simultaneous Dual Frequency 1H and 19F Open Coil Imaging of Arthritic Rabbit Knee at 3T. IEEE Trans. Med. Imaging 2011, 30, 22–27. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Song, H.; Zhang, J.; Dong, A.; Kong, D.; Wang, W.; Huang, P. 19F Magnetic Resonance Imaging Enabled Real-Time, Non-Invasive and Precise Localization and Quantification of the Degradation Rate of Hydrogel Scaffolds In Vivo. Biomater. Sci. 2020, 8, 3301–3309. [Google Scholar] [CrossRef]
- Kolouchova, K.; Groborz, O.; Slouf, M.; Herynek, V.; Parmentier, L.; Babuka, D.; Cernochova, Z.; Koucky, F.; Sedlacek, O.; Hruby, M.; et al. Thermoresponsive Triblock Copolymers as Widely Applicable 19F Magnetic Resonance Imaging Tracers. Chem. Mater. 2022, 34, 10902–10916. [Google Scholar] [CrossRef]
- Groborz, O.; Kolouchová, K.; Pankrác, J.; Keša, P.; Kadlec, J.; Krunclová, T.; Pierzynová, A.; Šrámek, J.; Hovořáková, M.; Dalecká, L.; et al. Pharmacokinetics of Intramuscularly Administered Thermoresponsive Polymers. Adv. Healthc. Mater. 2022, 11, 2201344. [Google Scholar] [CrossRef]
- Kolouchova, K.; Cernochova, Z.; Groborz, O.; Herynek, V.; Koucky, F.; Jaksa, R.; Benes, J.; Slouf, M.; Hruby, M. Multiresponsive Fluorinated Polymers as a Theranostic Platform Using 19F MRI. Eur. Polym. J. 2022, 175, 111381. [Google Scholar] [CrossRef]
- Markovic, M.; van Hoorick, J.; Hölzl, K.; Tromayer, M.; Gruber, P.; Nürnberger, S.; Dubruel, P.; van Vlierberghe, S.; Liska, R.; Ovsianikov, A. Hybrid Tissue Engineering Scaffolds by Combination of Three-Dimensional Printing and Cell Photoencapsulation. J. Nanotechnol. Eng. Med. 2015, 6, 021001. [Google Scholar] [CrossRef]
- Creff, J.; Courson, R.; Mangeat, T.; Foncy, J.; Souleille, S.; Thibault, C.; Besson, A.; Malaquin, L. Fabrication of 3D Scaffolds Reproducing Intestinal Epithelium Topography by High-Resolution 3D Stereolithography. Biomaterials 2019, 221, 119404. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer Structure-Property Requirements for Stereolithographic 3D Printing of Soft Tissue Engineering Scaffolds. In Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 170–188. [Google Scholar] [CrossRef]
- Gong, J.; Qian, Y.; Lu, K.; Zhu, Z.; Siow, L.; Zhang, C.; Zhou, S.; Gu, T.; Yin, J.; Yu, M.; et al. Digital Light Processing (DLP) in Tissue Engineering: From Promise to Reality, and Perspectives. Biomed. Mater. 2022, 17, 062004. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, Z.; Zheng, H.; Li, W.; Wang, M.; Cheng, F. Digital Light Processing (DLP)-based (Bio)Printing Strategies for Tissue Modeling and Regeneration. Aggregate 2022, 4, e270. [Google Scholar] [CrossRef]
- Ge, Q.; Jian, B.; Li, H. Shaping Soft Materials via Digital Light Processing-Based 3D Printing: A Review. Forces Mech. 2022, 6, 100074. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Verdile, N.; Szabó, A.; Pasquariello, R.; Brevini, T.A.L.; van Vlierberghe, S.; Gandolfi, F. Preparation of Biological Scaffolds and Primary Intestinal Epithelial Cells to Efficiently 3D Model the Fish Intestinal Mucosa; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of Carriers Controlling Phenotypic Expression in BMP-Induced Osteogenesis and Chondrogenesis. JBJS 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore Size of Porous Hydroxyapatite as the Cell-Substratum Controls BMP-Induced Osteogenesis1. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef]
- Li, Y.; Mao, Q.; Yin, J.; Wang, Y.; Fu, J.; Huang, Y. Theoretical Prediction and Experimental Validation of the Digital Light Processing (DLP) Working Curve for Photocurable Materials. Addit. Manuf. 2021, 37, 101716. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lin, S.; Gu, L. Influence of Crosslink Density and Stiffness on Mechanical Properties of Type I Collagen Gel. Materials 2015, 8, 551–560. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Szabó, A.; De Vlieghere, E.; Costa, P.F.; Geurs, I.; Dewettinck, K.; Van Vlierberghe, S. Increasing Hydrogel Complexity from 2D towards 3D towards Intestinal Tissue Engineering. Giant 2023, 16, 100198. [Google Scholar] [CrossRef]
- Štaffová, M.; Ondreáš, F.; Svatík, J.; Zbončák, M.; Jančář, J.; Lepcio, P. 3D Printing and Post-Curing Optimization of Photopolymerized Structures: Basic Concepts and Effective Tools for Improved Thermomechanical Properties. Polym. Test. 2022, 108, 107499. [Google Scholar] [CrossRef]
- Kolouchova, K.; Sedlacek, O.; Jirak, D.; Babuka, D.; Blahut, J.; Kotek, J.; Vit, M.; Trousil, J.; Konefał, R.; Janouskova, O.; et al. Self-Assembled Thermoresponsive Polymeric Nanogels for 19F MR Imaging. Biomacromolecules 2018, 19, 3515–3524. [Google Scholar] [CrossRef]
- Serafim, A.; Tucureanu, C.; Petre, D.-G.; Dragusin, D.-M.; Salageanu, A.; Van Vlierberghe, S.; Dubruel, P.; Stancu, I.-C. One-Pot Synthesis of Superabsorbent Hybrid Hydrogels Based on Methacrylamide Gelatin and Polyacrylamide. Effortless Control of Hydrogel Properties through Composition Design. New J. Chem. 2014, 38, 3112–3126. [Google Scholar] [CrossRef]
- Dragusin, D.-M.; Van Vlierberghe, S.; Dubruel, P.; Dierick, M.; Van Hoorebeke, L.; Declercq, H.A.; Cornelissen, M.M.; Stancu, I.-C. Novel Gelatin–PHEMA Porous Scaffolds for Tissue Engineering Applications. Soft Matter 2012, 8, 9589–9602. [Google Scholar] [CrossRef]
- Ma, C.; Li, W.; Li, D.; Chen, M.; Wang, M.; Jiang, L.; Mille, L.S.; Garciamendez, C.E.; Zhao, Z.; Zhou, Q.; et al. Photoacoustic imaging of 3D-printed vascular networks. Biofabrication 2022, 14, 025001. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Hao, J.; Gonzales, A.; Zhao, Z.; Flores, R.S.; Kuang, X.; Mu, X.; Ching, T.; Tang, G.; et al. Molecularly cleavable bioinks facilitate high-performance digital light processing-based bioprinting of functional volumetric soft tissues. Nat. Commun. 2022, 13, 3317. [Google Scholar] [CrossRef]
- Leulescu, M.; Rotaru, A.; Pălărie, I.; Moanţă, A.; Cioateră, N.; Popescu, M.; Morîntale, E.; Bubulică, M.V.; Florian, G.; Hărăbor, A.; et al. Tartrazine: Physical, thermal and biophysical properties of the most widely employed synthetic yellow food-colouring azo dye. J. Therm. Anal. Calorim. 2018, 134, 209–231. [Google Scholar] [CrossRef]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef]

| Material | cgel (w/v%) | 19F ± SD a* (wt. %) | nDFEA/nMA+AEMA Hydrogels * | SR ± SD * | GF ± SD * (%) | G′ (kPa) # | E′ (kPa) |
|---|---|---|---|---|---|---|---|
| Gel-MA-AEMA-F b | 15.0 | 13.1 | 15 | 2.5 ± 0.02 | 48.5 ± 1.1 | 12.3 ± 1.4 | 36.9 ± 4.1 |
| Gel-MA-AEMA c | 15.0 | 0.0 | 0 | 11.9 ± 0.1 # | 97.1 ± 0.1 # | 5.1 ± 2.1 | 15.4 ± 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, A.; Kolouchova, K.; Parmentier, L.; Herynek, V.; Groborz, O.; Van Vlierberghe, S. Digital Light Processing of 19F MRI-Traceable Gelatin-Based Biomaterial Inks towards Bone Tissue Regeneration. Materials 2024, 17, 2996. https://doi.org/10.3390/ma17122996
Szabó A, Kolouchova K, Parmentier L, Herynek V, Groborz O, Van Vlierberghe S. Digital Light Processing of 19F MRI-Traceable Gelatin-Based Biomaterial Inks towards Bone Tissue Regeneration. Materials. 2024; 17(12):2996. https://doi.org/10.3390/ma17122996
Chicago/Turabian StyleSzabó, Anna, Kristyna Kolouchova, Laurens Parmentier, Vit Herynek, Ondrej Groborz, and Sandra Van Vlierberghe. 2024. "Digital Light Processing of 19F MRI-Traceable Gelatin-Based Biomaterial Inks towards Bone Tissue Regeneration" Materials 17, no. 12: 2996. https://doi.org/10.3390/ma17122996
APA StyleSzabó, A., Kolouchova, K., Parmentier, L., Herynek, V., Groborz, O., & Van Vlierberghe, S. (2024). Digital Light Processing of 19F MRI-Traceable Gelatin-Based Biomaterial Inks towards Bone Tissue Regeneration. Materials, 17(12), 2996. https://doi.org/10.3390/ma17122996








