Functional Technical Textile-Based Polymer Nanocomposites with Adsorbent Properties of Toxins and Dyes also Have Antibacterial Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Chemical Modification of Cloisite 20A by Ultrasonic Tip
2.3. Nanocomposite Preparation
2.4. Preparation of Non-Woven Fabric Materials
2.5. Characterization
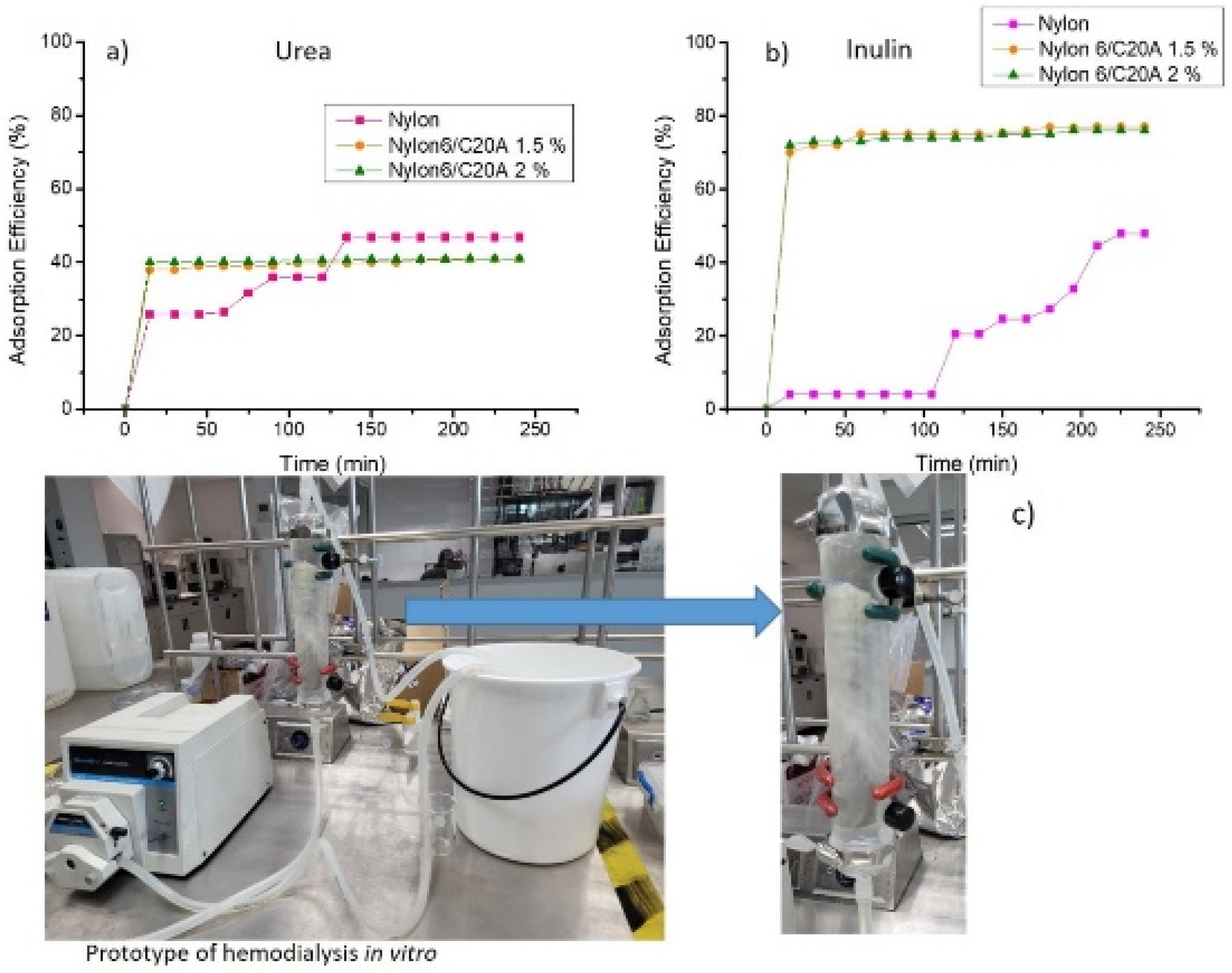
2.5.1. Adsorption of Uremic Toxins
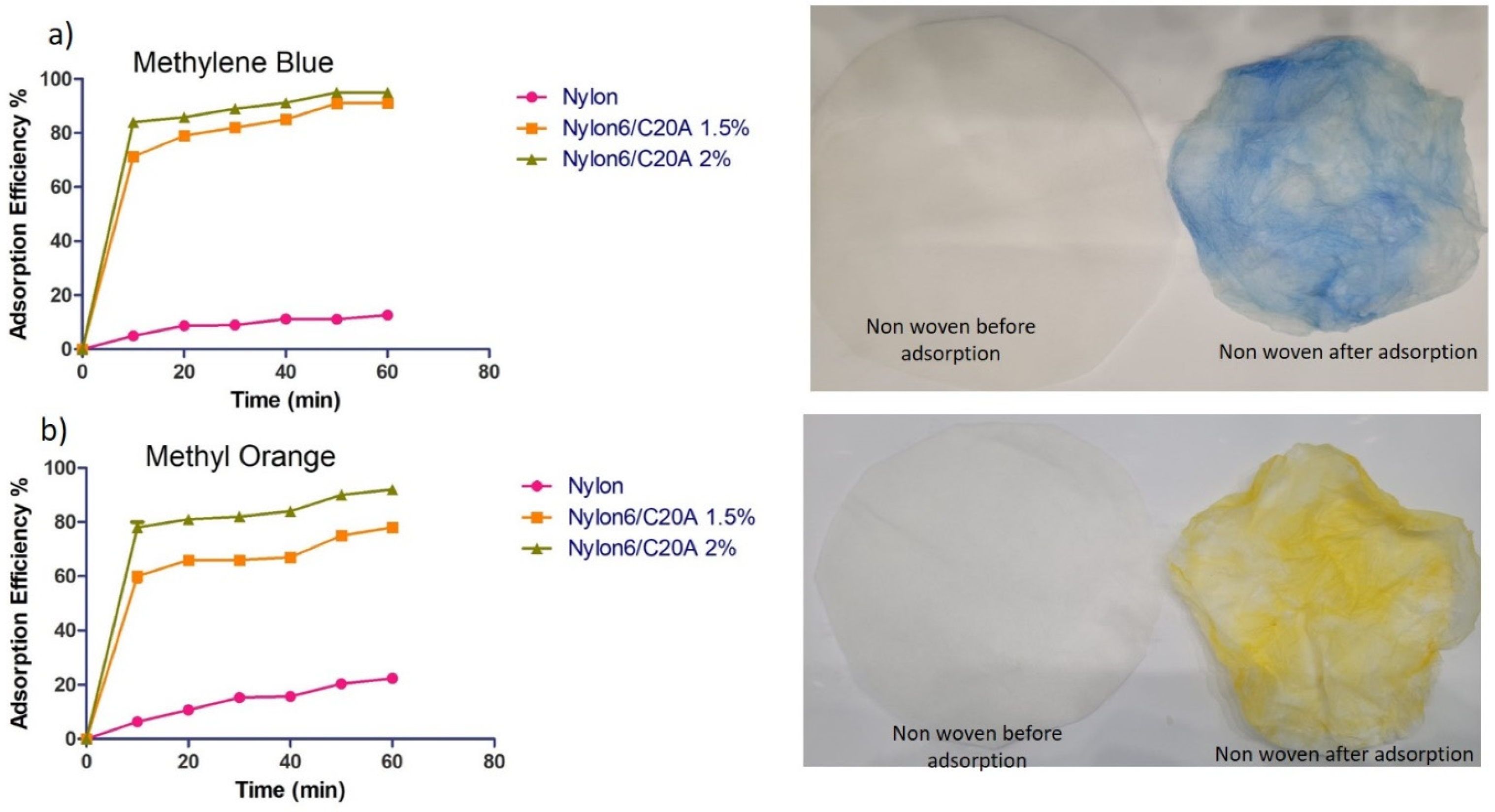
2.5.2. Adsorption of Dyes (Methylene Blue and Methyl Orange)
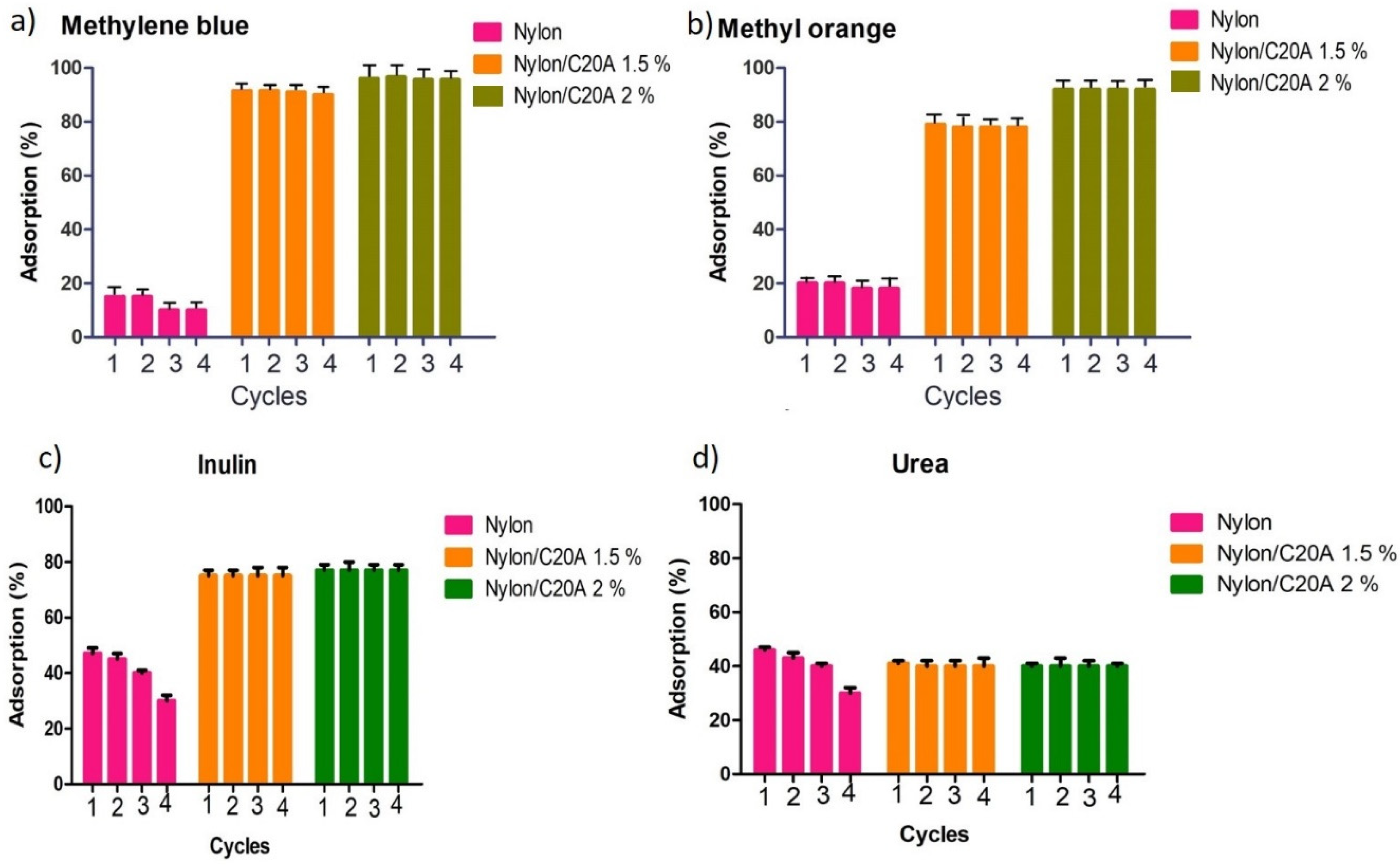
2.5.3. Reusability Study
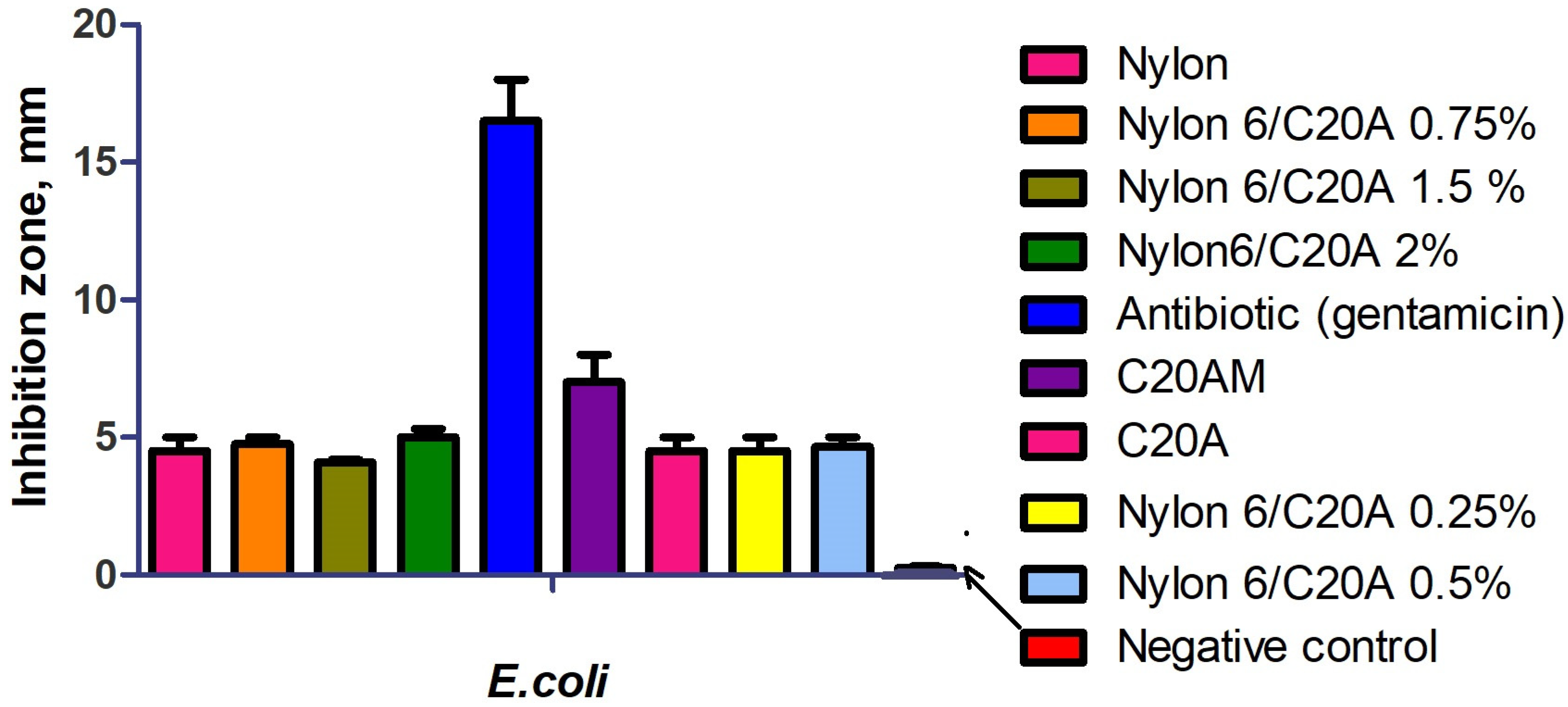
2.5.4. Antibacterial Activity
3. Results and Discussion
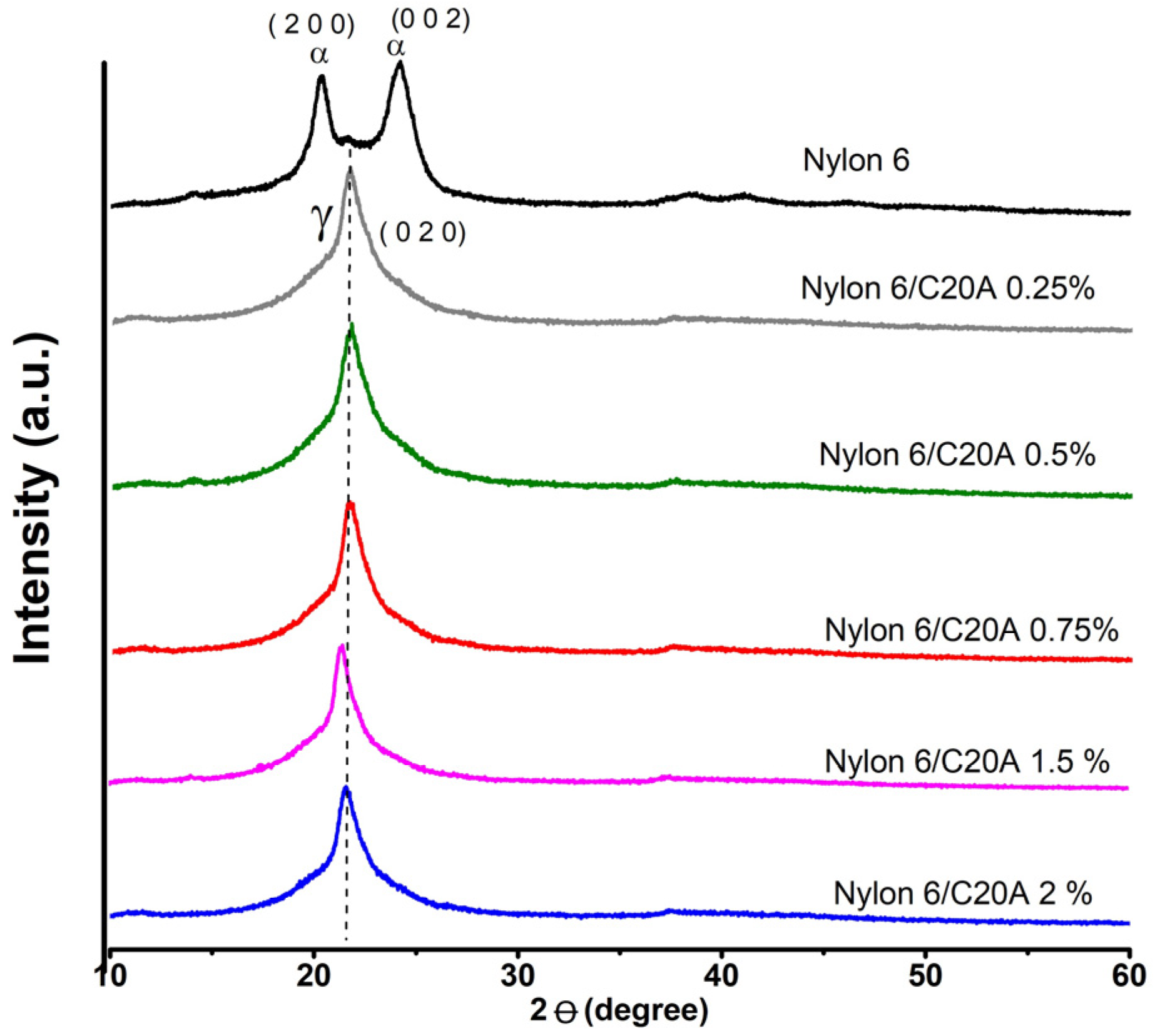
3.1. X-ray Diffraction (XRD)
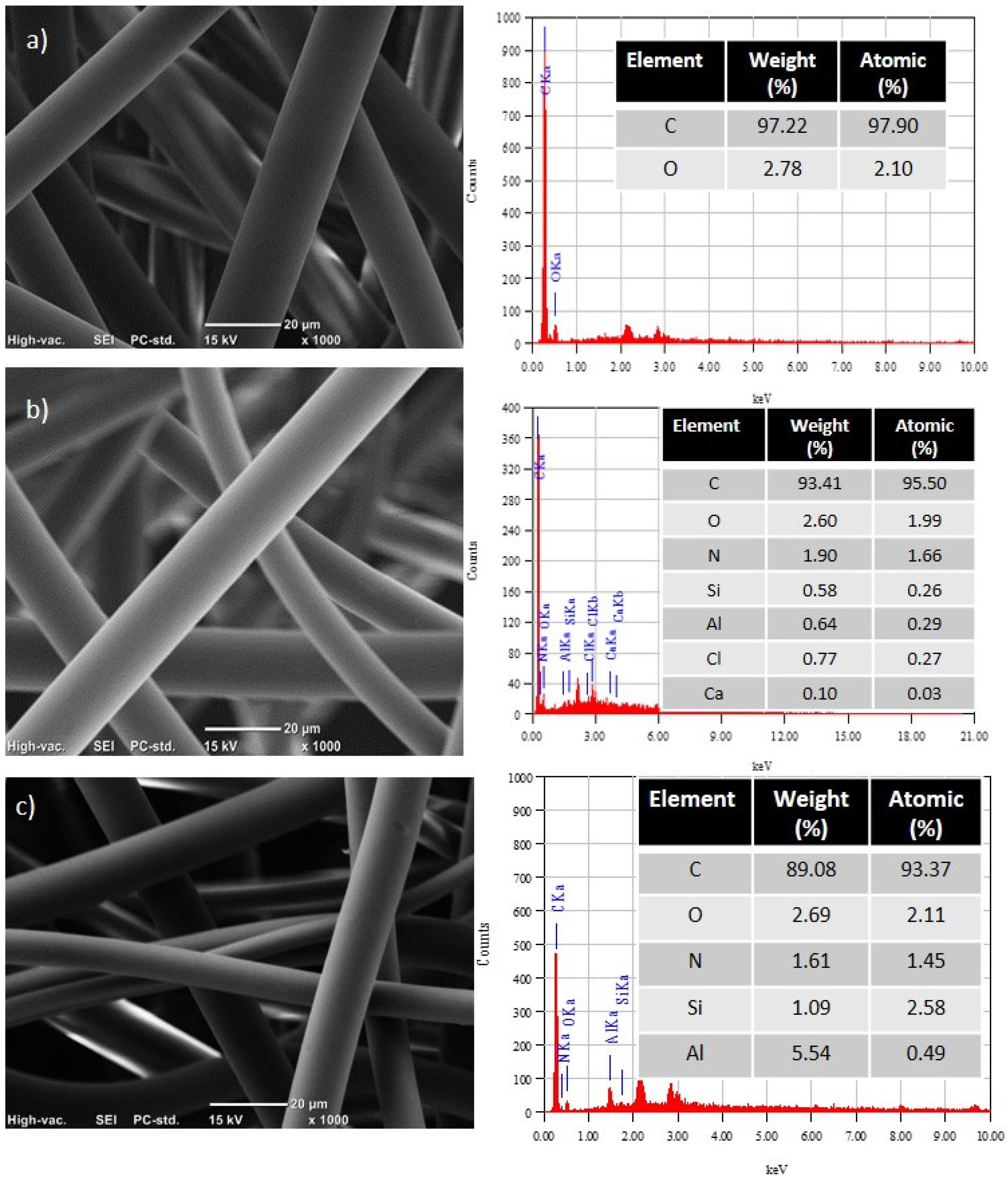
3.2. Scanning Electron Microscopy (SEM)
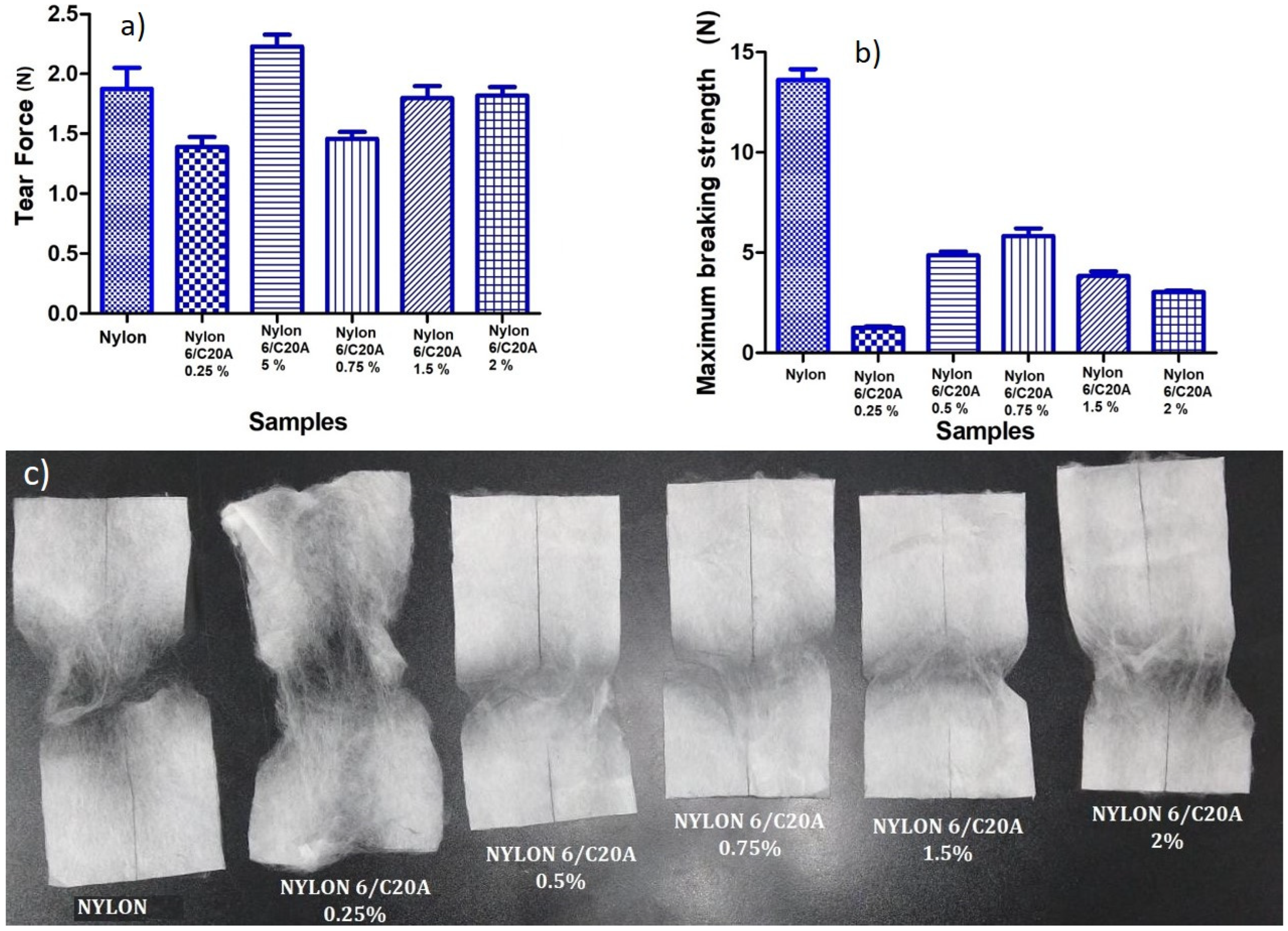
3.3. Mechanical Tests
3.4. Toxin Adsorption of Non-Woven Nylon 6/C20A
3.5. Dye Adsorption
3.6. Reusability of Non-Woven Fabric Nylon 6/C20A for the Adsorption of Dyes and Uremic Toxins
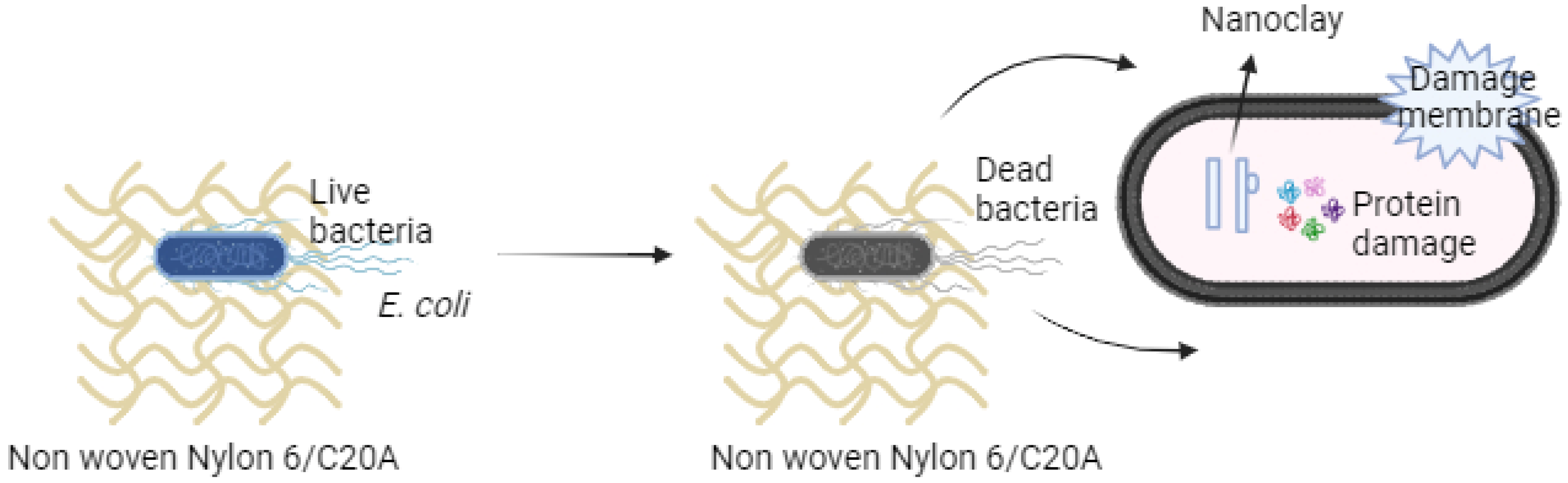
3.7. Antibacterial Activity
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and metal nanoparticles for functional textiles: A review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.J.; Andrade-Guel, M.L.; Medellín-Banda, D.I.; Melo-Lopez, L.; Ávila-Orta, C.A. Polymer Composites: Smart Synthetic Fibers Approach in Energy and Environmental Care. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Diao, Z.; Zhang, L.; Li, Q.; Gao, X.; Gao, X.; Seliem, M.K.; Dhaoudi, F.; Sellaoui, L.; Deng, S.; Bonilla-Petriciolet, A.; et al. Adsorption of food dyes from aqueous solution on a sweet potato residue-derived carbonaceous adsorbent: Analytical interpretation of adsorption mechanisms via adsorbent characterization and statistical physics modeling. Chem. Eng. J. 2024, 482, 148982. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Chen, M.; Seliem, M.K.; Mobarak, M.; Diao, Z.; Li, Z. Adsorption of azo dyes and Naproxen by few-layer MXene immobilized with dialdehyde starch nanoparticles: Adsorption properties and statistical physics modeling. Chem. Eng. J. 2023, 473, 145385. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Zhang, L.; Pei, L.; Xue, H.; Li, Z. Adsorption of methylene blue and Congo red from aqueous solution on 3D MXene/carbon foam hybrid aerogels: A study by experimental and statistical physics modeling. J. Environ. Chem. Eng. 2023, 11, 109206. [Google Scholar] [CrossRef]
- Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Antonello, A.B.; Salaün, F. Bio-functional textiles: Combining pharmaceutical nanocarriers with fibrous materials for innovative dermatological therapies. Pharmaceutics 2019, 11, 403. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a key functional requirement of next-generation medical devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef]
- Koh, E.; Lee, Y.T. Development of an embossed nanofiber hemodialysis membrane for improving capacity and efficiency via 3D printing and electrospinning technology. Sep. Purif. Technol. 2020, 241, 116657. [Google Scholar] [CrossRef]
- Yavuz, A.; Tetta, C.; Ersoy, F.F.; D’intini, V.; Ratanarat, R.; De Cal, M.; Bordoni, V.; Salvatori, G.; Andrikos, E.; Yakupoglu, G.; et al. Uremic toxins: A new focus on an old subject. Semin. Dial. 2005, 18, 203–211. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.S.; Said, N.; Ismail, A.F.; Othman, M.H.D.; Abdullah, M.S.; Ng, B.C.; Hasbullah, H.; Hamimah, S.; Abdul Kadir, S.; et al. Polysulfone/amino-silanized poly (methyl methacrylate) dual-layer hollow fiber membrane for uremic toxin separation. Sep. Purif. Technol. 2020, 236, 116216. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Reyes-Rodríguez, P.Y.; Cabello-Alvarado, C.J.; Cadenas-Pliego, G.; Ávila-Orta, C.A. Influence of Modified Carbon Black on Nylon 6 Nonwoven Fabric and Performance as Adsorbent Material. Nanomaterials 2022, 12, 4247. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Medellin-Banda, D.I.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Sáenz-Galindo, A.; Radillo-Radillo, R.; Lara-Sánchez, J.F.; Melo-Lopez, L. Non-woven fabrics based on Nylon 6/carbon black-graphene nanoplatelets obtained by melt-blowing for adsorption of urea, uric acid, and creatinine. Mater. Lett. 2022, 320, 132382. [Google Scholar] [CrossRef]
- Bajaj, P.; Sengupta, A.K. Industrial applications of textiles: Textiles for filtration and coated fabrics. Text. Prog. 1985, 14, 1–30. [Google Scholar] [CrossRef]
- Aldalbahi, A.; El-Naggar, M.E.; El-Newehy, M.H.; Rahaman, M.; Hatshan, M.R.; Khattab, T.A. Effects of technical textiles and synthetic nanofibers on environmental pollution. Polymers 2021, 13, 155. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; Rehman, A. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Dao, M.U.; Nguyen, T.T.T.; Le, V.T.; Hoang, H.Y.; Le, T.T.N.; Pham, T.N.; Nguyen, T.T.; Akhmadullin, R.M.; Le, H.S.; Tran, H.V.; et al. Non-woven polyester fabric-supported cuprous oxide/reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue. J. Mater. Sci. 2021, 56, 10353–10366. [Google Scholar] [CrossRef]
- Rana, M.S.; Rahman, N.; Chowdhury, T.A.; Dafader, N.C.; Sultana, S.; Sardar, M.N.; Kayser, M.N. Application of Sulfonated GMA-g-non Woven PE Fabric for the Efficient Removal of Methylene Blue Dye from Wastewater. Am. J. Polym. Sci. Technol. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Ávila-Orta, C.A.; Cabello-Alvarado, C.; Cadenas-Pliego, G.; Esparza-González, S.C.; Pérez-Alvarez, M.; Quiñones-Jurado, Z.V. Non-woven fabrics Based on Nanocomposite Nylon 6/ZnO Obtained by Ultrasound-Assisted Extrusion for Improved Antimicrobial and Adsorption Methylene Blue Dye Properties. Polymers 2021, 13, 1888. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Cabello-Alvarado, C.; Romero-Huitzil, R.L.; Rodríguez-Fernández, O.S.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Medellín-Banda, D.I.; Gallardo-Vega, C.; Cepeda-Garza, J. Nanocomposite PLA/C20A nanoclay by ultrasound-assisted melt extrusion for adsorption of uremic toxins and methylene blue dye. Nanomaterials 2021, 11, 2477. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Ávila-Orta, C.A.; Gamero-Melo, P.; Reyes-Rodríguez, P.Y.; Quiñones-Jurado, Z.V.; Cadenas-Pliego, G.; Bartolo-Pérez, P.; Soriano-Corral, F.; Covarrubias-Gordillo, C. Composites based on nylon 6/clinoptilolite by ultrasound-assisted extrusion for enhanced flame retardant and mechanical properties. Polym. Bull. 2022, 79, 1803–1819. [Google Scholar] [CrossRef]
- Reyes-Rodríguez, P.Y.; Ávila-Orta, C.A.; Andrade-Guel, M.; Cortés-Hernández, D.A.; Herrera-Guerrero, A.; Cabello-Alvarado, C.; Sánchez-Fuentes, J.; Ramos-Martínez, V.H.; Valdez-Garza, J.A.; Hurtado-López, G.F. Synthesis and characterization of magnetic nanoparticles Zn1-xMgxFe2O4 with partial substitution of Mg2+ (x = 0.0, 0.25, 0.5, 0.75, and 1.0) for adsorption of uremic toxins. Ceram. Int. 2020, 46, 27913–27921. [Google Scholar] [CrossRef]
- Rusu, G.; Ueda, K.; Rusu, E.; Rusu, M. Polyamides from lactams by centrifugal molding via anionic ring-opening polymerization. Polymer 2001, 42, 5669–5678. [Google Scholar] [CrossRef]
- Estrada-Flores, S.; Martínez-Luévanos, A.; Bartolo-Pérez, P.; García-Cerda, L.A.; Flores-Guia, T.E.; Aguilera-González, E.N. Facile synthesis of novel calcium silicate hydrated-nylon 6/66 nanocomposites by solution mixing method. RSC Adv. 2018, 8, 41818–41827. [Google Scholar] [CrossRef]
- Araujo, E.M.; Leite, A.M.D.; da Paz, R.A.; Medeiros, V.D.N.; de Melo, T.J.A.; Lira, H.D.L. Polyamide 6 nanocomposites with inorganic particles modified with three quaternary ammonium salts. Materials 2011, 4, 1956–1966. [Google Scholar] [CrossRef]
- Wu, T.M.; Liao, C.S. Polymorphism in nylon 6/clay nanocomposites. Macromol. Chem. Phys. 2000, 201, 2820–2825. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.J.; Quiñones-Jurado, Z.V.; Cruz-Delgado, V.J.; Avila-Orta, C.A. Pigmentation and degradative activity of TiO2 on polyethylene films using masterbatches fabricated using variable-frequency ultrasound-assisted melt-extrusion. Materials 2020, 13, 3855. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Camino, G. Poly (lactic acid)/clay nanocomposites: Effect of nature and content of clay on morphology, thermal and thermo-mechanical properties. Mater. Sci. Eng. C 2012, 32, 1790–1795. [Google Scholar] [CrossRef]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Cortés-Hernández, D.A.; Bartolo-Pérez, P.; Ávila-Orta, C.A.; Cruz-Delgado, V.J.; Zepeda-Pedreguera, A. Graphene nanoplatelets modified with amino-groups by ultrasonic radiation of variable frequency for potential adsorption of uremic toxins. Nanomaterials 2019, 9, 1261. [Google Scholar] [CrossRef]
- Chen, J.; Feng, J.; Yan, W. Influence of metal oxides on the adsorption characteristics of PPy/metal oxides for Methylene Blue. J. Colloid Interface Sci. 2016, 475, 26–35. [Google Scholar] [CrossRef]
- Muslim, M.; Ali, A.; Neogi, I.; Dege, N.; Shahid, M.; Ahmad, M. Facile synthesis, topological study, and adsorption properties of a novel Co (II)-based coordination polymer for adsorptive removal of methylene blue and methyl orange dyes. Polyhedron 2021, 210, 115519. [Google Scholar] [CrossRef]
- Le, T.; Esfahani, M.R. Thin-Film Nanocomposite Membranes Functionalized with Fe-Based Metal–Organic Frameworks for Enhanced Removal of Urea from Water. ACS EST Water 2024, 4, 2064–2075. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Lee, J.J.L.; Joo, T.C.; Ang, B.C.; Afifi, A.M. Adsorption study of methyl orange by chitosan/polyvinyl alcohol/zeolite electrospun composite nanofibrous membrane. Carbohydr. Polym. 2018, 191, 79–85. [Google Scholar] [CrossRef]
- Ali, H.; Mansor, E.S.; Taha, G.M. Microfiltration and adsorptive membranes for simultaneous removal of methyl orange and methylene blue using hybrid composites. Polym. Bull. 2022, 79, 7891–7908. [Google Scholar] [CrossRef]
- Łatwińska, M.; Sójka-Ledakowicz, J.; Chruściel, J.; Piórkowski, M. PLA and PP composite nonwoven with antimicrobial activity for filtration applications. Int. J. Polym. Sci. 2016, 2016, 2510372. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Gao, F.; Konovalova, V. Polymer-layered silicate nanocomposites in the design of antimicrobial materials. J. Mater. Sci. 2008, 43, 5728–5733. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.K.; Jeevanandam, J.; Muthalagu, M. Emerging nanomaterials for antibacterial textile fabrication. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1355–1382. [Google Scholar] [CrossRef]

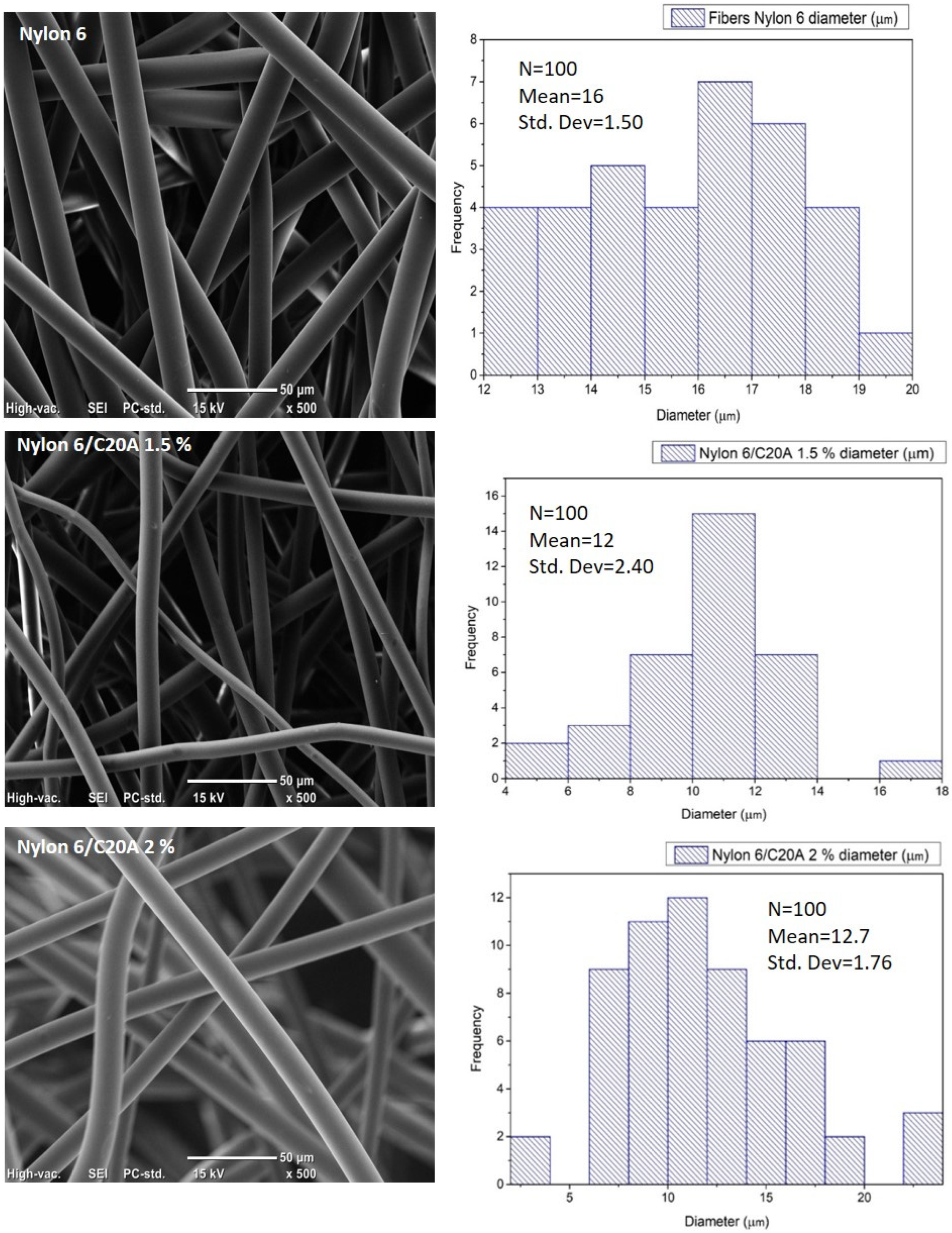
| Sample | Fiber Diameter (µm) |
|---|---|
| Nylon 6 | 16 and 17 ± 1.5 |
| Nylon 6/C20A 0.25% | 16 ± 0.7 |
| Nylon 6/C20A 0.5% | 15 ±1.8 |
| Nylon 6/C20A 0.75% | 13 ± 2.1 |
| Nylon 6/C20A 1.5% | 12 ± 2.4 |
| Nylon 6/C20A 2.0% | 12.7 ± 1.76 |
| Sample | Tear Force (N) | Maximum Breaking Strength (N) |
|---|---|---|
| Nylon 6 | 1.734 | 13.24 |
| Nylon 6/C20A 0.25% | 1.334 | 1.19 |
| Nylon 6/C20A 0.5% | 2.334 | 4.73 |
| Nylon 6/C20A 0.75% | 1.4243 | 5.56 |
| Nylon 6/C20A 1.5% | 1.7348 | 3.66 |
| Nylon 6/C20A 2.0% | 1.7792 | 3.08 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | KF | R2 | |
| Nylon | 0.02 | 3.67 | 0.9948 | 0.35 | 12.46 | 0.9994 |
| Nylon 6/C20A 0.25% | 0.04 | 8.11 | 0.9986 | 0.30 | 7.09 | 0.9989 |
| Nylon 6/C20A 0.5% | 0.4 | 8.10 | 0.9906 | 0.31 | 7.00 | 0.9989 |
| Nylon 6/C20A 0.75% | 0.11 | 1.44 | 0.9996 | 0.69 | 9.10 | 0.9997 |
| Nylon 6/C20A 1.5% | 0.01 | 1.60 | 0.9903 | 0.65 | 8.96 | 0.9995 |
| Nylon 6/C20A 2% | 0.01 | 1.47 | 0.9980 | 0.68 | 9.12 | 0.999 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | KF | R2 | |
| Nylon | 0.16 | 25.8 | 0.999 | 1.12 | 11.4 | 0.9996 |
| Nylon 6/C20A 0.25% | 0.06 | 0.94 | 0.9996 | 2.71 | 18.52 | 0.9994 |
| Nylon 6/C20A 0.5% | 0.006 | 0.7 | 0.9999 | 3.08 | 20.52 | 0.9992 |
| Nylon 6/C20A 0.75% | 0.04 | 1.13 | 0.9998 | 3.07 | 20.46 | 0.9994 |
| Nylon 6/C20A 1.5% | 0.059 | 66 | 0.9999 | 3.23 | 21.30 | 0.9994 |
| Nylon 6/C20A 2% | 0.042 | 1.24 | 0.9999 | 3.05 | 20.35 | 0.9995 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | KF | R2 | |
| Nylon | 9.3 | 312 | 0.9976 | 4.8 | 30.4 | 0.8799 |
| Nylon 6/C20A 0.25% | 8.3 | 113 | 0.9003 | 0.18 | 50.5 | 0.934 |
| Nylon 6/C20A 0.5% | 1.5 | 132 | 0.9317 | 4.9 | 52 | 0.9574 |
| Nylon 6/C20A 0.75% | 2.2 | 210 | 0.9886 | 4.0 | 51 | 0.9297 |
| Nylon 6/C20A 1.5% | 0.29 | 432 | 0.9994 | 2.6 | 8.11 | 0.9977 |
| Nylon 6/C20A 2% | 2 | 67.6 | 0.9998 | 6.5 | 14.77 | 0.9945 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | KF | R2 | |
| Nylon | 1.20 | 196.92 | 0.9387 | 9.61 | 52.17 | 0.9817 |
| Nylon 6/C20A 0.25% | 0.03 | 199 | 0.9990 | 0.52 | 2.77 | 0.9905 |
| Nylon 6/C20A 0.5% | 1.5 | 186 | 0.9953 | 1.25 | 9.47 | 0.9545 |
| Nylon 6/C20A 0.75% | 1.66 | 193 | 0.9973 | 1.4 | 6.09 | 0.9038 |
| Nylon 6/C20A 1.5% | 0.23 | 151.4 | 0.9933 | 4.6 | 11.32 | 0.9448 |
| Nylon 6/C20A 2% | 0.19 | 343 | 0.9985 | 9.7 | 20.11 | 0.9565 |
| Material | Uremic Toxins (Adsorption %) | Dyes (Adsorption %) | References | ||
|---|---|---|---|---|---|
| Urea | Inulin | Methylene Blue | Methyl Orange | ||
| Nylon 6/CB | 80–90 | 80–85 | ---- | ---- | [11] |
| PLA/C20A nanoclay | 65 | ---- | 97 | ---- | [20] |
| Nylon 6/ZnO | ---- | ---- | 93 | ---- | [19] |
| Polyamide functionalized with Fe-based metal–organic | 85 | ---- | ---- | ---- | [33] |
| Non-woven polyester fabric-supported cuprous oxide/reduced graphene oxide | ---- | ---- | 96 | ---- | [17] |
| Chitosan/polyvinyl alcohol/zeolite electrospun composite | ---- | ---- | ---- | 95 | [34] |
| Polyethylene oxide/bentonite/polyaniline | ---- | ---- | 96 | 94 | [35] |
| Nylon 6/C20A 1.5% | 40 | 75 | 90 | 78 | This study |
| Nylon 6/C20A 2% | 40 | 74 | 90 | 92 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Guel, M.; Cabello-Alvarado, C.J.; Ávila Orta, C.A.; Cadenas-Pliego, G.; Cruz-Ortiz, B. Functional Technical Textile-Based Polymer Nanocomposites with Adsorbent Properties of Toxins and Dyes also Have Antibacterial Behavior. Materials 2024, 17, 3007. https://doi.org/10.3390/ma17123007
Andrade-Guel M, Cabello-Alvarado CJ, Ávila Orta CA, Cadenas-Pliego G, Cruz-Ortiz B. Functional Technical Textile-Based Polymer Nanocomposites with Adsorbent Properties of Toxins and Dyes also Have Antibacterial Behavior. Materials. 2024; 17(12):3007. https://doi.org/10.3390/ma17123007
Chicago/Turabian StyleAndrade-Guel, Marlene, Christian J. Cabello-Alvarado, Carlos Alberto Ávila Orta, Gregorio Cadenas-Pliego, and Brenda Cruz-Ortiz. 2024. "Functional Technical Textile-Based Polymer Nanocomposites with Adsorbent Properties of Toxins and Dyes also Have Antibacterial Behavior" Materials 17, no. 12: 3007. https://doi.org/10.3390/ma17123007
APA StyleAndrade-Guel, M., Cabello-Alvarado, C. J., Ávila Orta, C. A., Cadenas-Pliego, G., & Cruz-Ortiz, B. (2024). Functional Technical Textile-Based Polymer Nanocomposites with Adsorbent Properties of Toxins and Dyes also Have Antibacterial Behavior. Materials, 17(12), 3007. https://doi.org/10.3390/ma17123007








