Effect of Wet Grinding Concrete Slurry Waste on Hydration and Hardening Properties of Cement: Micro-Nano-Scale Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Treatment and Characterization of CSW
2.3. Mix Design and Sample Preparation
2.4. Test Method
2.4.1. Particle Size Distribution (PSD)
2.4.2. Hydration Heat
2.4.3. XRD Analysis
2.4.4. SEM Analysis
2.4.5. TG Analysis
2.4.6. Mechanical Properties
3. Results
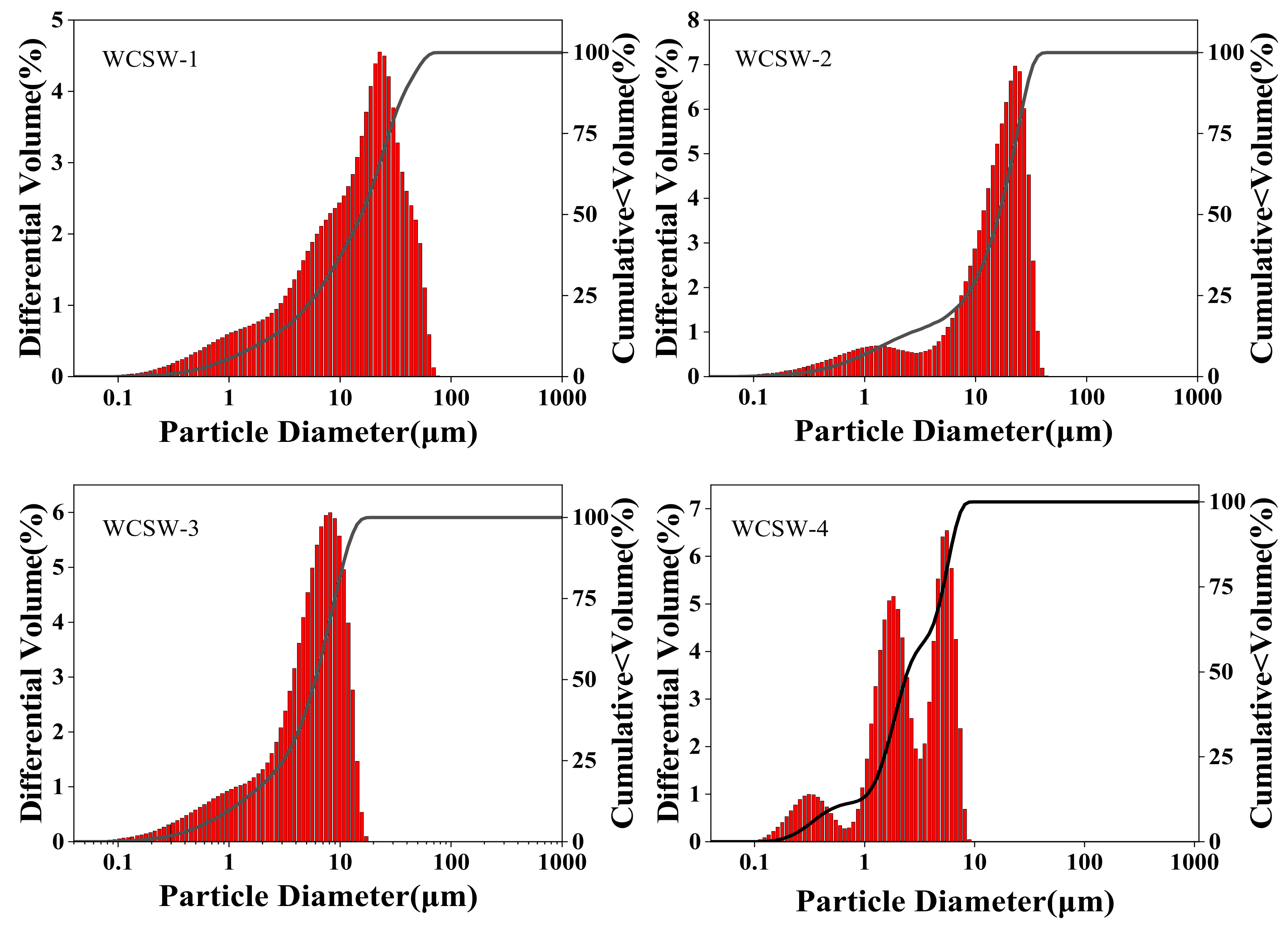
3.1. PSD of WCSW
3.2. Physical and Chemical Properties of WCSW-4
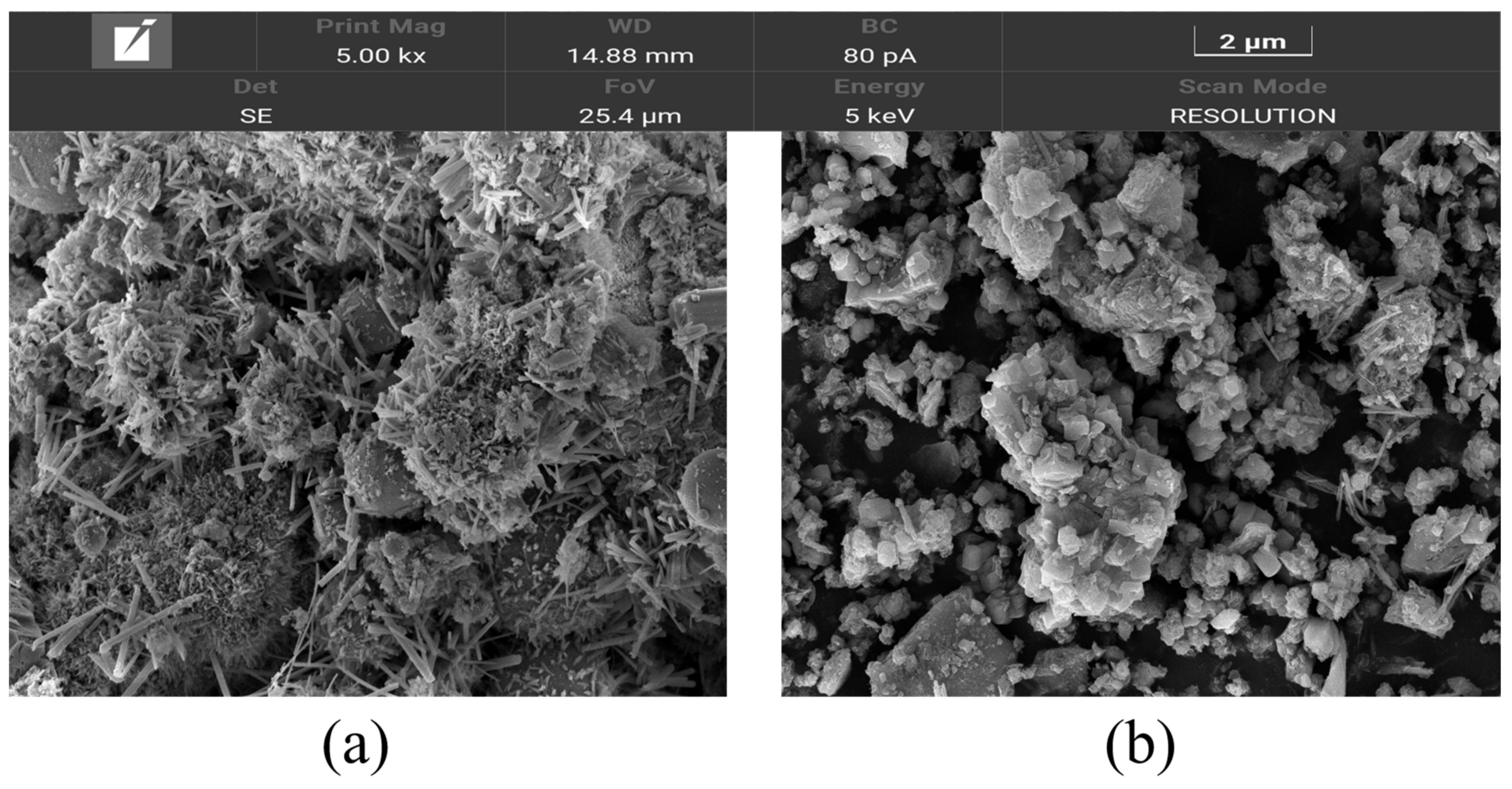
3.2.1. SEM Analysis
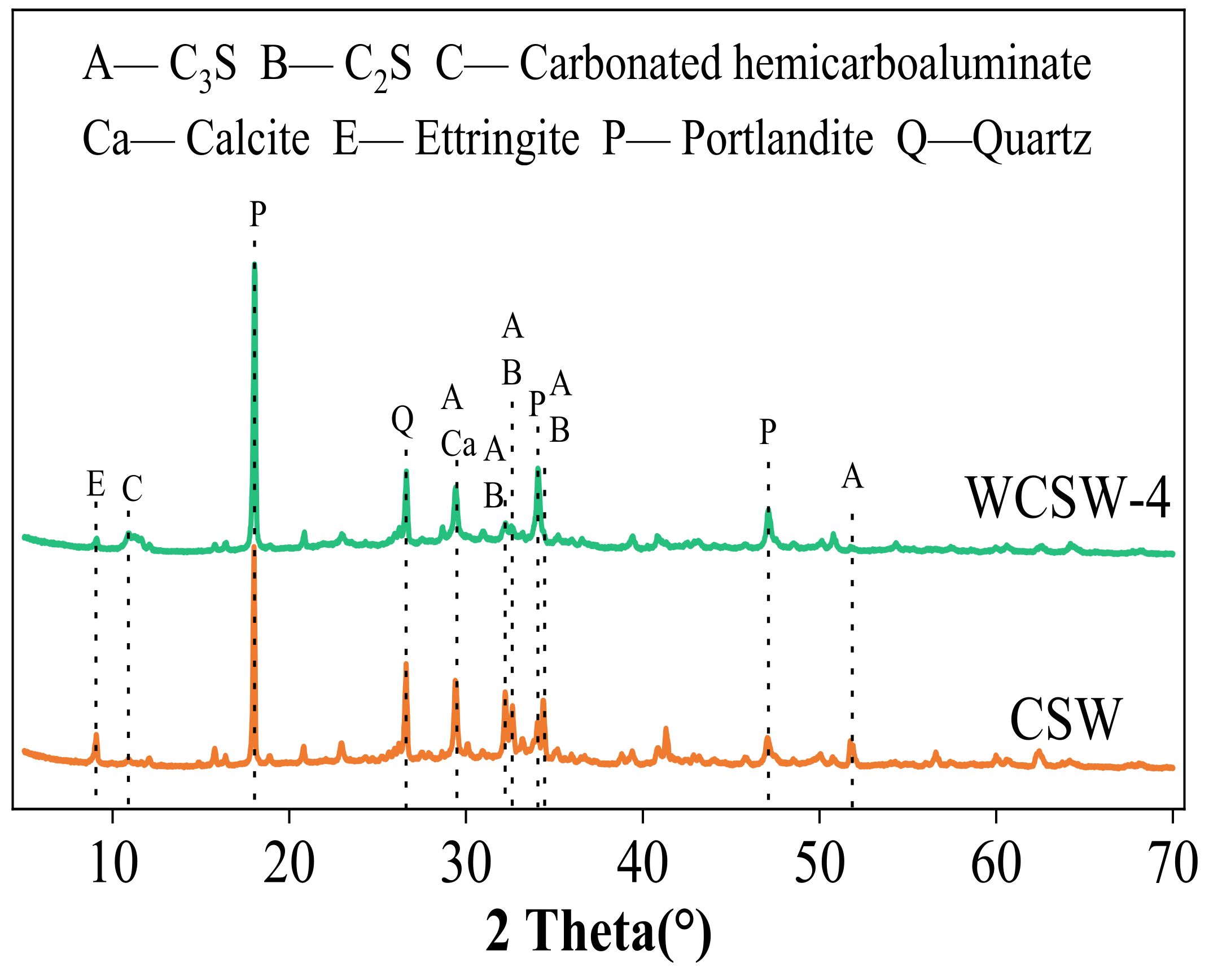
3.2.2. XRD Analysis
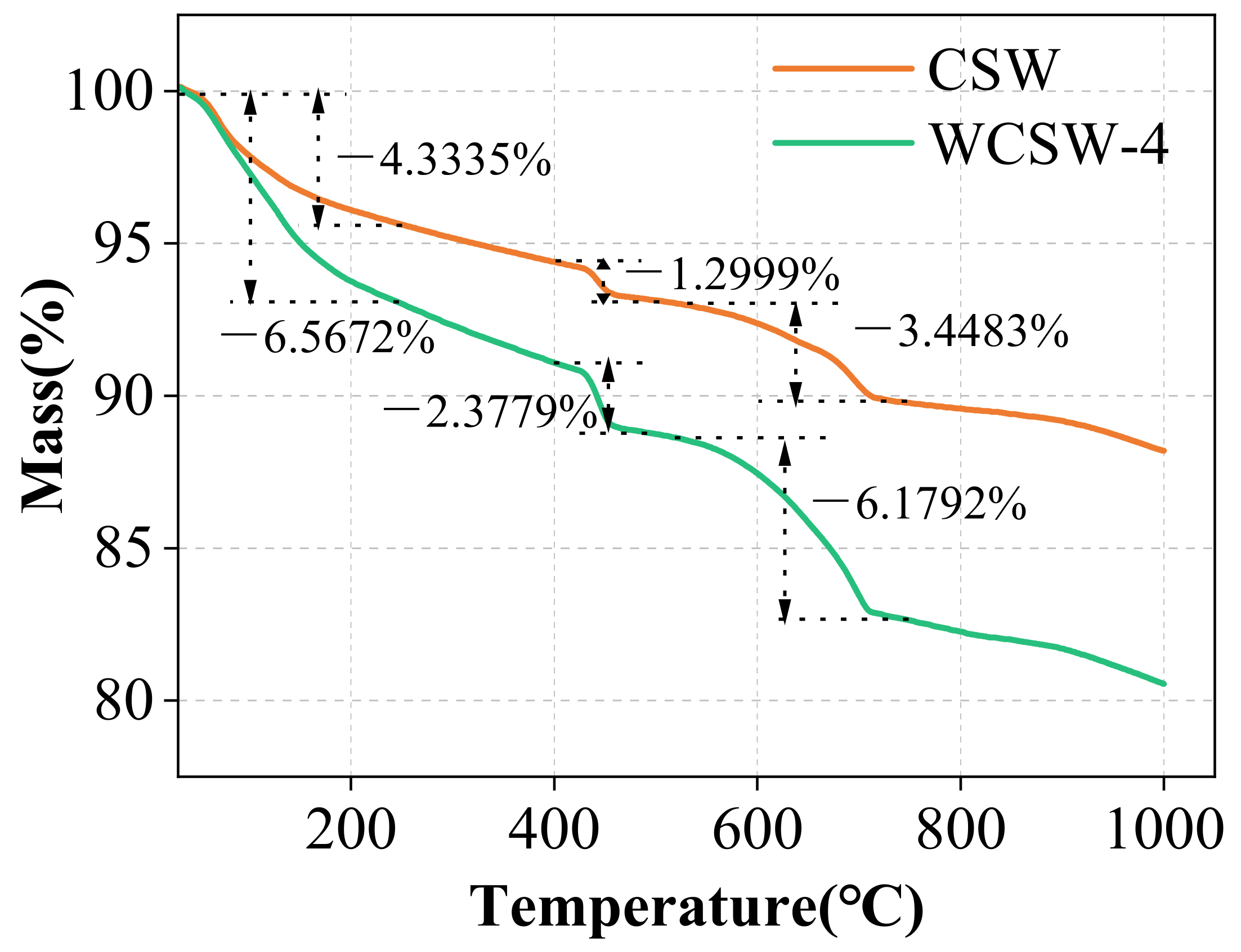
3.2.3. TG Analysis
3.3. Effect of WCSW-4 on Cement Hydration
3.3.1. Hydration Flow
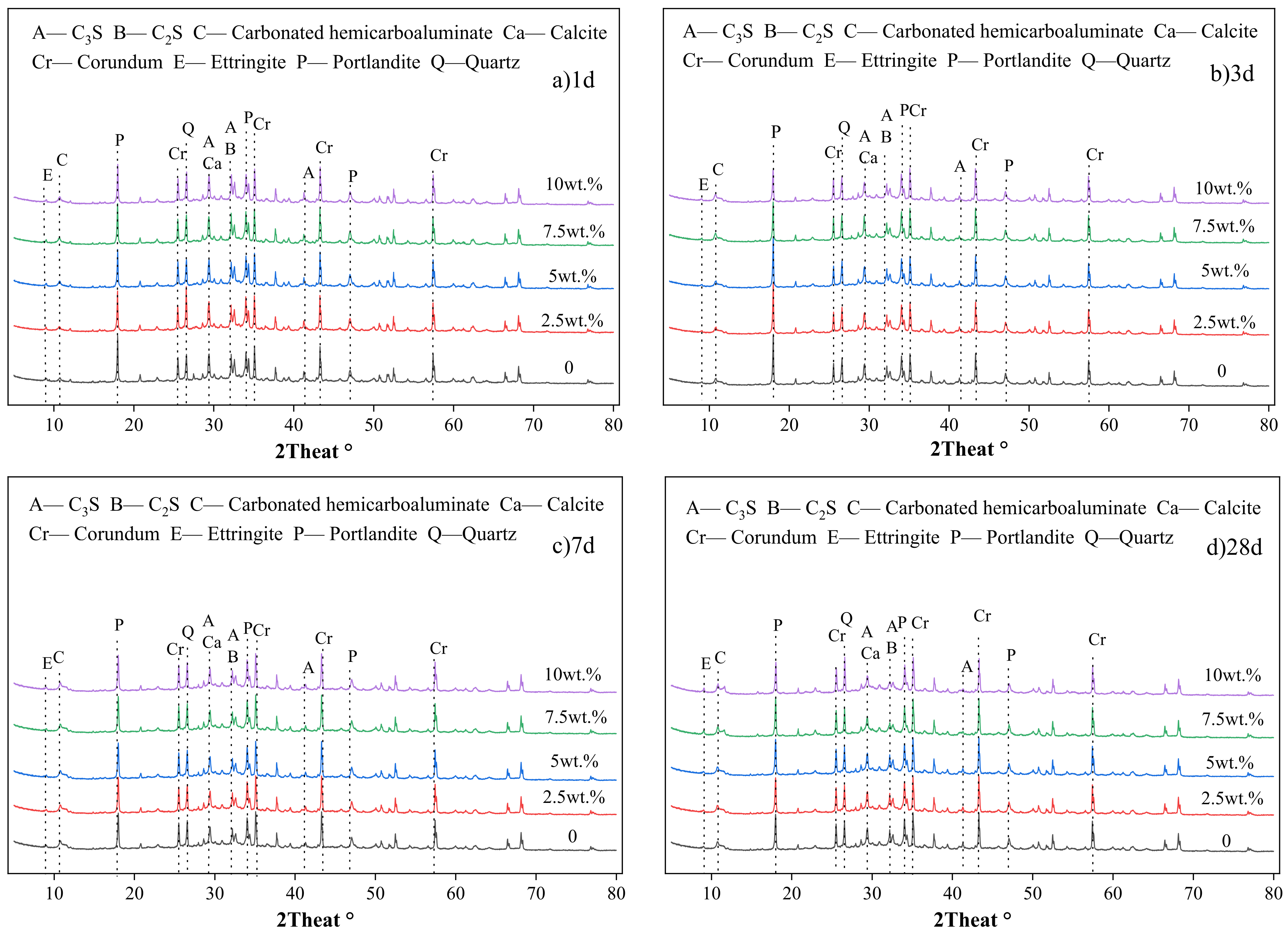
3.3.2. XRD Analysis
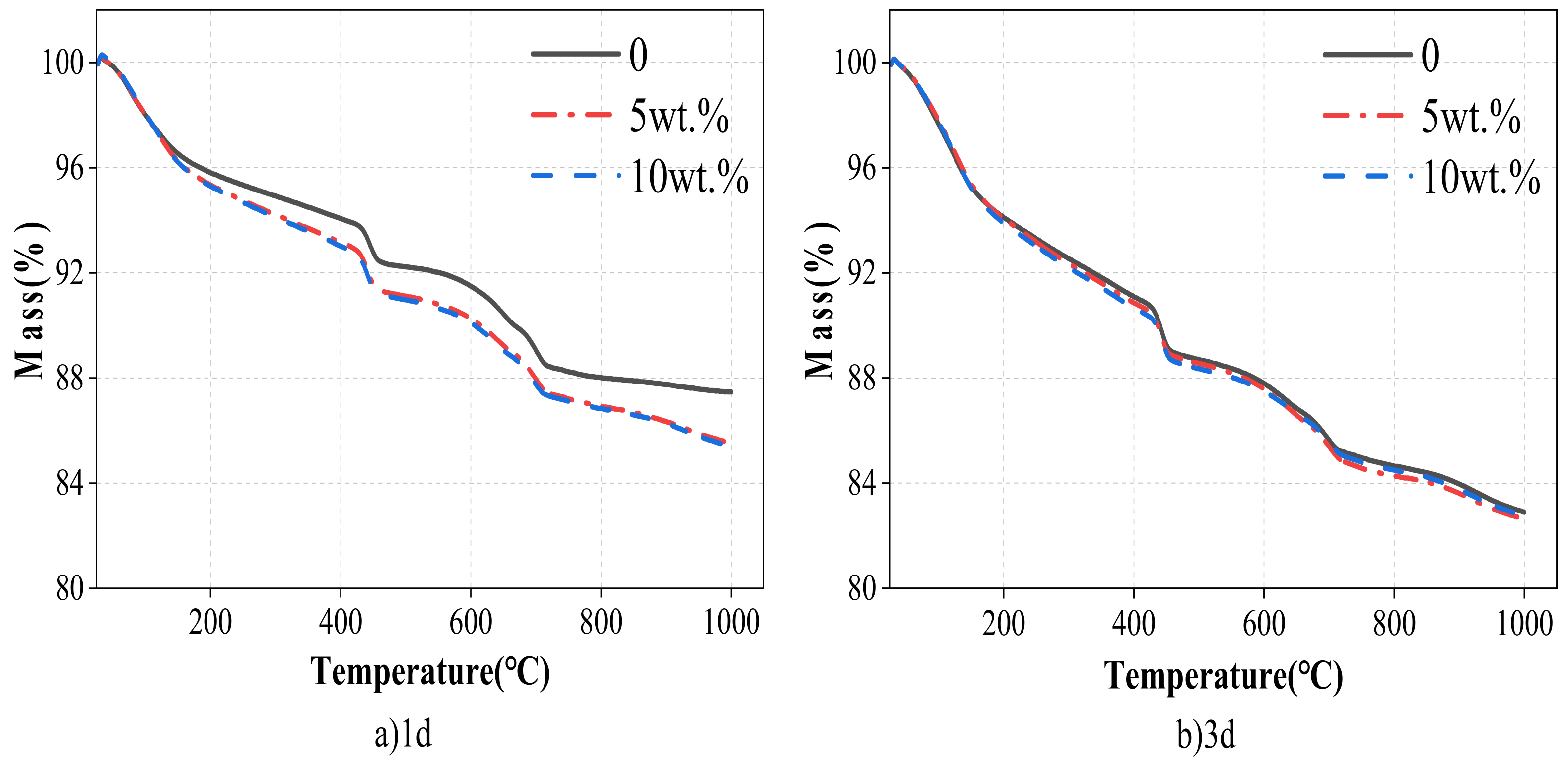
3.3.3. TG Analysis
3.4. Effect of WCSW-4 on the Mechanical Properties of Mortar
4. Discussion
5. Conclusions
- The wet-grinding process can refine CSW. When using zirconia balls with grinding media of 5 mm, 3 mm, and 1 mm, a ball grinding speed of 600 r/min, and a ball grinding time of 1 h, the particle size of WCSW particles is smaller, and the wet-grinding effect is better. Moreover, compared to CSW, the surface of WCSW-4 particles is smoother, with fewer unhydrated components and more hydration products.
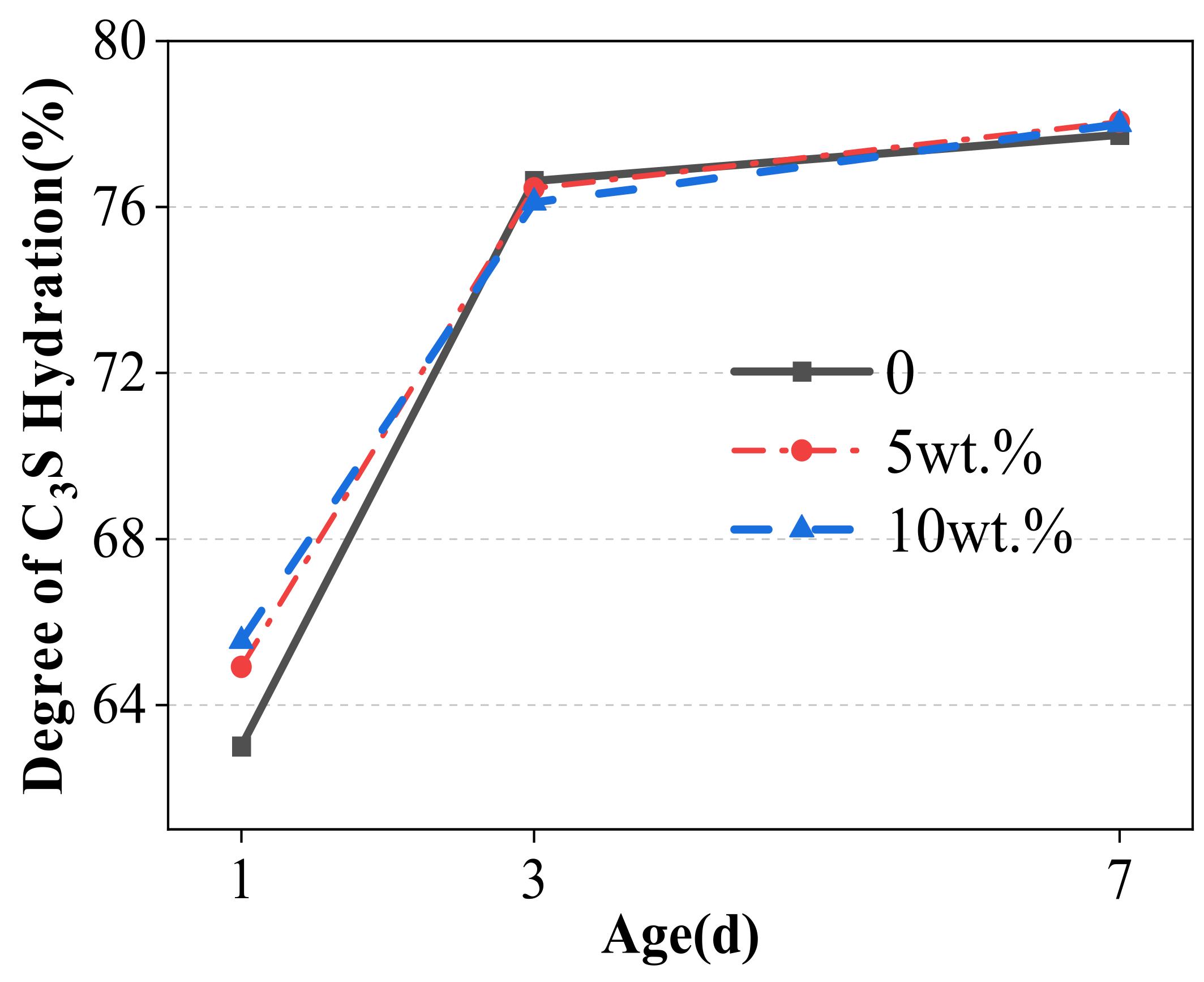
- Replacing cement with WCSW-4 particles can promote the early hydration of cement, and the higher the substitution amount, the more significant the promoting effect. When WCSW-4 particles replace cement, the hydration induction period of cement is shortened, the early hydration of C3S minerals is accelerated, and more hydration products are generated.
- After replacing cement with WCSW-4 particles, the strength of mortar can be significantly improved, and the strength of mortar increases first and then decreases with the increase in WCSW-4 particle substitution amount. At 1 d, the maximum increase in compressive and flexural strength is 43.67% and 45.29%; at 28 d, the maximum increase in compressive and flexural strength is 29.27% and 21.94% with more significant early strength growth.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vishwakarma, V.; Ramachandran, D. Green Concrete mix using solid waste and nanoparticles as alternatives—A review. Constr. Build. Mater. 2018, 162, 96–103. [Google Scholar] [CrossRef]
- Garikapati, K.P.; Sadeghian, P. Mechanical behavior of flax-lime concrete blocks made of waste flax shives and lime binder reinforced with jute fabric. J. Build. Eng. 2020, 29, 101187. [Google Scholar] [CrossRef]
- Xuan, D.X.; Zhan, B.J.; Poon, C.S.; Zheng, W. Carbon dioxide sequestration of concrete slurry waste and its valorisation in construction products. Constr. Build. Mater. 2016, 113, 664–672. [Google Scholar] [CrossRef]
- Audo, M.; Mahieux, P.; Turcry, P. Utilization of sludge from ready-mixed concrete plants as a substitute for limestone fillers. Constr. Build. Mater. 2016, 112, 799. [Google Scholar] [CrossRef]
- Yılmaz, A.H.; Engin, N.; Gökhan, K. Usage of ready-mixed concrete plant wastewater in concrete with superplasticizer: Effect on physico-mechanical properties. Constr. Build. Mater. 2022, 348, 128641. [Google Scholar] [CrossRef]
- Yao, X.; Xi, J.; Guan, J.; Liu, L.; Shangguan, L.; Xu, Z. A Review of Research on Mechanical Properties and Durability of Concrete Mixed with Wastewater from Ready-Mixed Concrete Plant. Materials 2022, 15, 1386. [Google Scholar] [CrossRef]
- Sandrolini, F.; Franzoni, E. Waste wash water recycling in ready-mixed concrete plants. Cem. Concr. Res. 2001, 31, 485–489. [Google Scholar] [CrossRef]
- Martin, K.; Vendula, D.; Barbora, D.; Lenka, S.; Pavel, R. Recycling of fresh concrete slurry waste as supplementary cementing material: Characterization, application and leaching of selected elements. Constr. Build. Mater. 2021, 300, 124061. [Google Scholar] [CrossRef]
- Chatveera, B.; Lertwattanaruk, P.; Makul, N. Effect of sludge water from ready-mixed concrete plant on properties and durability of concrete. Cem. Concr. Compos. 2006, 28, 441–450. [Google Scholar] [CrossRef]
- Kou, S.; Zhan, B.; Poon, C. Feasibility study of using recycled fresh concrete waste as coarse aggregates in concrete. Constr. Build. Mater. 2011, 28, 549–556. [Google Scholar] [CrossRef]
- Kou, S.; Zhan, B.; Poon, C. Properties of partition wall blocks prepared with fresh concrete wastes. Constr. Build. Mater. 2012, 36, 566–571. [Google Scholar] [CrossRef]
- Liang, Y. Fracture behaviors of sustainable recycled aggregate concrete under compression-shear loading: Laboratory test, numerical simulation, and environmental safety analysis. Case Stud. Constr. Mater. 2024, 20, e3328. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, X.; Mao, W.; Xin, S.; Chen, J.; Zhou, J. Carbonation resistance of recycled fine aggregate concrete reinforced by calcium sulfate whiskers. J. Build. Eng. 2024, 92, 109476. [Google Scholar] [CrossRef]
- Chen, X.D.; Wu, J.; Ning, Y.J.; Zhang, W. Experimental study on the effect of wastewater and waste slurry of mixing pla-nt on mechanical properties and microstructure of concrete. J. Build. Eng. 2022, 52, 104307. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, L.; He, X.; Su, Y.; Li, Y.; Tan, H.; Jiang, B.; Zhu, H.; Oh, S.-K. Improving durability of heat-cured high volume fly ash cement mor-tar by wet-grinding activation. Constr. Build. Mater. 2021, 289, 123157. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.; He, X.; Hu, H.; Su, Y.; Bai, H.; Tan, H. Eco-friendly UHPC prepared from high volume wet-grinded ultrafine GGBS slurry. Constr. Build. Mater. 2021, 308, 125057. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.; He, X.; Zhang, Y.; Su, Y.; Tan, H. Sustainable clinker-free solid waste binder produced from wet-ground granulated blast-furnace slag, phosphogypsum and carbide slag. Constr. Build. Mater. 2022, 330, 127218. [Google Scholar] [CrossRef]
- He, X.Y.; Zheng, Z.Q.; Ma, M.Y.; Su, Y.; Yang, J.; Tan, H.B.; Wang, Y.; Strnadel, B. New treatment technology: The use of wet-milling concrete slurry waste to substitute cement. J. Clean. Prod. 2020, 242, 118347. [Google Scholar] [CrossRef]
- Su, Y.; Wu, L.; He, X.; Zheng, Z.; Tan, H.; Yang, J.; Ma, Q.; Ding, J.; Bao, M. A novel early strength agent prepared by wet-grinding concrete waste slurry and its effect on early hydration and mechanical properties of cement based materials. Constr. Build. Mater. 2023, 362, 129673. [Google Scholar] [CrossRef]
- Ślusarek, J.; Nowoświat, A.; Olechowska, M. Logistic Model of Phase Transformation of Hardening Concrete. Materials 2022, 15, 4403. [Google Scholar] [CrossRef]
- Nazar, S.; Yang, J.; Thomas, B.S.; Azim, I.; Rehman, S.K.U. Rheological properties of cementitious composites with and without nano-materials: A comprehensive review. J. Clean. Prod. 2020, 272, 122701. [Google Scholar] [CrossRef]
- Meng, T.; Yu, Y.; Wang, Z. Effect of nano-CaCO3 slurry on the mechanical properties and micro-structure of concrete with and without fly ash. Compos. Part B Eng. 2017, 117, 124–129. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, Z.; Wu, J.; Yang, Q.; Li, Q.; Kong, X. Impact of triethanolamine on the hydration of Portland cement in the presence of high po-zzolanic activity supplementary cementitious materials. Cem. Concr. Compos. 2024, 147, 105435. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). The Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- Voicu, G.; Tiuca, G.; Badanoiu, A.; Holban, A. Nano and mesoscopic SiO2 and ZnO powders to modulate hydration, hardening and antibacterial properties of portland cements. J. Build. Eng. 2022, 57, 104862. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, Y.; Liu, M.; Guo, J.; Du, J.; Du, D. Hydration mechanism and phase assemblage of ternary blended cements based on sewage sludge ash and limestone: Modified by Na2SO4. Constr. Build. Mater. 2023, 364, 129982. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Q.; Xie, H.; Li, S.; Zhang, J.; Xia, C.; Ding, Y.; Chen, Y.; Gao, R.; Wei, Z.; et al. Effect of calcium alumina silicate hydrate nano-seeds on the hydration of low clinker cement. J. Build. Eng. 2023, 66, 105844. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Su, Y.; He, X.; Tan, H.; Yang, W.; Strnadel, B. Eco-friendly treatment of low-calcium coal fly ash for high pozzolanic reactivity: A step towards waste utilization in sustainable building material. J. Clean. Prod. 2019, 238, 117962. [Google Scholar] [CrossRef]
- Tan, H.; Kong, X.; He, X.; Li, M.; Su, Y.; Jian, S.; Yang, J. Effect of chemical additives on wet grinding and activation of fly ash. Mater. Rep. 2023, 38, 188–194. [Google Scholar]
- Gartner, E. Discussion of the paper “Dissolution theory applied to the induction period in alite hydration” by P. Juilland et al. Cem. Concr. Res. 2011, 41, 560–562. [Google Scholar] [CrossRef]
- Naber, C.; Bellmann, F.; Sowoidnich, T.; Goetz-Neunhoeffer, F.; Neubauer, J. Alite dissolution and C-S-H precipitation rates during hydration. Cem. Concr. Res. 2019, 115, 283–293. [Google Scholar] [CrossRef]
- Bergold, S.T.; Goetz-Neunhoeffer, F.; Neubauer, J. Mechanically activated alite: New insights into alite hydration. Cem. Concr. Res. 2015, 76, 202–211. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Bullard, J.W.; Scherer, G.W.; Thomas, J.J. Time dependent driving forces and the kinetics of tricalcium silicate hydration. Cem. Concr. Res. 2015, 74, 26–34. [Google Scholar] [CrossRef]
- Kumar, A.; Bishnoi, S.; Scrivener, K.L. Modelling early age hydration kinetics of alite. Cem. Concr. Res. 2012, 42, 903–918. [Google Scholar] [CrossRef]
- Ouyang, X.; Koleva, D.A.; Ye, G.; van Breugel, K. Understanding the adhesion mechanisms between CSH and fillers. Cem. Concr. Res. 2017, 100, 275–283. [Google Scholar] [CrossRef]
- John, E.; Matschei, T.; Stephan, D. Nucleation seeding with calcium silicate hydrate—A review. Cem. Concr. Res. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Guangwei, L.; Dayou, N.; Haoxin, L.; Biqin, D.; Zhenghong, Y. Synergistic effect of EVA, TEA and C-S-Hs-PCE on the hydration process and mechanical properties of Portland cement paste at early age. Constr. Build. Mater. 2021, 272, 121891. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M. Compressive strength and durability properties of high volume fly ash (HVFA) concretes containing ultrafine fly ash (UFFA). Constr. Build. Mater. 2015, 82, 192–205. [Google Scholar] [CrossRef]




| Components | Cement/% | FA/% |
|---|---|---|
| CaO | 59.26 | 4.58 |
| SiO2 | 20.28 | 44.61 |
| Al2O3 | 8.75 | 33.45 |
| SO3 | 3.94 | 1.68 |
| K2O | 0.61 | 1.09 |
| MgO | 2.56 | 0.35 |
| Fe2O3 | 2.41 | 8.85 |
| Na2O | 0.78 | 0.58 |
| Scheme | Grinding Medium | Grinding Speed | Grinding Time |
|---|---|---|---|
| 1 | 15 mm 10 mm 5 mm Agate ball | 80 r/min | 1 h |
| 2 | 15 mm 10 mm 5 mm Agate ball | 400 r/min | 1 h |
| 3 | 5 mm 3 mm 1 mm Zirconia ball | 600 r/min | 0.5 h |
| 4 | 5 mm 3 mm 1 mm Zirconia ball | 600 r/min | 1 h |
| Cement/g | FA/g | Sand/g | Water/g | Superplasticizer/g |
|---|---|---|---|---|
| 403.2 | 76.8 | 1350 | 153.6 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Sun, H.; Huang, Y.; Du, P. Effect of Wet Grinding Concrete Slurry Waste on Hydration and Hardening Properties of Cement: Micro-Nano-Scale Modification. Materials 2024, 17, 3010. https://doi.org/10.3390/ma17123010
Liu G, Sun H, Huang Y, Du P. Effect of Wet Grinding Concrete Slurry Waste on Hydration and Hardening Properties of Cement: Micro-Nano-Scale Modification. Materials. 2024; 17(12):3010. https://doi.org/10.3390/ma17123010
Chicago/Turabian StyleLiu, Guishan, Hao Sun, Yongbo Huang, and Peng Du. 2024. "Effect of Wet Grinding Concrete Slurry Waste on Hydration and Hardening Properties of Cement: Micro-Nano-Scale Modification" Materials 17, no. 12: 3010. https://doi.org/10.3390/ma17123010





