Yttria-Stabilized Zirconia Composite Coating as Barrier to Reduce Hydrogen Permeation into Steel
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Pretreatment of Matrix Q235 Mild Steel
2.3. Preparation of ZrO2 Coating and Yttria-Stabilized Zirconia Coating
2.4. Characterization of Materials
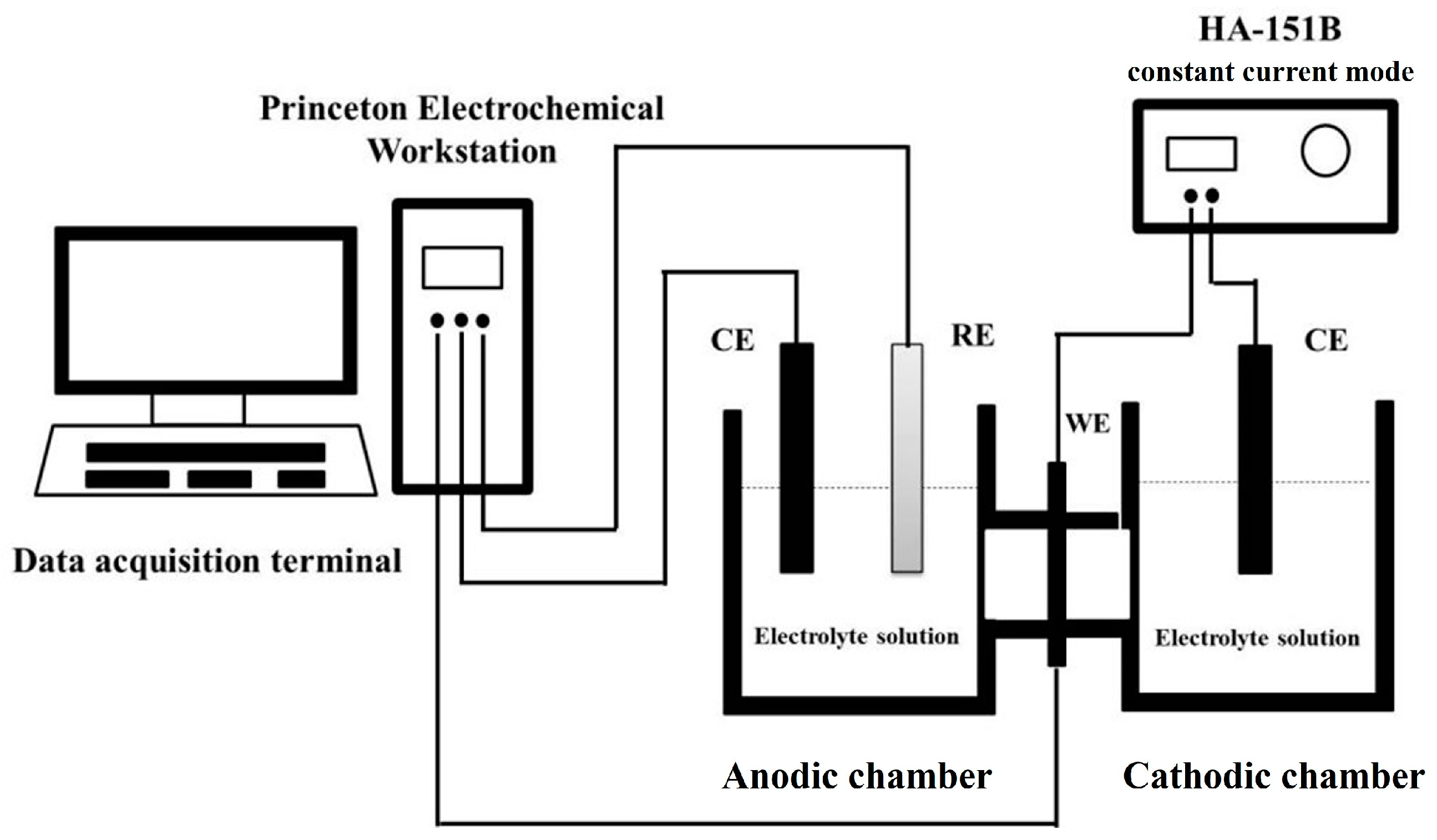
2.5. Hydrogen Barrier Performance Testing of Coatings
2.6. Hardness and Adhesion Testing of Coatings
2.7. Antioxidant Performance Test of Coatings
3. Results and Discussion
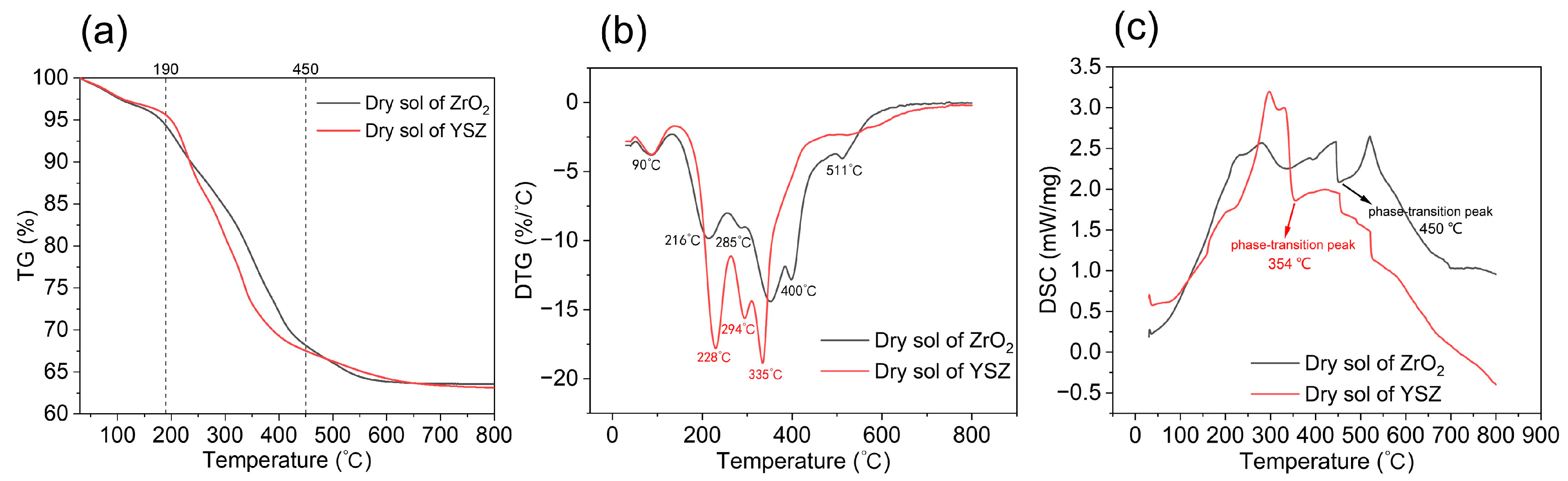
3.1. Thermal Transition Temperature Analysis of Different Types of Gel
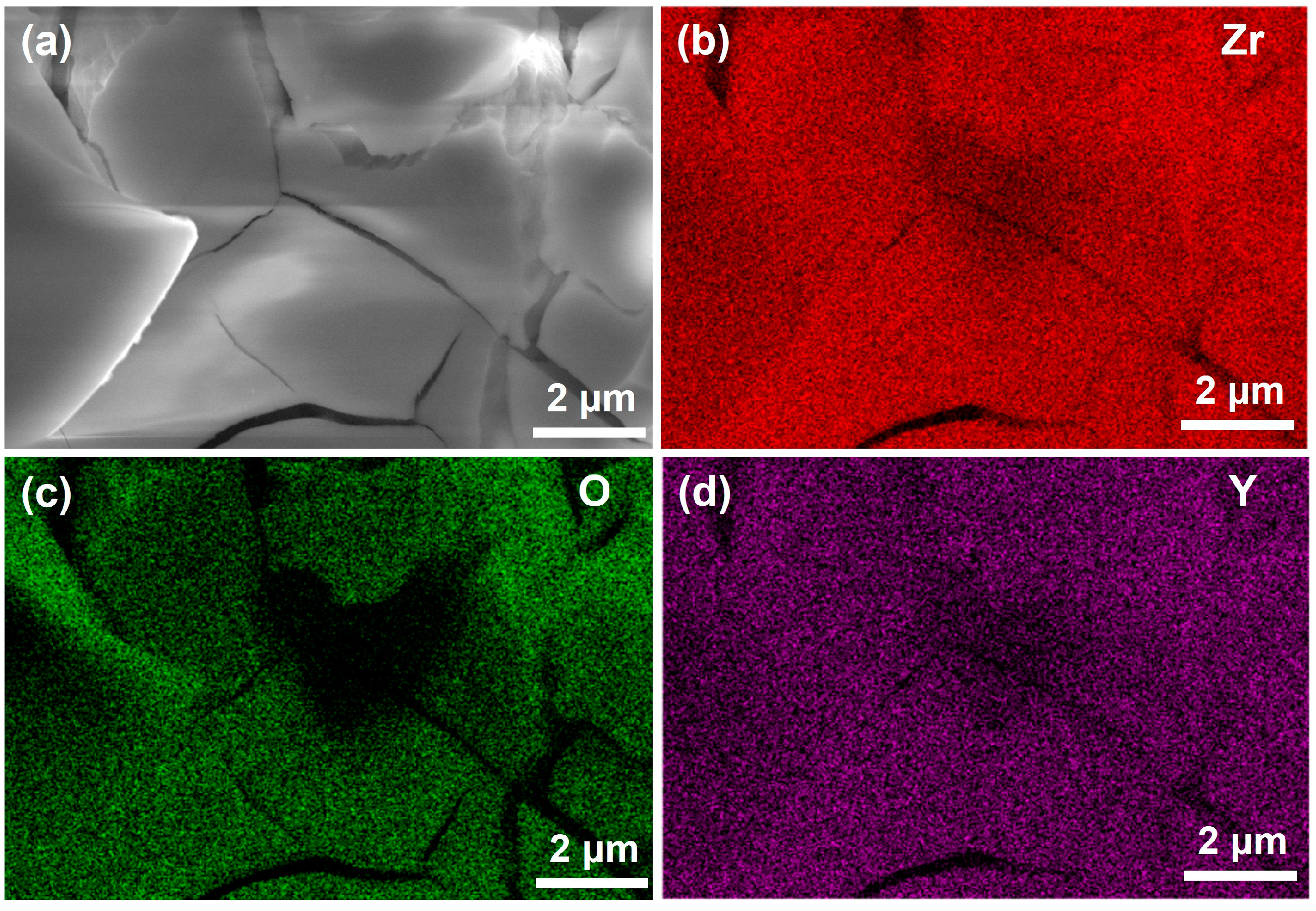
3.2. Surface Morphology Analysis of Coatings
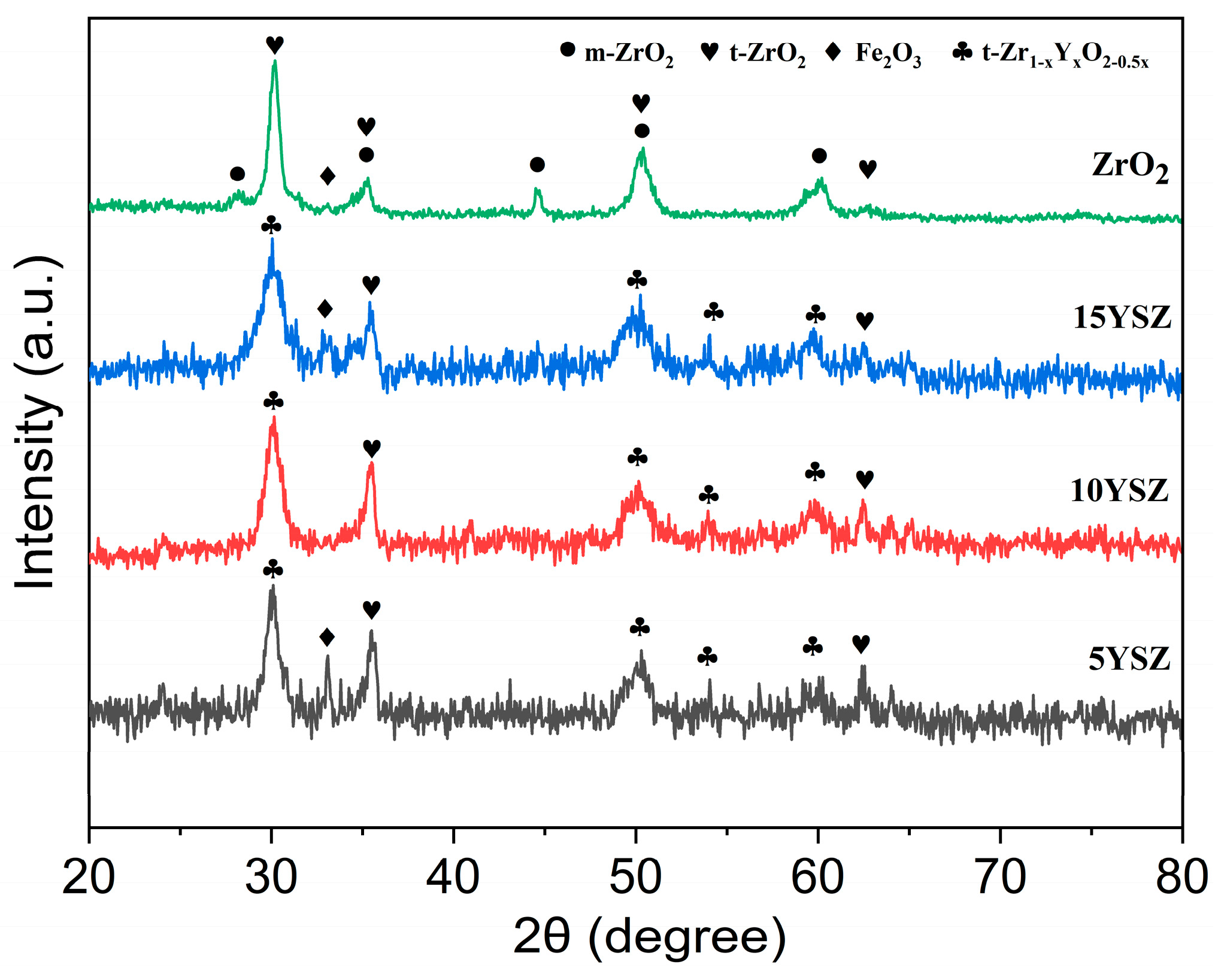
3.3. Phase Composition Analysis of Coatings
3.4. Evaluation of Physical Properties of Coatings
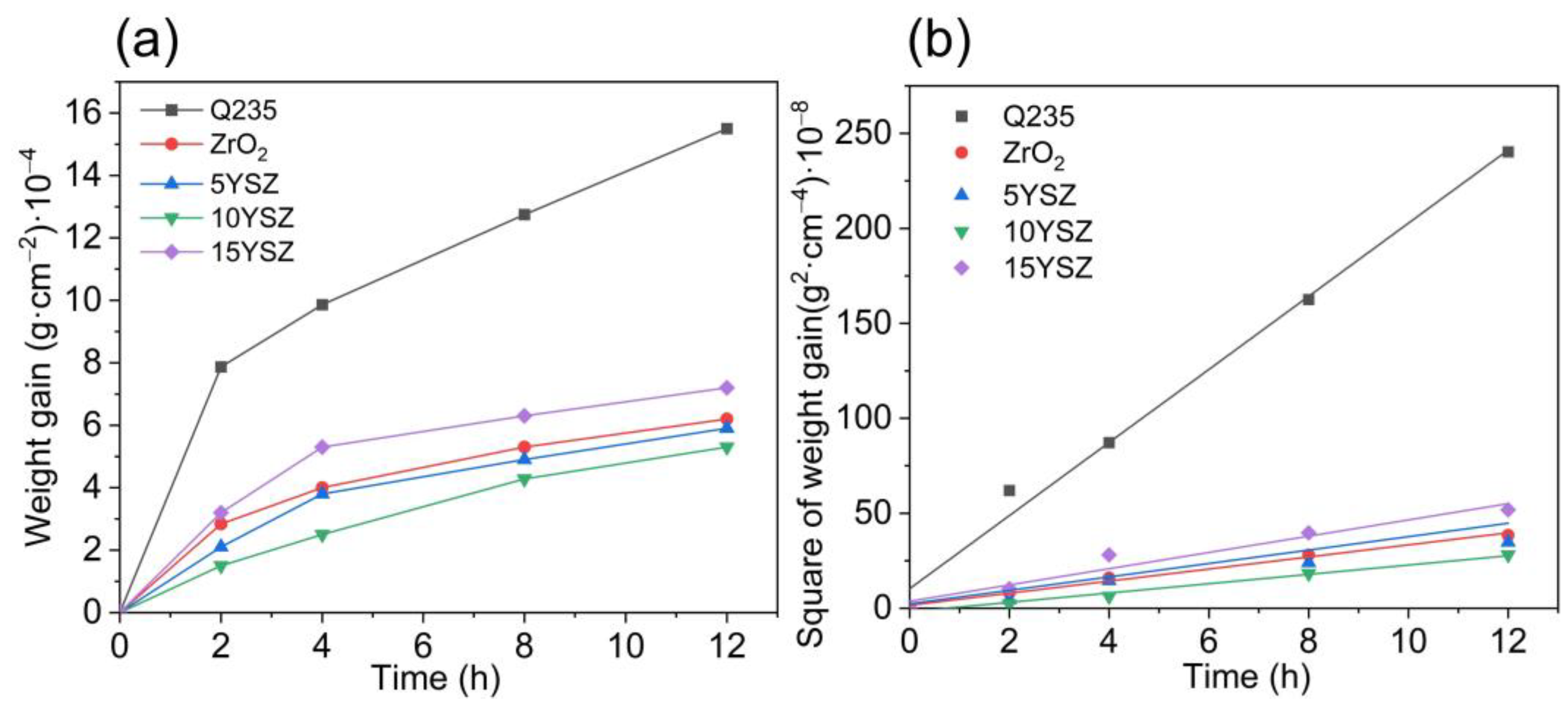
3.5. Antioxidant Behavior of Coatings
3.6. Electrochemical Hydrogen Permeation Performance of Coatings
4. Conclusions
- (1)
- XRD and SEM-EDS analyses show that the rare earth element Y was successfully doped into the ZrO2 coating. Y can stabilize the tetragonal phase zirconia existing at high temperatures to a room temperature environment, alleviate the cracking problem of the coating due to the volume change triggered by the crystalline transition, and improve the densification of the coating. Meanwhile, the addition of yttrium can refine the grain size of the oxide; as the Y doping increased from 5 wt% to 15 wt%, the average grain size of the oxides decreased by about 31%, 37%, and 60%, respectively.
- (2)
- Compared with the ZrO2 coating, the hardness, adhesion, and antioxidant performances of the YSZ coating show a tendency to increase and then decrease with the increase in Y doping, and the YSZ coating with a Y doping of 10 wt% has the best overall performance. When the Y doping amount was lower than 10 wt%, the uniformity and continuity of the coating surface gradually improved with the increase in yttrium doping, and the hardness was improved, with the hardness level increasing from B to 2H and the adhesion increasing from 4B to 5B level. The surface quality of the coating decreased and cracks and defects increased after the Y doping exceeded 10 wt%. Although the hardness level was further increased to 4H, the coating was more brittle at this time and was prone to peeling off, and the adhesion level decreased to 3B.
- (3)
- After heating at 500 °C for 12 h, the antioxidant performance of the ZrO2 coatings was enhanced by 60% compared to the pure substrate, and the antioxidant performance of the 5YSZ, 10YSZ, and 15YSZ coatings was enhanced by 61.9%, 65.8%, and 53.5%, respectively. Meanwhile, the steady-state hydrogen permeation current density of the ZrO2 coating was reduced by 62.5% compared to the matrix, and the steady-state hydrogen current density of the 5YSZ, 10YSZ, and 15YSZ coatings was reduced by 61.1%, 72.3%, and 53.5%, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondarenko, V.L.; Ilyinskaya, D.N.; Kazakova, A.A.; Kozlovtsev, P.S.; Lavrov, N.A.; Razenko, E.A. Introduction to Hydrogen Energy. Chem. Pet. Eng. 2022, 57, 1008–1014. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Yan, H.; Kang, J.; Li, J.; Zhang, H. Current Situation and Prospect of Global Hydrogen Energy Industry Policy. Electr. Power Inf. Commun. Technol. 2021, 19, 27–34. [Google Scholar]
- Huo, X.; Wang, J.; Jiang, L.; Xu, Q. Review on key technologies and applications of hydrogen energy storage system. Energy Storage Sci. Technol. 2016, 5, 197–203. [Google Scholar]
- Niu, M.; Xiao, Y.; Liu, F.; Zhao, P.; Zhao, B. Influences of Renewable Energy on Hydrogen Storage System and Its Control Strategy. Electr. Power Constr. 2018, 39, 28–34. [Google Scholar]
- Teng, X.; Zhang, G.; Hu, C.; Zhu, C.; Yu, D.; Liu, D.; Liu, S. Analysis on hydrogen energy economy and low cost of hydrogen source in typical cities of China. Chem. Ind. Eng. Prog. 2022, 41, 6295–6301. [Google Scholar]
- Sundaram, S.; Ram, G.D.J.; Amirthalingam, M. Metallurgical and mechanical properties of hydrogen charged carbide-free bainitic weld metals. Int. J. Hydrogen Energy 2023, 48, 18514–18525. [Google Scholar] [CrossRef]
- Guan, H.; Lin, Z.; Li, Y.; Liu, Q.; Xing, Y.; Wang, J.; Wang, X. Hydrogen embrittlement susceptibility of the X70 pipeline steel substrate and weld in simulated coal gas containing hydrogen environment. Chin. J. Eng. 2017, 39, 535–541. [Google Scholar]
- Zhao, W.; Zhang, T.; Zhao, Y.; Sun, J.; Wang, Y. Hydrogen permeation and embrittlement susceptibility of X80 welded joint under high-pressure coal gas environment. Corros. Sci. 2016, 111, 84–97. [Google Scholar] [CrossRef]
- Fowler, J.D.; Chandra, D.; Elleman, T.S.; Payne, A.W.; Verghese, K. Tritium diffusion in Al2O3 and BeO. J. Am. Ceram. Soc. 1977, 60, 155–161. [Google Scholar] [CrossRef]
- Yao, Z.; Suzuki, A.; Levchuk, D.; Chikada, T.; Tanaka, T.; Muroga, T.; Terai, T. Hydrogen permeation through steel coated with erbium oxide by sol-gel method. J. Nucl. Mater. 2009, 386–388, 700–702. [Google Scholar] [CrossRef]
- Heo, H.S.; Shin, D.H.; Kim, S.J. Effects of CrN and TiN Coating by Hydrogen Embrittlement of Aluminum Alloys for Hydrogen Valves of Hydrogen Fuel Cell Vehicles on Mechanical Properties. Corros. Sci. Technol. Korea 2023, 22, 232–241. [Google Scholar]
- Zhou, C.; He, M.; Xiao, S.; Shi, K.; Wu, H.; Jiang, S.; Chen, G.; Wu, C. Review on hydrogen permeation barrier coatings on stainless steels. Chem. Ind. Eng. Prog. 2020, 39, 3458–3468. [Google Scholar]
- Wang, B.; Sun, X.; Liu, E.; Liu, L.; Ma, W.; Shi, Y.; Huang, P.; Luo, Y. Preparation and Hydrogen Barrier Property of FexAly/Al/Al2O3 Composite Coating on X80 Steel Surface. Met. Mater. Int. 2024, 30, 77–88. [Google Scholar] [CrossRef]
- Huang, J.; Xie, H.; Luo, L.M.; Zan, X.; Liu, D.G.; Wu, Y.C. Preparation and properties of FeAl/Al2O3 composite tritium permeation barrier coating on surface of 316L stainless steel. Surf. Coat. Technol. 2020, 383, 125282. [Google Scholar] [CrossRef]
- Yao, Z.; Suzuki, A.; Levchuk, D.; Terai, T. Sic coating by rf sputtering as tritium permeation barrier for fusion blanket. Fusion Sci. Technol. 2007, 52, 865–869. [Google Scholar] [CrossRef]
- Wang, P.X.; Liu, J.; Wang, Y.; Shi, B.G. Investigation of SiC films deposited onto stainless steel and their retarding effects on tritium permeation. Surf. Coat. Technol. 2000, 128, 99–104. [Google Scholar] [CrossRef]
- Lakdhar, I.; Alhussein, A.; Capelle, J.; Creus, J. Al-Ti-W alloys deposited by magnetron sputtering: Effective barrier to prevent steel hydrogen embrittlement. Appl. Surf. Sci. 2021, 567, 150786. [Google Scholar] [CrossRef]
- Wang, J.; Ling, Y.; Lu, Z.; Zhang, M.; Zhou, Q.; Wang, R.; Li, Y.; Zhang, Z. Hydrogen interaction characteristics of a Cr2O3-Y2O3 coating formed on stainless steel in an ultra-low oxygen environment. Int. J. Hydrogen Energy 2019, 44, 8669–8679. [Google Scholar] [CrossRef]
- Kulsartov, T.V.; Hayashi, K.; Nakamichi, M.; Afanasyev, S.E.; Shestakov, V.P.; Chikhray, Y.V.; Kenzhin, E.A.; Kolbaenkov, A.N. Investigation of hydrogen isotope permeation through F82H steel with and without a ceramic coating of Cr2O3-SiO2 including CrPO4 (out-of-pile tests). Fusion Eng. Des. 2006, 81, 701–705. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Wang, S.; Jiang, L.; Liu, X. Influence of silicon on hot-dip aluminizing process and subsequent oxidation for preparing hydrogen/tritium permeation barrier. Int. J. Hydrogen Energy 2010, 35, 2689–2693. [Google Scholar] [CrossRef]
- He, D.; Li, S.; Liu, X.; Zhang, C.; Yu, Q.; Wang, S.; Jiang, L. Preparation of Cr2O3 film by MOCVD as hydrogen permeation barrier. Fusion Eng. Des. 2014, 89, 35–39. [Google Scholar] [CrossRef]
- Feng, J.; Dan, M.; Jin, F.; Chen, M.; Shen, L.; Tong, H.; Zhang, G. Preparation and Properties of Alumina Coatings as Tritium Permeation Barrier by Plasma Electrolytic Oxidation. Rare Met. Mater. Eng. 2016, 45, 315–320. [Google Scholar]
- Zhang, W.; Zhu, C.; Yang, J.; Chen, Q.; Shi, K.; Wang, L.; Feng, Y.; Feng, K.; Duan, Z.; Li, Y.; et al. Aluminum phosphate sealing to improve deuterium permeation resistance of α-Al2O3 coating prepared by MOD method. Surf. Coat. Technol. 2021, 419, 127298. [Google Scholar] [CrossRef]
- Wang, Z.G.; Chen, W.D.; Yan, S.F.; Fan, X.J.; Xu, Z.G. Characterization of ZrO2 ceramic coatings on ZrH1.8 prepared in different electrolytes by micro-arc oxidation. Rare Met. 2022, 41, 1043–1050. [Google Scholar] [CrossRef]
- Ju, H.; Chen, W.; Yan, S.; Liu, T.; Liu, F.; Ma, W. Zirconia Hydrogen Permeation Barrier Fabricated by Sol-Gel Method with Different Solvents. Chin. J. Rare Met. 2019, 43, 87–91. [Google Scholar]
- Siripurapu, R.K.; Szpunar, B.; Szpunar, J.A. Molecular Dynamics Study of Hydrogen in α-Zirconium. Int. J. Nucl. Energy 2014, 4, 912369. [Google Scholar] [CrossRef]
- Barbour, O.; Crocombette, J.P.; Schuler, T.; Tupin, M. Ab-initio calculations of hydrogen diffusion coefficient in monoclinic zirconia. J. Nucl. Mater. 2020, 539, 152333. [Google Scholar] [CrossRef]
- Haurat, E.; Crocombette, J.P.; Schuler, T.; Tupin, M. Hydrogen diffusion coefficient in monoclinic zirconia in presence of oxygen vacancies. Int. J. Hydrogen Energy 2022, 47, 33517–33529. [Google Scholar] [CrossRef]
- Hatano, Y.; Zhang, K.; Hashizume, K. Fabrication of ZrO2 coatings on ferritic steel by wet-chemical methods as a tritium permeation barrier. Phys. Scr. 2011, T145, 014044. [Google Scholar] [CrossRef]
- Meetei, S.D.; Singh, S.D.; Sudarsan, V. Polyol synthesis and characterizations of cubic ZrO2:Eu3+ nanocrystals. J. Alloys Compd. 2012, 514, 174–178. [Google Scholar] [CrossRef]
- Cong, Y.; Li, B.; Yue, S.; Fan, D.; Wang, X.J. Effect of Oxygen Vacancy on Phase Transition and Photoluminescence Properties of Nanocrystalline Zirconia Synthesized by the One-Pot Reaction. J. Phys. Chem. C 2009, 113, 13974–13978. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liu, W.M. Preparation and tribological properties of sol-gel zirconia thin films stabilized with ceria. Mater. Lett. 2002, 55, 407–413. [Google Scholar] [CrossRef]
- Araki, W.; Arai, Y. Oxygen diffusion in yttria-stabilized zirconia subjected to uniaxial stress. Solid State Ion. 2010, 181, 441–446. [Google Scholar] [CrossRef]
- Chen, D.; Mei, D.; Li, Y.; Chen, L.; Wang, H.; Huang, W.; Wang, L.; Zhu, S.; Guan, S. Protective nature of cerium-based oxides coating against Mg corrosion in Hanks’ balanced salt solution. Corros. Sci. 2023, 219, 111255. [Google Scholar] [CrossRef]
- Su, X.; Li, X.; Cai, T.; Zhang, X. Study on Preparation Process and Solid Solution Crystal Type of Yttria Stabilized Zirconia by Reverse Coprecipitation Method. China’s Ceram. 2017, 53, 41–45. [Google Scholar]
- Szajna, E.; Moskal, G.; Tupaj, M.; Dresner, J.; Dudek, A.; Szymański, K.; Tomaszewska, A.; Trzcionka-Szajna, A.; Mikuśkiewicz, M.; Łysiak, K. The influence of laser remelting on microstructural changes and hardness level of flame-sprayed NiCrBSi coatings with tungsten carbide addition. Surf. Coat. Technol. 2024, 478, 130403. [Google Scholar] [CrossRef]
- Veldhuis, S.A.; Brinks, P.; Ten Elshof, J.E. Rapid densification of sol-gel derived yttria-stabilized zirconia thin films. Thin Solid Film. 2015, 589, 503–507. [Google Scholar] [CrossRef]
- Criado, J.M.; Gonzalez, M.; Dianez, M.J.; Perez Maqueda, L.A.; Malek, J. Influence of environment and grinding on the crystallisation mechanism of ZrO2 gel. J. Phys. Chem. Solids 2007, 68, 824–829. [Google Scholar] [CrossRef]
- Mandal, S.; Kumar, C.J.D.; Kumar, D.; Syed, K.; Van Ende, M.A.; Jung, I.H.; Finkeldei, S.C.; Bowman, W.J. Designing environment-friendly chromium-free Spinel-Periclase-Zirconia refractories for Ruhrstahl Heraeus degasser. J. Am. Ceram. Soc. 2020, 103, 7095–7114. [Google Scholar] [CrossRef]
- Bartuli, C.; Bertamini, L.; Matera, S.; Sturlese, S. Investigation of the formation of an amorphous film at the ZrO2-Y2O3/nicocraly interface of thermal barrier coatings produced by plasma spraying. Mater. Sci. Eng. A 1995, 199, 229–237. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, H. Preparation and Structural Analysis of Y2O3 Stabilized ZrO2 Coating Material. Rare Met. Mater. Eng. 2015, 44, 2020–2023. [Google Scholar]
- Huang, W.; Yang, J.; Meng, X.; Cheng, Y.; Wang, C.; Zou, B.; Khan, Z.; Wang, Z.; Cao, X. Effect of the organic additions on crystal growth behavior of ZrO2 nanocrystals prepared via sol-gel process. Chem. Eng. J. 2011, 168, 1360–1368. [Google Scholar] [CrossRef]
- Chen, K.; Song, P.; Hua, C.; Zhou, Y.; Huang, T.; Li, C.; Lu, J. Effect of YSZ-dopant on microstructure and hardness property of the Al2O3-40%TiO2 plasma sprayed coating. Mater. Res. Express 2018, 5, 086504. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Adhikary, J.; Singh, T.B.; Singh, R. Preparation and characterization of sol-gel derived yttria doped zirconia coatings on AISI 316L. Thin Solid Film. 2009, 517, 4502–4508. [Google Scholar] [CrossRef]
- Yonggui, W.; Erni, Q.I.U.; Minghui, D. Characterization Analysis of Oxidation Kinetics of Aluminized Coating on Ni-base Superalloy. Hot Work. Technol. 2007, 36, 48–50. [Google Scholar]
- Li, Z.Y.; Zhang, J.Y.; Bao, R.; Hu, H.J.; Fei, B.J. Experimental study on adaptivity of oxidation kinetics equations in APS thermal barrier coatings. J. Aerosp. Power 2009, 24, 1726–1730. [Google Scholar]
- Hebert, V.; His, C.; Guille, J.; Vilminot, S.; Wen, T.L. Preparation and characterization of precursors of Y2O3 stabilized ZrO2 by metal-organic compounds. J. Mater. Sci. 1991, 26, 5184–5188. [Google Scholar] [CrossRef]
- Soo, M.T.; Kawamura, G.; Muto, H.; Matsuda, A.; Lockman, Z.; Cheong, K.Y. Design of hierarchically meso-macroporous tetragonal ZrO2 thin films with tunable thickness by spin-coating via sol-gel template route. Microporous Mesoporous Mater. 2013, 167, 198–206. [Google Scholar] [CrossRef]
- Zima, T.M.; Baklanova, N.I.; Belyaeva, E.I.; Lyakhov, N.Z. Preparation of ZrO2 and ZrO2-Y2O3 coatings on silicon carbide fibers. Inorg. Mater. 2006, 42, 647–653. [Google Scholar] [CrossRef]




| Element | C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|
| Contents (wt%) | 0.16 | 0.18 | 0.72 | 0.016 | 0.018 | Bal. |
| Temperature Range (°C) | Heating Rate (°C/min) | Terminal Holding Time (min) |
|---|---|---|
| RT–200 | 1 | 35 |
| 200–400 | 1 | 90 |
| 400–550 | 3 | 120 |
| Sample | Q235 | ZrO2 | 5YSZ | 10YSZ | 15YSZ |
|---|---|---|---|---|---|
| Iss (μA/cm2) | 33.01 | 12.36 | 12.84 | 9.13 | 15.34 |
| J∞ (mol/cm2·s) | 3.42 × 10−6 | 1.28 × 10−6 | 1.33 × 10−6 | 0.95 × 10−6 | 1.59 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Xie, J.; He, M.; Zhang, J.; Li, S. Yttria-Stabilized Zirconia Composite Coating as Barrier to Reduce Hydrogen Permeation into Steel. Materials 2024, 17, 3017. https://doi.org/10.3390/ma17123017
Wu J, Xie J, He M, Zhang J, Li S. Yttria-Stabilized Zirconia Composite Coating as Barrier to Reduce Hydrogen Permeation into Steel. Materials. 2024; 17(12):3017. https://doi.org/10.3390/ma17123017
Chicago/Turabian StyleWu, Jianmeng, Jiaqi Xie, Mengyuan He, Jingyi Zhang, and Songjie Li. 2024. "Yttria-Stabilized Zirconia Composite Coating as Barrier to Reduce Hydrogen Permeation into Steel" Materials 17, no. 12: 3017. https://doi.org/10.3390/ma17123017
APA StyleWu, J., Xie, J., He, M., Zhang, J., & Li, S. (2024). Yttria-Stabilized Zirconia Composite Coating as Barrier to Reduce Hydrogen Permeation into Steel. Materials, 17(12), 3017. https://doi.org/10.3390/ma17123017







