The Potential Risk of Nanoparticulate Release from Photocatalytic Pavement Concrete Surface Due to a Simulated Abrasion Load—An Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photocatalytic Cementitious Composites
2.2. Test Setup
2.2.1. Abrasion Test Procedure
- Analysis of the granulation of background aerosols in the testing chamber before abrasion of the mortar sample (of origin other than the tested mortar sample);
- Analysis of the granulation of aerosols mobilized from the mortar sample due to an abrasion load;
- Analysis of the granulation of background aerosols in the testing chamber after abrasion of the mortar sample.
- 10.4 nm–469.8 nm;
- 0.523 µm–19.810 µm.
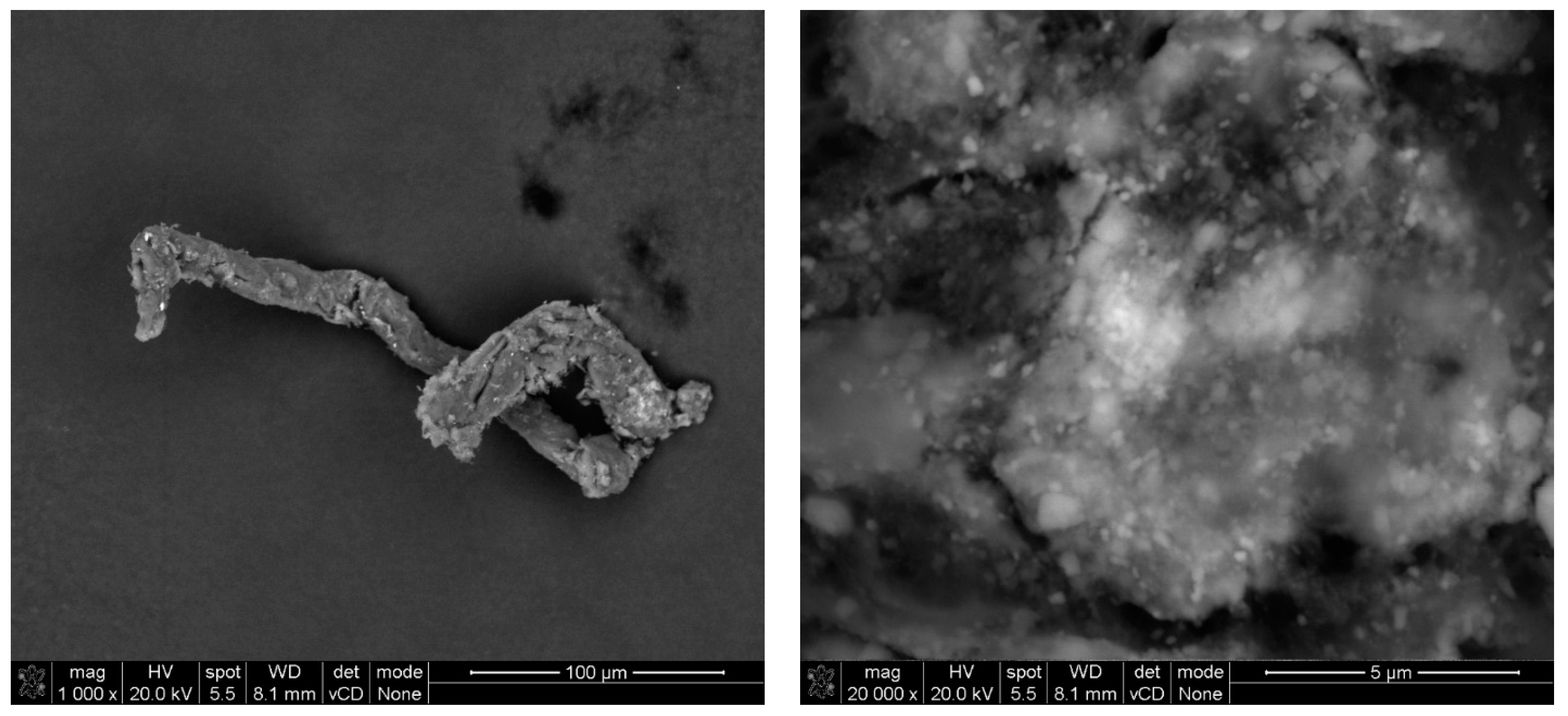
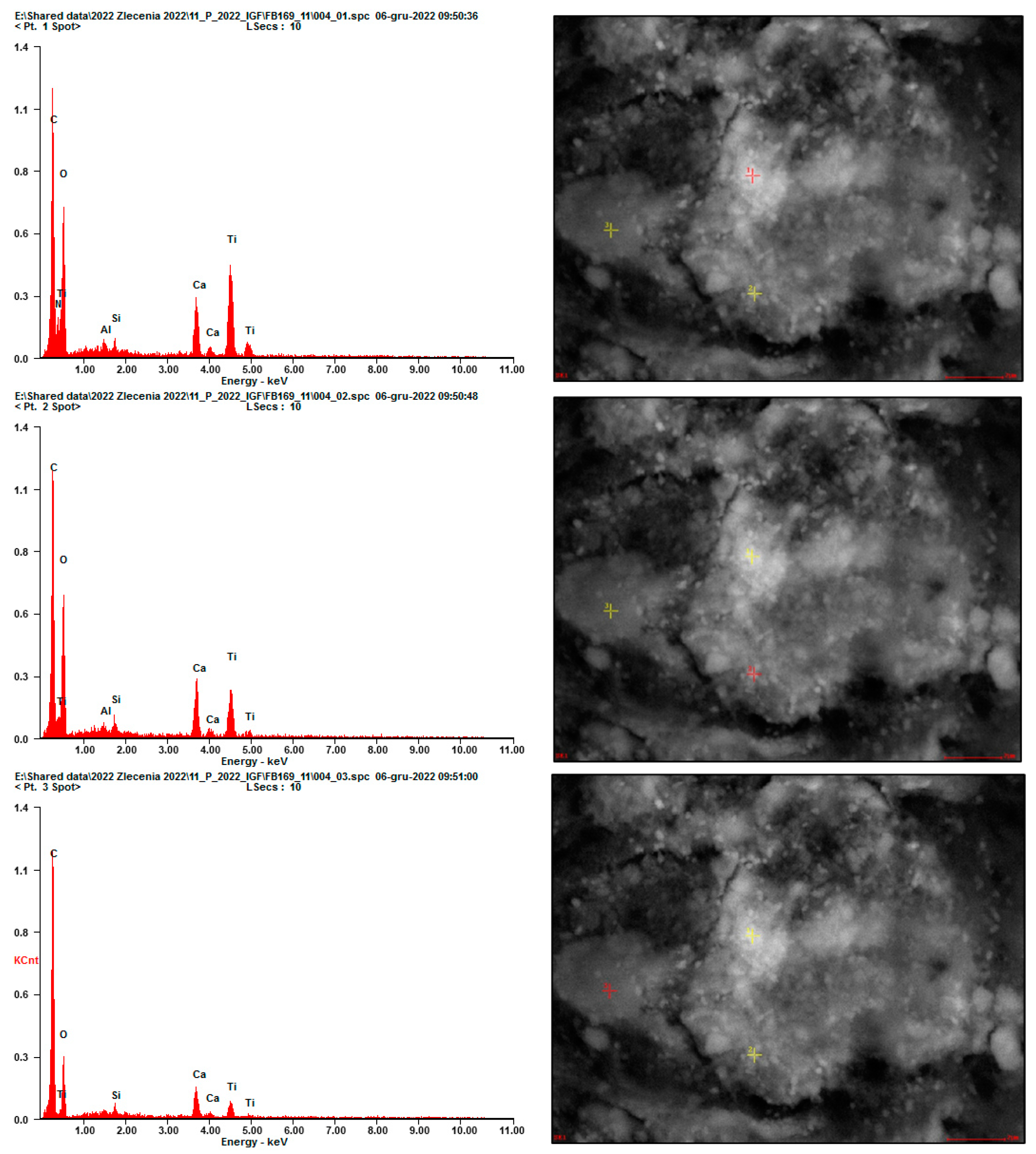
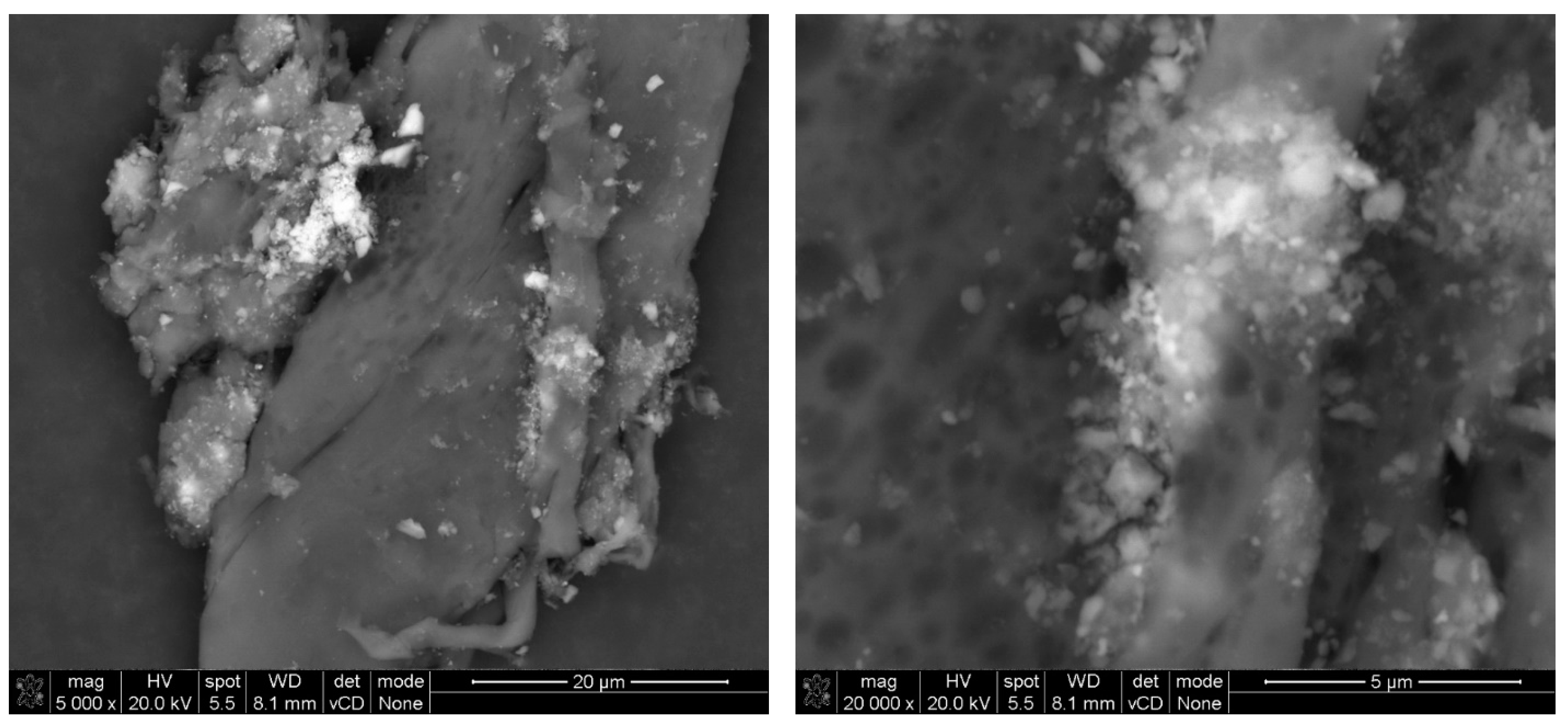
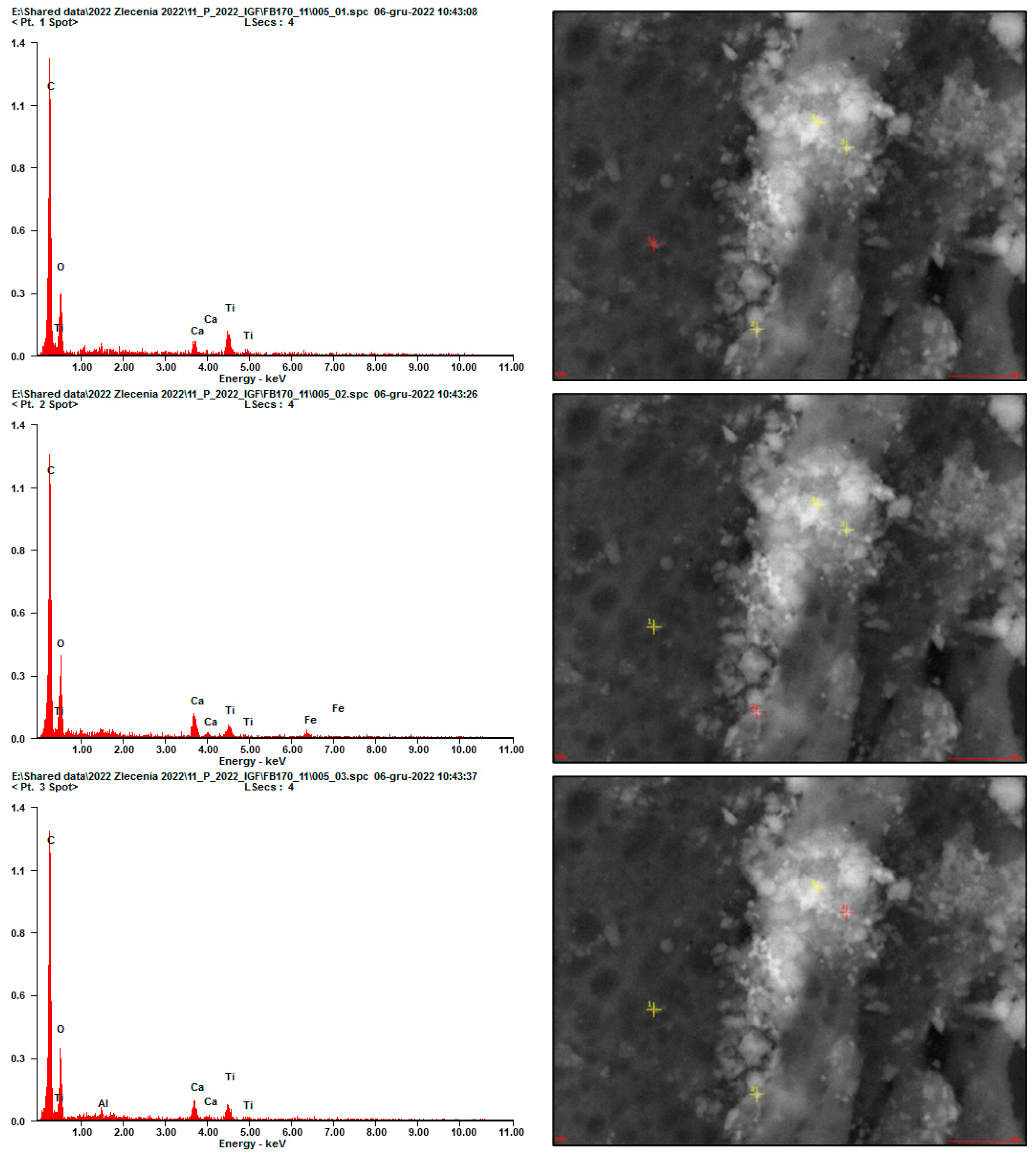
2.2.2. SEM Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dowling, A. Development of nanotechnologies. Mater. Today 2004, 7, 30–35. [Google Scholar] [CrossRef]
- Liang, S.; Xu, F.; Li, W.; Yang, W.; Cheng, S.; Yang, H.; Chen, J.; Yi, Z.; Jiang, P. Tunable smart mid infrared thermal control emitter based on phase change material VO2 thin film. Appl. Therm. Eng. 2023, 232, 121074. [Google Scholar] [CrossRef]
- Cui, S.; Lu, Y.; Kong, D.; Luo, H.; Peng, L.; Yang, G.; Yang, H.; Xu, K. Laser direct writing of Ga2O3/liquid metal-based flexible humidity sensors. Opto-Electron. Adv. 2023, 6, 220172. [Google Scholar] [CrossRef]
- Yalagala, B.P.; Dahiya, A.S.; Dahiya, R. ZnO nanowires based degradable high-performance photodetectors for eco-friendly green electronics. Opto-Electron. Adv. 2023, 6, 220020. [Google Scholar] [CrossRef]
- Kawashima, S.; Corr, P.H.D.J.; Shah, S.P. Modification of cement–based materials with nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef]
- Dudek, D.; Janus, M. Photoactive Cements: A Review. Materials 2022, 15, 5407. [Google Scholar] [CrossRef]
- Boonen, E.; Akylas, V.; Barmpas, F.; Boréave, A.; Bottalico, L.; Cazaunau, M.; Chen, H.; Daële, V.; De Marco, T.; Doussin, J.F.; et al. Construction of a photocatalytic de-polluting field site in the Leopold II tunnel in Brussels. J. Environ. Manag. 2015, 155, 136–144. [Google Scholar] [CrossRef]
- Guerrini, G.L. Photocatalytic performance in a city tunnel in Rome: NOx monitoring results. Constr. Build. Mater. 2021, 27, 165–175. [Google Scholar] [CrossRef]
- Georeg, C.; Beeldens, A.; Barmapss, F.; Doussin, J.-F.; Manganelli, G.; Herrmann, H.; Kleffmann, J.; Mellouki, A. Impact of photocatalytic remediation of pollutants on urban air quality. Front. Environ. Sci. Eng. 2016, 10, 2. [Google Scholar] [CrossRef]
- Martinez, T.; Dompoint, D.; Bertron, A.; Escadeillas, G.; Ringot, E. Photocatalytic coatings for building materials: Degradation of NOx and inhibition of algal growth. Int. J. 3 R’s 2013, 4, 520–533. [Google Scholar]
- Bertron, T.M.A.; Ringot, E.; Escadillas, G. Degradation of NO using photocatalytic coatings applied to different substrates. Build. Environ. 2011, 46, 1808–1816. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hong, S.J.; Kim, H.B.; Lee, S.W. Evaluation of In-Situ NOx Removal Efficiency of Photocatalytic Concrete in Expressways. KSCE J. Civ. Eng. 2018, 22, 2274–2280. [Google Scholar] [CrossRef]
- Maggos, T.; Plassais, A.; Bartzis, J.G.; Vasilakos, C.; Moussiopoulos, N.; Bonafous, L. Photocatalytic degradation of NOx in pilot street canyon configuration using TiO2-mortar panels. Environ. Monit. Assess. 2008, 136, 35–44. [Google Scholar] [CrossRef]
- Guerrini, G.L.; Beeldens, A.; Crispino, M.; D’Ambarosio, G.; Vismaro, S. Environmental benefits of innovative photocatalytic cementitous road material. In Proceedings of the 10th International Conference on Concrete Pavement, Quebeck, QC, Canada, 8–12 July 2012. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applicaions for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef]
- Witkowski, H.; Jarosławski, J.; Tryfon-Bojarska, A. Application of Photocatalytic Concrete Paving Blocks in Poland-Verification of Effectiveness of Nitric Oxides Reduction and Novel Test Method. Materials 2020, 13, 5183. [Google Scholar] [CrossRef]
- Guerrini, G.L. Some observations regarding in–service preformance Photocatalytic paving block surface. Betonw. Und Fert. Tech. BFT 2009, 5, 16–25. [Google Scholar]
- Witkowski, H.; Jackiewicz-Rek, W.; Chilmon, K.; Jarosławski, J.; Tryfon-Bojarska, A.; Gąsiński, A. Air Purification Performance of Photocatalytic Concrete Paving Blocks after Seven Years of Service. Appl. Sci. 2019, 9, 1735. [Google Scholar] [CrossRef]
- Kalinowski, M.; Chilmon, K.; Jackiewicz-Rek, W.; Rakowski, B. The Influence of Selected Material Variables of Photocatalytic Cementitious Composites on the Self-Cleaning Properties and Air Purification Efficiency from NOx Pollutants. Sustainability 2023, 15, 853. [Google Scholar] [CrossRef]
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent Progress in Nanomaterials for Modern Concrete Infrastructure: Advantages and Challenges. Materials 2019, 12, 3548. [Google Scholar] [CrossRef]
- Shah, S.N.A.; Shah, Z.; Hussain, M.; Khan, M. Hazardous Effects of Titanium Dioxide, Nanoprticles in Ecosystem. Bioinorg. Chem. Appl. 2017, 2017, 4101735. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends and Future Perspectives. Nanomicro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano–Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2015, 172, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Szwajger, D.; Oleszczuk, P. Winiarska-Mleczan. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Kalinowski, M.; Woyciechowski, P.; Jackiewicz-Rek, W. Surface Modification of Photocatalytic Cementitious Composites with Polyacrylic Superabsorbent Polymers (SAP). Mater. Proc. 2023, 13, 23. [Google Scholar] [CrossRef]
- Dylla, H.; Hassan, M.M. Characterization of nanparticles released during construction of photocatalytic pavements using nanoparticles. J. Nanoparticle Res. 2012, 14, 825. [Google Scholar] [CrossRef]
- Bossa, N.; Chaurand, P.; Levard, C.; Borschneck, D.; Miche, H.; Vicente, J.; Geantet, C.; Aguerre-Chariol, O.; Michel, F.M.; Rose, J. Environmental exposure to TiO2 nanomaterials incorporated in building material. Environ. Pollut. 2017, 220, 1160–1170. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A.; Dirkx, I.; Bams, V. Durability of Cementitious Photocatalytic Building Materials. Catal. Today 2017, 287, 196–202. [Google Scholar] [CrossRef]
- ISO 10545-7:1999; Ceramic Tiles—Part 7: Determination of Resistance to Surface Abrasion for Glazed Tiles. International Organization for Standarization: Geneve, Switzerland, 1999.
- EN 1338:2003; Concrete Paving Blocks—Requirements and Test Methods, ANNEX D: Determination of Freeze/Thaw Resistance with De-Icing Salt + ANNEX H: Measuring of Abrasion According to the Böhme Test. European Committee for Standardization: Brussels, Belgium, 2003.
- EN 197-1; Cement Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- CEN-EN 13263-1; Silica Fume for Concrete. Definitions, Requirements and Conformity Criteria. European Committee for Standardization: Brussels, Belgium, 2009.
- ISO/DIS 3262-13:2023; Specifications and Methods of Test. Part 13: Natural Quartz (Ground). International Organization for Standarization: Geneve, Switzerland, 2023.
- EN 13139:2003; Aggregates for Mortars. European Committee for Standardization: Brussels, Belgium, 2003.
- Witkowski, H.; Jackiewicz-Rek, W.; Jarosławski, J.; Chilmon, K.; Szkop, A. Ozone Formation during Photocatalytic Oxidation of Nitric Oxides under UV Irradiation with the Use of Commercial TiO2 Photocatalytic Powders. Materials 2022, 15, 5905. [Google Scholar] [CrossRef] [PubMed]
- EN 1008:2002; Mixing Water for Concrete. Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, as Mixing Water for Concrete. European Committee for Standardization: Brussels, Belgium, 2002.
- EN 934-2+A1:2012; Admixtures for Concrete, Mortar and Grout—Part 2: Concrete Admixtures for Masonry Mortar—Definitions, Requirements, Conformity and Marking and Labelling. European Committee for Standardization: Brussels, Belgium, 2012.
- EN 196-1:2016-07; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- Elyasigorji, F.; Farajiani, F.; Hajipour Manjili, M.; Lin, Q.; Elyasigorji, S.; Farhangi, V.; Tabatabai, H. Comprehensive Review of Direct and Indirect Pozzolanic Reactivity Testing Methods. Buildings 2023, 13, 2789. [Google Scholar] [CrossRef]
- Bagheri, A.; Samea, S.A. Role of non-reactive powder in strength enhancement of foamed concrete. Constr. Build. Mater. 2019, 203, 134–145. [Google Scholar] [CrossRef]
- Sudhanshu, S.P.; Gaurang, R.V. Effect of nano TiO2 on mechanical properties and microstructure of concrete. Mater. Today Proc. 2022, 65, 1915–1921. [Google Scholar] [CrossRef]
- Yang, F.; Xie, J.; Wang, W.; Wang, W.; Wang, Z. The role of nano-TiO2 on the mechanical properties and hydration behavior of C2S, C3S and C4A3S. Constr. Build. Mater. 2023, 388, 131558. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Yu, K.; Du, H.; Sioutas, C.; Wang, Q. Mechanics of abrasion-induced particulate matter emission. J. Mech. Phys. Solids 2024, 188, 105661. [Google Scholar] [CrossRef]
- Chen, K.; Qu, F.; Huang, Y.; Cai, J.; Wu, F.; Li, W. Advancing photocatalytic concrete technologies in design, performance and application for a sustainable future. Adv. Nanocomposites 2024, 1, 180–200. [Google Scholar] [CrossRef]
- Tryba, B.; Prowans, B.; Wróbel, R.J.; Szołdra, P.; Pichór, W. Application of TiO2 Supported on Nickel Foam for Limitation of NOx in the Air via Photocatalytic Processes. Molecules 2024, 29, 1766. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.; Castillo, A.; Castellote, M. Characteristics and efficiency of photocatalytic cementitious materials: Type of binder, roughness and microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, R.; Hu, L.; Li, B.; Chen, W.; Shen, J.; Wu, P.; Fang, J. Studying the mix design and investigaiting the photocatalytic performance of previous concrete containing TiO2-Soaked recycled aggregates. J. Clean. Prod. 2020, 248, 119281. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparision of photocatalytic efficiencies of TiO2 in suspended and immobilised form for the photocatalytic degradation of nitrobenzenesulfonic acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Sirota, V.V.; Vashchilin, V.S.; Ogurtsova, Y.N.; Gubareva, E.N.; Podgornyi, D.S.; Kovaleva, M.G. Structure and photocatalytic properties of the composite coating fabricated by detonation sprayed Ti powders. Ceram. Int. 2024, 50 Pt A, 739–749. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Wang, H.; Yu, H. Facile preparation of photocatalytic exposed aggregate concrete with highly efficient and stable catalytic performance. Chem. Eng. J. 2015, 264, 577–586. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Constr. Build. Mater. 2011, 27, 582–590. [Google Scholar] [CrossRef]
- Si, H.; Zhou, M.; Fang, Y.; He, J.; Yang, L.; Wang, F. Photocatalytic concrete for NOx degradation: Influence factors and durability. Constr. Build. Mater. 2021, 298, 123835. [Google Scholar] [CrossRef]
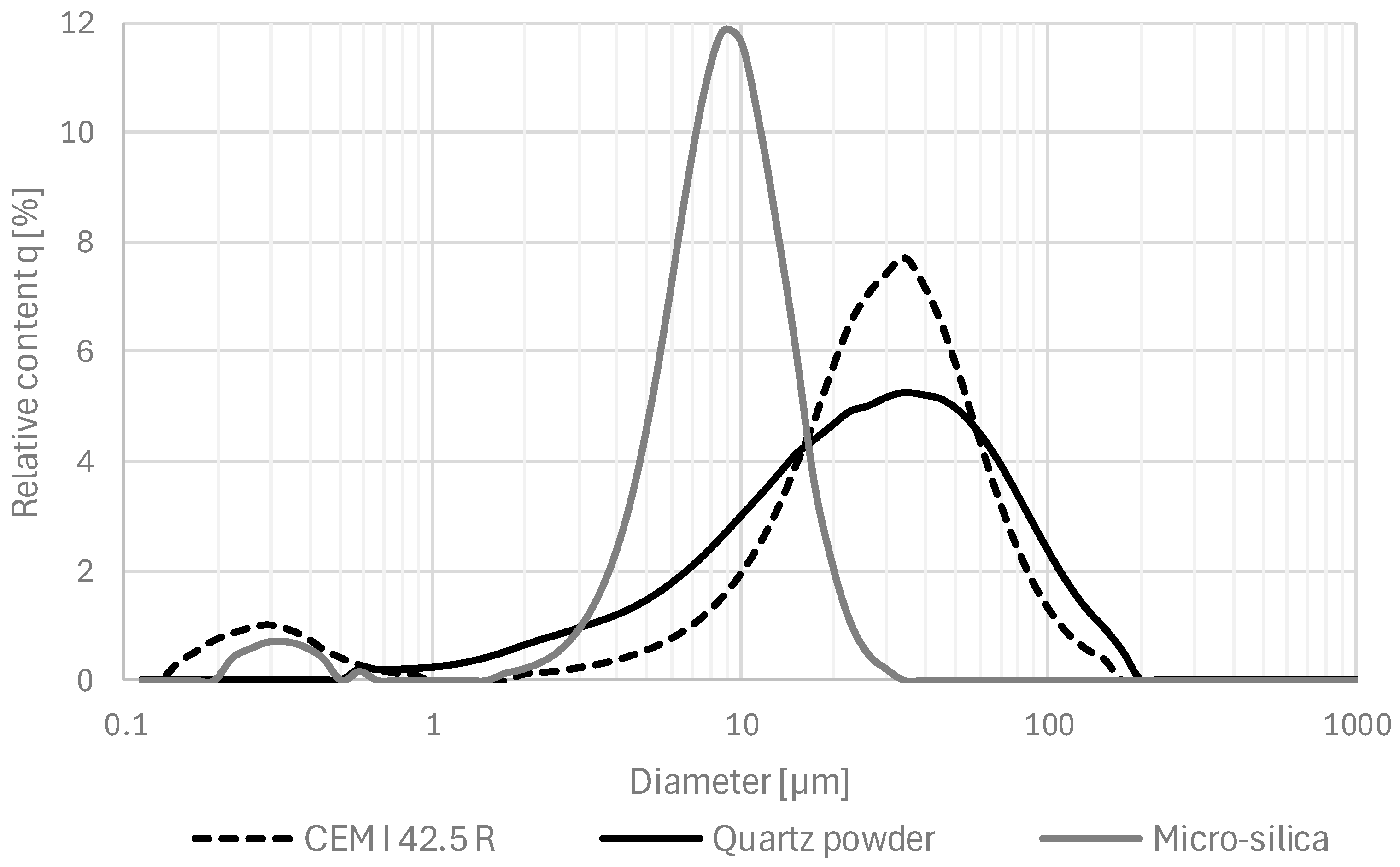


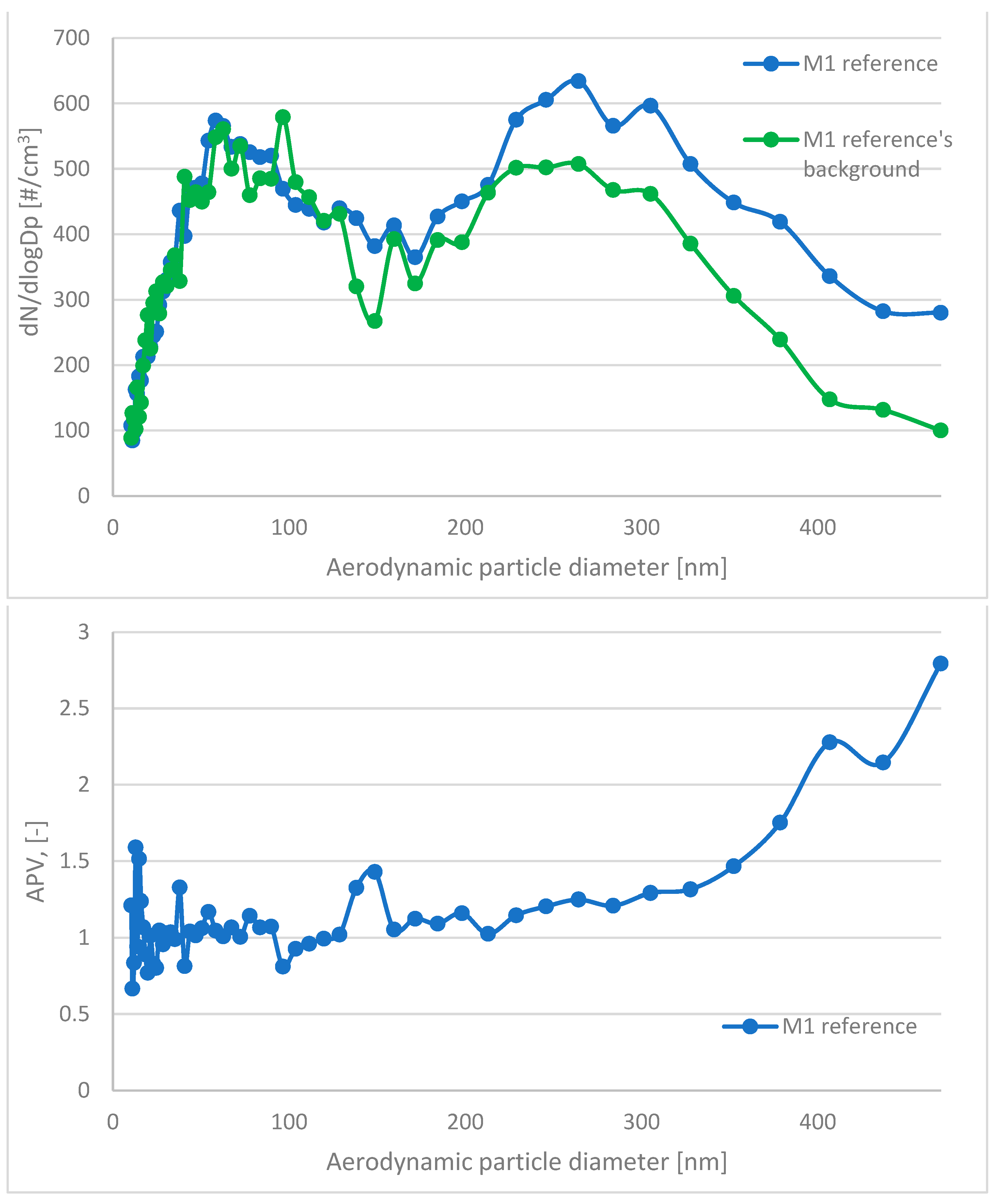
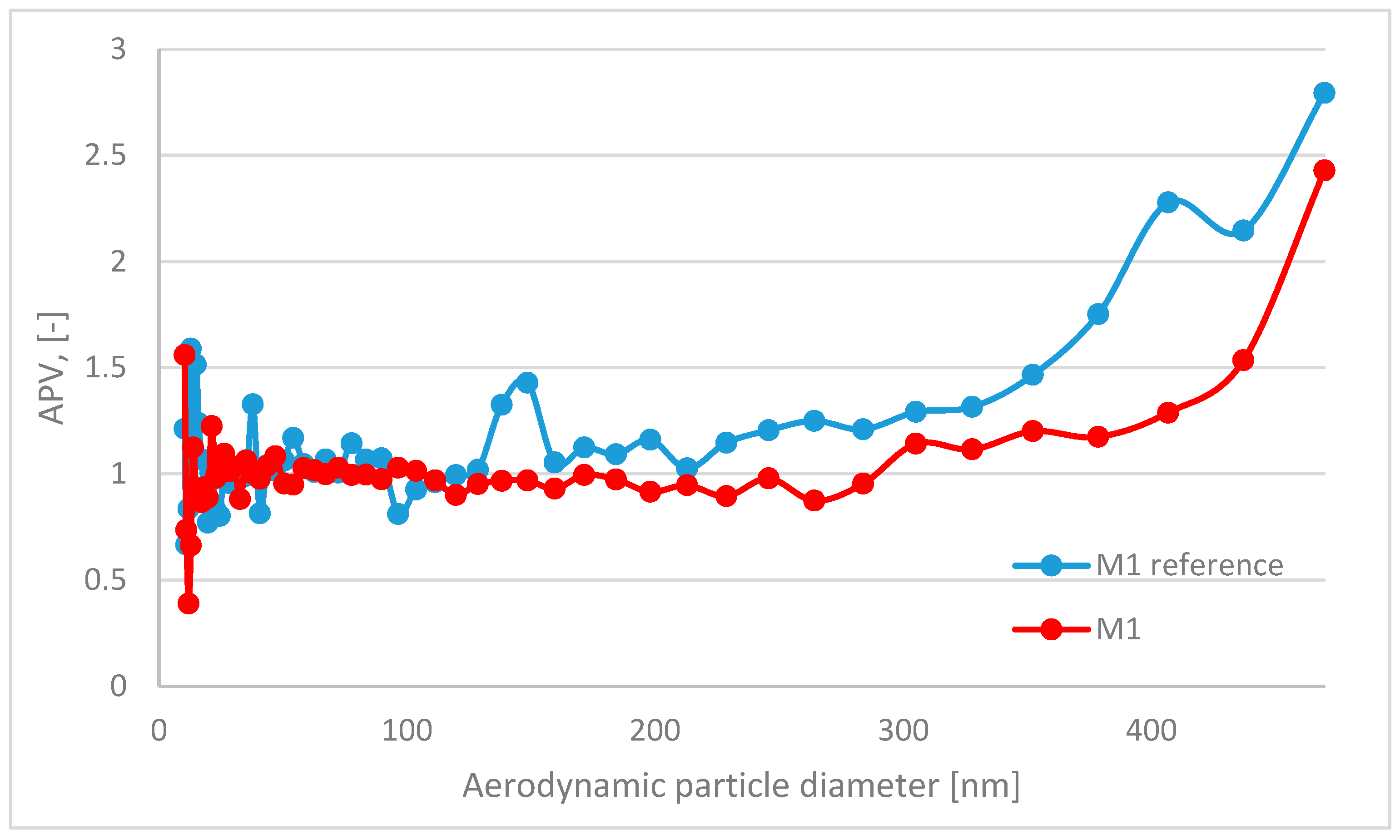


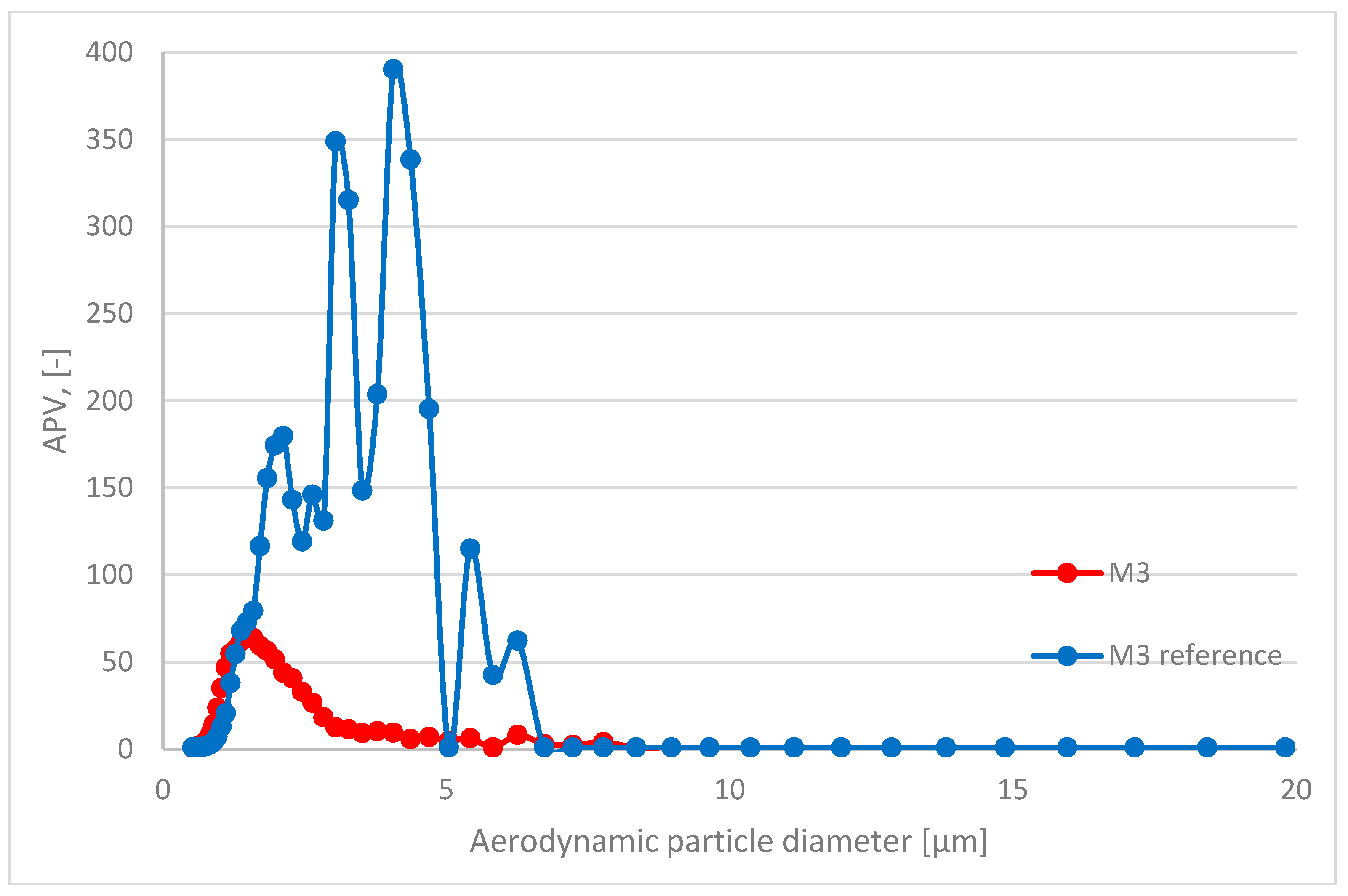

| Component/Series ID | M1 | M2 | M3 | M1 (Ref) | M2 (Ref) | M3 (Ref) |
|---|---|---|---|---|---|---|
| Content (kg/m3) | ||||||
| Cement | 794 | 812 | 837 | 794 | 812 | 837 |
| Silica | 10 | 10 | 10 | 10 | 10 | 10 |
| Quartz powder | 89 | 87 | 84 | 89 | 87 | 84 |
| Water | 286 | 292 | 301 | 286 | 292 | 301 |
| Sand 0.1/0.5 | 424 | 413 | 397 | 424 | 413 | 397 |
| Sand 0.5/1.2 | 679 | 661 | 635 | 679 | 661 | 635 |
| TiO2 (A) | 2.5 | 2.5 | 2.5 | - | - | - |
| TiO2 (B) | 10 | 10 | 10 | - | - | - |
| Superplasticizer | 4.76 | 4.87 | 5.02 | 4.21 | 4.54 | 4.89 |
| Mortar properties | ||||||
| w/c * | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| c/s ** | 0.72 | 0.76 | 0.81 | 0.72 | 0.76 | 0.81 |
| Slump flow, mm | 320 | 310 | 315 | 315 | 315 | 320 |
| Density, kg/m3 | 2245 | 2235 | 2220 | 2240 | 2235 | 2225 |
| Material | Oxide (wt. %) | LOI, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | P2O5 | Fe2O3 | MnO | ||
| Cement | 67.2 | 16.8 | 3.3 | 0.9 | 4.0 | 0.3 | 0.2 | 3.8 | 0.2 | 2.3 |
| Silica | 0.4 | 96.7 | 1.3 | <0.1 | - | <0.1 | <0.01 | 0.2 | <0.01 | 1.1 |
| Quartz powder | 0.1 | 94.3 | 0.2 | 1.2 | - | <0.1 | 0.1 | 0.6 | 0.1 | 2.5 |
| TiO2 (A) | - | 0.2 | - | - | 0.4 | 99.2 | 0.06 | - | - | n.d. * |
| TiO2 (B) | - | 0.7 | - | - | - | 99.1 | - | - | - | n.d. * |
| Photocatalyst | Phase Composition (%) | Size of Crystallites (nm) | Wettability | SSA (m2/g) | ||
|---|---|---|---|---|---|---|
| Rutile | Anatase | Rutile | Anatase | |||
| TiO2 (A) | - | 100 | - | 10 | Hydrophilic | 246.8 +/− 2.9 |
| TiO2 (B) | 13 | 87 | 54 | 33 | Hydrophobic | 53.8 +/− 0.2 |
| APV for Grain Diameter Range 10.4 ÷ 469.8 nm | Standard Deviation for APV in Range 10.4 ÷ 469.8 nm | APV for Grain Diameter Range 0.523 ÷ 19.810 μm | Standard Deviation for APV in Range 0.523 ÷ 19.810 μm | |
|---|---|---|---|---|
| M1 | 1.03 | 0.26 | 18.42 | 28.92 |
| M1 (ref) | 1.17 | 0.37 | 107.18 | 153.61 |
| M2 | 0.98 | 0.17 | 3.31 | 3.53 |
| M2 (ref) | 1.08 | 0.14 | 7.11 | 8.93 |
| M3 | 1.04 | 0.24 | 17.01 | 21.43 |
| M3 (ref) | 1.03 | 0.33 | 71.40 | 102.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowski, H.; Jarosławski, J.; Szkop, A.; Chilmon, K.; Kalinowski, M.; Jackiewicz-Rek, W. The Potential Risk of Nanoparticulate Release from Photocatalytic Pavement Concrete Surface Due to a Simulated Abrasion Load—An Experimental Study. Materials 2024, 17, 3022. https://doi.org/10.3390/ma17123022
Witkowski H, Jarosławski J, Szkop A, Chilmon K, Kalinowski M, Jackiewicz-Rek W. The Potential Risk of Nanoparticulate Release from Photocatalytic Pavement Concrete Surface Due to a Simulated Abrasion Load—An Experimental Study. Materials. 2024; 17(12):3022. https://doi.org/10.3390/ma17123022
Chicago/Turabian StyleWitkowski, Hubert, Janusz Jarosławski, Artur Szkop, Karol Chilmon, Maciej Kalinowski, and Wioletta Jackiewicz-Rek. 2024. "The Potential Risk of Nanoparticulate Release from Photocatalytic Pavement Concrete Surface Due to a Simulated Abrasion Load—An Experimental Study" Materials 17, no. 12: 3022. https://doi.org/10.3390/ma17123022
APA StyleWitkowski, H., Jarosławski, J., Szkop, A., Chilmon, K., Kalinowski, M., & Jackiewicz-Rek, W. (2024). The Potential Risk of Nanoparticulate Release from Photocatalytic Pavement Concrete Surface Due to a Simulated Abrasion Load—An Experimental Study. Materials, 17(12), 3022. https://doi.org/10.3390/ma17123022









