Abstract
Supercritical water gasification (SCWG) technology is highly promising for its ability to cleanly and efficiently convert biomass to hydrogen. This paper developed a model for the gasification of rice straw in supercritical water (SCW) to predict the direction and limit of the reaction based on the Gibbs free energy minimization principle. The equilibrium distribution of rice straw gasification products was analyzed under a wide range of parameters including temperatures of 400–1200 °C, pressures of 20–50 MPa, and rice straw concentrations of 5–40 wt%. Coke may not be produced due to the excellent properties of supercritical water under thermodynamic constraints. Higher temperatures, lower pressures, and biomass concentrations facilitated the movement of the chemical equilibrium towards hydrogen production. The hydrogen yield was 47.17 mol/kg at a temperature of 650 °C, a pressure of 25 MPa, and a rice straw concentration of 5 wt%. Meanwhile, there is an absorptive process in the rice straw SCWG process for high-calorific value hydrogen production. Energy self-sufficiency of the SCWG process can be maintained by adding small amounts of oxygen (ER < 0.2). This work would be of great value in guiding rice straw SCWG experiments.
1. Introduction
With the leapfrogging of global industry, the massive consumption of fossil energy has resulted in increasing emissions to the environment [1]. The Paris Agreement, a climate change agreement signed by 178 parties from around the world, aims to limit the increase in global average temperature to 2 °C over the pre-industrial period and to work towards limiting the temperature increase to 1.5 °C [2]. In response to this global challenge, China has set a dual-carbon goal of achieving carbon peaking by 2030 and carbon neutrality by 2060. The development and utilization of renewable and clean energy resources, such as biomass, to change the way energy is produced and consumed may be key for building a sustainable energy system to achieve the dual-carbon goal.
Biomass, as a renewable energy source with abundant production and easy access, is mainly composed of cellulose, hemicellulose, and lignin [3,4]. Cellulose is a polysaccharide consisting of glucose monomers linked by β (1,4) glycosidic bonds. Hemicellulose consists of various sugar monomers such as xylose, galactose, and glucose, which are easily hydrolyzed. Lignin is a polymer with a three-dimensional network structure formed by three kinds of phenylpropane units connected to each other through ether and carbon–carbon bonds. Rice straw, as a typical representative of agricultural biomass residues, has a global annual production of 800 million tons, of which China is the largest rice producer [5]. The efficient use and conversion of such abundant and accessible biomass resources has been a hot research topic. Resource utilization technologies for biomass include two broad categories: biochemical conversion and thermochemical methods [6,7,8,9]. Biochemical conversion, biogas technology, and hydrolytic fermentation use the metabolism of micro-organisms, such as bacteria, to produce combustible gases or liquid fuels to produce ethanol, hydrogen, etc. However, biochemical conversion has the shortcomings of low conversion efficiency, low end-product yield, difficulty in low-cost scale-up of the conversion process, and the need for secondary treatment of residual substrates. Conventional thermochemical conversion methods also suffer from treatment instability and low conversion efficiency because of the differences in the composition of different biomasses and their high water content. Therefore, there is an urgent need to develop new biomass conversion and utilization technologies.
Recently, the emerging supercritical water gasification (SCWG) technology has taken advantage of the unique physicochemical properties of supercritical water to explore a new highly efficient and clean biomass thermochemical conversion route [10,11,12]. Supercritical water (SCW) is water at temperatures and pressures exceeding 374.2 °C and 22.1 MPa, respectively. The physicochemical properties of supercritical water are significantly different from those of normal water. Supercritical water has a low viscosity and dielectric constant, and its solubility tends to be similar to that of organic solvents while its diffusion coefficient tends to be similar to that of gases [13,14]. The ability of supercritical water to be miscible with organics and gases in any ratio allows reactions to be conducted under homogeneous conditions, greatly facilitating reaction rates [15,16]. Supercritical water gasification biomass technology converts biomass into a highly concentrated hydrogen-rich gas mixture at lower temperatures and reducing conditions, and also avoids the generation of pollutants such as nitrogen compounds, sulfides, and suspended particles during combustion and conventional gasification processes [17,18]. The technology has attracted increasing attention at home and abroad due to many advantages such as high energy conversion efficiency and a wide range of feedstock adaptations.
Yanik et al. [19] conducted experiments using a batch high-pressure reactor at 500 °C with eight different biomasses, such as corn stover and cotton stalks, as feedstock. The amino acid, protein, and oil content of different crops affected the hydrogen yield, which ranged from 4.05 to 4.65 mol/kg of biomass. Williams et al. [20] investigated the SCWG characteristics of cellulose, starch, glucose, and cassava waste with a batch high-pressure reactor. Glucose had the highest hydrogen yield and cassava waste had the lowest. Although both starch and cellulose are polymers of glucose, cellulose produces more hydrocarbons during the gasification process, while starch produces more H2, CO, and oils. Lu et al. [21] used nickel catalysts to achieve efficient gasification of glucose in supercritical water. The addition of Ni/γAl2O3 and Ni/CeO2-γAl2O3 catalysts can significantly improve the hydrogen yield and selectivity, but carbon accumulation and coking may lead to catalyst deactivation. It was noted that Ce in Ni/CeO2-γAl2O3 catalysts had an inhibitory effect on carbon accumulation and coking. Experimental research can provide reliable data to advance technology, but it can also be expensive in terms of time and resources, especially to capture the effects of multiple parameters. The development of appropriate theoretical models may reveal SCWG reaction principles without being constrained by time and physical limitations.
So far, two approaches have been employed for theoretical modeling, namely the stoichiometric (kinetic) approach and the non-stoichiometric (thermodynamic) approach [22,23]. The stoichiometric approach relies on detailed and accurate reaction networks to quantify the reaction process by obtaining the defined reaction parameters. However, the complexity of biomass composition makes it difficult to determine its reaction network in the SCWG process. The non-stoichiometric approach based on the Gibbs free energy minimization principle is more advantageous in predicting the product distribution at reaction equilibrium than the stoichiometric approach. Gibbs free energy, a thermodynamic function introduced as a way of determining the direction in which a process proceeds, is an important concept and method in chemical thermodynamics. It refers to the portion of a system’s reduced internal energy that can be converted to external work in a given thermodynamic process. Tang et al. [24] analyzed the chemical equilibrium of methanol, glucose, cellulose, and real biomass in supercritical water based on the Gibbs free energy minimization principle and the choice of the Peng–Robinson (PM) equation of state. The developed model is a powerful tool for analyzing the SCWG process due to its ability to effectively predict the actual gasification process. Castello et al. [25] calculated the product distribution of biomass in supercritical water at the Gibbs free energy minimum using the PM equation. Total gasification of biomass in supercritical water is possible because coke may not be produced under actual biomass concentrations (<40 wt%) due to thermodynamic constraints.
Although progress has been made in current research on the thermodynamics of supercritical water gasification, more attention needs to be paid to the calculation of the thermodynamics of supercritical water gasification of biomass, especially complex real biomass. It is also attractive to focus on the reaction heat load of biomass supercritical water because the production of hydrogen from multiphase reaction processes at high temperatures and pressures may require heat absorption. The present work developed an SCWG thermodynamic model based on the Gibbs free energy minimization principle using rice straw as a typical representative of real biomass. The product distribution characteristics of rice straw SCWG in the equilibrium process were investigated for different operating parameters (temperature, pressure, and biomass concentration). The heat duty of the rice straw SCWG process and the variation of the calorific value of the products were investigated under different oxygen equivalence ratios.
2. Modeling Approach
2.1. Thermodynamic Equilibrium Method
It is extremely difficult to consider all liquid-phase products in a thermodynamic model because the liquid-phase intermediates of a real biomass supercritical water gasification process are numerous and complex [26,27]. Note that the liquid-phase organic products of gasification generally represent a relatively small mass fraction of the total gasification products, making them less critical and less necessary [28,29,30]. Therefore, the liquid-phase products after gasification were not considered in order to simplify the difficulty of model calculation by choosing H2O, H2, CO, CH4, CO2, and C as the final possible gasification products in this paper. A thermodynamic analytical model for the equilibrium composition of rice straw SCWG products was developed. The relevant physical properties of rice straw are shown in Table 1.

Table 1.
Properties of rice straw (a by difference).
When the chemical reaction reaches equilibrium, the Gibbs free energy of the whole reaction system reaches the minimum. Thus, the following thermodynamic model was established in this paper using the principle of minimum Gibbs free energy (G):
Objective function:
Subject to:
where is the number of atoms of the e element in the t compound; is the number of moles of the t compound when the reaction reaches equilibrium; is the number of moles of the e element in the reactant.
Ortiz et al. [31] used four equations of state, including the Predictive Soave–Redlich–Kwong Equation (PSRK), the Soave–Redlich–Kwong Equation (SRK), the Peng–Robinson Equation (PR), and the Peng–Robinson–Boston–Mathias Equation (PR-BM), for the calculation of supercritical fluid properties. The PR and the PR-BM equations can produce a very good fit. Qi et al. [32] employed the PR and PR-BM equations to perform the calculation of the products of supercritical water gasification equilibrium of black liquor (lignin). The results showed that the product equilibrium calculated by the PR-BM equation was supported by the experimental results. The PR-BM equation, suitable for both nonpolar and weakly polar systems in the supercritical and subcritical regions, is chosen to calculate the physical properties in this paper. The equation has also been widely used by many scholars in the calculation process of relevant thermodynamic parameters [33,34,35].
where v is the mole volume in m3/mol. a and b are the temperature-independent attraction and repulsion parameters, which are calculated by Equations (5) and (6).
where and are the critical temperature in K and critical pressure in Pa, respectively. is a temperature-dependent α-function. The equation for the Boston–Mathias α-function, a piecewise function, can be expressed as Equation (7) at supercritical conditions [36].
where ω is an acentric factor.
The supercritical water gasification process of rice straw was simulated in Aspen plus. An RStoic module and an RGibbs module were used to calculate the product composition. The RStoic module uses a FORTRAN subroutine to decompose rice straw based on its elemental composition. Substances decomposed in the RStoic module finish the SCWG process in the RGibbs module, which is based on the Gibbs free energy minimization principle to predict the product equilibrium composition.
2.2. Model Validation
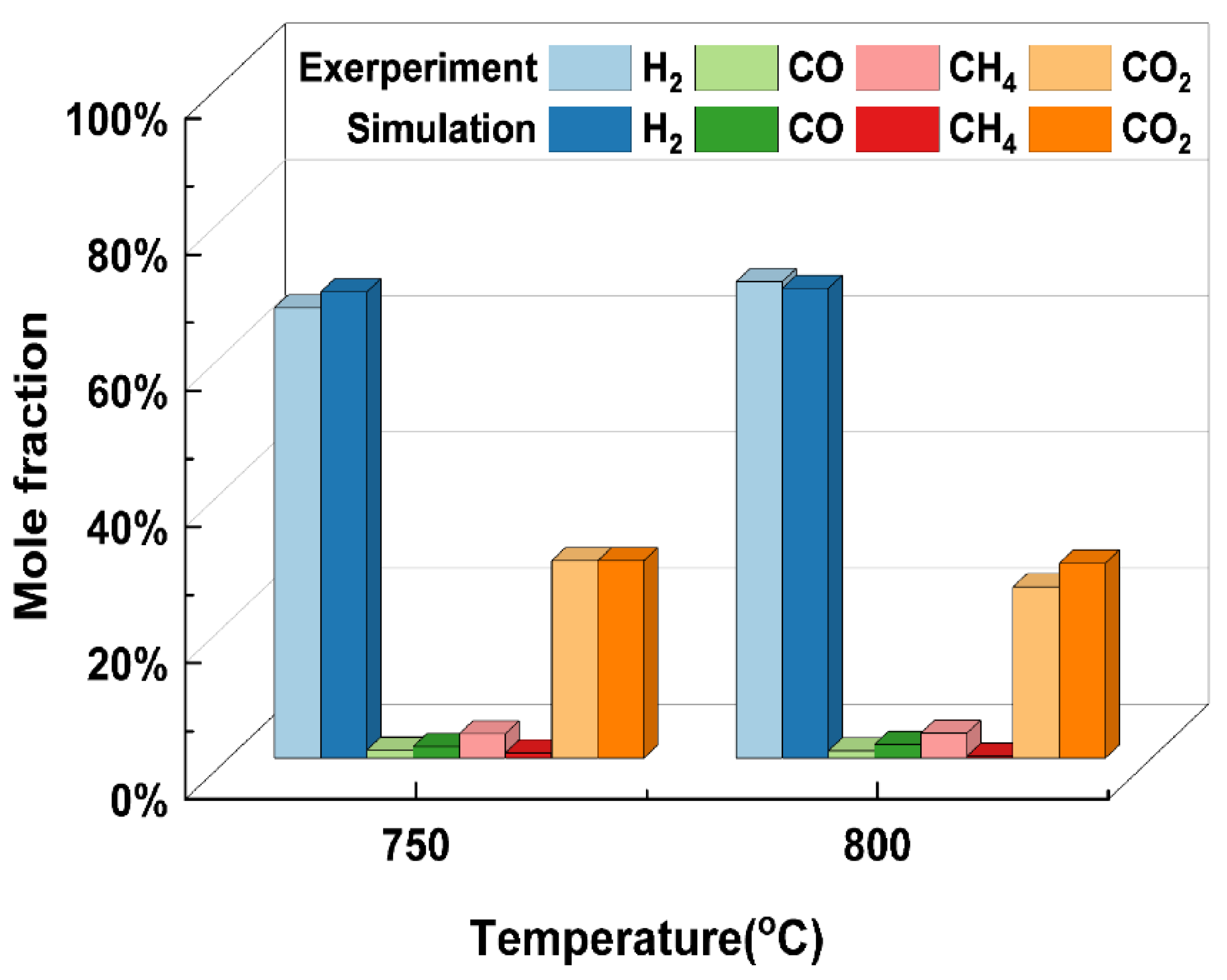
It is first necessary to verify the accuracy of the model before using the thermodynamic model developed above for further analyses of the reliability of the results obtained from the thermodynamic model. Many researchers have carried out supercritical water gasification experiments using glycerol as a model compound for biomass [31,37]. This paper selected glycerol SCWG conducted by Byrd et al. [38] as the material for validation due to the lack of access to experimental work on the SCWG of rice straw. Figure 1 shows that the simulation results can be well-supported by the experimental results to indicate the feasibility of the model. It is worth noting that liquid intermediates are ignored in the SCWG thermodynamic model developed in this paper. Glycerol with a simple structure is easily and totally gasified in supercritical water with few liquid phase products. For real biomass such as rice straw, the SCWG process might generate intermediate products. Therefore, the model may have some limitations, and more experimental data on rice straw need to be obtained for future improvements.
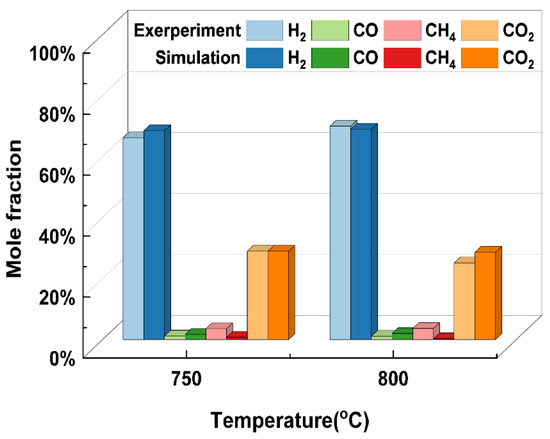
Figure 1.
Comparison of simulation results with the experimental results under different gasification temperatures (glycerol, 24.1 MPa, 5 wt%).
3. Results and Discussion
3.1. Effects of Operating Conditions on SCWG of Rice Straw
The supercritical water gasification process of water rice straw may undergo a series of reactions such as hydrolysis, pyrolysis, liquefaction, and gasification. The steam-reforming reaction, water–gas shift reaction, and methanation reaction during the SCWG of organics are accepted by researchers [39,40,41] as the main reactions, as shown in Equations (11)–(13). Gas molar fraction (14) and gas yield (15) were chosen as indicators to evaluate the gasification results in this paper.
3.1.1. Effect of Temperature
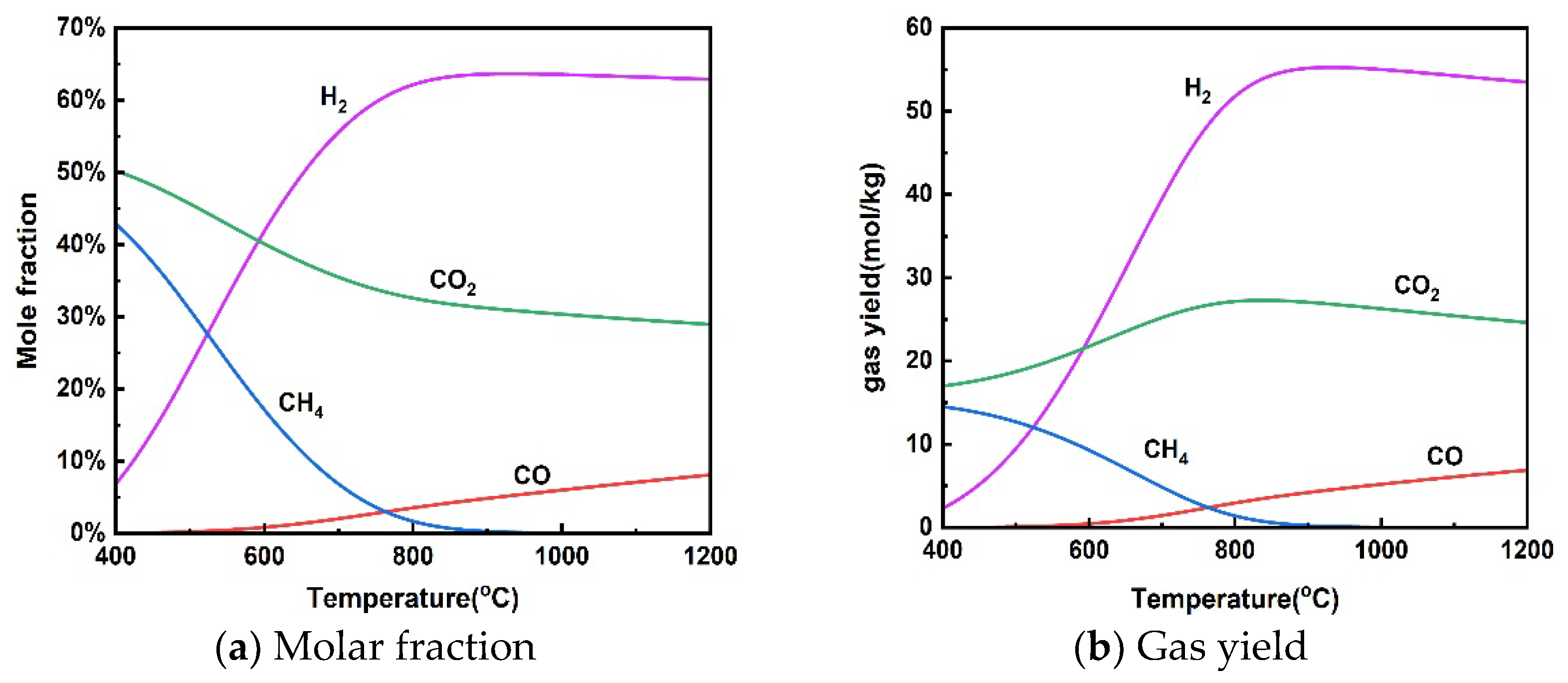
Figure 2 shows the effect of reaction temperatures in the range of 400–1200 °C on gas molar fraction and gas yield at a pressure of 25 MPa and a rice straw concentration of 10 wt%. The effect of temperature on H2 production from the supercritical water gasification of rice straw is roughly divided into two stages, including a rapid increase in H2 at 400–800 °C and a smooth change from 800–1200 °C. Kang et al. [42] found that the average hydrogen production of various biomass SCWGs increased from 0.32 mmol/g to 1.85 mmol/g as the temperature increased from 450–650, but the trend of the average hydrogen production with increasing temperature slowed down when the temperature was higher than 550 °C. There was a significant decrease in CH4 yield in the gasification process and an increase in H2 yield when the temperature was increased from 400 to 800 °C. This change in gas composition can be explained by the properties of the reactions performed in supercritical water gasification. The methanation reaction (13) was inhibited at higher temperatures because it is exothermic. On the other hand, the reforming of methane was enhanced because it is a heat-absorbing reaction. Both processes result in a decrease in CH4 production and an increase in H2 production. Meanwhile, the enhancement of steam reforming (11) of the heat-absorption reaction with increasing temperature leads to an increase in H2 production. In addition, the water–gas shift reaction (12) was also inhibited at high temperatures due to its exothermic properties, reducing the conversion of CO to CO2 and H2. Therefore, CO content increased with increasing temperature. Lu et al. [43] found that temperature has the greatest effect on biomass supercritical water gasification using the orthogonal method because high temperatures support free radical reactions.
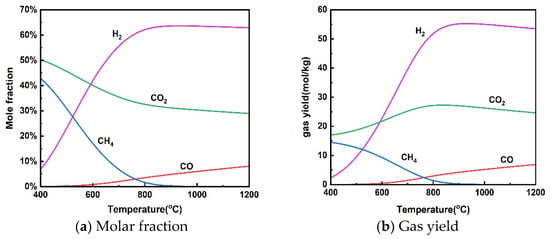
Figure 2.
Effect of temperature on product distribution (25 MPa, 10 wt% rice straw).
It can be seen from Figure 2 that the gas yield does not change much. In particular, H2 yield showed a slight decrease when the temperature was above 800 °C. It is also taken into account that overly high temperatures increase the cost of the gasification process and lead to material limitations. The supercritical water gasification temperature of rice straw should not exceed 800 °C.
3.1.2. Effect of Pressure
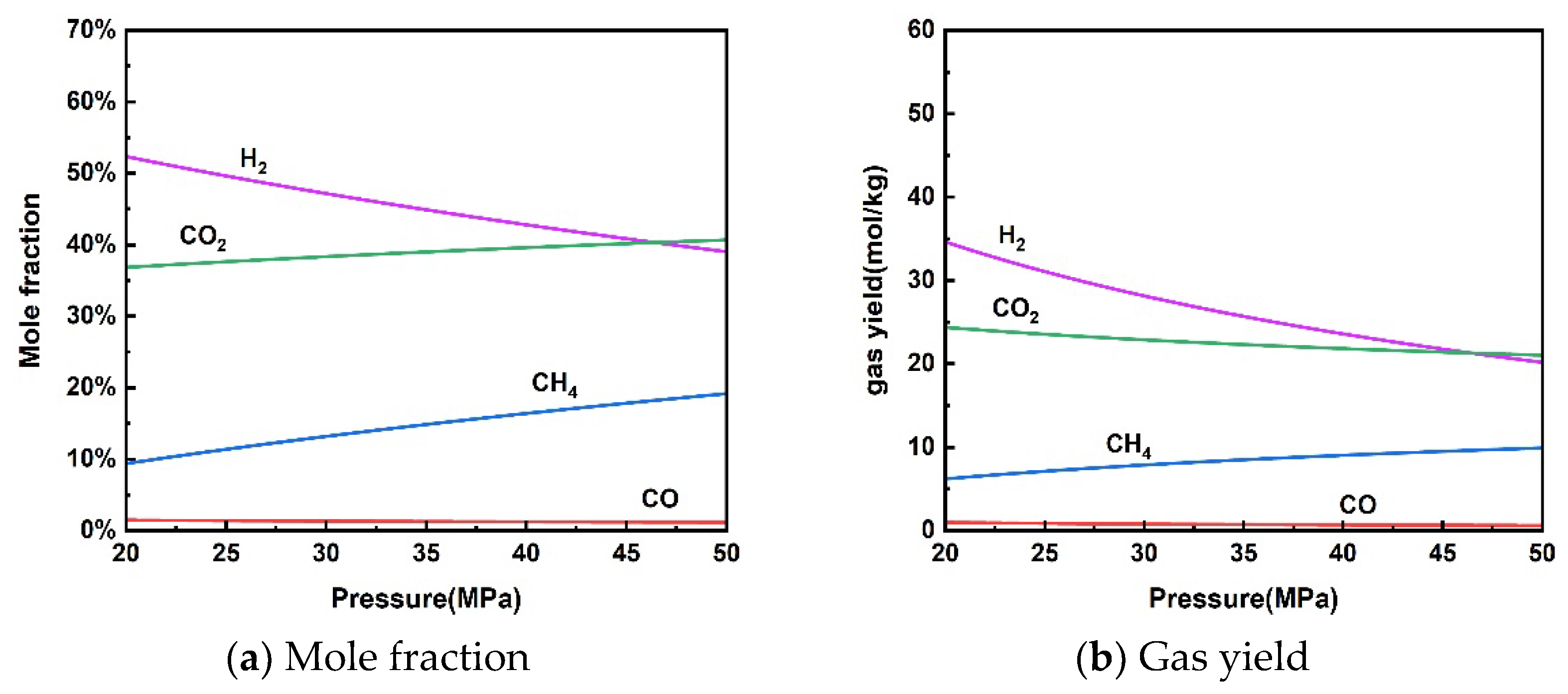
The effect of reaction pressure on the thermodynamic equilibrium of rice straw gasification in supercritical water is presented in Figure 3. As the pressure increased from 20 MPa to 50 MPa, the molar fraction of H2 and gas yield decreased from 52.33% and 34.62 mol/kg to 39.02% and 20.18 mol/kg, respectively. The molar fraction of CH4 and gas yield increased from 9.37% and 6.20 mol/kg to 19.16% and 9.91 mol/kg, respectively. Gas yields of CO and CO2 showed a slight decline. An increase in pressure promotes a shift in gas volume in the direction of decrease according to Le Chatelier’s principle of equilibrium shift. Therefore, the methanation reaction (13) moved in a positive direction making H2 production decrease and CH4 production increase. CO and CO2 changed very little due to the minimal impact on the water–gas shift reaction.
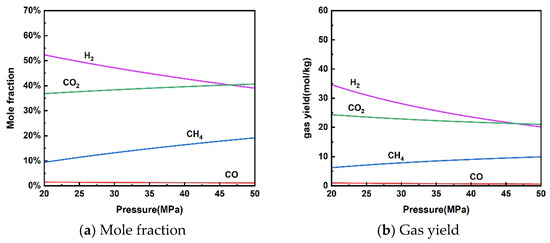
Figure 3.
Effect of pressure on product distribution (650 °C, 10 wt% rice straw).
The effect of pressure on the supercritical water gasification process is more complex due to two competing reactions [30]. Although increased pressure promotes the hydrolysis of organic matter in supercritical water, it also inhibits free radical reactions to produce abundant gases. Experiments by Bai et al. [44] and Fan et al. [45] showed no significant effect of pressure on the supercritical water gasification process. Note that high pressures can increase material costs and operational risks. Therefore, it is only necessary to ensure that the pressure is slightly higher than the critical point to utilize the performance of supercritical water during the actual SCWG process of rice straw.
3.1.3. Effect of Rice Straw Concentration
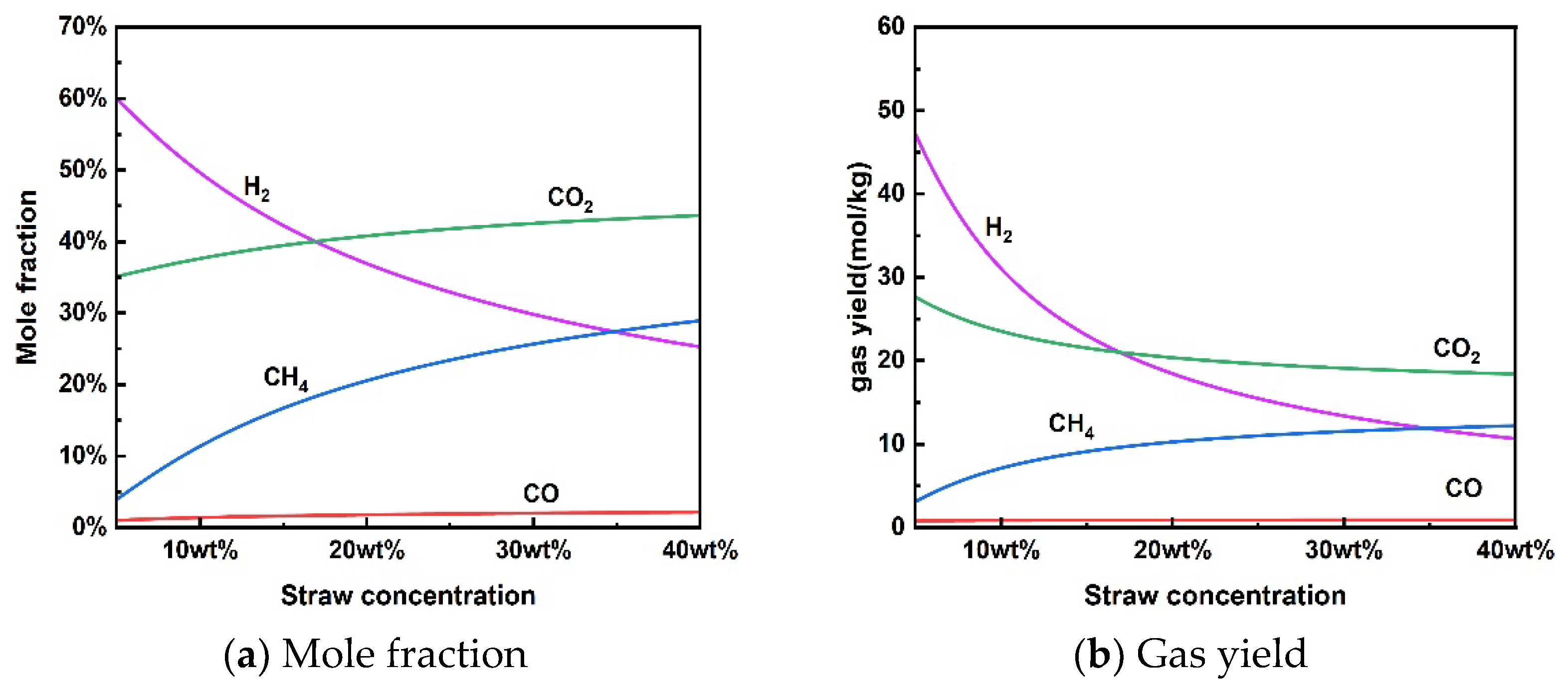
Figure 4 shows the reaction characteristics for the supercritical water gasification of rice straw at different concentrations. It is worth noting that no coke was produced in the concentration range examined under thermodynamic constraints. The results showed that the increase in concentration also had an inhibitory effect on H2 production by supercritical water gasification, which decreased the mole fraction and gas yield of H2. The opposite trend was observed for the molar fraction and gas yield of CH4. The increase in concentration increased the CH4 content in the produced gas. For a given temperature of 650 °C and pressure of 25 MPa, H2 was the most abundant gas at lower rice straw concentrations (<17 wt%). The gas yield of CH4 was higher than that of H2 when the concentration of rice straw exceeded 35 wt%. Thus, the increase in high rice straw concentration may facilitate the methanation reaction for the conversion of H2 to CH4 under thermodynamic constraints [46]. Huelsman et al. [47] investigated the gasification mechanism of different concentrations of phenol in supercritical water. High concentrations of phenol reduced hydrogen yield due to polymerization reactions, leading to deposition and coking. From a kinetic point of view, less water in the reactants may limit the contact between the material particles and water to reduce the reaction rate of SCWG, which is not conducive to total gasification [48].
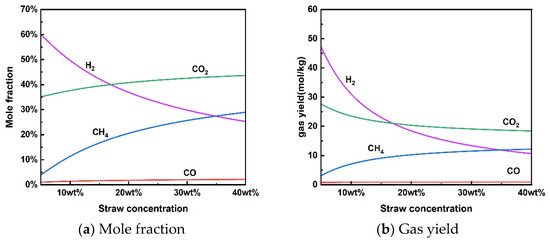
Figure 4.
Effect of rice straw concentration on product distribution (650 °C, 25 MPa).
Although rice straw SCWG can increase the treatment capacity at high rice concentrations, the steam-reforming reaction for hydrogen production is inhibited. At the same time, high-concentration rice straw slurry pulping is also a challenge due to the need for better fluidity and homogeneity in the input system. From the point of view of hydrogen production, the concentration of rice straw for supercritical water gasification of rice straw may not exceed 17 wt% at a temperature of 650 °C and a pressure of 25 MPa.
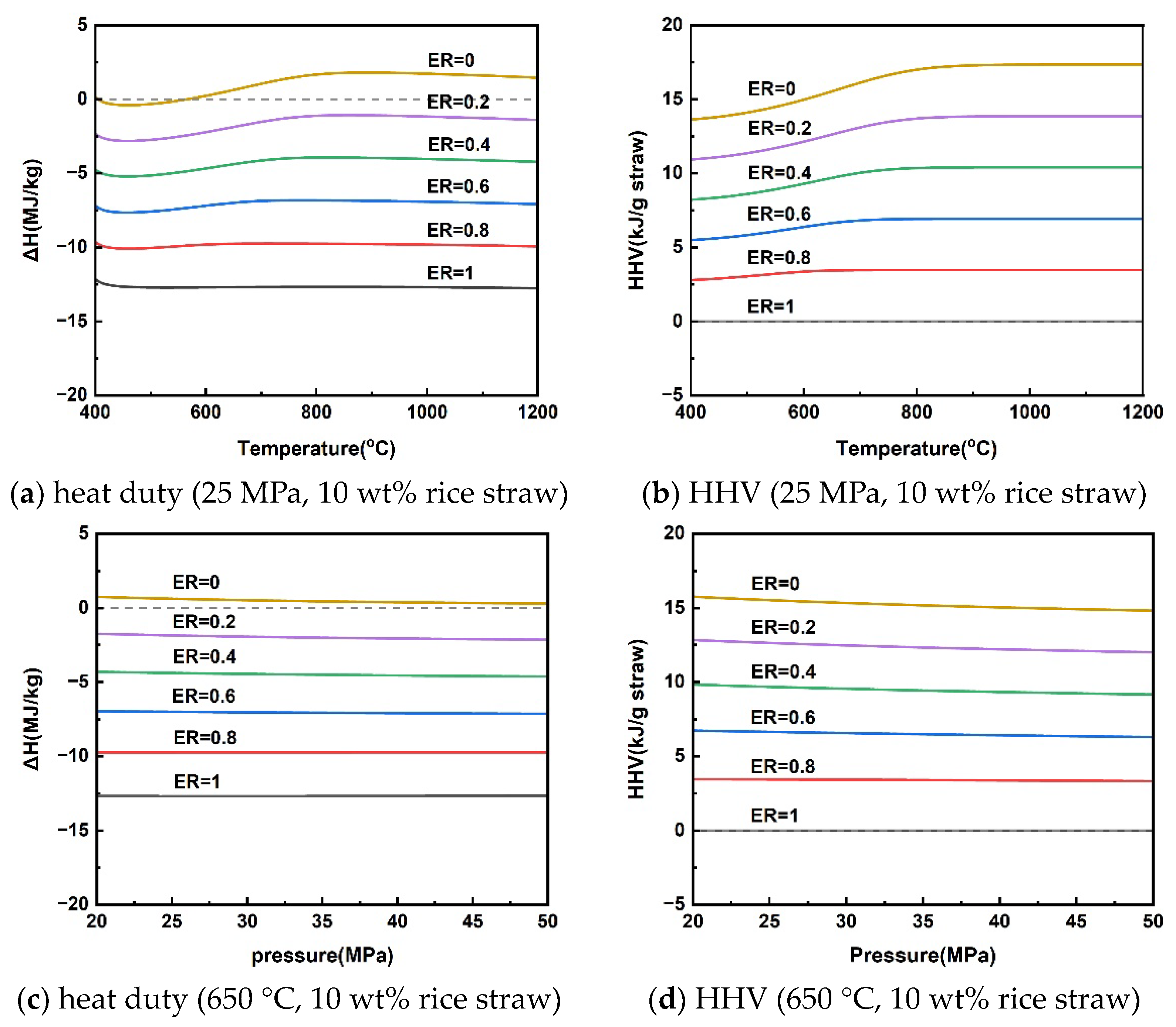
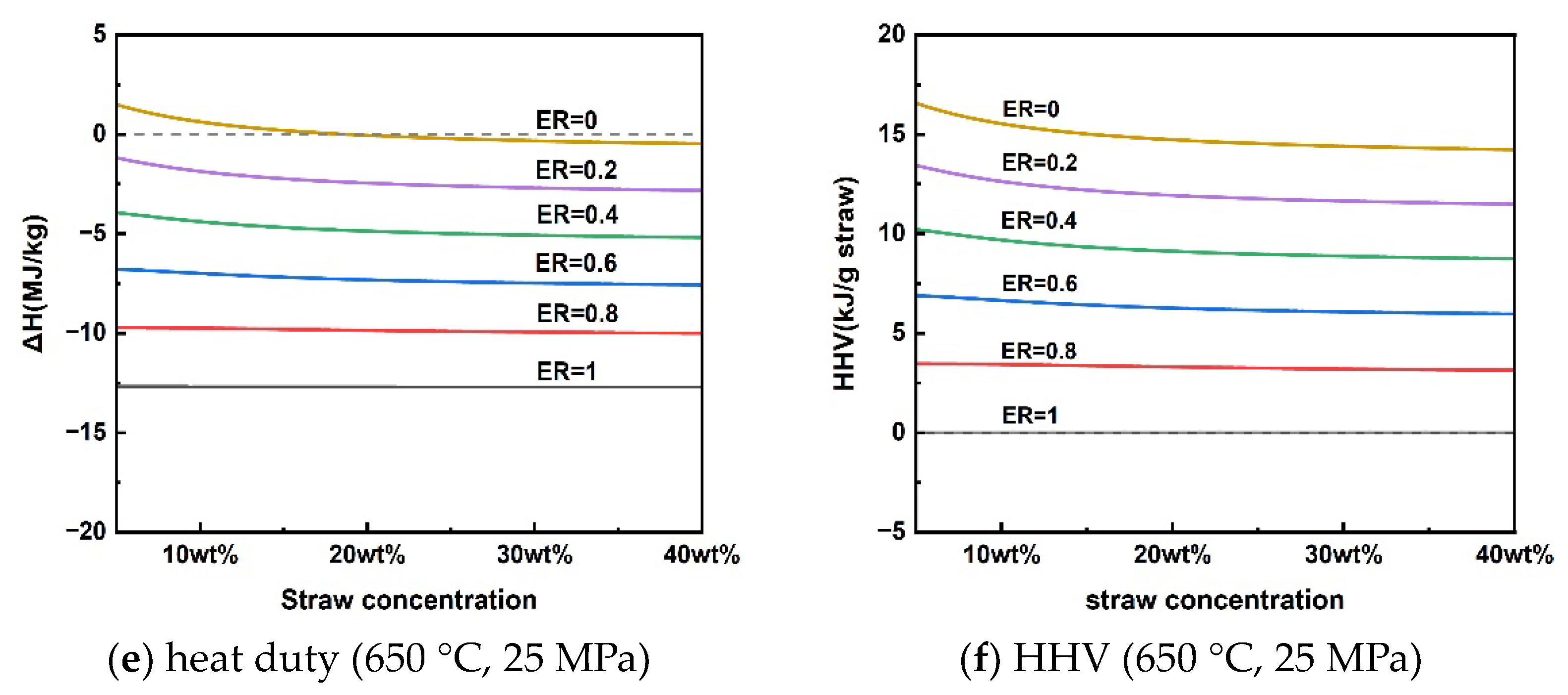
3.2. Analysis of Reaction Heat Duty and Higher Heating Value (HHV) of Gaseous Products
Clarifying the energy changes in the rice straw SCWG gasification process can facilitate energy matching to accelerate the reaction process, of which the reaction heat duty is an important indicator. Consumption of some of the hydrogen-rich gas can provide energy to the gasification process, as shown in Equations (16)–(18). This section analyzed the heat duty of the gasification process as well as the HHV of the gaseous products under different variations of reaction parameters by adding different levels of oxygen, i.e., the oxygen equivalence ratio (ER). The ER can be calculated using Equation (19), if the chemical formula of rice straw is assumed to be CxHyOz, according to elemental analysis of mass fractions. The simulation was calculated assuming an isothermal process and focusing only on the reaction process without including the heating process of the material and the preheated water.
The reaction heat duty of rice straw SCWG and the HHV of the gas products at different temperatures for a pressure of 25 MPa and a rice straw concentration of 10 wt% are given in Figure 5a,b. Rice straw supercritical water gasification reaction heat duty was subject to shifts in heat absorption and exothermic properties at different gasification temperatures. Rice straw SCWG was bounded by a reaction temperature of 565 °C; below 565 °C was an exothermic process, and above 565 °C was an absorptive process. This is because the increased gaseous products of high calorific value at high temperatures make it necessary for the rice straw SCWG to absorb more heat. As the pressure increased from 20 MPa to 50 MPa, the rice straw SCWG process was heat-absorbing, showing a decrease in the heat of reaction from 0.75 kJ/g to 0.30 kJ/g. The HHV of the gaseous products also decreased slowly due to the decrease in the production of hydrogen with a high calorific value. Figure 5e,f shows the trends of reaction heat duty and the HHV of gaseous products for rice straw concentrations in the range of 5–40 wt%. The low concentration can favor the production of H2 to increase the HHV of the gas products, making rice straw SCWG a heat-absorbing process, while the SCWG process was exothermic due to the inhibition of hydrogen production when the concentration of rice straw exceeds 18.56 wt%. A similar trend was observed in the results of reaction heat load calculations for the supercritical water gasification of diesel fuel by Xu et al. [49]. This depends mainly on the difference between the calorific value of the gas product and the calorific value of the feedstock in different conditions.
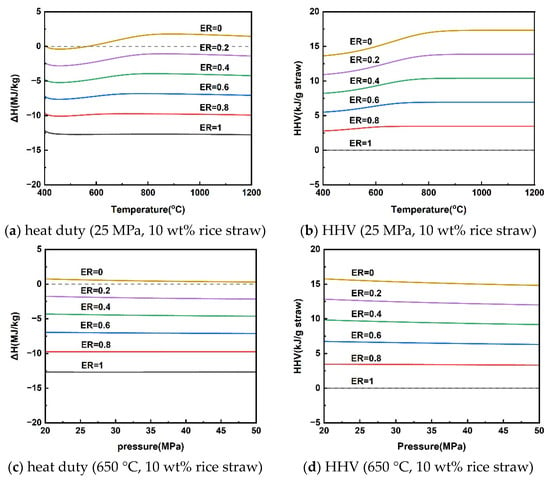
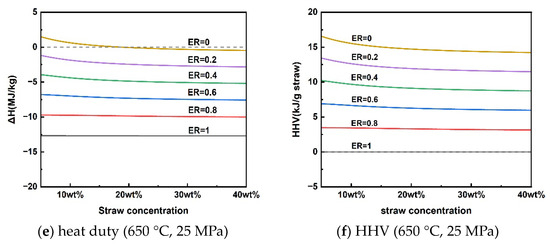
Figure 5.
Reaction heat duty and HHV of gaseous products under operating parameters.
From the above analysis, there is a conversion of heat absorption and exothermic processes in the supercritical water gasification of rice straw. The effect of these operating parameters on the absorptive and exothermic conversion depends mainly on the HHV of the gaseous products, where hydrogen is the most critical because of its high calorific value. In other words, the supercritical water gasification process of rice straw for the purpose of hydrogen production is heat-absorbing. It can be seen from Figure 5 that the conversion of the process from heat absorption to exothermic can be achieved by slightly inputting a little oxygen elimination into the supercritical water gasification process. It means that releasing part of the calorific value of the hydrogen-rich gas can achieve energy self-sufficiency for the SCWG process. The energy self-sufficiency of this approach may effectively reduce the consumption of high-quality electrical energy to sustain the SCWG gasification process [50,51].
4. Conclusions
This paper presented a thermodynamic analysis of the SCWG process for rice straw treatment. The chemical equilibrium calculations for the whole process were based on the Gibbs free energy minimization principle. The PR-BM equation of state was used to describe the physical properties of a substance in a supercritical state. The gas mole fraction, gas yield, reaction heat duty, and HHV of gas products were investigated for rice straw SCWG at temperatures of 400–1200 °C, pressures of 20–50 MPa, and rice straw concentrations of 5–40 wt%. Note that the absence of coke production under the conditions examined proves the feasibility of total gasification. Higher reaction temperatures can increase hydrogen yield and decrease methane yield due to the promotion of the steam-reforming reaction of methane. High reaction pressures may shift the equilibrium towards lower gas production, inhibiting free radical reactions to reduce hydrogen production. The increased concentration of rice straw was not favorable to SCWG for hydrogen production because the reduced water concentration limited the steam-reforming reaction. Rice straw SCWG targeting hydrogen production is overall a heat-absorbing process. Achieving energy self-sufficiency of the rice straw SCWG process can be performed with small amounts of oxygen (ER < 0.2) input by partial consumption of gas products to provide the heat duty required for gasification. Current thermodynamic models usually do not consider liquid-phase intermediates due to their complexity, but the efficient decomposition of intermediates is a bottleneck for total gasification. Future research may identify key kinetic models for the development of intermediates by experimental studies, combining the two models to improve their applicability and predictive robustness.
Author Contributions
Conceptualization, Z.P.; Methodology, Z.L., Z.P., L.Y., L.W. and J.C.; Validation, Z.L., L.W. and J.C.; Investigation, Z.L. and L.Y.; Writing—original draft, Z.L.; Writing—review & editing, Z.L.; Supervision, Z.P., B.C. and L.G.; Funding acquisition, Z.P. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Jiangxi Province major science & technology Research & development project (No. 20223AAG01009), Jiangxi University of Science and Technology (No. 205200100682), and Science and technology innovation project for carbon peak, carbon neutrality of Jiangxi Carbon Neutralization Research Center (No. 2022JXST02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Guo, L.; Jin, H. Boiling coal in water: Hydrogen production and power generation system with zero net CO2 emission based on coal and supercritical water gasification. Int. J. Hydrogen Energy 2013, 38, 12953–12967. [Google Scholar] [CrossRef]
- Heeley, K.; Orozco, R.L.; Macaskie, L.E.; Love, J.; Al-Duri, B. Supercritical water gasification of microalgal biomass for hydrogen production—A review. Int. J. Hydrogen Energy 2024, 49, 310–336. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mayanga, P.C.; Lachos-Perez, D.; Mudhoo, A.; Kumar, S.; Brown, A.B.; Tyufekchiev, M.; Dragone, G.; Mussatto, S.I.; Rostagno, M.A.; Timko, M.; et al. Production of biofuel precursors and value-added chemicals from hydrolysates resulting from hydrothermal processing of biomass: A review. Biomass Bioenergy 2019, 130, 105397. [Google Scholar] [CrossRef]
- Xu, J.; Niu, C.; Zhang, D.; Gen, Y.; Hou, Q.; Xie, Y.; Paul, B. Co-pyrolysis of rice straw and water hyacinth: Characterization of products, yields and biomass interaction effect. Biomass Bioenergy 2019, 127, 105281. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Biomass gasification as an industrial process with effective proof-of-concept: A comprehensive review on technologies, processes and future developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Hussain, S.Z.; Manzoor, S.; Naseer, B.; Taiwo, A.E.; Ayyash, M.; Al-Marzouqi, A.H.; Kamal-Eldin, A. A comprehensive review on the use of deep eutectic solvents for biomass processing, and the synergistic coupling with physical technology and biological method. Bioresour. Technol. Rep. 2023, 23, 101577. [Google Scholar] [CrossRef]
- Patel, B.; Guo, M.; Izadpanah, A.; Shah, N.; Hellgardt, K. A review on hydrothermal pre-treatment technologies and environmental profiles of algal biomass processing. Bioresour. Technol. 2016, 199, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Suriapparao, D.V.; Tejasvi, R. A review on role of process parameters on pyrolysis of biomass and plastics: Present scope and future opportunities in conventional and microwave-assisted pyrolysis technologies. Process Saf. Environ. Prot. 2022, 162, 435–462. [Google Scholar] [CrossRef]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefining 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Peng, Z.; Rong, S.; Xu, J.; Luo, K.; Zhang, J.; Jin, H.; Guo, L. Hydrogen production from oilfield wastewater by gasification in supercritical water with a continuous system. Fuel 2023, 344, 128094. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; He, C.; Liu, J.; Zhang, R.; Chen, Q. Oily sludge treatment in subcritical and supercritical water: A review. J. Hazard. Mater. 2022, 433, 128761. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Peng, Z.; Rong, S.; Xu, J.; Jin, H.; Zhang, J.; Shang, F.; Guo, L. Reaction pathways and kinetics for hydrogen production by oilfield wastewater gasification in supercritical water. Fuel 2022, 314, 123135. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A review. Renew. Sustain. Energy Rev. 2020, 118, 109529. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Cui, D.; Bai, J.; Wang, Z.; Xu, F.; Wang, Z. Advances in supercritical water gasification of lignocellulosic biomass for hydrogen production. J. Anal. Appl. Pyrolysis 2023, 170, 105934. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Y.; Song, Y.; Xu, A.; Zhu, G.; Jiang, J.; Hantoko, D. Comprehensive experimental study on energy conversion of household kitchen waste via integrated hydrothermal carbonization and supercritical water gasification. Energy 2022, 242, 123054. [Google Scholar] [CrossRef]
- Yanik, J.; Ebale, S.; Kruse, A.; Saglam, M.; Yuksel, M. Biomass gasification in supercritical water: Part 1. Effect of the nature of biomass. Fuel 2007, 86, 2410–2415. [Google Scholar] [CrossRef]
- Williams, P.T.; Onwudili, J. Subcritical and Supercritical Water Gasification of Cellulose, Starch, Glucose, and Biomass Waste. Energy Fuels 2006, 20, 1259–1265. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Guo, L. Hydrogen production by supercritical water gasification of glucose with Ni/CeO2/Al2O3: Effect of Ce loading. Fuel 2013, 103, 193–199. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Wang, H.; Han, X.; El-Sayed, H.; Zeng, Y.; Charles Xu, C. Supercritical water gasification of lignocellulosic biomass: Development of a general kinetic model for prediction of gas yield. Chem. Eng. J. 2022, 433, 133618. [Google Scholar] [CrossRef]
- Ruya, P.M.; Purwadi, R.; Lim, S.S. Supercritical water gasification of sewage sludge for power generation– thermodynamic study on auto-thermal operation using Aspen Plus. Energy Convers. Manag. 2020, 206, 112458. [Google Scholar] [CrossRef]
- Tang, H.; Kitagawa, K. Supercritical water gasification of biomass: Thermodynamic analysis with direct Gibbs free energy minimization. Chem. Eng. J. 2005, 106, 261–267. [Google Scholar] [CrossRef]
- Castello, D.; Fiori, L. Supercritical water gasification of biomass: Thermodynamic constraints. Bioresour. Technol. 2011, 102, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Guo, L.; Lu, Y. Thermodynamic analysis of hydrogen production from biomass gasification in supercritical water. Energy Convers. Manag. 2006, 47, 1515–1528. [Google Scholar] [CrossRef]
- Yoshida, T.; Oshima, Y. Partial Oxidative and Catalytic Biomass Gasification in Supercritical Water: A Promising Flow Reactor System. Ind. Eng. Chem. Res. 2004, 43, 4097–4104. [Google Scholar] [CrossRef]
- Kou, J.; Xu, J.; Jin, H.; Guo, L.; Zhang, D.; Cao, W. Evaluation of modified Ni/ZrO2 catalysts for hydrogen production by supercritical water gasification of oil-containing wastewater. Int. J. Hydrogen Energy 2018, 43, 13896–13903. [Google Scholar] [CrossRef]
- Ge, Z.; Jin, H.; Guo, L. Hydrogen production by catalytic gasification of coal in supercritical water with alkaline catalysts: Explore the way to complete gasification of coal. Int. J. Hydrogen Energy 2014, 39, 19583–19592. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, J.; Rong, S.; Zhang, M.; Wang, L.; Jin, H.; Guo, L. Clean treatment and resource utilization of oilfield wastewater using supercritical water gasification. J. Clean. Prod. 2023, 411, 137239. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Ollero, P.; Serrera, A.; Sanz, A. Thermodynamic study of the supercritical water reforming of glycerol. Int. J. Hydrogen Energy 2011, 36, 8994–9013. [Google Scholar] [CrossRef]
- Qi, X.; Chen, Y.; Zhao, J.; Su, D.; Liu, F.; Lu, L.; Jin, H.; Guo, L. Thermodynamic and environmental assessment of black liquor supercritical water gasification integrated online salt recovery polygeneration system. Energy 2023, 278, 127835. [Google Scholar] [CrossRef]
- Mathias, P.M.; Copeman, T.W. Extension of the Peng-Robinson equation of state to complex mixtures: Evaluation of the various forms of the local composition concept. Fluid Phase Equilibria 1983, 13, 91–108. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, L.; Yi, L.; Xu, J.; Liu, Z.; Jin, H.; Chen, B.; Guo, L. Performance assessment of an energetically self-sufficient system for hydrogen production from oilfield wastewater treated by supercritical water gasification. Int. J. Hydrogen Energy 2024, 53, 907–918. [Google Scholar] [CrossRef]
- Najafi, S.M.A.; Ghassemi, H. Supercritical water gasification of a heavy fuel oil. Pet. Sci. Technol. 2018, 36, 675–681. [Google Scholar] [CrossRef]
- Xu, J.; Miao, Q.; Peng, Z.; Liu, S.; Zhou, Y.; Yu, L. Thermodynamic analysis of using CO2 as a co-gasification agent in supercritical water gasification of glycerol. J. Environ. Chem. Eng. 2023, 11, 111359. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L.; Cao, C.; Yin, J.; Lu, Y.; Zhang, X. Hydrogen production from glycerol by supercritical water gasification in a continuous flow tubular reactor. Int. J. Hydrogen Energy 2012, 37, 5559–5568. [Google Scholar] [CrossRef]
- Byrd, A.; Pant, K.; Gupta, R. Hydrogen production from glycerol by reforming in supercritical water over Ru/Al2O3 catalyst. Fuel 2008, 87, 2956–2960. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Saeidi, S.; Lohi, A. A comparative thermodynamic analysis and experimental studies on hydrogen synthesis by supercritical water gasification of glucose. Clean Technol. Environ. Policy 2015, 17, 2267–2288. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Xu, P.; Liu, B.; Shuai, Y.; Li, B. Hydrogen production through biomass gasification in supercritical water: A review from exergy aspect. Int. J. Hydrogen Energy 2019, 44, 15727–15736. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, J.; Rong, S.; Luo, K.; Lu, L.; Jin, H.; Zhao, Q.; Guo, L. Thermodynamic and environmental analysis for multi-component supercritical thermal fluid generation by supercritical water gasification of oilfield wastewater. Energy 2023, 269, 126766. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, L.; Zhang, X.; Ji, C. Hydrogen production by supercritical water gasification of biomass: Explore the way to maximum hydrogen yield and high carbon gasification efficiency. Int. J. Hydrogen Energy 2012, 37, 3177–3185. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Zhang, H.; Zhou, F.; Han, X.; Wang, Q.; Jin, H. Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. Fuel 2020, 262, 116630. [Google Scholar] [CrossRef]
- Fan, Q. Petrochemical Wastewater Gasification with Na2CO3 and K2CO3. Pol. J. Environ. Stud. 2015, 24, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Cheng, Z.; Lu, L.; Guo, L.; Jin, H.; Zhang, D.; Wang, R.; Liu, S. Performance simulation and thermodynamics analysis of hydrogen production based on supercritical water gasification of coal. Int. J. Hydrogen Energy 2021, 46, 28474–28485. [Google Scholar] [CrossRef]
- Huelsman, C.M.; Savage, P.E. Intermediates and kinetics for phenol gasification in supercritical water. Phys. Chem. Chem. Phys. 2012, 14, 2900–2910. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, L.; Guo, J.; Ge, Z.; Cao, C.; Lu, Y. Study on gasification kinetics of hydrogen production from lignite in supercritical water. Int. J. Hydrogen Energy 2015, 40, 7523–7529. [Google Scholar] [CrossRef]
- Xu, J.; Peng, Z.; Rong, S.; Zhao, Q.; Jin, H.; Guo, L.; Zhou, T.; Zhang, X. Optimal retrofit of a novel multi-component supercritical thermal fluid generation system via thermodynamic analysis. Appl. Therm. Eng. 2022, 219, 119511. [Google Scholar] [CrossRef]
- Mokry, S.; Pioro, I.; Kirillov, P.; Gospodinov, Y. Supercritical-water heat transfer in a vertical bare tube. Nucl. Eng. Des. 2010, 240, 568–576. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Rasmussen, E.G.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Supercritical water gasification: Practical design strategies and operational challenges for lab-scale, continuous flow reactors. Heliyon 2019, 5, e01269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).